Genetic, Clinical, and Sociodemographic Profile of Individuals with Diagnosis or Family History of Hypertrophic Cardiomyopathy: Insights from a Prospective Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
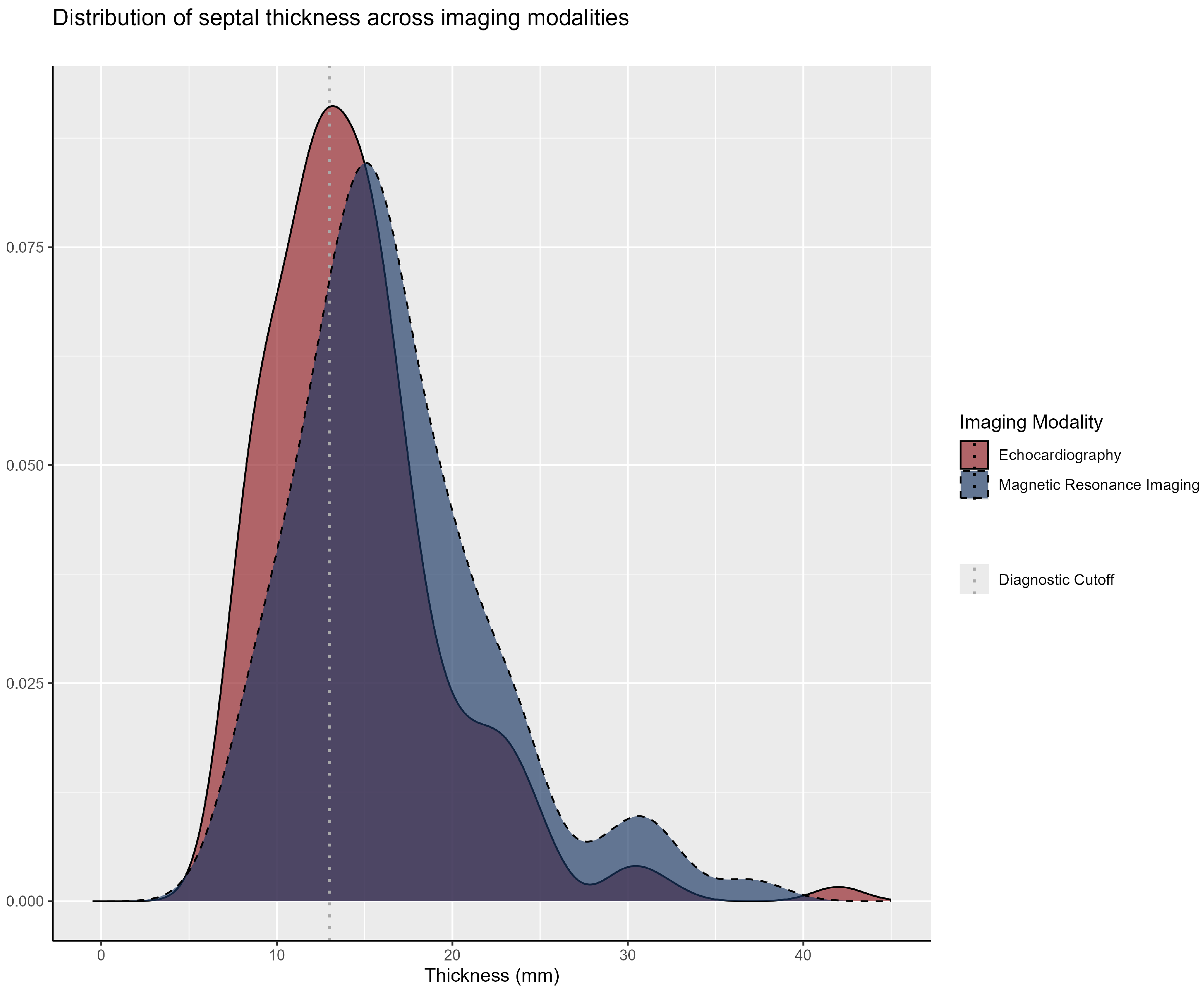
2.4. HCM Diagnosis Definition
2.5. Molecular Testing
2.6. Sample Characterization Variables
2.7. Statistical Analysis
2.8. Ethical and Legal Principles
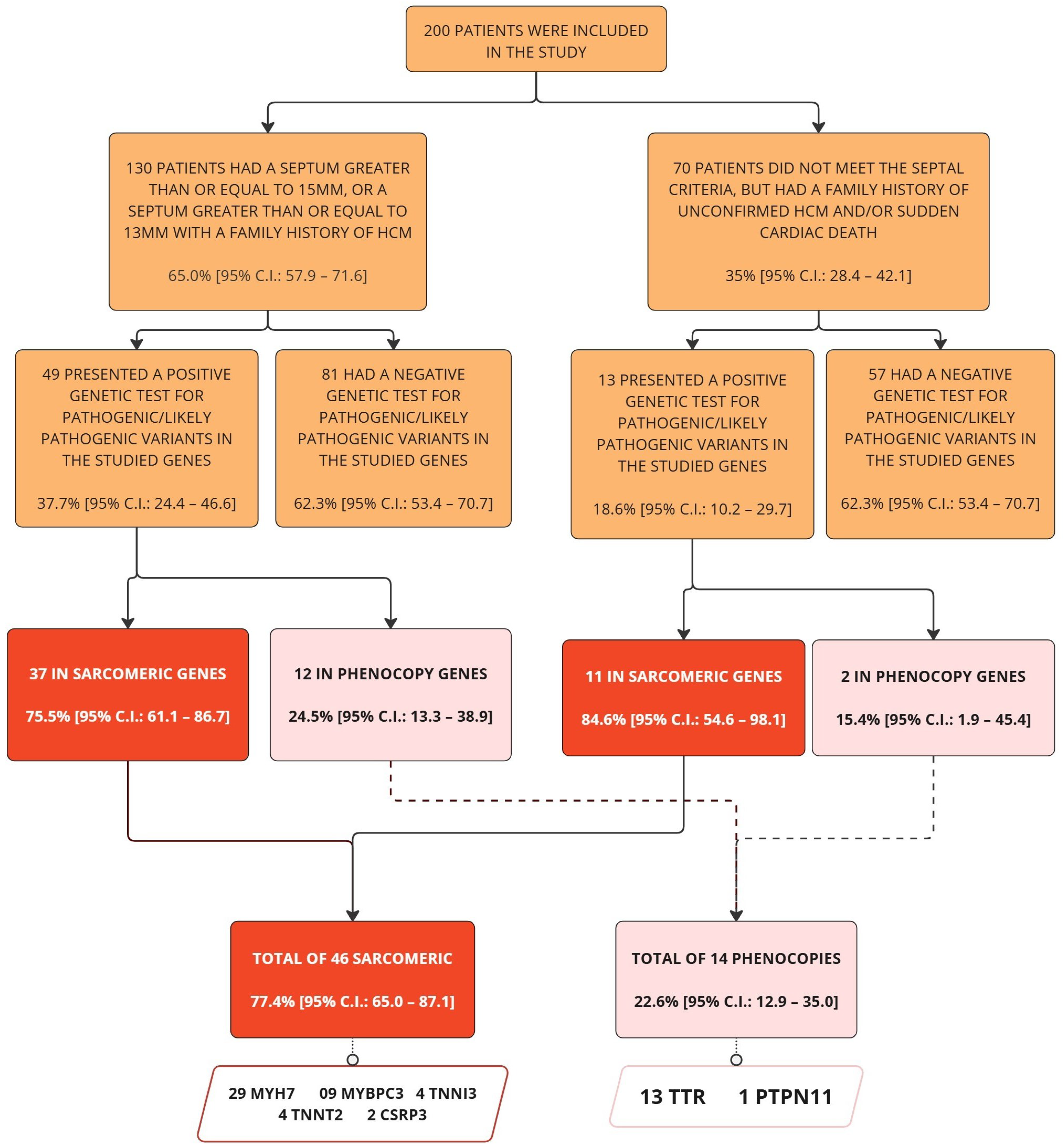
3. Results
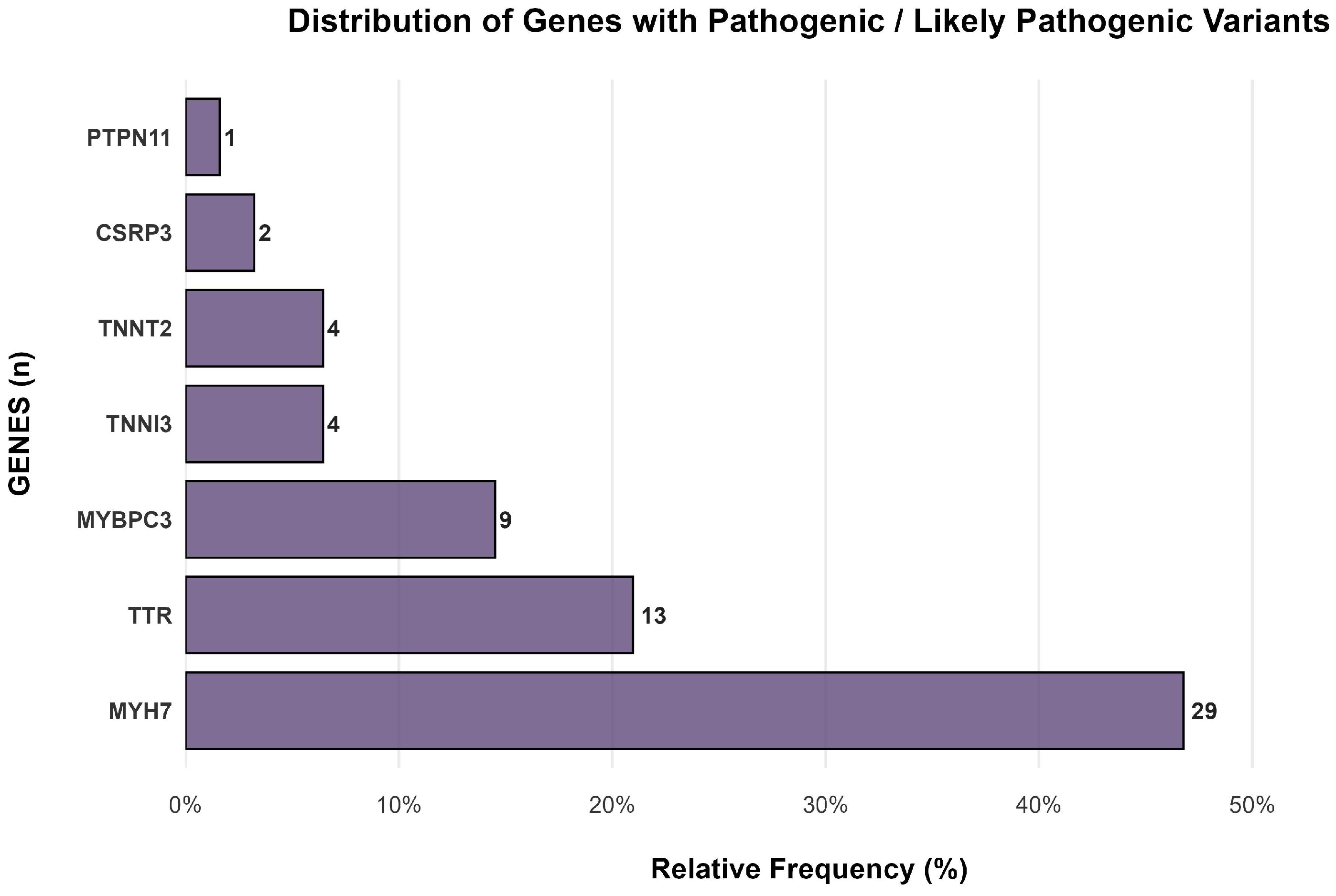
3.1. Genetic Analysis
3.2. Clinical Manifestations and Family History
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| ACEI | Angiotensin-Converting Enzyme Inhibitor |
| AHA | American Heart Association |
| AMSSM | American Medical Society for Sports Medicine |
| ARB | Angiotensin Receptor Blocker |
| BMI | Body Mass Index |
| DM | Diabetes Mellitus |
| ESC | European Society of Cardiology |
| GLA | Gene Associated with Fabry Disease |
| HCM | Hypertrophic Cardiomyopathy |
| HRS | Heart Rhythm Society |
| ICD | Implantable Cardioverter Defibrillator |
| IQR | Interquartile Range |
| LVH | Left Ventricular Hypertrophy |
| MLVWT | Maximum Left Ventricular Wall Thickness |
| MRI | Magnetic Resonance Imaging |
| MYBPC3 | Myosin-Binding Protein C3 |
| MYH7 | Myosin Heavy Chain 7 |
| NGS | Next-Generation Sequencing |
| P/LP | Pathogenic or Likely Pathogenic |
| PACES | Pediatric and Congenital Electrophysiology Society |
| RMC | Cardiac Magnetic Resonance |
| SCD | Sudden Cardiac Death |
| SCMR | Society for Cardiovascular Magnetic Resonance |
| TCLE | Termo de Consentimento Livre e Esclarecido (Informed Consent Form) |
| TTE | Transthoracic Echocardiography |
| TTR | Transthyretin |
| VUS | Variant of Uncertain Significance |
Appendix A
| Family | Gene | Variant | Amino Acid Change | ACMG Classification |
|---|---|---|---|---|
| CX | CSRP3 | 536C>T | Thr179Met | Likely Pathogenic |
| XX | CSRP3 | 715G>A | Asp239Asn | Pathogenic |
| CXIX | MYBPC3 | 3662del | Leu1221Argfs*16 | Pathogenic |
| CXXX | MYBPC3 | 3662del | Leu1221Argfs*16 | Pathogenic |
| CXXX | MYBPC3 | 3662del | Leu1221Argfs*16 | Pathogenic |
| I | MYBPC3 | 1484G>A | Arg495Gln | Pathogenic |
| I | MYBPC3 | 1484G>A | Arg495Gln | Pathogenic |
| LI | MYBPC3 | 1800del | Lys600Asnfs*2 | Pathogenic |
| LI | MYBPC3 | 1800del | Lys600Asnfs*2 | Likely Pathogenic |
| XLV | MYBPC3 | Arg453Cys | Leu1221Argfs*16 | Pathogenic |
| XXVIII | MYBPC3 | 3662del | Leu1221Argfs*16 | Pathogenic |
| CIII | MYH7 | 2389G>A | Ala797Thr | Pathogenic |
| CIII | MYH7 | 2389G>A | Ala797Thr | Pathogenic |
| CXII | MYH7 | 1357C>T | Arg453Cys | Pathogenic |
| CXX | MYH7 | 715G>A | Asp239Asn | Pathogenic |
| CXXI | MYH7 | 1357C>T | Arg453Cys | Pathogenic |
| CXXXI | MYH7 | 2167C>G | Arg723Gly | Pathogenic |
| LIX | MYH7 | 1357C>T | Arg453Cys | Pathogenic |
| LV | MYH7 | 715G>A | Asp239Asn | Pathogenic |
| LVII | MYH7 | 715G>A | Asp239Asn | Pathogenic |
| LVII | MYH7 | 1750G>C | Gly584Arg | Pathogenic |
| LVIII | MYH7 | 715G>A;p | Asp239Asn | Pathogenic |
| LXI | MYH7 | 746G>A | Arg249Gln | Pathogenic |
| LXXV | MYH7 | 2389G>A | Ala797Thr | Likely Pathogenic |
| LXXV | MYH7 | 2389G>A | Ala797Thr | Likely Pathogenic |
| LXXV | MYH7 | 2389G>A | Ala797Thr | Pathogenic |
| LXXXIV | MYH7 | 2389G>A | Ala797Thr | Likely Pathogenic |
| XCIX | MYH7 | 2012G>A | Arg671His | Pathogenic |
| XI | MYH7 | 1357C>T | Arg453Cys | Pathogenic |
| XII | MYH7 | 1357C>T | Arg453Cys | Pathogenic |
| XIX | MYH7 | 1750G>C | Gly584Arg | Pathogenic |
| XLIX | MYH7 | 2389G>A | Ala797Thr | Pathogenic |
| XLVI | MYH7 | 1750G>C | Gly584Arg | Pathogenic |
| XVII | MYH7 | 1750G>C | Gly584Arg | Pathogenic |
| XXXV | MYH7 | 715G>A | Asp239Asn | Pathogenic |
| XXXVII | MYH7 | 2389G>A | Ala797Thr | Pathogenic |
| III | PTPN11 | 836A>G | Tyr279Cys | Pathogenic |
| CIX | TNNI3 | 575G>A | Arg192His | Pathogenic |
| XVIII | TNNI3 | 470C>T | Ala157Val | Pathogenic |
| CXLI | TNNT2 | 856C>T | Arg286Cys | Pathogenic |
| CXXVIII | TNNT2 | 418C>T | Arg140Cys | Pathogenic |
| CXXXIX | TNNT2 | 877C>T | Arg293Cys | Pathogenic |
| LII | TNNT2 | 877C>T | Arg293Cys | Likely Pathogenic |
| CLI | TTR | 424G>A | Val142Ile | Pathogenic |
| CXXVII | TTR | 424G>A | Val142Ile | Pathogenic |
| L | TTR | 424G>A | Val142Ile | Pathogenic |
| LVI | TTR | 424G>A | Val142Ile | Pathogenic |
| LXIII | TTR | 424G>A | Val142Ile | Pathogenic |
| LXXIX | TTR | 424G>A | Val142Ile | Pathogenic |
| LXXXIII | TTR | 424G>A | Val142Ile | Pathogenic |
| XCII | TTR | 424G>A | Val142Ile | Pathogenic |
| XIV | TTR | 424G>A | Val142Ile | Pathogenic |
| XLVIII | TTR | 424G>A | Val142Ile | Pathogenic |
| XXV | TTR | 424G>A | Val142Ile | Pathogenic |
| XXV | TTR | 5330-2A>G | Splice acceptor | Likely Pathogenic |
References
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and evaluation of hypertrophic cardiomyopathy: State-of-the-art review of JACC. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Allouba, M.; Walsh, R.; Afify, A.; Hosny, M.; Halawa, S.; Galal, A.; Fathy, M.; Theotokis, P.I.; Boraey, A.; Ellithy, A.; et al. Ethnicity, consanguinity, and genetic architecture of hypertrophic cardiomyopathy. Eur. Heart J. 2023, 44, 5146–5158. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311, Erratum in Circulation 2024, 150. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. Hypertrophic cardiomyopathy: A brief overview. Am. J. Cardiol. 2024, 212, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Marian, A.J. Molecular genetic basis of hypertrophic cardiomyopathy. Circ. Res. 2021, 128, 1533–1553. [Google Scholar] [CrossRef]
- Bonaventura, J.; Polakova, E.; Vejtasova, V.; Veselka, J. Genetic testing in hypertrophic cardiomyopathy patients. Int. J. Mol. Sci. 2021, 22, 10401. [Google Scholar] [CrossRef]
- Walsh, R.; Offerhaus, J.A.; Tadros, R.; Bezzina, C.R. Minor hypertrophic cardiomyopathy genes, major insights into the genetics of cardiomyopathies. Nat. Rev. Cardiol. 2022, 19, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Licordari, R.; Trimarchi, G.; Teresi, L.; Restelli, D.; Lofrumento, F.; Perna, A.; Campisi, M.; de Gregorio, C.; Grimaldi, P.; Calabrò, D.; et al. Cardiac magnetic resonance in HCM phenocopies: From diagnosis to risk stratification and therapeutic management. J. Clin. Med. 2023, 12, 3481. [Google Scholar] [CrossRef]
- Cirino, A.L.; Ho, C. Hypertrophic Cardiomyopathy Overview. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993–2025; Updated 8 July 2021. [Google Scholar] [PubMed]
- Fogel, M.A.; Anwar, S.; Broberg, C.; Browne, L.; Chung, T.; Johnson, T.; Muthurangu, V.; Taylor, M.; Valsangiacomo-Buechel, E.; Wilhelm, C. Cardiovascular magnetic resonance/society for cardiovascular imaging/society of pediatric radiology/north american cardiovascular imaging society guidelines for the use of cardiac magnetic resonance in congenital and acquired pediatric heart disease: Endorsed by the American Heart Association. Circ. Cardiovasc. Imaging 2022, 15, e014415. [Google Scholar]
- Santos, E.d.S.; Castro, P.H.G.; Lima, L.P.S.; Pimentel, J.V.A.; Kuhn, G.d.C.; Sousa, A.C.S.; Oliveira, J.L.M. Mortality from hypertrophic cardiomyopathy in Brazil—Historical series. Int. J. Environ. Res. Public Health 2024, 21, 1498. [Google Scholar] [CrossRef]
- Scolari, F.L.; Garbin, H.I.; Beuren, T.M.A.; Matheus, F.C.; Mourilhe-Rocha, R.; Bittencourt, M.I. Genetics of the cardiomyopathies: A review for the cardiologist. ABC Heart Fail. Cardiomyop. 2024, 4, e20240047. [Google Scholar] [CrossRef]
- Wilde, A.A.M.; Semsarian, C.; Márquez, M.F.; Shamloo, A.S.; Ackerman, M.J.; Ashley, E.A.; Sternick, E.B.; Barajas-Martinez, H.; Behr, E.R.; Bezzina, C.R.; et al. Expert Consensus Statement from the European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia-Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) on the State of Genetic Testing for Cardiac Diseases. Heart Rhythm. 2022, 19, e1–e60. [Google Scholar] [CrossRef]
- RStudio Team. RStudio (Version 2024.09.0, Build 375) [Software]. Posit Software, PBC. Available online: https://posit.co/products/open-source/rstudio/?sid=1 (accessed on 5 February 2025).
- Irlanda, C.G.; Ho, C.Y. Genetic testing in hypertrophic cardiomyopathy. Am. J. Cardiol. 2024, 212, S4–S13. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Givertz, M.M.; Ho, C.Y.; Judge, D.P.; Kantor, P.F.; McBride, K.L.; Morales, A.; Taylor, M.R.; Vatta, M.; Ware, S.M. Genetic evaluation of cardiomyopathy—A Heart Failure Society of America Practice Guideline. J. Card Fail. 2018, 24, 281–302. [Google Scholar] [CrossRef]
- e Silva, S.M.; Chaves, A.V.F.; Antunes, M.; Costabel, J.P.; da Fonseca, A.A.; Furtado, A.; Gomez-Mesa, J.E.; Gutiérrez, F.J.M.; Caspi, O.; Maksimova, I.; et al. Multinational experience with next-generation sequencing: Opportunity to identify transthyretin cardiac amyloidosis and Fabry disease. Cardiovasc. Diagn. Ther. 2024, 14, 294–303. [Google Scholar] [CrossRef]
- Topriceanu, C.C.; Pereira, A.C.; Moon, J.C.; Captur, G.; Ho, C.Y. Meta-analysis of penetrance and systematic review on transition to disease in genetic hypertrophic cardiomyopathy. Circulation 2024, 149, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat-Hamedani, F.; Kayvanpour, E.; Tugrul, O.F.; Lai, A.; Amr, A.; Haas, J.; Proctor, T.; Ehlermann, P.; Jensen, K.; Katus, H.A.; et al. Clinical outcomes associated with sarcomere mutations in hypertrophic cardiomyopathy: A meta-analysis on 7675 individuals. Clin. Res. Cardiol. 2018, 107, 30–41. [Google Scholar] [CrossRef]
- Chiswell, K.; Zaininger, L.; Semsarian, C. Evolution of genetic testing and gene therapy in hypertrophic cardiomyopathy. Prog. Cardiovasc. Dis. 2023, 80, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.R.; Futema, M.; Akhtar, M.M.; Lorenzini, M.; Pittman, A.; Syrris, P.; Elliott, P.M. Prevalence of TTR variants detected by whole-exome sequencing in hypertrophic cardiomyopathy. Amyloid 2019, 26, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, A.M.C.; Janssen, A.; Boorsma, P.C.; Mannens, M.M.A.M.; Wilde, A.A.M.; Christiaans, I. Transthyretin amyloidosis: A phenocopy of hypertrophic cardiomyopathy. Amyloid 2017, 24, 87–91. [Google Scholar] [CrossRef]
- Barker, N.; Judge, D.P. Counseling Family Members and Monitoring for Evidence of Disease in Asymptomatic Carriers of Amyloid Transthyretin Cardiac Amyloidosis. Am. J. Cardiol. 2022, 185, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Reddi, H.V.; Jenkins, S.; Theis, J.; Thomas, B.C.; Connors, L.H.; Van Rhee, F.; Highsmith, W.E. Homozygosity for the V122I mutation in transthyretin is associated with earlier onset of cardiac amyloidosis in the African American population in the seventh decade of life. J. Mol. Diagn. 2014, 16, 68–74. [Google Scholar] [CrossRef]
- Nakashima, Y.; Kubo, T.; Sugiura, K.; Ochi, Y.; Takahashi, A.; Baba, Y.; Hirota, T.; Yamasaki, N.; Kimura, A.; Doi, Y.L.; et al. Lifelong clinical impact of the presence of sarcomere gene mutation in Japanese patients with hypertrophic cardiomyopathy. Circ. J. 2020, 84, 1846–1853. [Google Scholar] [CrossRef]
- de Marvao, A.; McGurk, K.A.; Zheng, S.L.; Thanaj, M.; Bai, W.; Duan, J.; Biffi, C.; Mazzarotto, F.; Statton, B.; Dawes, T.J.W.; et al. Phenotypic expression and outcomes in individuals with rare genetic variants of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2021, 78, 1097–1110. [Google Scholar] [CrossRef]
- van Velzen, H.G.; Vriesendorp, P.A.; Oldenburg, R.A.; van Slegtenhorst, M.A.; van der Velden, J.; Schinkel, A.F.L.; Michels, M. Value of genetic testing for the prediction of long-term outcome in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 2016, 118, 881–887. [Google Scholar] [CrossRef]
- Ho, C.Y.; Day, S.M.; Ashley, E.A.; Michels, M.; Pereira, A.C.; Jacoby, D.; Cirino, A.L.; Fox, J.C.; Lakdawala, N.K.; Ware, J.S.; et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: Insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018, 138, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.; de Brouwer, R.; Hassanzada, F.; Schoemaker, A.E.; Schmidt, A.F.; Kooijman-Reumerman, M.D.; Bracun, V.; Slieker, M.G.; Dooijes, D.; Vermeer, A.M.C.; et al. Penetrance and prognosis of MYH7 variant-associated cardiomyopathies: Results from a Dutch multicenter cohort study. JACC Heart Fail. 2024, 12, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Tudurachi, B.-S.; Zăvoi, A.; Leonte, A.; Țăpoi, L.; Ureche, C.; Bîrgoan, S.G.; Chiuariu, T.; Anghel, L.; Radu, R.; Sascău, R.A.; et al. An update on MYBPC3 gene mutation in hypertrophic cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 10510. [Google Scholar] [CrossRef]
- Viswanathan, S.K.; Sanders, H.K.; McNamara, J.W.; Jagadeesan, A.; Jahangir, A.; Tajik, A.J.; Sadayappan, S.; Ai, T. Hypertrophic cardiomyopathy clinical phenotype is independent of gene mutation and mutation dosage. PLoS ONE 2017, 12, e0187948. [Google Scholar] [CrossRef]
- Lipshultz, S.E.; Law, Y.M.; Asante-Korang, A.; Austin, E.D.; Dipchand, A.I.; Everitt, M.D.; Hsu, D.T.; Lin, K.Y.; Price, J.F.; Wilkinson, J.D.; et al. Cardiomyopathy in children: Classification and diagnosis: A scientific statement from the American Heart Association. Circulation 2019, 140, e9–e68. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, K.; Huang, J.; Chen, Y.; Liu, H.; Zhang, X.; Luo, W.; Xu, Z. Characteristics and outcomes associated with sarcomere mutations in patients with hypertrophic cardiomyopathy: A systematic review and meta-analysis. Int. J. Cardiol. 2024, 409, 132213. [Google Scholar] [CrossRef] [PubMed]
- Chumakova, O.S.; Baklanova, T.N.; Milovanova, N.V.; Zateyshchikov, D.A. Hypertrophic cardiomyopathy in underrepresented populations: Clinical and genetic landscape based on a Russian single-center cohort study. Genes 2023, 14, 2042. [Google Scholar] [CrossRef] [PubMed]
- Wasserstrum, Y.; Barriales-Villa, R.; Fernández-Fernández, X.; Adler, Y.; Lotan, D.; Peled, Y.; Klempfner, R.; Kuperstein, R.; Shlomo, N.; Sabbag, A.; et al. The impact of diabetes mellitus on the clinical phenotype of hypertrophic cardiomyopathy. Eur. Heart J. 2018, 40, 1671–1677. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Lee, H.-J.; Lee, H.; Park, J.-B.; Kim, Y.-J.; Han, K.; Kim, H.-K. Age-dependent association of metabolic dyslipidemia with clinical expression of hypertrophic cardiomyopathy. Int. J. Cardiol. 2023, 396, 131574. [Google Scholar] [CrossRef]
| Overall (N = 200) | Positive Genotype (n = 62) | Negative Genotype (n = 138) | p-Value 1 | |
|---|---|---|---|---|
| Sample Profile | ||||
| Median Age, (median [IQR]) | 52 (41–66) | 48 (38–67) | 54 (43–65) | 0.2 |
| Men, n (%) | 115 (58%) | 30 (48%) | 85 (62%) | 0.081 |
| Body Mass Index, kg/m2 (median [IQR]) | 27.4 (25.2–30.5) | 27.4 (25.5–30.7) | 27.3 (24.2–30.0) | 0.5 |
| Signs and Symptoms, n (%) | ||||
| Palpitations | 121 (61%) | 44 (71%) | 77 (56%) | 0.042 |
| Cornea Verticillata | 3 (1.5%) | 2 (3.3%) | 1 (0.7%) | 0.2 |
| Angiokeratomas | 11 (5.5%) | 5 (8.1%) | 6 (4.3%) | 0.3 |
| Dyspnea | 89 (45%) | 30 (48%) | 59 (43%) | 0.5 |
| Syncope | 52 (27%) | 17 (28%) | 35 (26%) | 0.7 |
| Precordial Pain | 87 (44%) | 25 (40%) | 62 (46%) | 0.5 |
| Acroparesthesias | 74 (37%) | 25 (40%) | 49 (36%) | 0.5 |
| Comorbidity, n (%) | ||||
| Hypertension | 120 (61%) | 34 (55%) | 86 (63%) | 0.3 |
| Diabetes | 40 (20%) | 8 (13%) | 32 (23%) | 0.093 |
| Dyslipidemia | 114 (58%) | 32 (52%) | 82 (60%) | 0.3 |
| ICD Carrier | 9 (5.1%) | 6 (11%) | 3 (2.5%) | 0.026 |
| Hypothyroidism | 17 (9.0%) | 8 (15%) | 9 (6.7%) | 0.10 |
| Heart Failure | 52 (27%) | 18 (31%) | 34 (26%) | 0.5 |
| Renal Failure | 12 (6.6%) | 3 (5.2%) | 9 (7.3%) | 0.8 |
| Medications, n (%) | ||||
| Beta-Blockers | 115 (68%) | 42 (78%) | 73 (63%) | 0.054 |
| ACE Inhibitors/ARBs | 88 (52%) | 19 (37%) | 69 (58%) | 0.011 |
| Statins | 101 (59%) | 27 (52%) | 74 (63%) | 0.2 |
| Amiodarone | 17 (11%) | 9 (20%) | 8 (7.3%) | 0.026 |
| Diuretics | 67 (41%) | 20 (41%) | 47 (41%) | >0.9 |
| Aspirin (ASA) | 35 (22%) | 7 (15%) | 28 (25%) | 0.15 |
| Habits, n (%) | ||||
| Physical Inactivity | 71 (38%) | 23 (38%) | 48 (38%) | >0.9 |
| Smoking | 29 (16%) | 6 (10%) | 23 (18%) | 0.2 |
| Alcohol Consumption | 35 (19%) | 10 (17%) | 25 (20%) | 0.7 |
| Family History, n (%) | ||||
| Parental Consanguinity | 22 (11%) | 4 (6.7%) | 18 (14%) | 0.13 |
| Sudden Cardiac Death | 101 (53%) | 41 (68%) | 60 (46%) | 0.004 |
| Image-Derived Measurements, n (IQR) | ||||
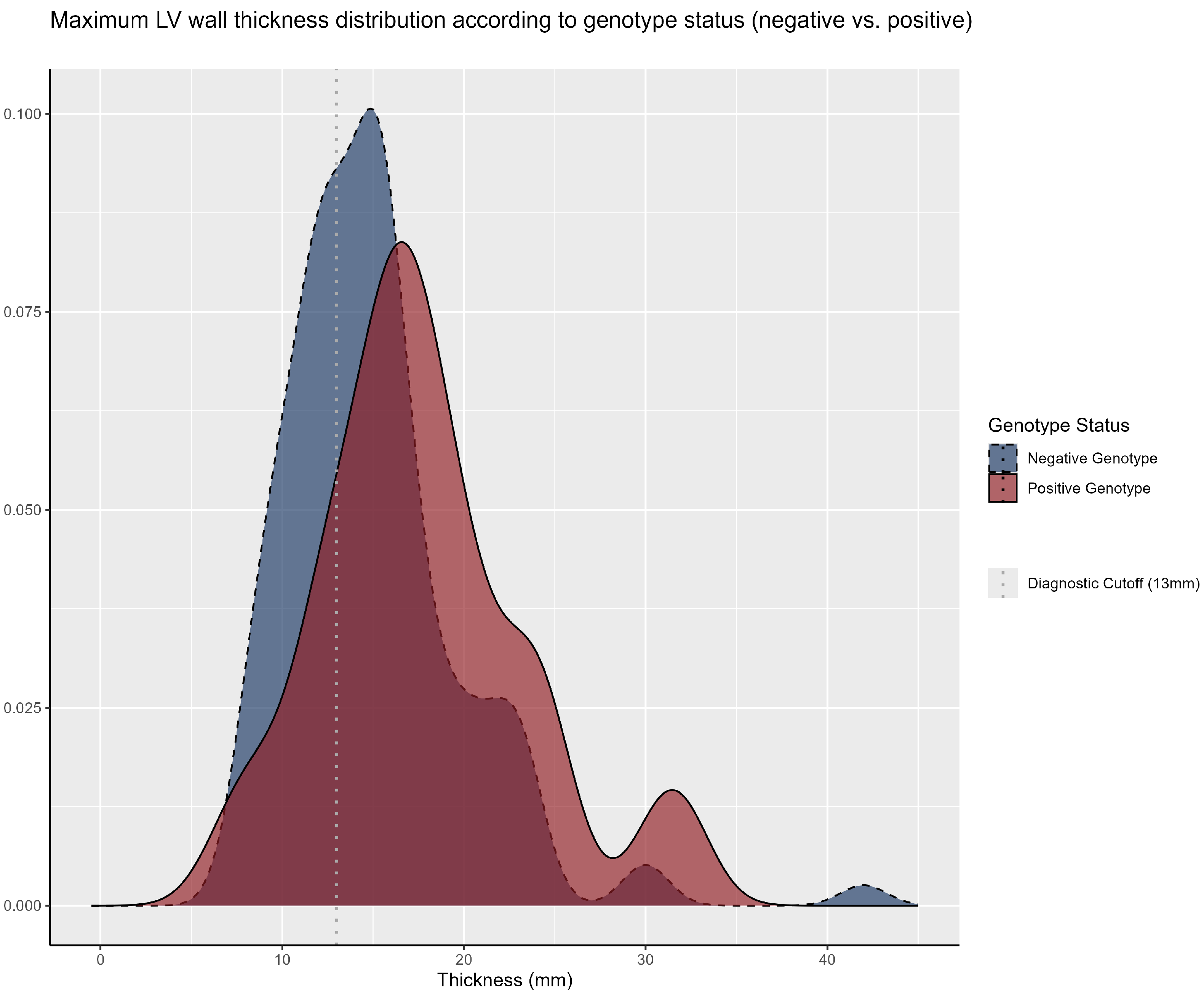
| LV Wall Thickness | 15.0 (12.0–18.0) | 17.0 (14.2–20.6) | 15.0 (12.0–17.0) | <0.001 |
| Ejection Fraction 2 | 66 (61–71) | 67 (62–72) | 65 (61–70) | 0.2 |
| Left Ventricular Diastolic Diameter 2 | 48 (43–53) | 46 (42–52) | 48 (44–55) | 0.030 |
| Left Atrial Diameter 2 | 42 (37–48) | 45 (39–49) | 41 (36–46) | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, E.d.S.; Kuhn, G.d.C.; de Almeida, A.G.C.; Pimentel, J.V.A.; Figueiredo Neto, N.V.; Tavares, L.R.d.S.; dos Santos, B.L.L.; Aragão, A.B.L.; Pereira, B.C.d.A.; Ferreira, C.d.S.; et al. Genetic, Clinical, and Sociodemographic Profile of Individuals with Diagnosis or Family History of Hypertrophic Cardiomyopathy: Insights from a Prospective Cohort. Genes 2025, 16, 1100. https://doi.org/10.3390/genes16091100
Santos EdS, Kuhn GdC, de Almeida AGC, Pimentel JVA, Figueiredo Neto NV, Tavares LRdS, dos Santos BLL, Aragão ABL, Pereira BCdA, Ferreira CdS, et al. Genetic, Clinical, and Sociodemographic Profile of Individuals with Diagnosis or Family History of Hypertrophic Cardiomyopathy: Insights from a Prospective Cohort. Genes. 2025; 16(9):1100. https://doi.org/10.3390/genes16091100
Chicago/Turabian StyleSantos, Emerson de Santana, Gabriel da Costa Kuhn, Antônio Guilherme Cunha de Almeida, João Victor Andrade Pimentel, Newton Vital Figueiredo Neto, Larissa Rebeca da Silva Tavares, Bárbara Letícia Lima dos Santos, Ana Beatriz Leite Aragão, Beatriz Carolina de Araujo Pereira, Caio da Silva Ferreira, and et al. 2025. "Genetic, Clinical, and Sociodemographic Profile of Individuals with Diagnosis or Family History of Hypertrophic Cardiomyopathy: Insights from a Prospective Cohort" Genes 16, no. 9: 1100. https://doi.org/10.3390/genes16091100
APA StyleSantos, E. d. S., Kuhn, G. d. C., de Almeida, A. G. C., Pimentel, J. V. A., Figueiredo Neto, N. V., Tavares, L. R. d. S., dos Santos, B. L. L., Aragão, A. B. L., Pereira, B. C. d. A., Ferreira, C. d. S., Silva, W. M. L. e., Cardiogenetics Research Group of Sergipe, Melo, E. V. d., Tavares, I. d. S., Sousa, A. C. S., & Oliveira, J. L. M. (2025). Genetic, Clinical, and Sociodemographic Profile of Individuals with Diagnosis or Family History of Hypertrophic Cardiomyopathy: Insights from a Prospective Cohort. Genes, 16(9), 1100. https://doi.org/10.3390/genes16091100






