Abstract
The Eph family of receptor tyrosine kinases (RTKs) has been implicated in the regulation of many aspects of mammalian development. Recent analyses have revealed that the EphA2 receptor is a key modulator for a wide variety of cellular functions. This review focuses on the roles of EphA2 in both development and disease.
1. Introduction
Receptor tyrosine kinases (RTKs) are membrane spanning proteins that play an important role in a wide range of signaling processes in development [1,2]. The largest group of RTKs is the Eph family of molecules, which comprises of 14 members (EphA1–EphA8, EphA10; EphB1–EphB4, EphB6) in mammals [3,4,5,6]. Eph receptors and their ligands, the ephrins, have been shown to play several key roles in embryonic development [7,8], including tissue boundary formation [9,10,11], neural crest cell migration [12,13,14], axon guidance [15,16], central nervous system patterning [17,18], bone remodeling [19,20,21,22] and vascular organization [23,24,25,26,27,28,29]. In general, Eph and ephrin proteins are highly expressed during development while declining in later stages [30]. However, there is increasing evidence that these molecules can be re-expressed in specific circumstances such as tumorigenesis [30,31].
Recent investigations have shown that EphA2 has many important and diverse biological functions. EphA2 expression has been detected in a wide assortment of tissues including the brain, skin, bone marrow, lung, thymus, spleen, liver, small intestine, colon, urinary bladder, kidney, uterus, testis and prostate [32]. Most tissues express low levels of EphA2. Overexpression and dysregulation of this receptor have been associated with carcinogenesis, metastasis, and poor clinical prognosis [31,33,34]. In addition, EphA2 has attracted special attention in the field of lens [35,36,37,38,39,40,41], kidney [42,43], bone [22], mammary gland [34,44,45,46,47], and ear [48] development. Here, we highlight recent findings addressing the crucial roles of EphA2 receptor and ephrin-A ligands in tissue morphogenesis and disease. This review focuses on EphA2 functions that have not been reviewed previously, and its roles in tumorigenesis and cancer are not discussed since the several acceptant reviews in this area have been published recently [31,49,50,51,52,53,54,55,56].
2. Eph Receptors and Ephrins
The Eph receptors are named after erythropoietin-producing hepatocellular carcinoma cell lines in which the first member of the family, EphA1, was isolated [57]. Their respective ligands, the membrane-bound ephrins [58], were first identified in 1994 with the discovery of ephrin-A1 [59]. Both the Eph receptors and ephrin ligands are divided into the A (EphA and ephrin-A) and B (EphB and ephrin-B) subgroups. The categorization was based on sequence homology within subfamilies and binding affinities between Eph-ephrin pairs. In the case of the ligands, the mode of membrane attachment is different, as ephrin-A ligands are attached to the cell surface through a glycophosphotidylinositol (GPI) anchor, while the ephrin-B ligands are anchored by a hydrophobic transmembrane region [6,60].
In general, the EphA members (EphA1–EphA10) primarily interact with the ephrin-A ligands (ephrin-A1-ephrin-A6), while the EphB receptors (EphB1-EphB6) bind to the ephrin-B ligands (ephrin-B1-ephrin-B3) [5,6,60,61]. However, interactions between receptor and ligand subgroups have been previously reported; for instance, EphA4 has been shown to interact with both ephrin-B2 [62] and ephrin-B3 [63], and EphB2 has been found to be activated by ephrin-A5 [64].
2.1. Domain Configuration
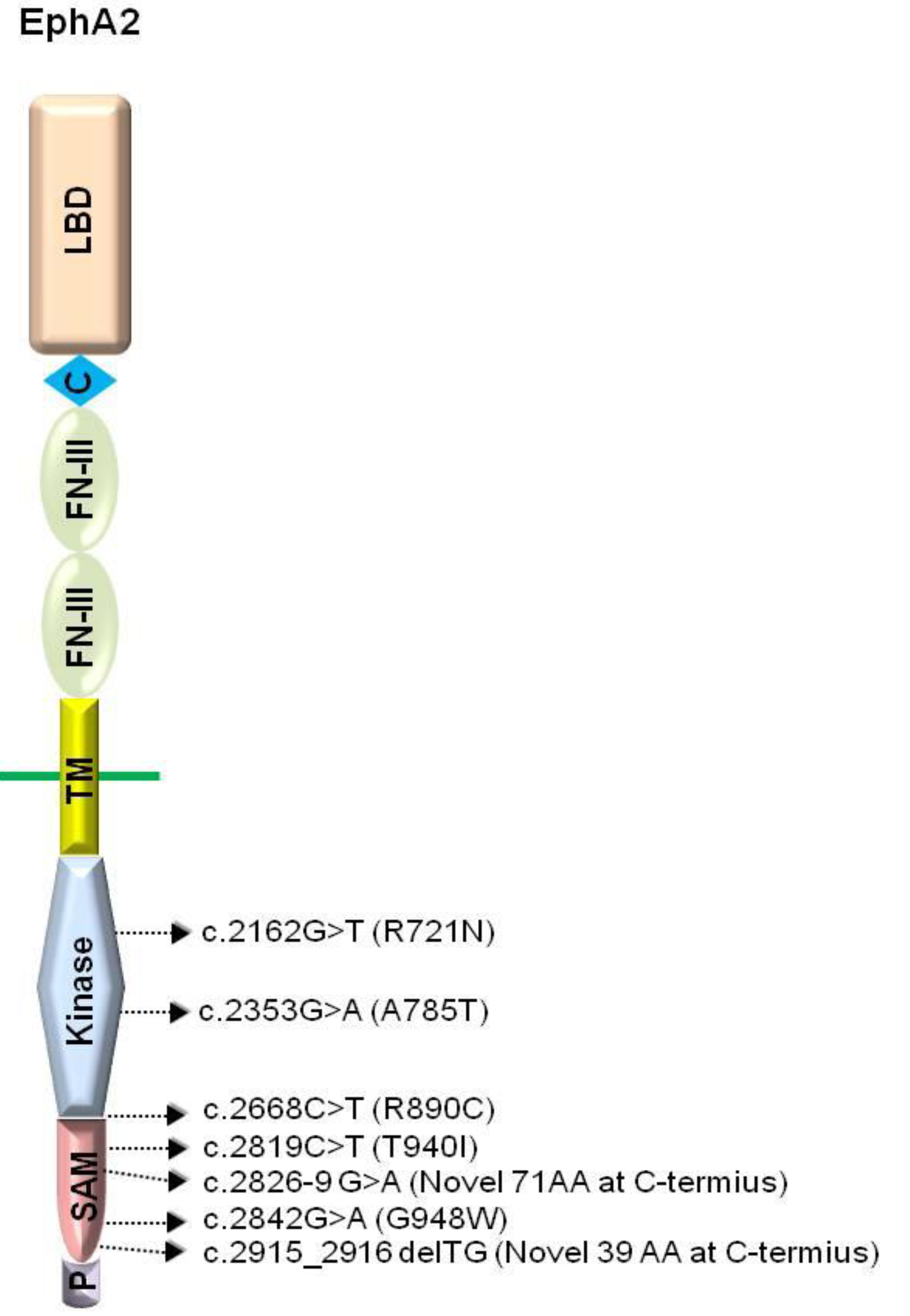
The Eph receptors comprise several distinctive domains required for their signaling capabilities. The extracellular domain contains an ephrin ligand-binding domain in its N-terminal-most region, followed by a cysteine-rich region and two fibronectin type-III repeats [65]. The intracellular region comprises of the signaling components which include a tyrosine kinase domain, a SAM (Sterile Alpha Motif) domain, and a PDZ (Postsynaptic density protein, Disks large, Zona occludens)-binding motif (Figure 1) [65]. Both the SAM and the PDZ domains have been shown to mediate protein-protein interactions [66,67,68,69,70,71].
2.2. Signaling
Eph receptors regulate a diverse range of biological processes including cell proliferation, differentiation, migration and tissue morphogenesis [7,30,31,72,73,74]. Signal transduction by the Eph family is a multistep process leading to the assembly of higher-order signaling clusters in the interacting cells [75]. The Eph family is capable of bidirectional signaling; upon interaction between receptor-ligand pairs, signaling events may be initiated by receptor-expressing cells (forward signaling), ligand-expressing cells (reverse signaling), or both (bidirectional signaling) [31,74,76,77,78]. These events result in the reorganization of the actin cytoskeleton, which leads to contact-dependent cell-cell attraction or repulsion and early embryonic cell motility and migration [15,72,79,80,81,82].
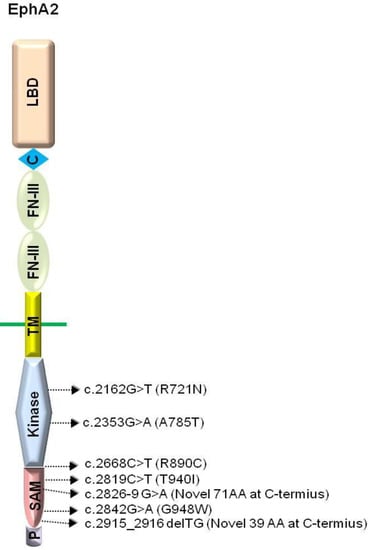
Figure 1.
Domain structure of EphA2 and the locations of human cataract mutations. LBD, ligand binding domain; C, cystein rich; FN-III, fibronectin type-III domain; TM, transmembrane domain; Kinase, protein tyrosine kinase domain; SAM, sterile alpha motif domain; P, PDZ-binding motif. Arrows indicate relative location of human cataract mutations.
Figure 1.
Domain structure of EphA2 and the locations of human cataract mutations. LBD, ligand binding domain; C, cystein rich; FN-III, fibronectin type-III domain; TM, transmembrane domain; Kinase, protein tyrosine kinase domain; SAM, sterile alpha motif domain; P, PDZ-binding motif. Arrows indicate relative location of human cataract mutations.
A major consequence of Eph-ephrin interaction is intercellular repulsion. This function occurs, in part, by proteolytic cleavage of the ephrin ligand at the juxtamembrane domain by the ADAM-10/Kuzbanian metalloproteinase leading to the disengagement of cells [83]. Eph-ephrin signaling can also elicit endocytosis and degradation through the Cbl ubiquitin ligase [84,85,86], which binds to activated receptors and acts also as an adaptor for signal tranducers [87].
Numerous downstream molecules mediate signal transmission by activated Eph receptors, including the Src kinase family [88,89] and the Ras and Rho family of small GTP-binding proteins (G-proteins) [76,90,91,92,93,94,95,96]. EphB4 activation results in Rac activation and assembly of actin and lammelipodia formation in the cells expressing the receptor [91]. In addition, Eph receptors have been shown to interact with receptors of other families including ErbB2 [31,97]. For reverse signaling, ephrin-B ligands are capable of activating Src family kinases (SFKs) [88] and recruiting cytoplasmic SH2 and PDZ domain containing proteins, such as Grb4 adaptor protein [98], glutamate receptor interacting protein (GRIP) [99], phospho-tyrosine phosphatase PTP-BL [88] and PDZ-regulator of heterotrimeric G protein signaling (RGS) 3 [92]. Ephrin-As collaborate with the ADAM10/Kuzbanian [83], the p75 (NTR) protein [79] and the NF-κB and Ret tyrosine kinase receptors to send signals [93]. The ephrin ligands can also initiate reverse signaling through SH2 or PDZ domain-independent associations [92,98,99].
The juxtamembrane region of Eph receptors has two highly conserved tyrosine residues (Tyr588 and Tyr594 in EphA2; Tyr596 and Tyr602 in EphA4; Tyr594 and Tyr600 in EphB1; Tyr604 and Tyr610 in EphB2). These tyrosine residues are major sites for autophosphorylation [100,101,102] and interact with a number of SH2 domain-containing cytoplasmic proteins including the human growth factor receptor bound protein (Grb7) [103], the Ras GTPase-activating protein (RasGAP) [104], the p85 subunit of phosphatidylinositol 3-kinase (PI3K), the adaptor protein NCK [102,104,105], and the R-Ras- and Rap1A-interacting protein SHEP-1 [90]. These SH2 domain-containing adaptor partners can modify several aspects of cellular behavior including cell adhesion and cytoskeletal structures [90,102,103,104]. The SH2 domain of Grb7 binds to the tyrosine phosphorylated SAM domain of EphB1, and this interaction affects regulation of cell migration [103,106]. In PC-3 prostate carcinoma cells, activated EphA2 caused dissociation of focal adhesion kinase (FAK) and the transient recruitment of the phosphotyrosine phosphatase Shp2 [82]. This event also correlates with inhibition of integrin-mediated adhesion, cell spreading and cell migration. In contrast, ligand activation of EphA2 can increase FAK phosphorylation in NIH3T3 cells and enhance cell spreading in a FAK-dependent manner [82]. The mechanisms of Eph receptor forward signaling and ephrin ligand reverse signaling are complex and have been well reviewed previously [31,52,55,72,76,78,81,107,108].
3. EphA2
EphA2 was identified in 1990 by the screening of the human epithelial HeLa cell cDNA library using degenerate probes designed to hybridize highly conserved regions of protein tyrosine kinases [109]. Originally named epithelial cell kinase (Eck) for its expression in the majority of epithelial cells, it resides on human chromosome 1p36.1 [110] and encodes a 130 kDa Type-1 glycoprotein composed of 976 amino acid residues [109,111]. EphA2 is localized on the cell membrane [111] and binds to ephrin-A1, -A2, -A3, -A4, and -A5 [14,22,60,112,113], but does not require ligand binding for some of its activities [114].
Like other receptor tyrosine kinases, the extracellular domain of EphA2 mediates ligand binding, which results in the autophosphorylation of its tyrosine residues [100,115]. The intracellular domain possesses intrinsic enzymatic activity, with the juxtamembrane region and the kinase domain containing many tyrosine residues that serve as docking sites for interactions with other signaling proteins containing SH2/SH3 domains [116]. The SAM domain is involved with receptor homo- or hetero-dimerization [66,69,70,71], and the PDZ-binding motif binds to other PDZ domain-containing proteins [67,68]. In our multiple sequence alignment analyses, EphA2 shows 55–70% sequence homologies and 40–50% sequence identities with other Eph receptors at the amino acid level, and the tyrosine residues are conserved within the juxtamembrane and kinase domains (data not shown).
EphA2 plays key roles in several developmental processes. Recent studies indicate that EphA2 regulates lens transparency, kidney repair following renal injury, bone remodeling, mammary gland branch morphogenesis, and inner ear development, as well as cell transformation in a variety of tumors.
3.1. Lens Development
Cataracts, or the opacification of the crystalline lens, are a major cause of visual impairments, accounting for up to 48% of all eye diseases [117]. Congenital cataract, in which lens deficits occur prior to or during childhood, has an estimated prevalence of 3–5/1,000 live births [118]. These early-onset cataracts may have a genetic basis, as genetic linkage studies have defined numerous mutations associated with deficits in lens development and maintenance, including those associated with connexins [119,120], crystallines [121,122,123,124,125,126,127,128,129], and intermediate-filament-like factors [130]. More recent investigations have identified the Eph family to play pivotal roles in lens development and maintenance [35,36,37,38,39,40,41,112,131,132,133,41,112,131].
3.1.1. EphA2 Expression in the Lens
The first evidence that the Eph family play a role in maintaining lens clarity came from our analyses of the role of ephrin-A5 in mouse development [112,133]. EphA2 was found to be expressed in the lens fiber cell layer, most notably within the subcortical region and throughout the epithelial cell layer [35,112,131,132]. In the mature lens, EphA2 protein is detected on the shorter edges of lens fiber cells when cut into cross-sections [131]. A recent report by Son et al. has identified that both EphA2 and ephrin-A5 are expressed during early ocular development and continue to be expressed into postnatal stages, and that both EphA2 and ephrin-A5 also co-localize in similar locations [112,133]. Our laboratory has also observed expression of several other Eph receptors and ephrin ligands, and their extensive expression has been shown throughout lens development as early as embryonic day 12 (E12), indicating a potential role during early lens development [133].
3.1.2. EphA2 Mutations and Cataractogenesis
Genetic analyses have shown that the 1p36 locus encodes mutated EPHA2 in human populations with cataracts [35,36,37,38,39,40,41]. The cataract mutations in EPHA2 reside in the intracellular compartment of the receptor, specifically within the kinase and SAM domains (Figure 1) [35,36,37,38,41]. The loss of EPHA2 function may directly or indirectly affect structural integrity, cell-cell communication, and protein stability, leading to congenital and age-related cataracts.
The EPHA2 mutations in the kinase domain have been identified in Caucasian populations [35] and a Pakistani family [37], leading to age-related cortical cataract or autosomal recessive congenital cataract, respectively. One of the cataract mutations residing in the protein kinase domain of EPHA2, the missense mutation Arg721Gln (R721N), significantly alters EPHA2 signaling and cellular regulation, with significantly greater growth inhibition by ephrin-A1 [35]. Another kinase domain mutation, c.2353G > A, results in the change of alanine to threonine at codon 785 (A785T), and leads to the formation of a nuclear cataract [37]. In a recent study, a missense mutation, c.2668C > T (p.R890C), was found between the kinase domain and the SAM domain [41]. However, the underlying mechanisms by which this mutation causes cataracts are still unknown.
Additionally, four other cataract mutations have been identified in the SAM domain of EPHA2 [36,38]. A missense mutation, c.2842G > T, which substitutes a glycine with tryptophan at codon 948 (GGG > TGG: p.G948W) is associated with autosomal dominant posterior polar cataracts in Caucasians [36]. Other SAM domain mutations include a missense mutation [c.2819C > T (p.T940I) in a Chinese family], a frameshift mutation [c.2915_2916delTG (p.V972GfsX39) in a British family] and a splicing mutation [c.2826-9G > A in an Australian family] (Figure 1) [38]. Interestingly, these SAM domain mutations are autosomal dominant, suggesting that they interfere with the wild-type EPHA2 receptor functions in lens development (Table 1).

Table 1.
EPHA2 mutations in cataractogenesis.
| Mutants | Domain | Mutation | Effect | Phenotype | Ref. |
|---|---|---|---|---|---|
| c.2842G > T | SAM | Missense | G948W | Autosomal dominant posterior polar cataract | [36] |
| c.2819C > T | SAM | Missense | T940I | Autosomal dominant posterior polar cataract | [38] |
| c.2826-9G > A | SAM | Splicing | novel 71 AA | Autosomal dominant total cataract | [38] |
| c.2915_2916delTG | SAM | Frameshift | novel 39 AA | Autosomal dominant posterior polar cataract | [38] |
| c.2162G > T | Kinase | Missense | R721N | Autosomal dominant cortical cataract | [35] |
| c.2353G > A | Kinase | Missense | A785T | Autosomal recessive nuclear cataract | [37] |
| c.2668C > T | Between the kinase and the SAM | Missense | R890C | Autosomal dominant posterior cataract | [41] |
3.1.3. Cataract Mouse Models
The first evidence that deficiencies in Eph-ephrin signaling lead to cataract formation came from the observation that the loss of ephrin-A5 in mice resulted in lens deformations [112,133]. Ephrin-A5 knockout lenses form vacuoles in the subcortical areas starting as early as postnatal day 6 (P6), eventually becoming exacerbated at later stages and leading to lens rupture by two months of age [112]. Histological analysis of early postnatal ephrin-A5 knockout lenses before the development of serious lens pathologies show distinct alterations in the shape of lens fiber cells at P6. In the wild type, cross-sectioned fiber cells are hexagonally shaped and tightly packed into regular rows radiating from the center of the lens. In contrast, ephrin-A5 knockout lens fiber cells are mostly cuboidal and have lost this organized packing arrangement. Eph receptor expression analyses showed that EphA2 co-localized with ephrin-A5, and that the absence of ephrin-A5 reduced the tyrosine phosphorylation of EphA2, suggesting that EphA2 is one of the receptors mediating ephrin-A5 function in the lens. This notion is strongly supported by the observations both EPHA2 mutations in humans and EphA2 inactivation in mice also result in cataracts [35]. Biochemical analysis further implicated a role of ephrin-A5 in regulating adherens junction functions and the loss of ephrin-A5 could cause a reduction in adherens junction-mediated cell-cell adhesion, which may contribute to loose fiber cell packing in the lens [112].
Jun et al. [35] had observed that EphA2 knockout mice developed using either a secretory gene trapping strategy of partial EphA2 ectodomain fused to β-gal on FVB/NJ genetic background [134,135] or a retroviral insertion into the first intron of EphA2 gene on C57BL/6 genetic background [136] developed age-related cortical cataracts. In both strains, pathological alterations were observed one month after birth with the onset of subcapsular vacuoles in the anterior cortex, followed by lens opacity and rupture between six to eight months of age. Cataract formation in the homozygous mutant mice was over 80% by 12 months of age; in contrast, no cataract formation was observed in wild-type or heterozygous lens. EphA2 became upregulated when lens epithelial cells underwent differentiation into cortical lens fiber cells, and the amount of expression progressively decreased with age. In addition, Jun et al. found that the signaling molecule HSP25, a marker for oxidative stress that is overexpressed in lenses undergoing cataract formation, was upregulated in the EphA2 knockout mice [35].
Structural deformations in the surface patterning of individual fiber cells in EphA2 knockout lenses have been reported by Shi et al. [131]. In this study, the loss of EphA2 resulted in abnormalities in lens shape and composition, resulting in inaccurate light refraction and sutural defects in the surface patterning of individual fiber cells, indicating possible problems in lens cell migration. However, these EphA2 knockout mice were developed using an in-frame translational stop codon at exon 5 under a 129/Sv and C57BL/6 mixed background and did not develop severe cataracts, though small flecklike opacities were observed within the lens nucleus. The discrepancy of lens phenotypes in between this EphA2 knockout strain and the two others observed by Jun et al. [35] remains unclear, though the differences in mouse genetic background may contribute to the variability in phenotypes.
3.1.4. Signaling and Molecular Mechanisms
The recent findings on EphA2 in cataractogenesis reveal several intriguing aspects of EphA2 function and activity. Of particular interest has been the finding that four of the total known seven cataract mutations in EPHA2 are located within the SAM domain, suggesting that this domain plays a critical role in the regulation of EPHA2 function and lens development. Interestingly, all of the identified SAM domain mutations resulted in autosomal dominant phenotypes [36,38].
The SAM domain is a conserved protein module in several key transcription factors, scaffolding proteins and regulatory proteins that are capable of forming homo- and hetero-oligomer [71]. Previous studies have shown that the SAM domain in the Eph receptors may have multiple functions [69,70,137,138,139]. Since SAM domains facilitate protein-protein interactions, it is possible that the EphA2 SAM domain mutations may interfere with receptor oligomerization or clustering into particular complexes essential for biological signaling [65]. Both the frameshift mutation c.2915_2916delTG and splicing mutation c.2826-9G > A, which affect the EPHA2 SAM domain, significantly enhance protein-protein interactions between EPHA2 and low molecular weight protein-tyrosine phosphatase (LMW-PTP) [38], which normally associates with the C-terminus of the EPHA2 and negatively regulates its signaling [140,141].
Further analysis of the EPHA2 SAM domain mutations has shown that these mutations induce degradation of EPHA2 through the ubiquitin-mediated proteasomal pathway and affect their solubility [47]. The EPHA2 mutants are also incapable of promoting cell migration. However, although the mutations within the SAM domain affect EPHA2 stability and solubility, how these mutants produce autosomal dominant effects is still unknown. In addition, the role of the PDZ-binding motif on the EPHA2 C-terminus may also play a role in regulating receptor function, as two of the mutants, c.2915_2916delTG and c.2826-9G > A, also lack the PDZ-binding domain [38].
The EPHA2 kinase domain also plays an important role in regulating lens development. The R721N mutation within the kinase domain is a risk allele for cataractogenesis in human populations [35]. Biochemical analysis has shown that the R721N mutation results in higher basal activation of the EPHA2 receptor compared to wild-type EPHA2, which leads to enhanced inhibition of extracellular signal-regulated kinase (ERK) 1/2. In addition, the kinase domain missense mutation c.2353G > A has been found to cause autosomal recessive congenital cataract, though the mechanism of this mutation remains to be elucidated [37].
3.2. Retinal Angiogenesis
The retina contains an intricate network of vasculature whose development, organization, and maturation are tightly regulated. Initial vascularization occurs in late prenatal stages in humans and early postnatal stages in mice, with vessels forming on the superficial retinal layers from the optic nerve head and extending outward to the peripheral edges of the organ [142,143]. This peripheral expansion is followed by selective remodeling of the vascular network [142,143]. Misregulation of this process has devastating consequences that leads to pathological retinal angiogenesis, in which aberrant and deficient vessels are formed, resulting in visual impairment and blindness [142,144,145,146]. Several diseases are associated with aberrant retinal angiogenesis, including proliferative diabetic retinopathy [147,148], age-related macular degeneration (AMD) [149,150], and retinopathy of prematurity (ROP) [151], amongst others, making the understanding of this process of particular importance [145].
EphA2 has been found to be upregulated during the angiogenic process of several cancers [152,153]. Inhibition of receptor activity using an EphA2-Fc fusion protein, in which the extracellular domain of EphA2 is fused to the IgG1 Fc chain and binds with ephrin-A ligands to prevent the activation of endogenous receptors, also has been shown to inhibit vascular endothelial growth factor (VEGF)-mediated angiogenesis [154,155,156,157]. In addition, the Eph family, including members of the EphA receptor and ephrin-A ligand subgroups, has been implicated in the process of neovascularization of the retina [158].
Recent work by several groups has found EphA2 to be a promising therapeutic molecule for inhibiting retinal angiogenesis. Initial work by Chen et al. had found that inhibiting EphA2 activity through injection of EphA2-Fc into the rat retina inhibited retinal neovascularization, while not affecting normal retinal vascular development [159]. The targeting of EphA2 has been found to inhibit neovascularization in both the retina [29] and choroid [160]. Interestingly, ephrin-A1-Fc treatment in the eye had also been found to inhibit neovascularization, although this study implies that the inhibition is brought on by EphA2 activation and not its inhibition [161].
These results point to the targeting of EphA2 and its signaling pathway as a promising approach for the treatment of retinal angiogenesis. In particular, EphA2-Fc, which specifically targets neovascular structures while seemingly not affecting normal retinal vascular development, may hold great promise for the treatment of diseases related to retinal angiogenesis. Future studies to examine the effectiveness of targeting EphA2 and its putative ligands in the eye in the context of other retinal neovascular diseases, including diabetic retinopathy and AMD, may provide additional treatments for these debilitating diseases. Further elucidation of the mechanism by which EphA2 regulates angiogenic formation is also required.
3.3. Kidney
In mouse kidney organogenesis, the initiation of branching morphogenesis occurs when the membrane protrusions from the tip of the ureteric bud (UB) invades into the metanephric mesenchyme [162,163]. During development, renal epithelia arise from two distinct sources: the collecting ducts and the nephrons. The collecting ducts develop by repeated branching of the ureteric bud, and the nephrons develop by the mesenchymal-to-epithelial transition (MET) of the metanephric mesenchyme [163].
EphA2 has been shown to be expressed at high levels in the developing kidney [43]. Miao et al. has reported that EphA2 is expressed in the ureteric buds of embryonic kidneys using an in vitro three-dimensional culture system [42]. In this study, EphA2 negatively regulated hepatocyte growth factor (HGF)-induced branch morphogenesis of Madin-Darby Canine Kidney (MDCK) cells [42]. Moreover, activation of EphA2 by its ligand ephrin-A1 caused a collapse of existing branch structures and prevented new branches from forming. HGF alone induced an epithelial-to-mesenchymal transition (EMT) which is required for the rearrangement and remodeling of MDCK cells during branch morphogenesis. In contrast, EphA2 reversed this process, which ensured that branch morphogenesis occurred within the correct location. Consistent with this observation, treatment with ephrin-A1 also binds to MDCK cell compaction [164].
In addition to kidney development, EphA2 has been found to play roles in Renal Ischemia-Reperfusion Injury (IRI), which is a major cause of acute kidney injuries in both native kidneys and renal allografts. Studies in several models have suggested that one of the key events in the pathogenesis of IRI is the disruption of the tubular epithelial actin cytoskeleton [165,166,167,168]. In previous studies, Eph receptors have been found to be a key developmental regulators of cytoskeletal remodeling during embryonic development, particularly in respect to the vascular and central nervous systems [12,13,14,23,24,25,26,27,28,29,169]. However, the contribution of surface receptors that orchestrate cytoskeletal repair is not fully understood in the repair process of kidney following IRI.
Baldwin et al. [89] was the first to show that EphA2 is a critical regulator of actin dynamics in a mouse model of renal IRI. While basal expression of EphA2 protein was observed in distal tubular segments, IRI resulted in more intense and generalized up-regulation of EphA2 protein expression. This increase in EphA2 expression appears to be through a Src kinase-dependent pathway, as the enhanced expression of EphA2 was inhibited by the Src kinase inhibitor PP2. Src kinases also strongly activated the human EphA2 promoter, suggesting that this up-regulation occurs through a transcriptional mechanism. In addition, ephrin-A1 treatment led to tyrosine phosphorylation of EphA2, and this particular interaction between up-regulated EphA2 and its putative ligand may serve to enhance cell contact-dependent signaling for cytoskeletal repair in renal IRI.
Recent studies have also reported the increase of EphA2 expression under stressful conditions. Specifically, EphA2 up-regulation has been investigated in cultured Inner Medullar Collecting Duct (IMCD-3) cells and in the renal medulla in response to urea stress and hypertonicity [43,170]. EphA2 was expressed in high levels within the collecting ducts of the renal papilla and the renal medulla but only in low levels within rat renal cortex [43]. An enhanced expression of EphA2 has also been observed in the livers of rats stimulated with bacterial lipopolysaccharides [171] and in epithelial cells exposed to the detergent deoxycholic acid [172]. Together, these studies suggest that EphA2 has a critical role in the tissue repair process of disease models, such as IRI. More specifically, EphA2 may behave as an injury- and stress-responsive regulator.
3.4. Bone
Ephs and ephrins are expressed in chondrocytes, osteoclasts and osteoblasts, as well as endothelial cells and neurons in the bones and in the bone marrow [19,20]. Bones are constantly remodeled throughout life, and the coupling of bone resorption and formation is tightly regulated by communication between osteoclasts and osteoblasts [173]. Osteoclasts are multinucleated cells responsible for bone resorption through cell-cell fusion [174]. Unlike osteoclasts, bone-forming osteoblasts are mononuclear cells derived from mesenchymal progenitors and express the two major membrane-bound proteins, macrophage-colony stimulating factor (M-CSF) and the receptor activator of NF-κB ligand (RANKL) [175,176]. During differentiation of cultured osteoclasts induced by RANKL, the EphA1, A2 and A4 receptors, and the ephrin-A2, -B1, and -B2 ligands, are clearly expressed [20,21,22].
Irie et al. have recently found that bidirectional EphA2-ephrin-A2 signaling is critical in bone remodeling at the initiation phase [22]. The EphA2-ephrin-A2 interaction promoted bone resorption and concomitantly suppressed osteoblastogenesis in a manner distinct from EphB4-ephrin-B2 interaction [21]. In addition, the loss of EphA2 enhanced osteoblastogenesis by a decrease in GTP-RhoA, suggesting that signaling through EphA2 into osteoblasts suppresses osteoblast differentiation by activating RhoA.
3.5. Mammary Gland Branch Morphogenesis
Mammary gland branching morphogenesis is a developmental process during which an extensive complex of the growing ducts forms from a rudimentary epithelial bud [177,178]. Expressions of some Eph family receptors and their ligands have been reported in the mammary gland [34,45,46,51,179,180,181,182,183]. In initial developmental studies, two receptor protein tyrosine kinases, EphB4 and EphA2, were originally isolated at the RNA level from the mature mouse mammary glands [34]. The EphB4 receptor is expressed in myoepithelial luminal cells and developmentally regulated in a hormone-dependent manner during normal mammary gland development, with misregulation resulting in the formation of invasive mouse mammary tumors. Further studies have found that ephrin-B2 is expressed on the luminal cells in the mammary gland and acts as a cognate ligand of EphB4 [183]. Estrogen acts as one of the mediators to induce the expression of EphB4 and ephrin-B2 [183].
More recently, a genome-wide transcript analysis has identified only the Eph family molecules EphA2 and ephrin-B1 in the terminal end buds forming at the tips of the ducts [44]. EphA2 expression was also shown to be developmentally controlled in the growth and branch morphogenesis of normal mammary epithelium [34,44]. Loss of EphA2 resulted in reduction of mammary epithelial proliferation and marked inhibition of epithelial branching necessary for complete fat pad filling [46]. Further in vitro analyses have shown that EphA2 expression was negatively regulated by estrogen and c-myc [184]. EphA2 expression was also detected in human mammary epithelial cells [45,153,185]. EphA2 leads to growth arrest and differentiation in normal human mammary epithelial cells in three-dimensional cultures, and its level was significantly decreased in the differentiated cells [186], whereas the expression increased in breast cancer [187]. Interestingly, enhanced EphA2 expression in human breast cancer is associated with a poor patient prognosis [188].
Epithelial branch morphogenesis is a fundamental biological process by which the endocrine hormones and local paracrine interaction between the developing epithelial ducts and their adjacent mesenchymal stroma drives mammary gland development [46,51]. Some of the cytockines and growth factors, such as FGF, HGF and TGF-β, act as critical molecules in the local regulation of branch morphogenesis [182,189]. Vaught et al. [46] had found that HGF-induced mammary epithelial branch morphogenesis was significantly reduced in EphA2-deficient cells relative to wild-type controls, which correlated with increased RhoA activity. Taken together, these results indicate that EphA2 functions as a positive regulator in mammary gland morphogenesis.
3.6. Ear
The mammalian inner ear initially forms an epithelial sac-like otocyst, and differentiates into a highly complex structure. They are subdivided into the auditory component (the coiled, snail-shaped cochlea, that regulates hearing), and five vestibular regions (consisting of the saccular and utricular maculae, and the ampullar cristae of the three semicircular canals whose primary role is to regulate balance) [190]. Several studies have reported the expression of the Eph family in structures of the inner ear [48,190,191,192]. Ephrin-A1 and ephrin-A2 were detected in the epithelial cells lining the fluid filled ducts and in connective tissue regions of the inner ear, respectively [190]. EphA4 was expressed in vestibular hair cells, and EphA5 and EphA7 were exhibited in cochlear and vestibular supporting cells, suggesting that these Eph receptors have a role in establishing the formation and cellular organization of the inner ear [190]. A later study by Pickles et al. had also identified the expression of EphA4 and its ligand ephrin-A2 in cochlear tissues [191]. In addition, disruption of EphB1 or EphB3 receptors in mice lead to deficits in cochlear function [193]. These results indicate that specific Eph receptors are necessary for cochlear function in the inner ear.
Recent examinations of the function of the Eph family during development have identified the expression of EphA2 during otic placode formation between E8.5 and E10.5 [48]. The vertebrate inner ear develops from the otic placode, an ectodermal thickening located adjacent to the posterior hindbrain [194]. Saeger et al. first observed EphA2 expression in the otic region in the ventral and posterior ectoderm; the ventral, posterior and subplacodal mesenchyme; and the anterior neural tube (rhombomere r4) [48]. However, EphA2 involvement in inner ear development remains to be functionally demonstrated.
4. Conclusions and Perspectives
The Eph receptors and ephrin ligands are expressed during the development of a range of vertebrate species. Critical roles of EphA2 have been shown in lens development and renal repair. However, key questions remain in terms of how EphA2 and its ephrin-A ligands cooperate for tissue patterning. The role of EphA2 in the lens has been of particular interest in the past several years, resulting in several advances in understanding its role in lens biology. Among the members of the Eph family, only EphA2 and the ligand ephrin-A5 have thus far been identified as critical regulators in lens development. Both the EphA2 kinase and SAM domains are required for function. In particular, the analysis of human EPHA2 SAM domain mutations reveals a novel mode of action of SAM domain, namely the regulation of protein stability and solubility. Additionally, molecular and signaling mechanisms underlying EphA2 function within the lens are beginning to come to light. However, many important questions remain. Thus far, most of the signaling pathway studies have utilized in vitro models, but how this applies to lens tissues remain to be determined. Functional studies using transgenic knock-in mouse models expressing various EPHA2 signaling mutations may provide further insights. The importance of EphA2 in other tissues, including the retina, kidney, bone and ear development, has also been recently reported, and many of these studies are still in their infancy. The questions concerning the intracellular pathways linking EphA2 receptor and ephrin-A signaling still need to be analyzed, especially in relation to other signals in organ development.
Acknowledgments
This research was supported by the National Institutes of Health (NIH) grant (1RO1EY019012).
Conflicts of Interest
The author declares no conflict of interest.
References
- Van der Geer, P.; Hunter, T.; Lindberg, R.A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu. Rev. Cell. Biol. 1994, 10, 251–337. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Fox, G.M.; Holst, P.L.; Chute, H.T.; Lindberg, R.A.; Janssen, A.M.; Basu, R.; Welcher, A.A. cDNA cloning and tissue distribution of five human EPH-like receptor protein-tyrosine kinases. Oncogene 1995, 10, 897–905. [Google Scholar]
- Gurniak, C.B.; Berg, L.J. A new member of the Eph family of receptors that lacks protein tyrosine kinase activity. Oncogene 1996, 13, 777–786. [Google Scholar]
- Pandey, A.; Lindberg, R.A.; Dixit, V.M. Cell signalling. Receptor orphans find a family. Curr. Biol. 1995, 5, 986–989. [Google Scholar] [CrossRef]
- Zhou, R. The Eph family receptors and ligands. Pharmacol. Ther. 1998, 77, 151–181. [Google Scholar] [CrossRef]
- Holder, N.; Klein, R. Eph receptors and ephrins: Effectors of morphogenesis. Development 1999, 126, 2033–2044. [Google Scholar]
- Flenniken, A.M.; Gale, N.W.; Yancopoulos, G.D.; Wilkinson, D.G. Distinct and overlapping expression patterns of ligands for Eph-related receptor tyrosine kinases during mouse embryogenesis. Dev. Biol. 1996, 179, 382–401. [Google Scholar] [CrossRef]
- Rohani, N.; Canty, L.; Luu, O.; Fagotto, F.; Winklbauer, R. EphrinB/EphB signaling controls embryonic germ layer separation by contact-induced cell detachment. PLoS Biol. 2011, 9, e1000597. [Google Scholar]
- Mellitzer, G.; Xu, Q.; Wilkinson, D.G. Eph receptors and ephrins restrict cell intermingling and communication. Nature 1999, 400, 77–81. [Google Scholar] [CrossRef]
- Xu, Q.; Mellitzer, G.; Robinson, V.; Wilkinson, D.G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature 1999, 399, 267–271. [Google Scholar]
- Robinson, V.; Smith, A.; Flenniken, A.M.; Wilkinson, D.G. Roles of Eph receptors and ephrins in neural crest pathfinding. Cell Tissue Res. 1997, 290, 265–274. [Google Scholar] [CrossRef]
- Smith, A.; Robinson, V.; Patel, K.; Wilkinson, D.G. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. 1997, 7, 561–570. [Google Scholar] [CrossRef]
- Flanagan, J.G.; Vanderhaeghen, P. The ephrins and Eph receptors in neural development. Annu. Rev. Neurosci. 1998, 21, 309–345. [Google Scholar] [CrossRef]
- Orioli, D.; Klein, R. The Eph receptor family: Axonal guidance by contact repulsion. Trends Genet. 1997, 13, 354–359. [Google Scholar] [CrossRef]
- Egea, J.; Klein, R. Bidirectional Eph-ephrin signaling during axon guidance. Trends Cell. Biol. 2007, 17, 230–238. [Google Scholar] [CrossRef]
- Wilkinson, D.G. Multiple roles of EPH receptors and ephrins in neural development. Nat. Rev. Neurosci. 2001, 2, 155–164. [Google Scholar] [CrossRef]
- Palmer, A.; Klein, R. Multiple roles of ephrins in morphogenesis, neuronal networking, and brain function. Genes Dev. 2003, 17, 1429–1450. [Google Scholar] [CrossRef]
- Edwards, C.M.; Mundy, G.R. Eph receptors and ephrin signaling pathways: A role in bone homeostasis. Int. J. Med. Sci. 2008, 5, 263–272. [Google Scholar] [CrossRef]
- Matsuo, K.; Otaki, N. Bone cell interactions through Eph/ephrin: Bone modeling, remodeling and associated diseases. Cell Adhes. Migr. 2012, 6, 148–156. [Google Scholar] [CrossRef]
- Zhao, C.; Irie, N.; Takada, Y.; Shimoda, K.; Miyamoto, T.; Nishiwaki, T.; Suda, T.; Matsuo, K. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab. 2006, 4, 111–121. [Google Scholar] [CrossRef]
- Irie, N.; Takada, Y.; Watanabe, Y.; Matsuzaki, Y.; Naruse, C.; Asano, M.; Iwakura, Y.; Suda, T.; Matsuo, K. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J. Biol. Chem. 2009, 284, 14637–14644. [Google Scholar] [CrossRef]
- Gale, N.W.; Yancopoulos, G.D. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999, 13, 1055–1066. [Google Scholar] [CrossRef]
- Pandey, A.; Shao, H.; Marks, R.M.; Polverini, P.J.; Dixit, V.M. Role of B61, the ligand for the Eck receptor tyrosine kinase, in TNF-alpha-induced angiogenesis. Science 1995, 268, 567–569. [Google Scholar]
- Wang, H.U.; Chen, Z.F.; Anderson, D.J. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998, 93, 741–753. [Google Scholar] [CrossRef]
- Adams, R.H.; Wilkinson, G.A.; Weiss, C.; Diella, F.; Gale, N.W.; Deutsch, U.; Risau, W.; Klein, R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: Demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999, 13, 295–306. [Google Scholar] [CrossRef]
- Adams, R.H.; Diella, F.; Hennig, S.; Helmbacher, F.; Deutsch, U.; Klein, R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell 2001, 104, 57–69. [Google Scholar] [CrossRef]
- Brantley-Sieders, D.M.; Chen, J. Eph receptor tyrosine kinases in angiogenesis: From development to disease. Angiogenesis 2004, 7, 17–28. [Google Scholar] [CrossRef]
- Shen, J.; Xie, B.; Hatara, C.M.; Hackett, S.F.; Campochiaro, P.A. Vegf or EphA2 antisense polyamide-nucleic acids; vascular localization and suppression of retinal neovascularization. Mol. Ther. 2007, 15, 1924–1930. [Google Scholar] [CrossRef]
- Coulthard, M.G.; Duffy, S.; Down, M.; Evans, B.; Power, M.; Smith, F.; Stylianou, C.; Kleikamp, S.; Oates, A.; Lackmann, M.; et al. The role of the Eph-ephrin signalling system in the regulation of developmental patterning. Int. J. Dev. Biol. 2002, 46, 375–384. [Google Scholar]
- Pasquale, E.B. Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nat. Rev. Cancer 2010, 10, 165–180. [Google Scholar] [CrossRef]
- Hafner, C.; Schmitz, G.; Meyer, S.; Bataille, F.; Hau, P.; Langmann, T.; Dietmaier, W.; Landthaler, M.; Vogt, T. Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin. Chem. 2004, 50, 490–499. [Google Scholar] [CrossRef]
- Andres, A.C.; Zuercher, G.; Djonov, V.; Flueck, M.; Ziemiecki, A. Protein tyrosine kinase expression during the estrous cycle and carcinogenesis of the mammary gland. Int. J. Cancer 1995, 63, 288–296. [Google Scholar] [CrossRef]
- Andres, A.C.; Reid, H.H.; Zurcher, G.; Blaschke, R.J.; Albrecht, D.; Ziemiecki, A. Expression of two novel eph-related receptor protein tyrosine kinases in mammary gland development and carcinogenesis. Oncogene 1994, 9, 1461–1467. [Google Scholar]
- Jun, G.; Guo, H.; Klein, B.E.; Klein, R.; Wang, J.J.; Mitchell, P.; Miao, H.; Lee, K.E.; Joshi, T.; Buck, M.; et al. EPHA2 is associated with age-related cortical cataract in mice and humans. PLoS Genet. 2009, 5, e1000584. [Google Scholar] [CrossRef]
- Shiels, A.; Bennett, T.M.; Knopf, H.L.; Maraini, G.; Li, A.; Jiao, X.; Hejtmancik, J.F. The EPHA2 gene is associated with cataracts linked to chromosome 1p. Mol. Vis. 2008, 14, 2042–2055. [Google Scholar]
- Kaul, H.; Riazuddin, S.A.; Shahid, M.; Kousar, S.; Butt, N.H.; Zafar, A.U.; Khan, S.N.; Husnain, T.; Akram, J.; Hejtmancik, J.F.; et al. Autosomal recessive congenital cataract linked to EPHA2 in a consanguineous Pakistani family. Mol. Vis. 2010, 16, 511–517. [Google Scholar]
- Zhang, T.; Hua, R.; Xiao, W.; Burdon, K.P.; Bhattacharya, S.S.; Craig, J.E.; Shang, D.; Zhao, X.; Mackey, D.A.; Moore, A.T.; et al. Mutations of the EPHA2 receptor tyrosine kinase gene cause autosomal dominant congenital cataract. Hum. Mutat. 2009, 30, E603–E611. [Google Scholar] [CrossRef]
- Tan, W.; Hou, S.; Jiang, Z.; Hu, Z.; Yang, P.; Ye, J. Association of EPHA2 polymorphisms and age-related cortical cataract in a Han Chinese population. Mol. Vis. 2011, 17, 1553–1558. [Google Scholar]
- Sundaresan, P.; Ravindran, R.D.; Vashist, P.; Shanker, A.; Nitsch, D.; Talwar, B.; Maraini, G.; Camparini, M.; Nonyane, B.A.; Smeeth, L.; et al. EPHA2 polymorphisms and age-related cataract in India. PLoS One 2012, 7, e33001. [Google Scholar] [CrossRef]
- Shentu, X.C.; Zhao, S.J.; Zhang, L.; Miao, Q. A novel p.R890C mutation in EPHA2 gene associated with progressive childhood posterior cataract in a Chinese family. Int. J. Ophthalmol. 2013, 6, 34–38. [Google Scholar]
- Miao, H.; Nickel, C.H.; Cantley, L.G.; Bruggeman, L.A.; Bennardo, L.N.; Wang, B. EphA kinase activation regulates HGF-induced epithelial branching morphogenesis. J. Cell. Biol. 2003, 162, 1281–1292. [Google Scholar] [CrossRef]
- Xu, H.; Tian, W.; Lindsley, J.N.; Oyama, T.T.; Capasso, J.M.; Rivard, C.J.; Cohen, H.T.; Bagnasco, S.M.; Anderson, S.; Cohen, D.M. EphA2: Expression in the renal medulla and regulation by hypertonicity and urea stress in vitro and in vivo. Am. J. Physiol. Renal Physiol. 2005, 288, F855–F866. [Google Scholar]
- Kouros-Mehr, H.; Werb, Z. Candidate regulators of mammary branching morphogenesis identified by genome-wide transcript analysis. Dev. Dyn. 2006, 235, 3404–3412. [Google Scholar] [CrossRef]
- Zelinski, D.P.; Zantek, N.D.; Stewart, J.C.; Irizarry, A.R.; Kinch, M.S. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001, 61, 2301–2306. [Google Scholar]
- Vaught, D.; Chen, J.; Brantley-Sieders, D.M. Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol. Biol. Cell 2009, 20, 2572–2581. [Google Scholar] [CrossRef]
- Park, J.E.; Son, A.I.; Hua, R.; Wang, L.; Zhang, X.; Zhou, R. Human cataract mutations in EPHA2 SAM domain alter receptor stability and function. PLoS One 2012, 7, e36564. [Google Scholar]
- Saeger, B.M.; Suhm, M.; Neubuser, A. Ephrin/ephrin receptor expression during early stages of mouse inner ear development. Dev. Dyn. 2011, 240, 1578–1585. [Google Scholar] [CrossRef]
- Lin, S.; Wang, B.; Getsios, S. Eph/ephrin signaling in epidermal differentiation and disease. Semin. Cell Dev. Biol. 2012, 23, 92–101. [Google Scholar] [CrossRef]
- Arvanitis, D.; Davy, A. Eph/ephrin signaling: Networks. Genes Dev. 2008, 22, 416–429. [Google Scholar] [CrossRef]
- Vaught, D.; Brantley-Sieders, D.M.; Chen, J. Eph receptors in breast cancer: Roles in tumor promotion and tumor suppression. Breast Cancer Res. 2008, 10, 217. [Google Scholar]
- Chen, J. Regulation of tumor initiation and metastatic progression by Eph receptor tyrosine kinases. Adv. Cancer Res. 2012, 114, 1–20. [Google Scholar]
- Janes, P.W.; Nievergall, E.; Lackmann, M. Concepts and consequences of Eph receptor clustering. Semin. Cell Dev. Biol. 2012, 23, 43–50. [Google Scholar] [CrossRef]
- Funk, S.D.; Orr, A.W. Ephs and ephrins resurface in inflammation, immunity, and atherosclerosis. Pharmacol. Res. 2013, 67, 42–52. [Google Scholar] [CrossRef]
- Chen, J.; Zhuang, G.; Frieden, L.; Debinski, W. Eph receptors and Ephrins in cancer: Common themes and controversies. Cancer Res. 2008, 68, 10031–10033. [Google Scholar] [CrossRef]
- Wykosky, J.; Debinski, W. The EphA2 receptor and ephrinA1 ligand in solid tumors: Function and therapeutic targeting. Mol. Cancer Res. 2008, 6, 1795–1806. [Google Scholar] [CrossRef]
- Hirai, H.; Maru, Y.; Hagiwara, K.; Nishida, J.; Takaku, F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science 1987, 238, 1717–1720. [Google Scholar]
- Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell 1997, 90, 403–404. [CrossRef]
- Bartley, T.D.; Hunt, R.W.; Welcher, A.A.; Boyle, W.J.; Parker, V.P.; Lindberg, R.A.; Lu, H.S.; Colombero, A.M.; Elliott, R.L.; Guthrie, B.A.; et al. B61 is a ligand for the ECK receptor protein-tyrosine kinase. Nature 1994, 368, 558–560. [Google Scholar] [CrossRef]
- Pasquale, E.B. The Eph family of receptors. Curr. Opin. Cell. Biol. 1997, 9, 608–615. [Google Scholar] [CrossRef]
- Gale, N.W.; Holland, S.J.; Valenzuela, D.M.; Flenniken, A.; Pan, L.; Ryan, T.E.; Henkemeyer, M.; Strebhardt, K.; Hirai, H.; Wilkinson, D.G.; et al. Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 1996, 17, 9–19. [Google Scholar] [CrossRef]
- Qin, H.; Noberini, R.; Huan, X.; Shi, J.; Pasquale, E.B.; Song, J. Structural characterization of the EphA4-Ephrin-B2 complex reveals new features enabling Eph-ephrin binding promiscuity. J. Biol. Chem. 2010, 285, 644–654. [Google Scholar]
- Takemoto, M.; Fukuda, T.; Sonoda, R.; Murakami, F.; Tanaka, H.; Yamamoto, N. Ephrin-B3-EphA4 interactions regulate the growth of specific thalamocortical axon populations in vitro. Eur. J. Neurosci. 2002, 16, 1168–1172. [Google Scholar]
- Himanen, J.P.; Chumley, M.J.; Lackmann, M.; Li, C.; Barton, W.A.; Jeffrey, P.D.; Vearing, C.; Geleick, D.; Feldheim, D.A.; Boyd, A.W.; et al. Repelling class discrimination: Ephrin-A5 binds to and activates EphB2 receptor signaling. Nat. Neurosci. 2004, 7, 501–509. [Google Scholar] [CrossRef]
- Himanen, J.P.; Nikolov, D.B. Eph signaling: A structural view. Trends Neurosci. 2003, 26, 46–51. [Google Scholar] [CrossRef]
- Qiao, F.; Bowie, J.U. The many faces of SAM. Sci. STKE 2005, 2005, re7. [Google Scholar] [CrossRef]
- Hui, S.; Xing, X.; Bader, G.D. Predicting PDZ domain mediated protein interactions from structure. BMC Bioinformatics 2013, 14, 27. [Google Scholar] [CrossRef]
- Hock, B.; Bohme, B.; Karn, T.; Yamamoto, T.; Kaibuchi, K.; Holtrich, U.; Holland, S.; Pawson, T.; Rubsamen-Waigmann, H.; Strebhardt, K. PDZ-domain-mediated interaction of the Eph-related receptor tyrosine kinase EphB3 and the ras-binding protein AF6 depends on the kinase activity of the receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 9779–9784. [Google Scholar] [CrossRef]
- Smalla, M.; Schmieder, P.; Kelly, M.; Ter Laak, A.; Krause, G.; Ball, L.; Wahl, M.; Bork, P.; Oschkinat, H. Solution structure of the receptor tyrosine kinase EphB2 SAM domain and identification of two distinct homotypic interaction sites. Protein Sci. 1999, 8, 1954–1961. [Google Scholar] [CrossRef]
- Thanos, C.D.; Goodwill, K.E.; Bowie, J.U. Oligomeric structure of the human EphB2 receptor SAM domain. Science 1999, 283, 833–836. [Google Scholar] [CrossRef]
- Slaughter, B.D.; Huff, J.M.; Wiegraebe, W.; Schwartz, J.W.; Li, R. SAM domain-based protein oligomerization observed by live-cell fluorescence fluctuation spectroscopy. PLoS One 2008, 3, e1931. [Google Scholar] [CrossRef]
- Poliakov, A.; Cotrina, M.; Wilkinson, D.G. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev. Cell. 2004, 7, 465–480. [Google Scholar] [CrossRef]
- Pasquale, E.B. Eph receptor signalling casts a wide net on cell behaviour. Nat. Rev. Mol. Cell Biol. 2005, 6, 462–475. [Google Scholar] [CrossRef]
- Kullander, K.; Klein, R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell. Biol. 2002, 3, 475–486. [Google Scholar] [CrossRef]
- Himanen, J.P.; Yermekbayeva, L.; Janes, P.W.; Walker, J.R.; Xu, K.; Atapattu, L.; Rajashankar, K.R.; Mensinga, A.; Lackmann, M.; Nikolov, D.B.; et al. Architecture of Eph receptor clusters. Proc. Natl. Acad. Sci. USA 2010, 107, 10860–10865. [Google Scholar] [CrossRef]
- Noren, N.K.; Pasquale, E.B. Eph receptor-ephrin bidirectional signals that target Ras and Rho proteins. Cell. Signal. 2004, 16, 655–666. [Google Scholar] [CrossRef]
- Klein, R. Bidirectional modulation of synaptic functions by Eph/ephrin signaling. Nat. Neurosci. 2009, 12, 15–20. [Google Scholar] [CrossRef]
- Murai, K.K.; Pasquale, E.B. 'Eph'ective signaling: Forward, reverse and crosstalk. J. Cell. Sci. 2003, 116, 2823–2832. [Google Scholar] [CrossRef]
- Lim, Y.S.; McLaughlin, T.; Sung, T.C.; Santiago, A.; Lee, K.F.; O’Leary, D.D. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron 2008, 59, 746–758. [Google Scholar] [CrossRef]
- Yamazaki, T.; Masuda, J.; Omori, T.; Usui, R.; Akiyama, H.; Maru, Y. EphA1 interacts with integrin-linked kinase and regulates cell morphology and motility. J. Cell. Sci. 2009, 122, 243–255. [Google Scholar] [CrossRef]
- Lee, H.S.; Daar, I.O. EphrinB reverse signaling in cell-cell adhesion: Is it just par for the course? Cell Adhes. Migr. 2009, 3, 250–255. [Google Scholar] [CrossRef]
- Carter, N.; Nakamoto, T.; Hirai, H.; Hunter, T. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas). Nat. Cell Biol. 2002, 4, 565–573. [Google Scholar]
- Hattori, M.; Osterfield, M.; Flanagan, J.G. Regulated cleavage of a contact-mediated axon repellent. Science 2000, 289, 1360–1365. [Google Scholar] [CrossRef]
- Sharfe, N.; Freywald, A.; Toro, A.; Roifman, C.M. Ephrin-A1 induces c-Cbl phosphorylation and EphA receptor down-regulation in T cells. J. Immunol. 2003, 170, 6024–6032. [Google Scholar]
- Walker-Daniels, J.; Riese, D.J., 2nd; Kinch, M.S. c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol. Cancer Res. 2002, 1, 79–87. [Google Scholar]
- Wang, Y.; Ota, S.; Kataoka, H.; Kanamori, M.; Li, Z.; Band, H.; Tanaka, M.; Sugimura, H. Negative regulation of EphA2 receptor by Cbl. Biochem. Biophys. Res. Commun. 2002, 296, 214–220. [Google Scholar] [CrossRef]
- Marmor, M.D.; Yarden, Y. Role of protein ubiquitylation in regulating endocytosis of receptor tyrosine kinases. Oncogene. 2004, 23, 2057–2070. [Google Scholar] [CrossRef]
- Palmer, A.; Zimmer, M.; Erdmann, K.S.; Eulenburg, V.; Porthin, A.; Heumann, R.; Deutsch, U.; Klein, R. EphrinB phosphorylation and reverse signaling: Regulation by Src kinases and PTP-BL phosphatase. Mol. Cell 2002, 9, 725–737. [Google Scholar] [CrossRef]
- Baldwin, C.; Chen, Z.W.; Bedirian, A.; Yokota, N.; Nasr, S.H.; Rabb, H.; Lemay, S. Upregulation of EphA2 during in vivo and in vitro renal ischemia-reperfusion injury: Role of Src kinases. Am. J. Physiol. Renal Physiol. 2006, 291, F960–F971. [Google Scholar] [CrossRef]
- Dodelet, V.C.; Pazzagli, C.; Zisch, A.H.; Hauser, C.A.; Pasquale, E.B. A novel signaling intermediate, SHEP1, directly couples Eph receptors to R-Ras and Rap1. J. Biol. Chem. 1999, 274, 31941–31946. [Google Scholar] [CrossRef]
- Marston, D.J.; Dickinson, S.; Nobes, C.D. Rac-dependent trans-endocytosis of ephrinBs regulates Eph-ephrin contact repulsion. Nat. Cell Biol. 2003, 5, 879–888. [Google Scholar] [CrossRef]
- Lu, Q.; Sun, E.E.; Klein, R.S.; Flanagan, J.G. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell 2001, 105, 69–79. [Google Scholar] [CrossRef]
- Feng, Y.X.; Zhao, J.S.; Li, J.J.; Wang, T.; Cheng, S.Q.; Yuan, Y.; Wang, F.; Wang, X.F.; Xie, D. Liver cancer: EphrinA2 promotes tumorigenicity through Rac1/Akt/NF-kappaB signaling pathway 120. Hepatology 2010, 51, 535–544. [Google Scholar] [CrossRef]
- Zhuang, G.; Hunter, S.; Hwang, Y.; Chen, J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-Kinase-dependent Rac1 activation. J. Biol. Chem. 2007, 282, 2683–2694. [Google Scholar] [CrossRef]
- Fang, W.B.; Ireton, R.C.; Zhuang, G.; Takahashi, T.; Reynolds, A.; Chen, J. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J. Cell Sci. 2008, 121, 358–368. [Google Scholar] [CrossRef]
- Margolis, S.S.; Salogiannis, J.; Lipton, D.M.; Mandel-Brehm, C.; Wills, Z.P.; Mardinly, A.R.; Hu, L.; Greer, P.L.; Bikoff, J.B.; Ho, H.Y.; et al. EphB-mediated degradation of the RhoA GEF Ephexin5 relieves a developmental brake on excitatory synapse formation. Cell 2010, 143, 442–455. [Google Scholar] [CrossRef]
- Brantley-Sieders, D.M.; Zhuang, G.; Hicks, D.; Fang, W.B.; Hwang, Y.; Cates, J.M.; Coffman, K.; Jackson, D.; Bruckheimer, E.; Muraoka-Cook, R.S.; et al. The receptor tyrosine kinase EphA2 promotes mammary adenocarcinoma tumorigenesis and metastatic progression in mice by amplifying ErbB2 signaling. J. Clin. Invest. 2008, 118, 64–78. [Google Scholar] [CrossRef]
- Cowan, C.A.; Henkemeyer, M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals. Nature 2001, 413, 174–179. [Google Scholar] [CrossRef]
- Bruckner, K.; Pablo Labrador, J.; Scheiffele, P.; Herb, A.; Seeburg, P.H.; Klein, R. EphrinB ligands recruit GRIP family PDZ adaptor proteins into raft membrane microdomains. Neuron 1999, 22, 511–524. [Google Scholar] [CrossRef]
- Ellis, C.; Kasmi, F.; Ganju, P.; Walls, E.; Panayotou, G.; Reith, A.D. A juxtamembrane autophosphorylation site in the Eph family receptor tyrosine kinase, Sek, mediates high affinity interaction with p59fyn. Oncogene 1996, 12, 1727–1736. [Google Scholar]
- Kalo, M.S.; Pasquale, E.B. Multiple in vivo tyrosine phosphorylation sites in EphB receptors. Biochemistry 1999, 38, 14396–14408. [Google Scholar] [CrossRef]
- Stein, E.; Huynh-Do, U.; Lane, A.A.; Cerretti, D.P.; Daniel, T.O. Nck recruitment to Eph receptor, EphB1/ELK, couples ligand activation to c-Jun kinase. J. Biol. Chem. 1998, 273, 1303–1308. [Google Scholar]
- Han, D.C.; Shen, T.L.; Miao, H.; Wang, B.; Guan, J.L. EphB1 associates with Grb7 and regulates cell migration. J. Biol. Chem. 2002, 277, 45655–45661. [Google Scholar]
- Holland, S.J.; Gale, N.W.; Gish, G.D.; Roth, R.A.; Songyang, Z.; Cantley, L.C.; Henkemeyer, M.; Yancopoulos, G.D.; Pawson, T. Juxtamembrane tyrosine residues couple the Eph family receptor EphB2/Nuk to specific SH2 domain proteins in neuronal cells. EMBO J. 1997, 16, 3877–3888. [Google Scholar] [CrossRef]
- Hu, Q.; Milfay, D.; Williams, L.T. Binding of NCK to SOS and activation of ras-dependent gene expression. Mol. Cell. Biol. 1995, 15, 1169–1174. [Google Scholar]
- Han, D.C.; Shen, T.L.; Guan, J.L. The Grb7 family proteins: Structure, interactions with other signaling molecules and potential cellular functions. Oncogene 2001, 20, 6315–6321. [Google Scholar] [CrossRef]
- Cheng, N.; Brantley, D.M.; Chen, J. The ephrins and Eph receptors in angiogenesis. Cytokine Growth Factor Rev. 2002, 13, 75–85. [Google Scholar] [CrossRef]
- Singh, A.; Winterbottom, E.; Daar, I.O. Eph/ephrin signaling in cell-cell and cell-substrate adhesion. Front. Biosci. 2012, 17, 473–497. [Google Scholar] [CrossRef]
- Lindberg, R.A.; Hunter, T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol. Cell Biol. 1990, 10, 6316–6324. [Google Scholar]
- Sulman, E.P.; Tang, X.X.; Allen, C.; Biegel, J.A.; Pleasure, D.E.; Brodeur, G.M.; Ikegaki, N. ECK, a human EPH-related gene, maps to 1p36.1, a common region of alteration in human cancers. Genomics 1997, 40, 371–374. [Google Scholar] [CrossRef]
- Ruiz, J.C.; Robertson, E.J. The expression of the receptor-protein tyrosine kinase gene, eck, is highly restricted during early mouse development. Mech. Dev. 1994, 46, 87–100. [Google Scholar] [CrossRef]
- Cooper, M.A.; Son, A.I.; Komlos, D.; Sun, Y.; Kleiman, N.J.; Zhou, R. Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc. Natl. Acad. Sci. USA 2008, 105, 16620–16625. [Google Scholar]
- Noberini, R.; Koolpe, M.; Peddibhotla, S.; Dahl, R.; Su, Y.; Cosford, N.D.; Roth, G.P.; Pasquale, E.B. Small molecules can selectively inhibit ephrin binding to the EphA4 and EphA2 receptors. J. Biol. Chem. 2008, 283, 29461–29472. [Google Scholar] [CrossRef]
- Walker-Daniels, J.; Hess, A.R.; Hendrix, M.J.; Kinch, M.S. Differential regulation of EphA2 in normal and malignant cells. Am. J. Pathol. 2003, 162, 1037–1042. [Google Scholar] [CrossRef]
- Davis, S.; Gale, N.W.; Aldrich, T.H.; Maisonpierre, P.C.; Lhotak, V.; Pawson, T.; Goldfarb, M.; Yancopoulos, G.D. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science 1994, 266, 816–819. [Google Scholar]
- Pawson, T.; Nash, P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000, 14, 1027–1047. [Google Scholar]
- Foster, A.; Resnikoff, S. The impact of Vision 2020 on global blindness. Eye (Lond.) 2005, 19, 1133–1135. [Google Scholar] [CrossRef]
- Rahi, J.S.; Dezateux, C. Measuring and interpreting the incidence of congenital ocular anomalies: Lessons from a national study of congenital cataract in the UK. Invest. Ophthalmol. Vis. Sci. 2001, 42, 1444–1448. [Google Scholar]
- Shiels, A.; Mackay, D.; Ionides, A.; Berry, V.; Moore, A.; Bhattacharya, S. A missense mutation in the human connexin50 gene (GJA8) underlies autosomal dominant “zonular pulverulent” cataract, on chromosome 1q. Am. J. Hum. Genet. 1998, 62, 526–532. [Google Scholar] [CrossRef]
- Mackay, D.; Ionides, A.; Kibar, Z.; Rouleau, G.; Berry, V.; Moore, A.; Shiels, A.; Bhattacharya, S. Connexin46 mutations in autosomal dominant congenital cataract. Am. J. Hum. Genet. 1999, 64, 1357–1364. [Google Scholar] [CrossRef]
- Litt, M.; Kramer, P.; LaMorticella, D.M.; Murphey, W.; Lovrien, E.W.; Weleber, R.G. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum. Mol. Genet. 1998, 7, 471–474. [Google Scholar] [CrossRef]
- Berry, V.; Francis, P.; Reddy, M.A.; Collyer, D.; Vithana, E.; MacKay, I.; Dawson, G.; Carey, A.H.; Moore, A.; Bhattacharya, S.S.; et al. Alpha-B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am. J. Hum. Genet. 2001, 69, 1141–1145. [Google Scholar] [CrossRef]
- Mackay, D.S.; Boskovska, O.B.; Knopf, H.L.; Lampi, K.J.; Shiels, A. A nonsense mutation in CRYBB1 associated with autosomal dominant cataract linked to human chromosome 22q. Am. J. Hum. Genet. 2002, 71, 1216–1221. [Google Scholar] [CrossRef]
- Litt, M.; Carrero-Valenzuela, R.; LaMorticella, D.M.; Schultz, D.W.; Mitchell, T.N.; Kramer, P.; Maumenee, I.H. Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human beta-crystallin gene CRYBB2. Hum. Mol. Genet. 1997, 6, 665–668. [Google Scholar] [CrossRef]
- Riazuddin, S.A.; Yasmeen, A.; Yao, W.; Sergeev, Y.V.; Zhang, Q.; Zulfiqar, F.; Riaz, A.; Riazuddin, S.; Hejtmancik, J.F. Mutations in betaB3-crystallin associated with autosomal recessive cataract in two Pakistani families. Invest. Ophthalmol. Vis. Sci. 2005, 46, 2100–2106. [Google Scholar] [CrossRef]
- Kannabiran, C.; Rogan, P.K.; Olmos, L.; Basti, S.; Rao, G.N.; Kaiser-Kupfer, M.; Hejtmancik, J.F. Autosomal dominant zonular cataract with sutural opacities is associated with a splice mutation in the betaA3/A1-crystallin gene. Mol. Vis. 1998, 4, 21. [Google Scholar]
- Billingsley, G.; Santhiya, S.T.; Paterson, A.D.; Ogata, K.; Wodak, S.; Hosseini, S.M.; Manisastry, S.M.; Vijayalakshmi, P.; Gopinath, P.M.; Graw, J.; Heon, E. CRYBA4, a novel human cataract gene, is also involved in microphthalmia. Am. J. Hum. Genet. 2006, 79, 702–709. [Google Scholar] [CrossRef]
- Sun, H.; Ma, Z.; Li, Y.; Liu, B.; Li, Z.; Ding, X.; Gao, Y.; Ma, W.; Tang, X.; Li, X.; et al. Gamma-S crystallin gene (CRYGS) mutation causes dominant progressive cortical cataract in humans. J. Med. Genet. 2005, 42, 706–710. [Google Scholar] [CrossRef]
- Heon, E.; Priston, M.; Schorderet, D.F.; Billingsley, G.D.; Girard, P.O.; Lubsen, N.; Munier, F.L. The gamma-crystallins and human cataracts: A puzzle made clearer. Am. J. Hum. Genet. 1999, 65, 1261–1267. [Google Scholar] [CrossRef]
- Muller, M.; Bhattacharya, S.S.; Moore, T.; Prescott, Q.; Wedig, T.; Herrmann, H.; Magin, T.M. Dominant cataract formation in association with a vimentin assembly disrupting mutation. Hum. Mol. Genet. 2009, 18, 1052–1057. [Google Scholar] [CrossRef]
- Shi, Y.; de Maria, A.; Bennett, T.; Shiels, A.; Bassnett, S. A role for epha2 in cell migration and refractive organization of the ocular lens. Invest. Ophthalmol. Vis. Sci. 2012, 53, 551–559. [Google Scholar] [CrossRef]
- Cheng, C.; Gong, X. Diverse roles of Eph/ephrin signaling in the mouse lens. PLoS One 2012, 6, e28147. [Google Scholar] [CrossRef]
- Son, A.I.; Cooper, M.A.; Sheleg, M.; Sun, Y.; Kleiman, N.J.; Zhou, R. Further analysis of the lens of ephrin-A5(-/-) mice: Development of postnatal defects. Mol. Vis. 2013, 19, 254–266. [Google Scholar]
- Guo, H.; Miao, H.; Gerber, L.; Singh, J.; Denning, M.F.; Gilliam, A.C.; Wang, B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006, 66, 7050–7058. [Google Scholar] [CrossRef]
- Mitchell, K.J.; Pinson, K.I.; Kelly, O.G.; Brennan, J.; Zupicich, J.; Scherz, P.; Leighton, P.A.; Goodrich, L.V.; Lu, X.; Avery, B.J.; et al. Functional analysis of secreted and transmembrane proteins critical to mouse development. Nat. Genet. 2001, 28, 241–249. [Google Scholar] [CrossRef]
- Naruse-Nakajima, C.; Asano, M.; Iwakura, Y. Involvement of EphA2 in the formation of the tail notochord via interaction with ephrinA1. Mech. Dev. 2001, 102, 95–105. [Google Scholar] [CrossRef]
- Fang, W.B.; Brantley-Sieders, D.M.; Hwang, Y.; Ham, A.J.; Chen, J. Identification and functional analysis of phosphorylated tyrosine residues within EphA2 receptor tyrosine kinase. J. Biol. Chem. 2008, 283, 16017–16026. [Google Scholar] [CrossRef]
- Dufour, A.; Egea, J.; Kullander, K.; Klein, R.; Vanderhaeghen, P. Genetic analysis of EphA-dependent signaling mechanisms controlling topographic mapping in vivo. Development 2006, 133, 4415–4420. [Google Scholar] [CrossRef]
- Park, E.K.; Warner, N.; Bong, Y.S.; Stapleton, D.; Maeda, R.; Pawson, T.; Daar, I.O. Ectopic EphA4 receptor induces posterior protrusions via FGF signaling in Xenopus embryos. Mol. Biol. Cell 2004, 15, 1647–1655. [Google Scholar] [CrossRef]
- Kikawa, K.D.; Vidale, D.R.; van Etten, R.L.; Kinch, M.S. Regulation of the EphA2 kinase by the low molecular weight tyrosine phosphatase induces transformation. J. Biol. Chem. 2002, 277, 39274–39279. [Google Scholar]
- Parri, M.; Buricchi, F.; Taddei, M.L.; Giannoni, E.; Raugei, G.; Ramponi, G.; Chiarugi, P. EphrinA1 repulsive response is regulated by an EphA2 tyrosine phosphatase. J. Biol. Chem. 2005, 280, 34008–34018. [Google Scholar]
- Saint-Geniez, M.; D’Amore, P.A. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int. J. Dev. Biol. 2004, 48, 1045–1058. [Google Scholar] [CrossRef]
- Gariano, R.F.; Kalina, R.E.; Hendrickson, A.E. Normal and pathological mechanisms in retinal vascular development. Surv. Ophthalmol. 1996, 40, 481–490. [Google Scholar] [CrossRef]
- Gariano, R.F.; Gardner, T.W. Retinal angiogenesis in development and disease. Nature 2005, 438, 960–966. [Google Scholar] [CrossRef]
- Stahl, A.; Connor, K.M.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Invest. Ophthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef]
- Miller, J.W.; Le Couter, J.; Strauss, E.C.; Ferrara, N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 2013, 120, 106–114. [Google Scholar] [CrossRef]
- Kempen, J.H.; O’Colmain, B.J.; Leske, M.C.; Haffner, S.M.; Klein, R.; Moss, S.E.; Taylor, H.R.; Hamman, R.F. The prevalence of diabetic retinopathy among adults in the United States. Arch. Ophthalmol. 2004, 122, 552–563. [Google Scholar]
- Klein, B.E. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 2007, 14, 179–183. [Google Scholar] [CrossRef]
- Congdon, N.; O’Colmain, B.; Klaver, C.C.; Klein, R.; Munoz, B.; Friedman, D.S.; Kempen, J.; Taylor, H.R.; Mitchell, P. Causes and prevalence of visual impairment among adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef]
- Friedman, D.S.; O’Colmain, B.J.; Munoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J. Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [CrossRef]
- Mechoulam, H.; Pierce, E.A. Retinopathy of prematurity: Molecular pathology and therapeutic strategies. Am. J. Pharmacogenomics 2003, 3, 261–277. [Google Scholar] [CrossRef]
- Hess, A.R.; Seftor, E.A.; Gardner, L.M.; Carles-Kinch, K.; Schneider, G.B.; Seftor, R.E.; Kinch, M.S.; Hendrix, M.J. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: Role of epithelial cell kinase (Eck/EphA2). Cancer Res. 2001, 61, 3250–3255. [Google Scholar]
- Ogawa, K.; Pasqualini, R.; Lindberg, R.A.; Kain, R.; Freeman, A.L.; Pasquale, E.B. The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene. 2000, 19, 6043–6052. [Google Scholar] [CrossRef]
- Brantley, D.M.; Cheng, N.; Thompson, E.J.; Lin, Q.; Brekken, R.A.; Thorpe, P.E.; Muraoka, R.S.; Cerretti, D.P.; Pozzi, A.; Jackson, D.; et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo . Oncogene 2002, 21, 7011–7026. [Google Scholar] [CrossRef]
- Cheng, N.; Brantley, D.M.; Liu, H.; Lin, Q.; Enriquez, M.; Gale, N.; Yancopoulos, G.; Cerretti, D.P.; Daniel, T.O.; Chen, J. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol. Cancer Res. 2002, 1, 2–11. [Google Scholar] [CrossRef]
- Cheng, N.; Brantley, D.; Fang, W.B.; Liu, H.; Fanslow, W.; Cerretti, D.P.; Bussell, K.N.; Reith, A.; Jackson, D.; Chen, J. Inhibition of VEGF-dependent multistage carcinogenesis by soluble EphA receptors. Neoplasia 2003, 5, 445–456. [Google Scholar]
- Dobrzanski, P.; Hunter, K.; Jones-Bolin, S.; Chang, H.; Robinson, C.; Pritchard, S.; Zhao, H.; Ruggeri, B. Antiangiogenic and antitumor efficacy of EphA2 receptor antagonist. Cancer Res. 2004, 64, 910–919. [Google Scholar] [CrossRef]
- Recchia, F.M.; Xu, L.; Penn, J.S.; Boone, B.; Dexheimer, P.J. Identification of genes and pathways involved in retinal neovascularization by microarray analysis of two animal models of retinal angiogenesis. Invest. Ophthalmol. Vis. Sci. 2010, 51, 1098–1105. [Google Scholar] [CrossRef]
- Chen, J.; Hicks, D.; Brantley-Sieders, D.; Cheng, N.; McCollum, G.W.; Qi-Werdich, X.; Penn, J. Inhibition of retinal neovascularization by soluble EphA2 receptor. Exp. Eye Res. 2006, 82, 664–673. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, Y.L.; Li, Y.; Dai, W.B.; Guo, Z.M.; Wang, Z.H.; Zhang, Q. EphA2 targeted doxorubicin stealth liposomes as a therapy system for choroidal neovascularization in rats. Invest. Ophthalmol. Vis. Sci. 2012, 53, 7348–7357. [Google Scholar]
- Ojima, T.; Takagi, H.; Suzuma, K.; Oh, H.; Suzuma, I.; Ohashi, H.; Watanabe, D.; Suganami, E.; Murakami, T.; Kurimoto, M.; et al. EphrinA1 inhibits vascular endothelial growth factor-induced intracellular signaling and suppresses retinal neovascularization and blood-retinal barrier breakdown. Am. J. Pathol. 2006, 168, 331–339. [Google Scholar] [CrossRef]
- Miao, H.; Wang, B. Eph/ephrin signaling in epithelial development and homeostasis. Int. J. Biochem. Cell Biol. 2009, 41, 762–770. [Google Scholar] [CrossRef]
- Costantini, F.; Kopan, R. Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Dev. Cell 2010, 18, 698–712. [Google Scholar] [CrossRef]
- Wakayama, Y.; Miura, K.; Sabe, H.; Mochizuki, N. EphrinA1-EphA2 signal induces compaction and polarization of Madin-Darby canine kidney cells by inactivating Ezrin through negative regulation of RhoA. J. Biol. Chem. 2011, 286, 44243–44253. [Google Scholar] [CrossRef]
- Kellerman, P.S.; Norenberg, S.L.; Jones, G.M. Early recovery of the actin cytoskeleton during renal ischemic injury in vivo. Am. J. Kidney Dis. 1996, 27, 709–714. [Google Scholar] [CrossRef]
- Molitoris, B.A. Actin cytoskeleton in ischemic acute renal failure. Kidney Int. 2004, 66, 871–883. [Google Scholar] [CrossRef]
- Molitoris, B.A. Ischemia-induced loss of epithelial polarity: Potential role of the actin cytoskeleton. Am. J. Physiol. 1991, 260, F769–F778. [Google Scholar]
- Molitoris, B.A.; Dahl, R.; Geerdes, A. Cytoskeleton disruption and apical redistribution of proximal tubule Na(+)-K(+)-ATPase during ischemia. Am. J. Physiol. 1992, 263, F488–F495. [Google Scholar]
- Murai, K.K.; Pasquale, E.B. New exchanges in eph-dependent growth cone dynamics. Neuron 2005, 46, 161–163. [Google Scholar] [CrossRef]
- Nahm, O.; Woo, S.K.; Handler, J.S.; Kwon, H.M. Involvement of multiple kinase pathways in stimulation of gene transcription by hypertonicity. Am. J. Physiol. Cell Physiol. 2002, 282, C49–C58. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Steiner, A.A.; Scheck, A.C.; Romanovsky, A.A. Expression of Eph receptors and their ligands, ephrins, during lipopolysaccharide fever in rats. Physiol. Genomics 2005, 21, 152–160. [Google Scholar] [CrossRef]
- Li, Z.; Tanaka, M.; Kataoka, H.; Nakamura, R.; Sanjar, R.; Shinmura, K.; Sugimura, H. EphA2 up-regulation induced by deoxycholic acid in human colon carcinoma cells, an involvement of extracellular signal-regulated kinase and p53-independence. J. Cancer Res. Clin. Oncol. 2003, 129, 703–708. [Google Scholar] [CrossRef]
- Matsuo, K.; Irie, N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef]
- Matsuo, K.; Ray, N. Osteoclasts, mononuclear phagocytes, and c-Fos: New insight into osteoimmunology. Keio J. Med. 2004, 53, 78–84. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Yasuda, H.; Shima, N.; Nakagawa, N.; Yamaguchi, K.; Kinosaki, M.; Mochizuki, S.; Tomoyasu, A.; Yano, K.; Goto, M.; Murakami, A.; et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. USA 1998, 95, 3597–3602. [Google Scholar] [CrossRef]
- Hennighausen, L.; Robinson, G.W. Information networks in the mammary gland. Nat. Rev. Mol. Cell. Biol. 2005, 6, 715–725. [Google Scholar] [CrossRef]
- Watson, C.J.; Khaled, W.T. Mammary development in the embryo and adult: A journey of morphogenesis and commitment. Development 2008, 135, 995–1003. [Google Scholar] [CrossRef]
- Andres, A.-C.; Ziemiecki, A. Eph and ephrin signaling in mammary gland morphogenesis and cancer. J. Mammary Gland Biol. Neoplasia 2003, 8, 475–485. [Google Scholar] [CrossRef]
- Munarini, N.; Jager, R.; Abderhalden, S.; Zuercher, G.; Rohrbach, V.; Loercher, S.; Pfanner-Meyer, B.; Andres, A.C.; Ziemiecki, A. Altered mammary epithelial development, pattern formation and involution in transgenic mice expressing the EphB4 receptor tyrosine kinase. J. Cell. Sci. 2002, 115, 25–37. [Google Scholar]
- Haldimann, M.; Custer, D.; Munarini, N.; Stirnimann, C.; Zurcher, G.; Rohrbach, V.; Djonov, V.; Ziemiecki, A.; Andres, A.C. Deregulated ephrin-B2 expression in the mammary gland interferes with the development of both the glandular epithelium and vasculature and promotes metastasis formation. Int. J. Oncol. 2009, 35, 525–536. [Google Scholar]
- Sternlicht, M.D.; Kouros-Mehr, H.; Lu, P.; Werb, Z. Hormonal and local control of mammary branching morphogenesis. Differentiation 2006, 74, 365–381. [Google Scholar] [CrossRef]
- Nikolova, Z.; Djonov, V.; Zuercher, G.; Andres, A.C.; Ziemiecki, A. Cell-type specific and estrogen dependent expression of the receptor tyrosine kinase EphB4 and its ligand ephrin-B2 during mammary gland morphogenesis. J. Cell. Sci. 1998, 111, 2741–2751. [Google Scholar]
- Zelinski, D.P.; Zantek, N.D.; Walker-Daniels, J.; Peters, M.A.; Taparowsky, E.J.; Kinch, M.S. Estrogen and Myc negatively regulate expression of the EphA2 tyrosine kinase. J. Cell. Biochem. 2002, 85, 714–720. [Google Scholar] [CrossRef]
- Martin, K.J.; Patrick, D.R.; Bissell, M.J.; Fournier, M.V. Prognostic breast cancer signature identified from 3D culture model accurately predicts clinical outcome across independent datasets. PLoS One 2008, 3, e2994. [Google Scholar] [CrossRef]
- Fournier, M.V.; Martin, K.J.; Kenny, P.A.; Xhaja, K.; Bosch, I.; Yaswen, P.; Bissell, M.J. Gene expression signature in organized and growth-arrested mammary acini predicts good outcome in breast cancer. Cancer Res. 2006, 66, 7095–7102. [Google Scholar] [CrossRef]
- Ireton, R.C.; Chen, J. EphA2 receptor tyrosine kinase as a promising target for cancer therapeutics. Curr. Cancer Drug Targets 2005, 5, 149–157. [Google Scholar] [CrossRef]
- Kamat, A.A.; Coffey, D.; Merritt, W.M.; Nugent, E.; Urbauer, D.; Lin, Y.G.; Edwards, C.; Broaddus, R.; Coleman, R.L.; Sood, A.K. EphA2 overexpression is associated with lack of hormone receptor expression and poor outcome in endometrial cancer. Cancer 2009, 115, 2684–2692. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-stromal interactions. Transforming growth factor-beta isoforms and hepatocyte growth factor/scatter factor in mammary gland ductal morphogenesis. Breast Cancer Res. 2001, 3, 230–237. [Google Scholar] [CrossRef]
- Bianchi, L.M.; Liu, H. Comparison of ephrin-A ligand and EphA receptor distribution in the developing inner ear. Anat. Rec. 1999, 254, 127–134. [Google Scholar] [CrossRef]
- Pickles, J.O.; Claxton, C.; van Heumen, W.R. Complementary and layered expression of Ephs and ephrins in developing mouse inner ear. J. Comp. Neurol. 2002, 449, 207–216. [Google Scholar] [CrossRef]
- Van Heumen, W.R.; Claxton, C.; Pickles, J.O. Expression of EphA4 in developing inner ears of the mouse and guinea pig. Hear. Res. 2000, 139, 42–50. [Google Scholar] [CrossRef]
- Howard, M.A.; Rodenas-Ruano, A.; Henkemeyer, M.; Martin, G.K.; Lonsbury-Martin, B.L.; Liebl, D.J. Eph receptor deficiencies lead to altered cochlear function. Hear. Res. 2003, 178, 118–130. [Google Scholar] [CrossRef]
- Torres, M.; Giraldez, F. The development of the vertebrate inner ear. Mech. Dev. 1998, 71, 5–21. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
