Arrest of Viral Proliferation by Ectopic Copies of Its Cognate Replication Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial, Plasmids, and Phage Strains
2.2. Bacterial Growth and Phage Proliferation Studies
2.3. Isolation of Intracellular λ DNA
2.4. Detection of Intracellular λ DNA by CsCl Equilibrium Density Gradient Centrifugation
2.5. Electron Microscopy of DNA, Measuring, and Computation of Data
2.6. RNase Treatment of λ Replicative Intermediates
3. Results
3.1. E. coli Harboring a Plasmid Containing the λ Origin of DNA Replication is Resistant to λ Infection
3.2. Phage Proliferation Is Impaired in Bacteria Harboring Ectopic Copies of λOri
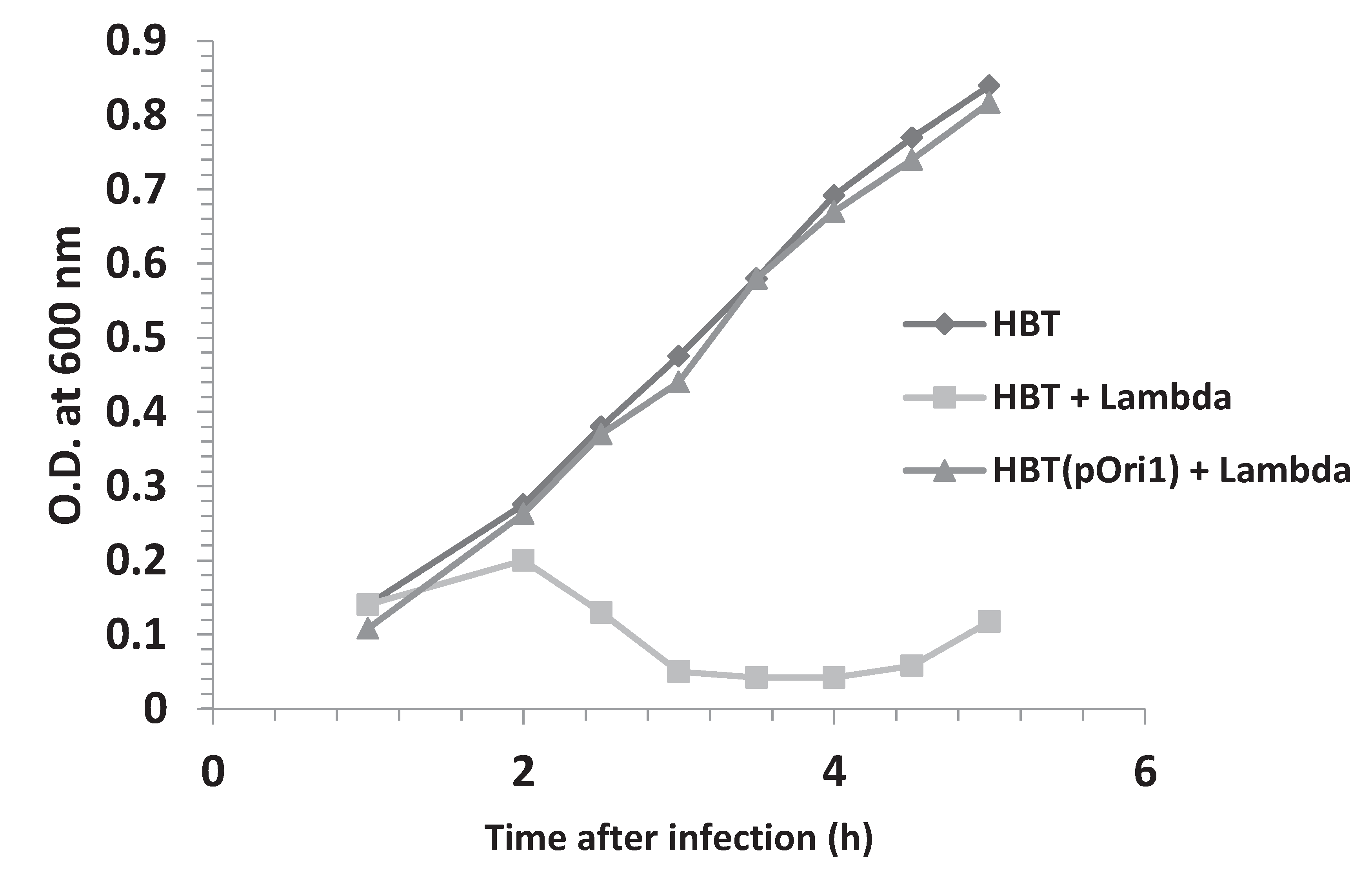

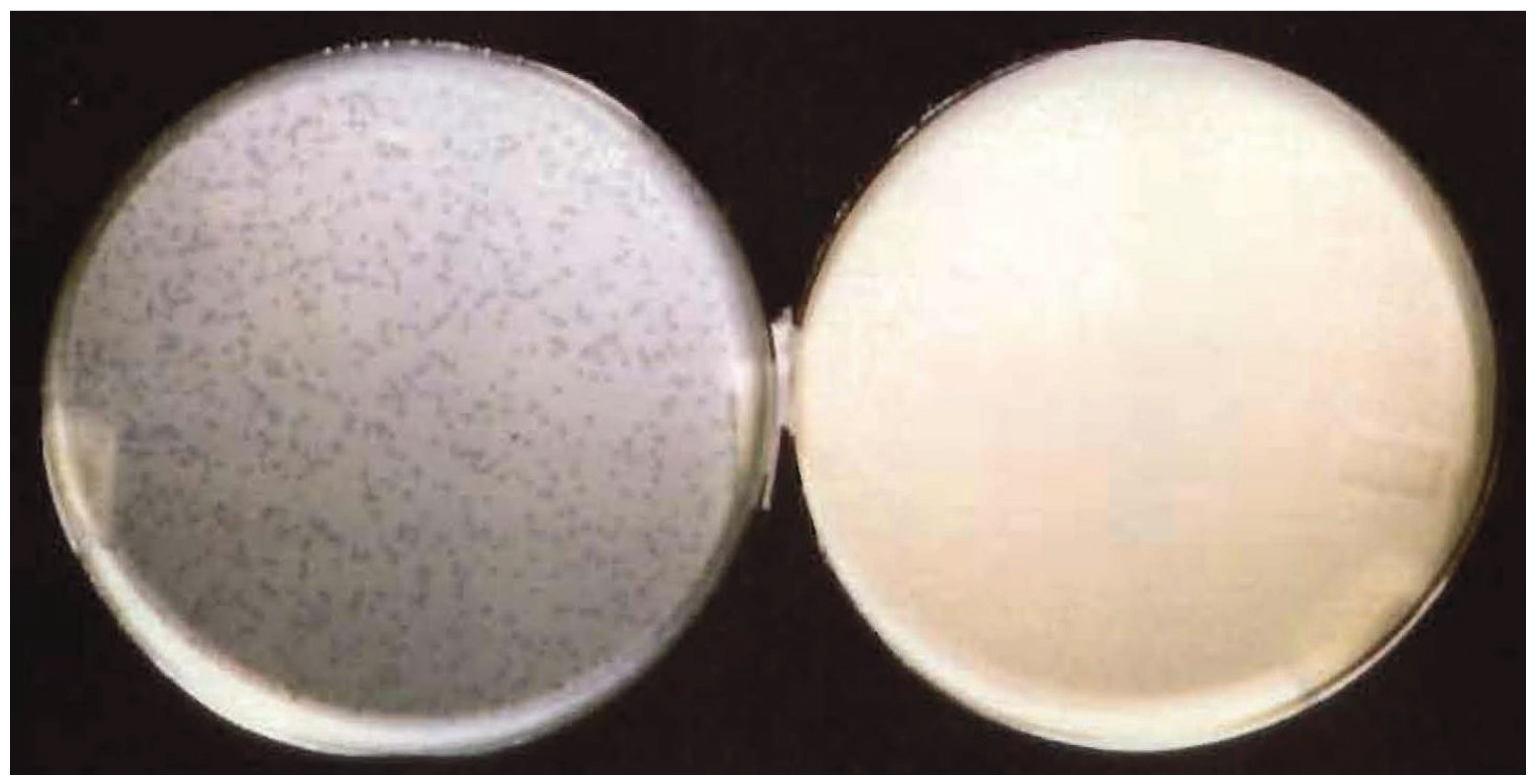
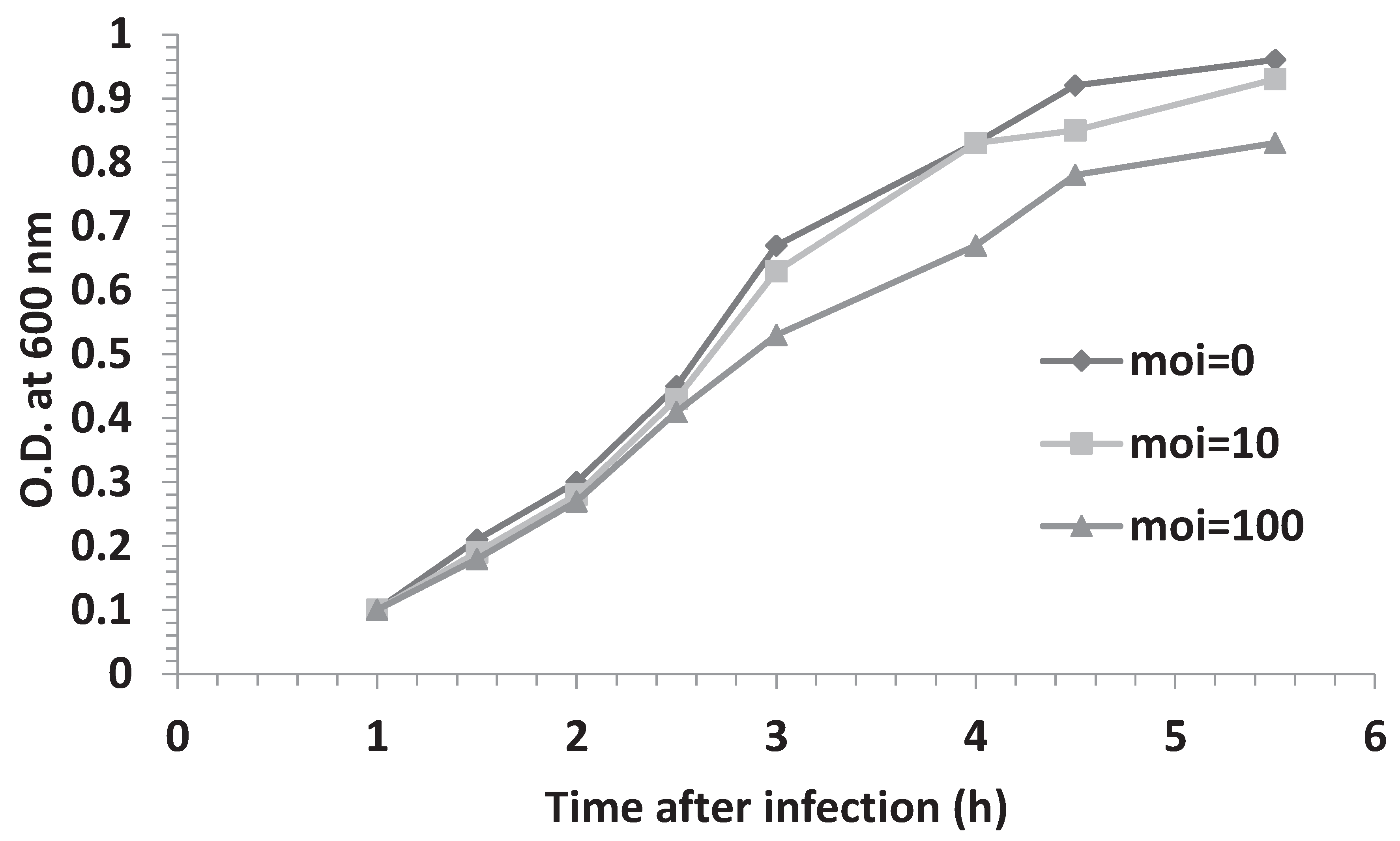
3.3. Effect of Multiplicity of Infection and λOri Variants on Bacterial Resistance
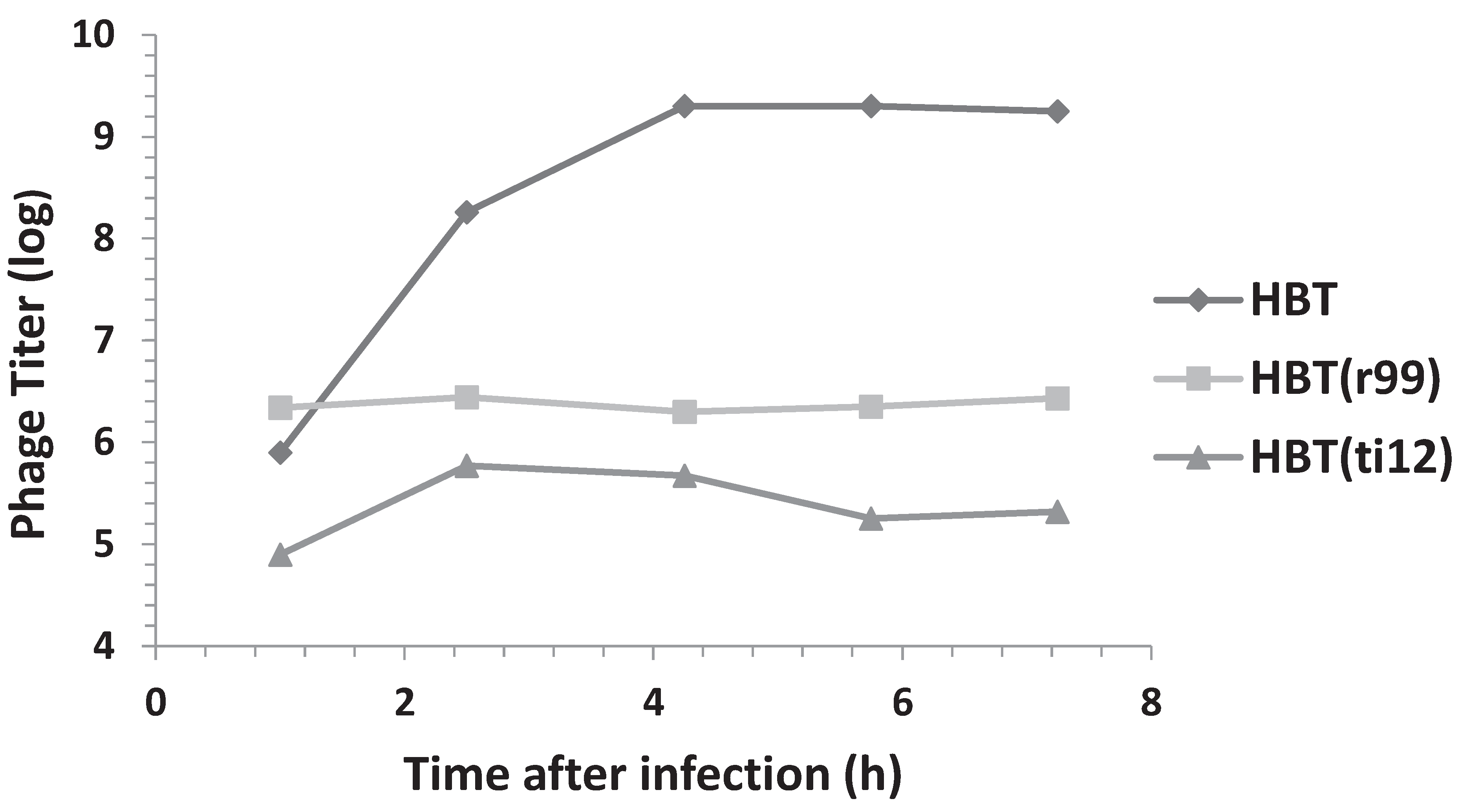
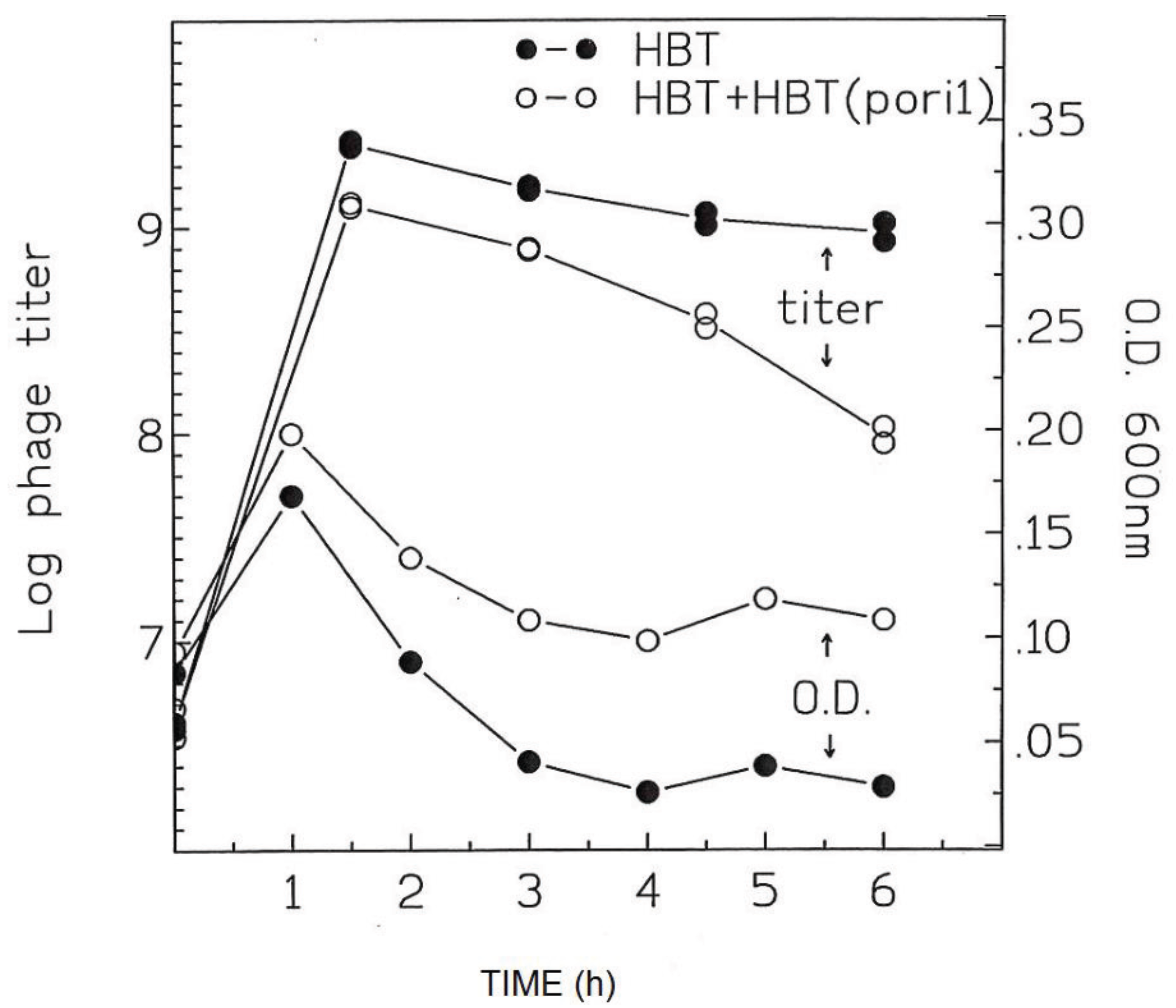
3.4. Addition of HBT(pOri1) to λ-Infected HBT Relieves the Sensitivity of HBT to λ Infection
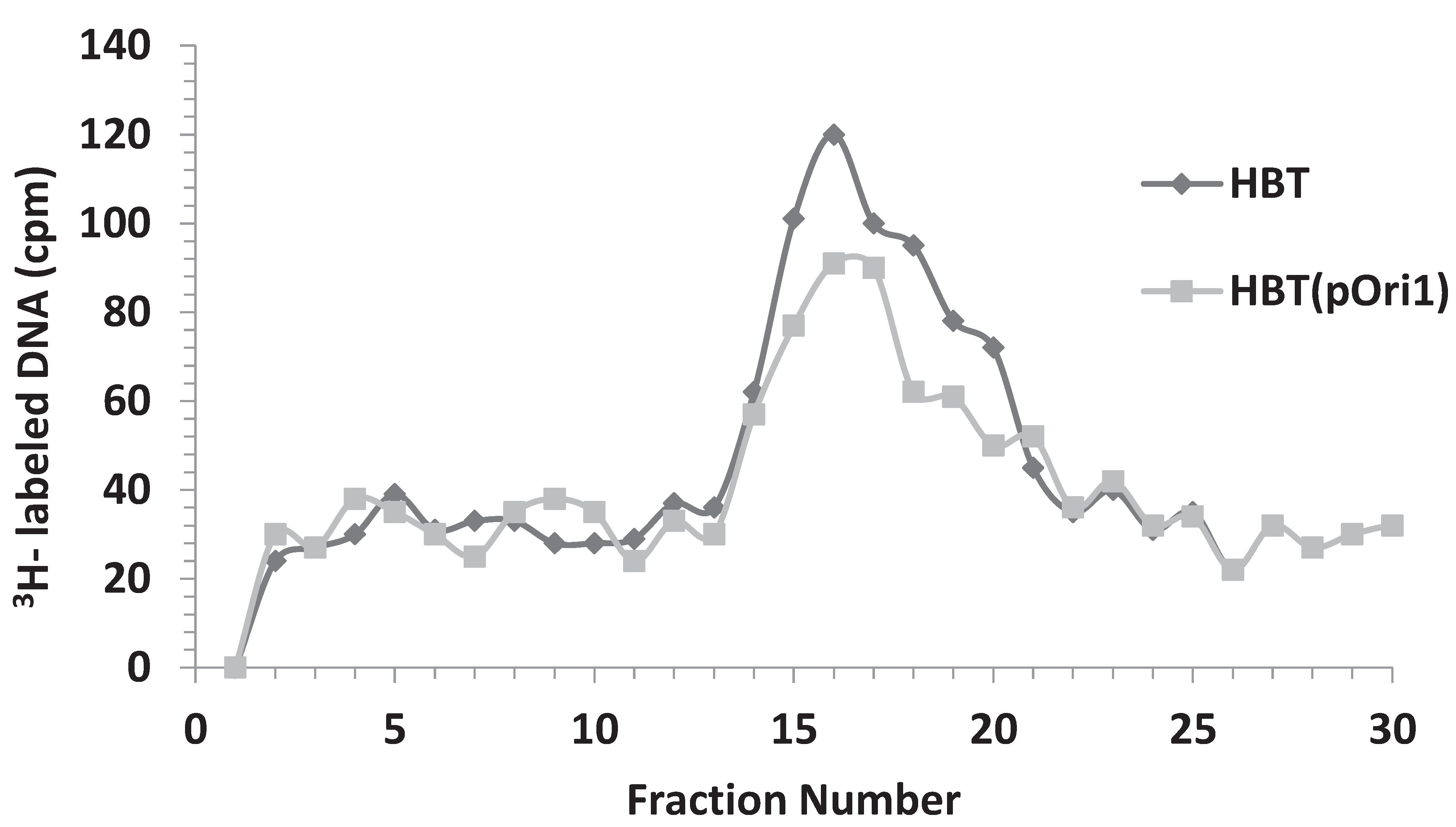
3.5. Replicative Intermediates Found in λ-Infected, HBT(pOri1) or Variant Strains, Are Reminiscent of Intermediates Isolated from λO− Mutants

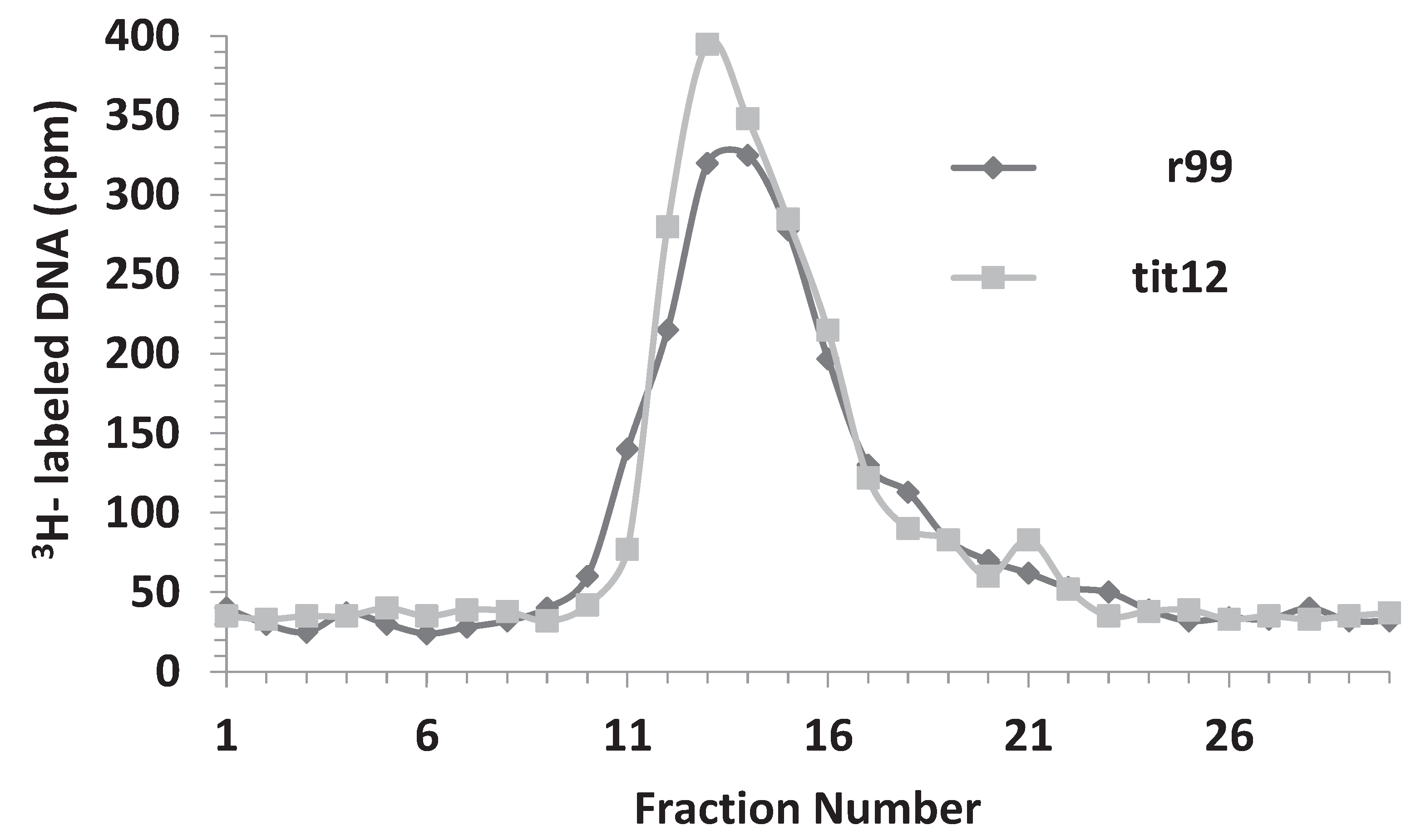
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacob, F.; Brenner, S.; Cuzin, F. On the regulation of DNA synthesis in bacteria: The hypothesis of the replicon. Cold Spring Harb. Symp. Quant. Biol. 1963, 256, 298–300. [Google Scholar]
- Furth, M.E.; Wickner, S.H. Lambda DNA replication. In Lambda II; Hendrix, R.W., Roberts, J.W., Weisberg, R.A., Eds.; Cold Spring Harbor Laboratory: New York, NY, USA, 1983; pp. 145–173. [Google Scholar]
- Ogawa, T.; Tomizawa, J. Replication of bacteriophage DNA I. Replication of DNA of lambdoid phages defective in early functions. J. Mol. Biol. 1968, 38, 217–225. [Google Scholar] [CrossRef]
- Matsubara, K.; Kaiser, A. λdv: An autonomously replicating fragment. Cold Spring Harb. Symp. Quant. Biol. 1968, 33, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K. Replication control system in λdv. Plasmid 1981, 5, 32–52. [Google Scholar] [CrossRef]
- Tsurimoto, T.; Matsubara, K. Replication of λdv plasmid in vitro promoted by purified λ O and P proteins. Proc. Natl. Acad. Sci. USA 1982, 79, 7639–7643. [Google Scholar] [CrossRef] [PubMed]
- Furth, M.E.; Mcleester, C.; Dove, W.F. Specificity determinants for bacteriophage lambda replication. I. A chain of interactions that control the initiation of replication. J. Mol. Biol. 1978, 126, 195–225. [Google Scholar] [CrossRef]
- Furth, M.; Yates, J. Specific determinants for bacteriophage λ DNA replication. II. Structure of O proteins of λ-θ80 and λ-82 hybrid phages and of a λ mutant defective in the origin of replication. J. Mol. Biol. 1978, 126, 227–240. [Google Scholar] [CrossRef]
- Dodson, M.; Roberts, J.; McMacken, R.; Echols, H. Specialized nucleoprotein structures at the origin of replication of bacteriophage λ: Complexes with λ O protein and with λ O, λ P, and E. coli DNAB proteins. Proc. Natl. Acad. Sci. USA 1985, 82, 4678–4682. [Google Scholar] [CrossRef] [PubMed]
- Schnӧs, M.; Zahn, K.; Inman, R.B.; Blattner, F.R. Initiation protein induced helix destabilization at the λ origin: A prepriming step in DNA replication. Cell 1988, 52, 385–395. [Google Scholar] [CrossRef]
- Zahn, K.; Blattner, F.R. Binding and bending of the λ replication origin by the phage O protein. EMBO J. 1985, 4, 3605–3618. [Google Scholar] [PubMed]
- Zahn, K.; Blattner, F.R. Sequence-induced DNA curvature at the bacteriophage λ origin of replication. Nature 1985, 317, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Zahn, K.; Blattner, F.R. Direct evidence for DNA bending at the lambda replication origin. Science 1987, 236, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.S.; Meharry Medical College, Nashville, TN, USA. Unpublished work. 2015.
- Valenzuela, M.S. Preparation of high titer radioactive phage stocks on agar plates. Microbios Lett. 1981, 17, 121–124. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Bacteriophage λ growth, purification, and DNA extraction. In Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory: New York, NY, USA, 1989; Volume 2, pp. 60–81. [Google Scholar]
- Valenzuela, M.S.; Inman, R.B. Direction of bacteriophage λ DNA replication in a thymine requiring Escherichia coli k-12 strain. Effect of thymidine concentration. Nucleic Acids Res. 1981, 9, 6975–6984. [Google Scholar] [CrossRef] [PubMed]
- Schnӧs, M.; Inman, R.B. Position of branch points in replicating lambda DNA. J. Mol. Biol. 1970, 51, 61–73. [Google Scholar] [CrossRef]
- Scherer, G. Nucleotide sequence of the O gene and the origin of replication in bacteriophage λ DNA. Nucleic Acids Res. 1978, 5, 3141–3156. [Google Scholar] [CrossRef] [PubMed]
- Dove, W.H.; Inokuchi, H.; Stevens, W. Replication control in phage Lambda. In The Bacteriophage Lambda; Hershey, A.D., Ed.; Cold Spring harbor Laboratory: New York, NY, USA, 1971; p. 747. [Google Scholar]
- Rambach, A. Replicator mutants of bacteriophage λ: Characterization of two subclasses. Virology 1973, 54, 270–277. [Google Scholar] [CrossRef]
- Chattoraj, D.K.; Stahl, F.W. Evidence of RNA in D loops of intracellular λ DNA. Proc. Natl. Acad. Sci. USA 1980, 77, 2153–2157. [Google Scholar] [CrossRef] [PubMed]
- Schnӧs, M.; Inman, R.B. Caffeine-induced reinitiation of phage λ DNA replication. J. Mol. Biol. 1982, 159, 457–465. [Google Scholar] [CrossRef]
- Valenzuela, M.S.; Freifelder, D.; Inman, R.B. Lack of a unique termination site for the first round of bacteriophage lambda DNA replication. J. Mol. Biol. 1976, 102, 569–581. [Google Scholar] [CrossRef]
- Takahashi, S. The rolling-circle replicative structure of a bacteriophage lambda DNA. Biochem. Biophys. Res. Commun. 1974, 61, 657–663. [Google Scholar] [CrossRef]
- Ogawa, T.; Tomizawa, J.I.; Fuke, M. Replication of bacteriophage DNA, II. Structure of replicating DNA of phage lambda. Proc. Natl. Acad. Sci. USA 1968, 60, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, M.S.; Inman, R.B. Multiply branched DNA molecules from bacteriophage λ: Putative post-replicational repair DNA intermediates. Biochem. Biophys. Res. Commun. 1986, 137, 869–875. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela, M.S.; Sharan, C. Arrest of Viral Proliferation by Ectopic Copies of Its Cognate Replication Origin. Genes 2015, 6, 436-450. https://doi.org/10.3390/genes6020436
Valenzuela MS, Sharan C. Arrest of Viral Proliferation by Ectopic Copies of Its Cognate Replication Origin. Genes. 2015; 6(2):436-450. https://doi.org/10.3390/genes6020436
Chicago/Turabian StyleValenzuela, Manuel S., and Chakradhari Sharan. 2015. "Arrest of Viral Proliferation by Ectopic Copies of Its Cognate Replication Origin" Genes 6, no. 2: 436-450. https://doi.org/10.3390/genes6020436
APA StyleValenzuela, M. S., & Sharan, C. (2015). Arrest of Viral Proliferation by Ectopic Copies of Its Cognate Replication Origin. Genes, 6(2), 436-450. https://doi.org/10.3390/genes6020436




