Cardiac Phenotypes in Fabry Disease: Genetic Variability and Clinical Severity Staging Correlation in a Reference Center Cohort
Abstract
1. Introduction
1.1. The Genetic Landscape of GLA Variants
1.2. Cardiac Involvement and Its Clinical Impact
1.3. Toward a Structured Stratification: Three Complementary Models
1.4. What Is Missing: Knowledge Gap and Challenges in Stratification and Aims of the Study
2. Materials and Methods
2.1. Study Design and Population
- Cardiac: ECG, echocardiogram; CMR and Holter ECG were prescribed to all patients but not everyone accepted to undergo these examinations;
- Neurological: MRI, MR angiography, electromyography, visual evoked potentials, autonomic function tests;
- Renal: serum creatinine, urine analysis, renal biopsy if indicated by the specialist;
- Audiological: audiometry, brainstem auditory evoked potentials;
- Pulmonary: chest X-ray/CT scan, spirometry;
- Gastrointestinal: abdominal ultrasound, CT if needed;
- Ophthalmologic: slit-lamp and fundus examination;
- Vascular: Doppler ultrasound, nailfold capillaroscopy;
- Dermatologic: clinical identification of angiokeratomas;
- Psychological issues and quality of life.
2.2. Clinical Endpoints
2.3. Cardiac Staging and Classification
- General: systemic symptoms such as fever, fatigue, and weight loss.
- Neurological: includes acroparesthesias, hypohidrosis, and other neurological signs.
- Cardiac: assesses arrhythmias, ventricular hypertrophy, and other cardiac findings.
- Renal: evaluates renal function via proteinuria and serum creatinine.
2.4. Genetic Analysis and Multidisciplinary Management
2.5. Ethical Considerations
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Clinical Outcomes and Cardiovascular Events
- Cardiovascular death (n = 2, 3%);
- Stroke, transient ischemic attack (TIA), or arterial thromboembolism (n = 11, 16.9%);
- Atrial fibrillation (AF) (n = 3, 4.5%);
- Progression to NYHA class III–IV (n = 1, 1.5%);
- Hospitalization for HF (n = 1, 1.5%);
- Myocardial infarction with non-obstructive coronary arteries (MINOCA) (n = 1, 1.5%);
- Device implantation (1 PM and 1 ICD) (n = 2, 3%);
- Non-sustained ventricular tachycardias (NSVT) (n = 4, 6%), although none progressed to sustained arrhythmia or required ICD intervention.
3.3. Cardiac Staging and Risk Stratification
3.4. Arrhythmic Burden and Conduction Abnormalities
3.5. Multiorgan Involvement and MSSI Correlation
3.6. Genetics
3.6.1. Genetic Mutations in the GLA Gene
3.6.2. Genotype–Phenotype Correlation and Outcome Analysis
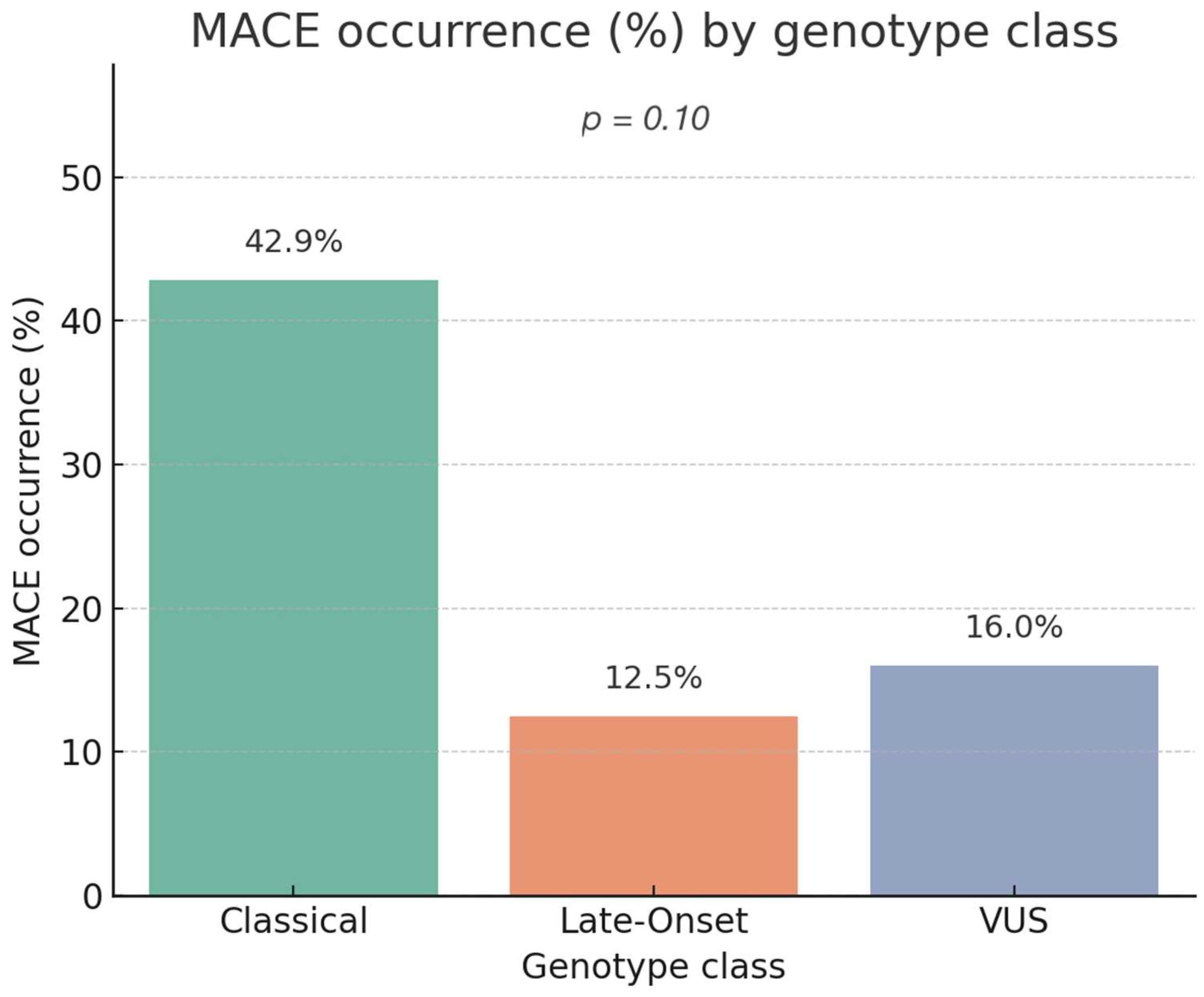
3.6.3. Association Between Genotype Class and Composite Cardiovascular Events
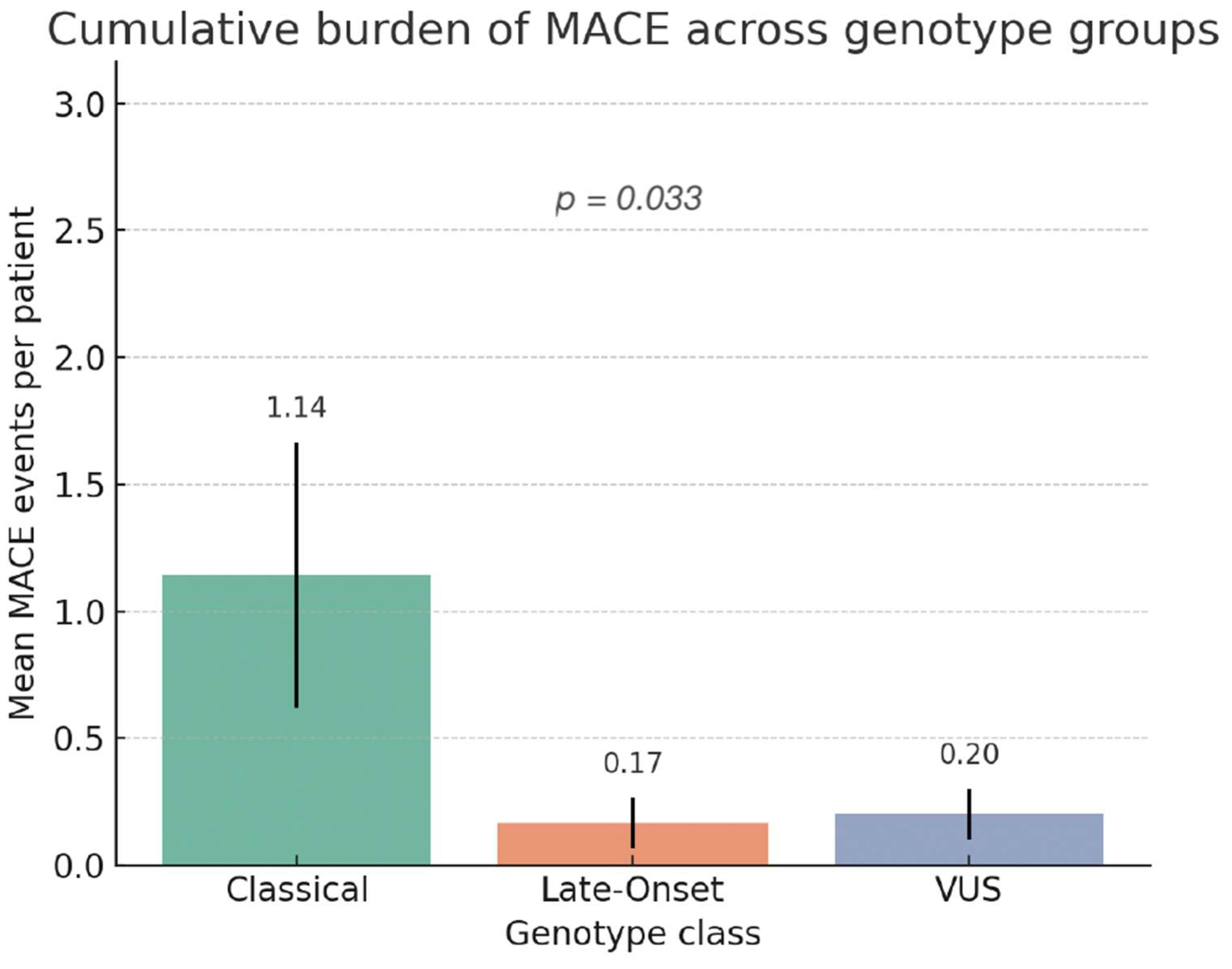
3.6.4. Association Between Genotype Class and Burden (Number) of MACE
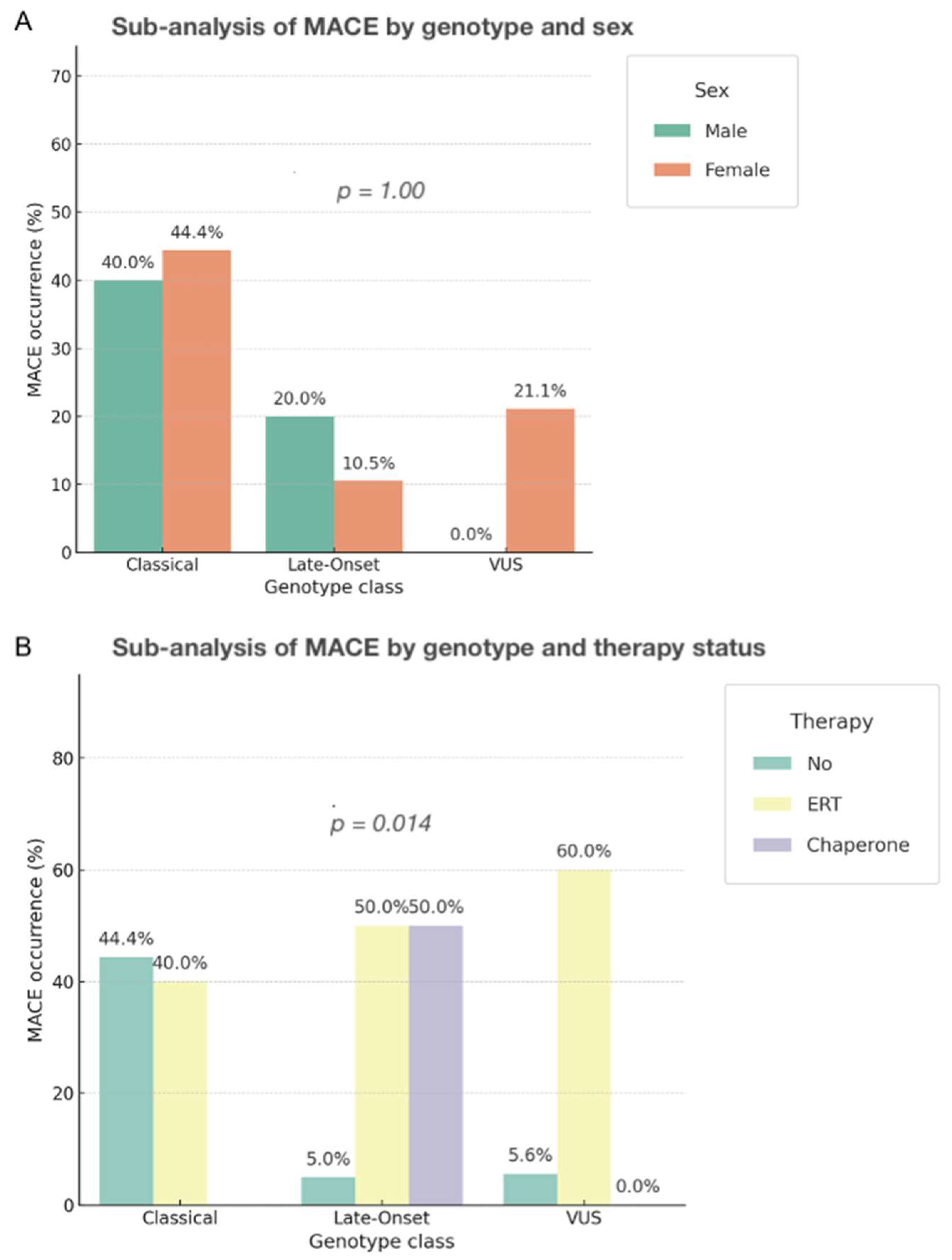
3.6.5. Stratified Analyses by Sex and Therapy
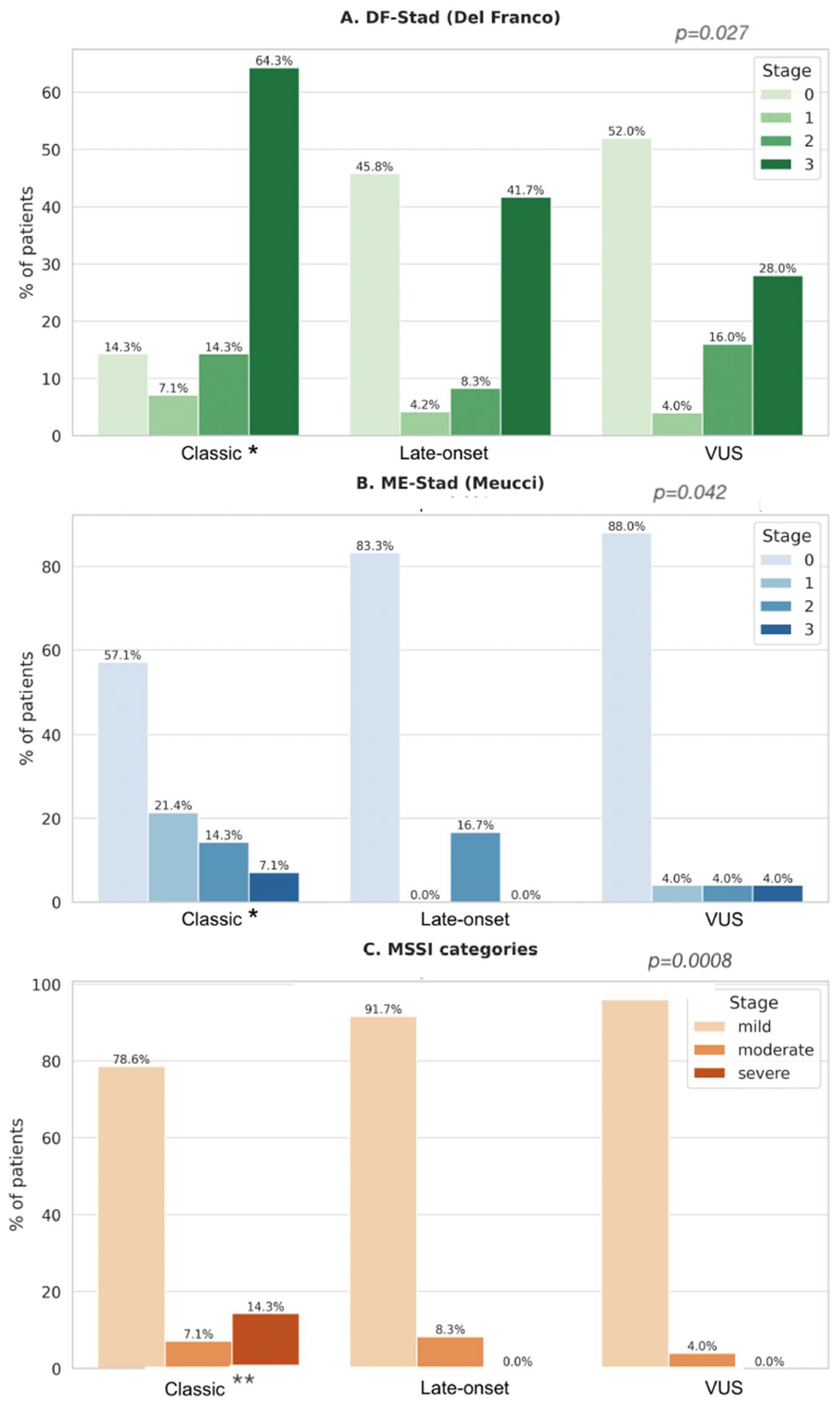
3.6.6. Association Between Genotype Class and Clinical Staging (ME-Stad, DF-Stad, MSSI)
- DF-Stad: 85% of classical carriers were in stage ≥ 2 vs. 29% of Late-Onset and 25% of VUS.
- ME-Stad: 80% of classical carriers reached stage ≥ 2 vs. 29% of Late-Onset and 12.5% of VUS.
3.6.7. Sensitivity Analysis in Patients with Available CMR Data
4. Discussion
4.1. Genotype, Cardiac Involvement, and Staging Severity
4.2. Genotype–MACE Association and Risk Stratification
4.3. Staging Systems and Independent Prognostic Role of Genotype
4.4. Sex-Related Differences and Their Clinical Implications in Fabry Disease
4.5. Summary and Translational Relevance
4.6. Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial Fibrillation |
| AFD | Anderson-Fabry disease |
| CMR | Cardiac Magnetic Resonance |
| GLA | Alpha-Galactosidase |
| DF-Stad | Del Franco Stadiation |
| ERT | Enzyme Replacement Therapy |
| GLS | Global longitudinal strain |
| LGE | Late gadolinium enhancement |
| LVH | Left Ventricle Hypertrophy |
| MACE | Major Adverse Cardiovascular Event(s) |
| ME-Stad | Meucci Stadiation |
| MSSI | Meinz Severity |
| NSVT | Non-sustained Ventricular Tachycardia |
| VUS | Variant of unknown significance |
References
- Gal, A.; Schäfer, E.; Rohard, I. The genetic basis of Fabry disease. In Fabry Disease: Perspectives from 5 Years of FOS; Mehta, A., Beck, M., Sunder-Plassmann, G., Eds.; Oxford PharmaGenesis: Oxford, UK, 2006. [Google Scholar]
- Faro, D.C.; Di Pino, F.L.; Monte, I.P. Inflammation, Oxidative Stress, and Endothelial Dysfunction in the Pathogenesis of Vascular Damage: Unraveling Novel Cardiovascular Risk Factors in Fabry Disease. Int. J. Mol. Sci. 2024, 25, 8273. [Google Scholar] [CrossRef] [PubMed]
- Lenders, M.; Menke, E.R.; Brand, E. Progress and Challenges in the Treatment of Fabry Disease. BioDrugs 2025, 39, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Perrone Filardi, P.; Indolfi, C.; Limongelli, G.; Sinagra, G. Cardiomiopatie Rare; Edizioni, I., Ed.; Italian Society of Cardiology: Napoli, Italy, 2023. [Google Scholar]
- Germain, D.P. Fabry disease. Orphanet J. Rare Dis. 2010, 5, 30. [Google Scholar] [CrossRef]
- Lenders, M.; Brand, E. Fabry Disease: The Current Treatment Landscape. Drugs 2021, 81, 635–645. [Google Scholar] [CrossRef]
- Spada, M.; Pagliardini, S.; Yasuda, M.; Tukel, T.; Thiagarajan, G.; Sakuraba, H.; Ponzone, A.; Desnick, R.J. High incidence of later-onset fabry disease revealed by newborn screening. Am. J. Hum. Genet. 2006, 79, 31–40. [Google Scholar] [CrossRef]
- Germain, D.P.; Fouilhoux, A.; Decramer, S.; Tardieu, M.; Pillet, P.; Fila, M.; Rivera, S.; Deschênes, G.; Lacombe, D. Consensus recommendations for diagnosis, management and treatment of Fabry disease in paediatric patients. Clin. Genet. 2019, 96, 107–117. [Google Scholar] [CrossRef]
- Burlina, A.B.; Polo, G.; Salviati, L.; Duro, G.; Zizzo, C.; Dardis, A.; Bembi, B.; Cazzorla, C.; Rubert, L.; Zordan, R.; et al. Newborn screening for lysosomal storage disorders by tandem mass spectrometry in North East Italy. J. Inherit. Metab. Dis. 2018, 41, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Levade, T.; Hachulla, E.; Knebelmann, B.; Lacombe, D.; Seguin, V.L.; Nguyen, K.; Noël, E.; Rabès, J.P. Challenging the traditional approach for interpreting genetic variants: Lessons from Fabry disease. Clin. Genet. 2022, 101, 390–402. [Google Scholar] [CrossRef]
- Ortiz, A.; Germain, D.P.; Desnick, R.J.; Politei, J.; Mauer, M.; Burlina, A.; Eng, C.; Hopkin, R.J.; Laney, D.; Linhart, A.; et al. Fabry disease revisited: Management and treatment recommendations for adult patients. Mol. Genet. Metab. 2018, 123, 416–427. [Google Scholar] [CrossRef]
- HGMD. The Human Gene Mutation Database. Available online: http://www.hgmd.cf.ac.uk/ac/gene.php?gene=GLA (accessed on 17 July 2025).
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Lavalle, L.; Thomas, A.S.; Beaton, B.; Ebrahim, H.; Reed, M.; Ramaswami, U.; Elliott, P.; Mehta, A.B.; Hughes, D.A. Phenotype and biochemical heterogeneity in late onset Fabry disease defined by N215S mutation. PLoS ONE 2018, 13, e0193550. [Google Scholar] [CrossRef]
- McCarron, E.P.; Chinnadurai, R.; Meyer, J.; Anderson, T.; Stepien, K.M.; Sharma, R.; Woolfson, P.; Jovanovic, A. Real-world clinical outcomes in adult patients with Fabry disease: A 20-year retrospective observational cohort study from a single centre. Mol. Genet. Metab. Rep. 2025, 43, 101229. [Google Scholar] [CrossRef]
- Brand, E.; Linhart, A.; Deegan, P.; Jurcut, R.; Pisani, A.; Torra, R.; Feldt-Rasmussen, U. Clinical management of female patients with Fabry disease based on expert consensus. Orphanet J. Rare Dis. 2025, 20, 7. [Google Scholar] [CrossRef]
- Faro, D.C.; Losi, V.; Rodolico, M.S.; Torrisi, E.M.; Colomba, P.; Duro, G.; Monte, I.P. Sex Differences in Anderson-Fabry Cardiomyopathy: Clinical, Genetic, and Imaging Analysis in Women. Genes 2023, 14, 1804. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.P.; Ferreira, S. Multiple phenotypic domains of Fabry disease and their relevance for establishing genotype-phenotype correlations. Appl. Clin. Genet. 2019, 12, 35–50. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, D.X.; Junior, H.V. (Eds.) Amyloidosis and Fabry Disease: A Clinical Guide; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Pieroni, M.; Moon, J.C.; Arbustini, E.; Barriales-Villa, R.; Camporeale, A.; Vujkovac, A.C.; Elliott, P.M.; Hagege, A.; Kuusisto, J.; Linhart, A.; et al. Cardiac Involvement in Fabry Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 922–936. [Google Scholar] [CrossRef]
- Pieroni, M.; Ciabatti, M.; Graziani, F.; Camporeale, A.; Saletti, E.; Lillo, R.; Figliozzi, S.; Bolognese, L. The Heart in Fabry Disease: Mechanisms Beyond Storage and Forthcoming Therapies. RCM 2022, 23, 196. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; de Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bhalla, J.S.; Erwin, A.L.; Jaber, W.; Wang, T.K.M. Contemporary Multimodality Imaging for Diagnosis and Management of Fabry Cardiomyopathy. J. Clin. Med. 2024, 13, 4771. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Pieroni, M.; Pastore, M.C.; Brucato, A.; Castelletti, S.; Crotti, L.; Dweck, M.; Frustaci, A.; Gimelli, A.; Klingel, K.; et al. The role of cardiovascular multimodality imaging in the evaluation of Anderson-Fabry disease: From early diagnosis to therapy monitoring. Eur. Heart J. Cardiovasc. Imaging 2025, 26, 814–829. [Google Scholar] [CrossRef]
- Del Franco, A.; Iannaccone, G.; Meucci, M.C.; Lillo, R.; Cappelli, F.; Zocchi, C.; Pieroni, M.; Graziani, F.; Olivotto, I. Clinical staging of Anderson-Fabry cardiomyopathy: An operative proposal. Heart Fail. Rev. 2024, 29, 431–444. [Google Scholar] [CrossRef]
- Meucci, M.C.; Lillo, R.; Del Franco, A.; Monda, E.; Iannaccone, G.; Baldassarre, R.; Di Nicola, F.; Parisi, V.; Lombardo, A.; Spinelli, L.; et al. Prognostic Implications of the Extent of Cardiac Damage in Patients With Fabry Disease. J. Am. Coll. Cardiol. 2023, 82, 1524–1534. [Google Scholar] [CrossRef]
- Lillo, R.; Meucci, M.C.; Del Franco, A.; Monda, E.; Iannaccone, G.; Ditaranto, R.; Di Nicola, F.; Serratore, S.; Lombardo, A.; Spinelli, L.; et al. Evolution of Cardiac Damage Staged With Echocardiography in Fabry Disease. J. Am. Heart Assoc. 2025, 14, e041627. [Google Scholar] [CrossRef]
- Whybra, C.; Kampmann, C.; Krummenauer, F.; Ries, M.; Mengel, E.; Miebach, E.; Baehner, F.; Kim, K.; Bajbouj, M.; Schwarting, A.; et al. The Mainz Severity Score Index: A new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin. Genet. 2004, 65, 299–307. [Google Scholar] [CrossRef]
- Fuller, M.; Mehta, A. Fabry cardiomyopathy: Missing links from genotype to phenotype. Heart 2020, 106, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.; Gal, A. Genotype-phenotype correlation in Fabry disease. In Fabry Disease: Perspectives from 5 Years of FOS; Mehta, A., Beck, M., Sunder-Plassmann, G., Eds.; Oxford PharmaGenesis: Oxford, UK, 2006. [Google Scholar]
- Blanco, R.; Rico-Ramírez, Y.; Hermida-Ameijeiras, Á.; Abdullah, I.M.S.; Lau, K.; Alvarez-Rubio, J.; Fortuny, E.; Martínez-Monzonís, A.; Nowak, A.; Nordbeck, P.; et al. Phenotypic Expression and Outcomes in Patients with the p.Arg301Gln GLA Variant in Anderson-Fabry Disease. Int. J. Mol. Sci. 2024, 25, 4299. [Google Scholar] [CrossRef]
- Houge, G.; Langeveld, M.; Oliveira, J.-P. GLA insufficiency should not be called Fabry disease. Eur. J. Hum. Genet. 2025, 33, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Tuttolomondo, A.; Chimenti, C.; Cianci, V.; Gallieni, M.; Lanzillo, C.; La Russa, A.; Limongelli, G.; Mignani, R.; Olivotto, I.; Pieruzzi, F.; et al. Females with Fabry disease: An expert opinion on diagnosis, clinical management, current challenges and unmet needs. Front. Cardiovasc. Med. 2025, 12, 1536114. [Google Scholar] [CrossRef] [PubMed]
- Faro, D.C.; Di Pino, F.L.; Rodolico, M.S.; Costanzo, L.; Losi, V.; Di Pino, L.; Monte, I.P. Relationship between Capillaroscopic Architectural Patterns and Different Variant Subgroups in Fabry Disease: Analysis of Cases from a Multidisciplinary Center. Genes 2024, 15, 1101. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Nowak, A.; Barbey, F.; Torres, M.; Nunes, J.P.; Teixeira-e-Costa, F.; Carvalho, F.; Sampaio, S.; Tavares, I.; Pereira, O.; et al. Fabry disease caused by the GLA p.Phe113Leu (p.F113L) variant: Natural history in males. Eur. J. Med. Genet. 2020, 63, 103703. [Google Scholar] [CrossRef]
- Linhart, A.; Germain, D.P.; Olivotto, I.; Akhtar, M.M.; Anastasakis, A.; Hughes, D.; Namdar, M.; Pieroni, M.; Hagège, A.; Cecchi, F.; et al. An expert consensus document on the management of cardiovascular manifestations of Fabry disease. Eur. J. Heart Fail. 2020, 22, 1076–1096. [Google Scholar] [CrossRef]
- Pieroni, M.; Namdar, M.; Olivotto, I.; Desnick, R.J. Anderson-Fabry disease management: Role of the cardiologist. Eur. Heart J. 2024, 45, 1395–1409. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sniderman King, L.; Vengoechea, E.; Liu, R.; Chen-Deutsch, X.; Smith, S.; Wang, Y.; da Silva, C.; Chakraborty, P.; Kallu, E.; et al. Enhancing Fabry disease screening and diagnostic efficiency: Integration of enzyme, biomarker, and next-generation sequencing testing. Mol. Genet. Metab. 2025, 145, 109082. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Cosyns, B.; Edvardsen, T.; Cardim, N.; Delgado, V.; Di Salvo, G.; Donal, E.; Sade, L.E.; Ernande, L.; Garbi, M.; et al. Standardization of adult transthoracic echocardiography reporting in agreement with recent chamber quantification, diastolic function, and heart valve disease recommendations: An expert consensus document of the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Germain, D.P.; Altarescu, G.; Barriales-Villa, R.; Mignani, R.; Pawlaczyk, K.; Pieruzzi, F.; Terryn, W.; Vujkovac, B.; Ortiz, A. An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol. Genet. Metab. 2022, 137, 49–61. [Google Scholar] [CrossRef]
- Azevedo, O.; Gal, A.; Faria, R.; Gaspar, P.; Miltenberger-Miltenyi, G.; Gago, M.F.; Dias, F.; Martins, A.; Rodrigues, J.; Reimão, P.; et al. Founder effect of Fabry disease due to p.F113L mutation: Clinical profile of a late-onset phenotype. Mol. Genet. Metab. 2020, 129, 150–160. [Google Scholar] [CrossRef]
- Hsu, T.R.; Hung, S.C.; Chang, F.P.; Yu, W.C.; Sung, S.H.; Hsu, C.L.; Dzhagalov, I.; Yang, C.F.; Chu, T.H.; Lee, H.J.; et al. Later Onset Fabry Disease, Cardiac Damage Progress in Silence: Experience With a Highly Prevalent Mutation. J. Am. Coll. Cardiol. 2016, 68, 2554–2563. [Google Scholar] [CrossRef]
- Aladağ, N.; Ali Barman, H.; Şipal, A.; Akbulut, T.; Özdemir, M.; Ceylaner, S. Difficulties in Diagnosing Fabry Disease in Patients with Unexplained Left Ventricular Hypertrophy (LVH): Is the Novel GLA Gene Mutation a Pathogenic Mutation or Polymorphism? Balkan J. Med. Genet. 2023, 26, 43–50. [Google Scholar] [CrossRef]
- Iemolo, F.; Pizzo, F.; Albeggiani, G.; Zizzo, C.; Colomba, P.; Scalia, S.; Bartolotta, C.; Duro, G. De novo mutation in a male patient with Fabry disease: A case report. BMC Res. Notes 2014, 7, 11. [Google Scholar] [CrossRef]
- Koulousios, K.; Stylianou, K.; Pateinakis, P.; Zamanakou, M.; Loules, G.; Manou, E.; Kyriklidou, P.; Katsinas, C.; Ouzouni, A.; Kyriazis, J.; et al. Fabry disease due to D313Y and novel GLA mutations. BMJ Open 2017, 7, e017098. [Google Scholar] [CrossRef]
- Valtola, K.; Nino-Quintero, J.; Hedman, M.; Lottonen-Raikaslehto, L.; Laitinen, T.; Maria, M.; Kantola, I.; Naukkarinen, A.; Laakso, M.; Kuusisto, J. Cardiomyopathy associated with the Ala143Thr variant of the α-galactosidase A gene. Heart 2020, 106, 609–615. [Google Scholar] [CrossRef]
- Lenders, M.; Weidemann, F.; Kurschat, C.; Canaan-Kühl, S.; Duning, T.; Stypmann, J.; Schmitz, B.; Reiermann, S.; Krämer, J.; Blaschke, D.; et al. Alpha-Galactosidase A p.A143T, a non-Fabry disease-causing variant. Orphanet J. Rare Dis. 2016, 11, 54. [Google Scholar] [CrossRef]
- Monda, E.; Diana, G.; Graziani, F.; Rubino, M.; Bakalakos, A.; Linhart, A.; Germain, D.P.; Scarpa, M.; Biagini, E.; Pieroni, M.; et al. Impact of GLA Variant Classification on the Estimated Prevalence of Fabry Disease: A Systematic Review and Meta-Analysis of Screening Studies. Circ. Genom. Precis. Med. 2023, 16, e004252. [Google Scholar] [CrossRef]
- Colastra Ugena, E.; Peña Cabia, A.; Calero Paniagua, I.; Rodríguez Díaz, S.; Rincón Ruiz, B.; de Oña, J.G. Fabry disease: Importance of genetic counseling in the reclassification of variants of uncertain clinical significance. Med. Clin. 2025, 165, 106965. [Google Scholar] [CrossRef]
- Germain, D.P.; Oliveira, J.P.; Bichet, D.G.; Yoo, H.W.; Hopkin, R.J.; Lemay, R.; Politei, J.; Wanner, C.; Wilcox, W.R.; Warnock, D.G. Use of a rare disease registry for establishing phenotypic classification of previously unassigned GLA variants: A consensus classification system by a multispecialty Fabry disease genotype-phenotype workgroup. J. Med. Genet. 2020, 57, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Smid, B.E.; van der Tol, L.; Cecchi, F.; Elliott, P.M.; Hughes, D.A.; Linthorst, G.E.; Timmermans, J.; Weidemann, F.; West, M.L.; Biegstraaten, M.; et al. Uncertain diagnosis of Fabry disease: Consensus recommendation on diagnosis in adults with left ventricular hypertrophy and genetic variants of unknown significance. Int. J. Cardiol. 2014, 177, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Urtis, M.; Cavaliere, C.; Vilardo, V.; Paganini, C.; Smirnova, A.; Giorgianni, C.; Di Toro, A.; Chiapparini, L.; Pellegrini, C.; Grasso, M.; et al. Unambiguous Interpretation of the Pathogenicity of the GLA c.547+3A>G Variant Causing Fabry Disease. Genes 2024, 15, 1212. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Klug, K.; Breyer, M.; Grüner, J.; Medala, V.K.; Nordbeck, P.; Wanner, C.; Klopocki, E.; Üçeyler, N. Genetic variants of unknown significance in alpha-galactosidase A: Cellular delineation from Fabry disease. J. Inherit. Metab. Dis. 2024, 47, 805–817. [Google Scholar] [CrossRef]
- Rychlík, I.; Francová, L.; Dostálová, G.; Pešková, M.; Pešičková, S.; Ryba, M.; Švára, F.; Nemcová, Z.; Dusilová Sulková, S.; Viklický, O.; et al. Czech nationwide screening for Fabry disease in patients on maintenance dialysis: A call for evaluation of population-enriched GLA gene variants of uncertain significance. Clin. Kidney J. 2025, 18, sfaf167. [Google Scholar] [CrossRef]
- Migeon, B.R. X-linked diseases: Susceptible females. Genet. Med. 2020, 22, 1156–1174. [Google Scholar] [CrossRef]
- Echevarria, L.; Benistan, K.; Toussaint, A.; Dubourg, O.; Hagege, A.A.; Eladari, D.; Jabbour, F.; Beldjord, C.; De Mazancourt, P.; Germain, D.P. X-chromosome inactivation in female patients with Fabry disease. Clin. Genet. 2016, 89, 44–54. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, S.J.; Hollak, C.E.M.; van Kuilenburg, A.B.P.; Langeveld, M. Developments in the treatment of Fabry disease. J. Inherit. Metab. Dis. 2020, 43, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Wu, C.; Yanagisawa, H.; Miyajima, T.; Akiyama, K.; Eto, Y. Future clinical and biochemical predictions of Fabry disease in females by methylation studies of the GLA gene. Mol. Genet. Metab. Rep. 2019, 20, 100497. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria | Clinical Endpoints (MACE) |
|---|---|
| Age ≥ 18 years Confirmed genetic diagnosis of AFD Signed informed consent | Cardiovascular death Heart failure hospitalization Worsening HF (NYHA 3–4) |
| Availability of ECG, echocardiography, and (when available) CMR | Arrhythmias (AF, major VAs, bradyarrhythmias requiring pacing, e.g., AVBs) |
| Sufficient clinical and laboratory data for staging Optimal acoustic window for echo images analysis | Device implantation (ICD, PM) Appropriate device interventions MINOCA Stroke |
| Vascular Thromboembolic events |
| Del Franco | |
| Stage 0 (Non-hypertrophic) | LV wall thickness (LVWT) ≤ 12 mm; normal systolic/diastolic function; absence of fibrosis or imaging abnormalities. |
| Stage 1 (Hypertrophic, pre-fibrotic) | LV wall thickness (LVWT) ≤ 12 mm; normal systolic/diastolic function; absence of fibrosis or imaging abnormalities. |
| Stage 2 (Hypertrophic, fibrotic) | LVWT > 12 mm; evidence of myocardial fibrosis on CMR (LGE positive); possibly altered LAVi and E/e′. |
| Stage 3 (Overt dysfunction) | Reduced LVEF < 50%, E/e′ ≥ 15, LAVi > 34 mL/m2, TAPSE < 17 mm, extensive LGE, and advanced clinical symptoms. |
| Meucci | |
| Stage 0 | No structural/functional cardiac involvement; |
| Stage 1 | Isolated LVH |
| Stage 2 | Presence of left atrial dilation |
| Stage 3 | Overt ventricular dysfunction (LVEF < 50%, E/e′ ≥ 15, or TAPSE < 17 mm). |
| (A) Demographic and Clinical | |
| Parameter | AFD n = 65 |
| Age at diagnosis (years) | 40.6 ± 15.1 |
| Sex | |
| -Male | 16 (24.6%) |
| -Female | 49 (75.4%) |
| GLA variant | |
| -Classic | 14 (21.5%) |
| -Late onset | 24 (36.9%) |
| -VUS | 25 (28.5%) |
| -Polymorphism | 2 (3.1%) |
| Baseline specific therapy | |
| -No | 49 (75.4%) |
| -ERT | 12 (18.5%) |
| -Migalastat | 4 (6.2%) |
| BMI (kg/m2) | 24.2 [21; 27] |
| A-Gal -A (nmol/mL/h) | 7.35 ± 5.21 |
| Lyso-Gb3 (nmol/L) | 6.13 ± 14.19 |
| NYHA-baseline I | 43 (66.2%) |
| NYHA-baseline II | 22 (33.8%) |
| Syncope | 5 (7.7%) |
| Comorbidities | |
| Hypertension | 29 (44.6%) |
| Diabetes | 7 (10.8%) |
| Cerebral ischemic disease | 9 (13.8%) |
| Coronary and peripheral vasculopathy | 11 (16.9%) |
| Dyslipidaemia | 12 (18.5%) |
| Smoking history | 10 (15.4%) |
| Creatinine (mg/dL) | 0.71 [0.6; 0.9] |
| Proteinuria | 4 (6.2%) |
| Chronic kidney disease | 4 (6.2%) |
| Dialysis | 5 (7.7%) |
| Kidney transplant | 5 (7.7%) |
| (B) Cardiac Imaging Parameters | |
| Parameter | AFD n = 65 |
| Sinus rhythm | 63 (96.9%) |
| Atrial fibrillation | 1 (1.5%) |
| PM rhythm | 1 (1.5%) |
| Heart rate (bpm) | 71 [66; 77] |
| PR interval (ms) | 148 ± 21.3 |
| QRS duration (ms) | 88 [84; 98] |
| Right bundle branch block (RBBB) | 4 (6.2%) |
| Left bundle branch block (LBBB) | 0 (0%) |
| LVH by ECG criteria | 5 (7.7%) |
| Max wall thickness (mm) | 8 [7; 10.5] |
| LVMi (g/m2) | 70 [58; 91] |
| LVH by echo criteria | 11 (17%) |
| LVEF (%) | 65 [61; 66.5] |
| LV-GLS (%) | −18.5 [−21; −16] |
| E/e′ | 6.6 [5.7; 9.3] |
| LAVi (mL/m2) | 25 [20; 31] |
| TAPSE (mm) | 22 [20; 31] |
| TRV-max (m/s) | 2.1 ± 0.5 |
| Cardiac Magnetic Resonance performed | 13 (20%) |
| T1 increased | 1 (1.5%) |
| LGE present | 9 (13.8%) |
| LGE extension (>3 segments) | 2 (3.1%) |
| Event | n (%) |
|---|---|
| Cardiovascular death | 2 (3.1%) |
| Ischemic stroke (after enrolment) | 2 (3.1%) |
| New-onset atrial fibrillation | 2 (3.1%) |
| NYHA class III–IV progression | 1 (1.5%) |
| Hospitalization for cardiac causes | 1 (1.5%) |
| MINOCA | 1 (1.5%) |
| Ventricular major arrhythmias or ICD therapy | 0 (0.0%) |
| Total number of patients with at least one MACE | 12 (18.5%) |
| Total number of MACE | 25 events |
| Staging System | Stage | n (%) |
|---|---|---|
| Meucci | 0 | 52 (80%) |
| 1 | 4 (6.2%) | |
| 2 | 7 (10.8%) | |
| 3 | 2 (3.1%) | |
| Del Franco | 0 | 27 (41.5%) |
| 1 | 3 (4.6%) | |
| 2 | 8 (12.3%) | |
| 3 | 27 (41.5%) | |
| MSSI | Median score | 10.0 [5; 14] |
| mild | 60 (92.3%) | |
| moderate | 4 (6.2%) | |
| severe | 1 (1.5%) |
| Variant Class | OR | 95% CI | p-Value |
|---|---|---|---|
| Classical | 4.71 | 1.125; 17.74 | 0.022 |
| Late-Onset | 0.44 | 0.11; 1.80 | 0.256 |
| VUS | 0.66 | 0.18; 2.41 | 0.526 |
| Staging | Variant | Coeff. | 95% CI | p-Value |
|---|---|---|---|---|
| ME-Stad | CL | 3.795 | 1.052; 13.684 | 0.041 |
| DF-Stad | CL | 3.835 | 1.169; 12.578 | 0.027 |
| MSSI | CL | β +9.11 | 4.08; 14.15 | 0.0008 |
| ME-Stad | LO | 0.705 | 0.192; 2.586 | 0.598 |
| DF-Stad | LO | 0.862 | 0.330; 2.252 | 0.762 |
| MSSI | LO | β −3.75 | −8.39; 0.89 | 0.118 |
| ME-Stad | VUS | 0.394 | 0.097; 1.602 | 0.193 |
| DF-Stad | VUS | 0.445 | 0.170; 1.162 | 0.098 |
| MSSI | VUS | β −2.88 | −7.53; 1.76 | 0.229 |
| Genotype | n | DF | ME | MSSI | MACE (%) | MACE | LGE (%) |
|---|---|---|---|---|---|---|---|
| Classical | 8 | 2.5 | 0.75 | 21.9 | 50.0 | 3.25 | 62.0 |
| Late-onset | 3 | 2.6 | 1.33 | 16.0 | 33.0 | 2.0 | 67.0 |
| VUS | 2 | 2.0 | 0.0 | 15.0 | 50.0 | 2.0 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faro, D.C.; Di Salvo, S.; Rodolico, M.S.; Losi, V.; Capodanno, D.; Monte, I.P. Cardiac Phenotypes in Fabry Disease: Genetic Variability and Clinical Severity Staging Correlation in a Reference Center Cohort. Genes 2025, 16, 1086. https://doi.org/10.3390/genes16091086
Faro DC, Di Salvo S, Rodolico MS, Losi V, Capodanno D, Monte IP. Cardiac Phenotypes in Fabry Disease: Genetic Variability and Clinical Severity Staging Correlation in a Reference Center Cohort. Genes. 2025; 16(9):1086. https://doi.org/10.3390/genes16091086
Chicago/Turabian StyleFaro, Denise Cristiana, Serena Di Salvo, Margherita Stefania Rodolico, Valentina Losi, Davide Capodanno, and Ines Paola Monte. 2025. "Cardiac Phenotypes in Fabry Disease: Genetic Variability and Clinical Severity Staging Correlation in a Reference Center Cohort" Genes 16, no. 9: 1086. https://doi.org/10.3390/genes16091086
APA StyleFaro, D. C., Di Salvo, S., Rodolico, M. S., Losi, V., Capodanno, D., & Monte, I. P. (2025). Cardiac Phenotypes in Fabry Disease: Genetic Variability and Clinical Severity Staging Correlation in a Reference Center Cohort. Genes, 16(9), 1086. https://doi.org/10.3390/genes16091086






