Resistance Mutations in CLL: Genetic Mechanisms Shaping the Future of Targeted Therapy
Abstract
1. Introduction
2. Emergence of Targeted Therapies
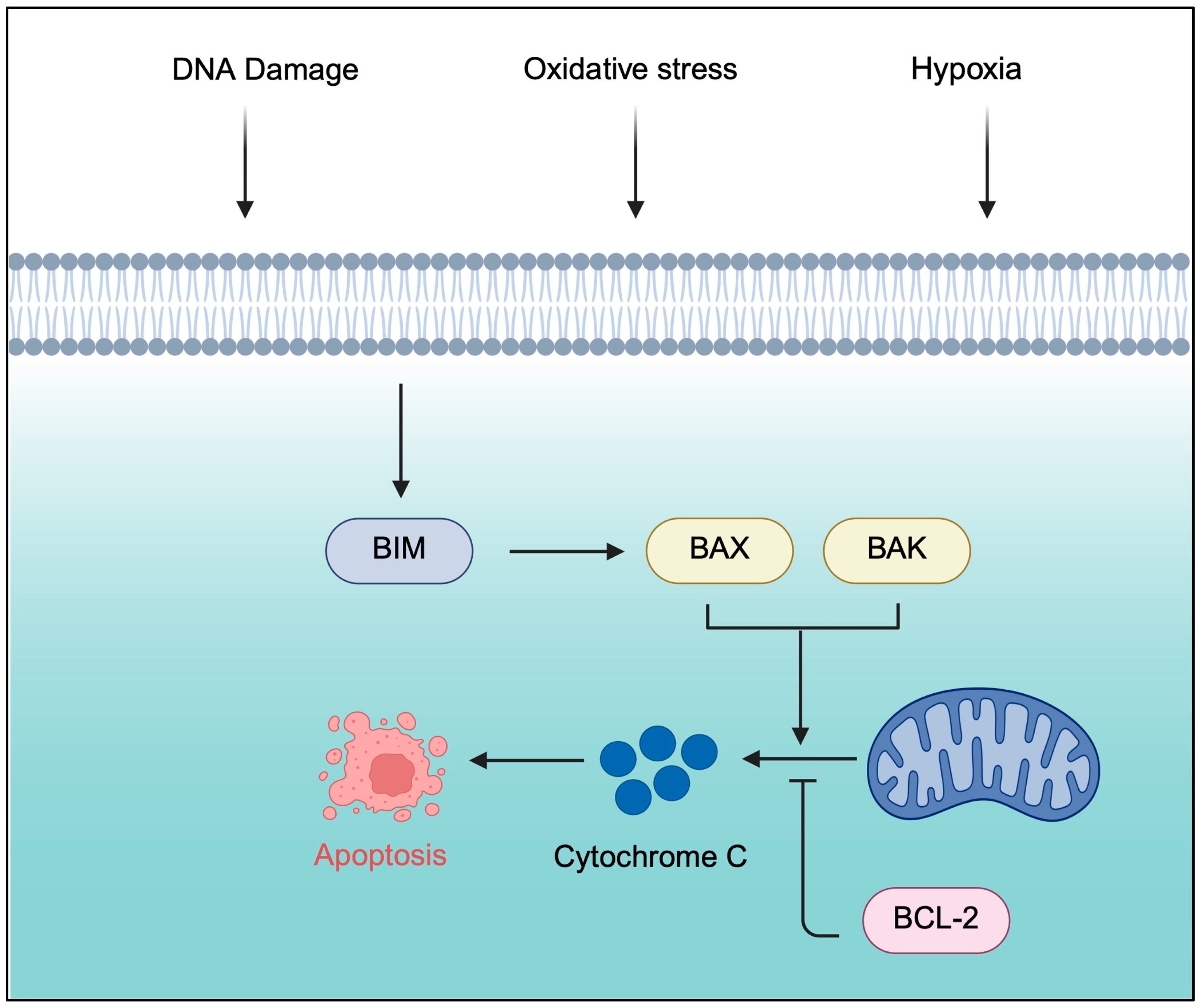
2.1. B-Cell Lymphoma 2 Inhibitors
2.2. Bruton’s Tyrosine Kinase Inhibitors
3. Evolving Therapies
3.1. Combination Therapies
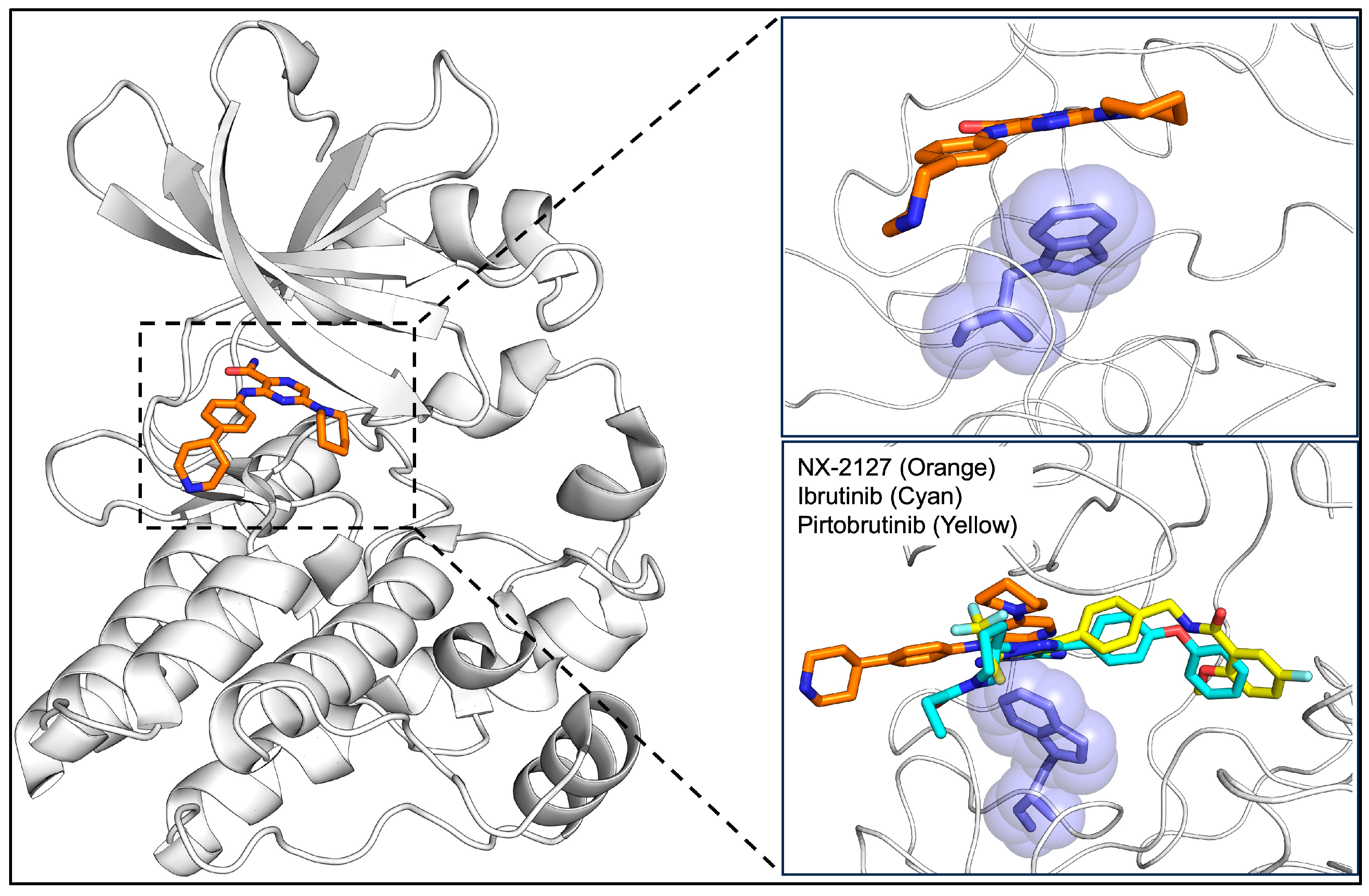
3.2. BTK PROTAC Degraders

4. Conclusions and Future Directions
5. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLL | Chronic lymphocytic leukemia |
| CIT | Chemoimmunotherapy |
| BR | Bendamustine and rituximab |
| BTKi | Bruton’s tyrosine kinase inhibitor |
| BCL2i | B-cell lymphoma 2 inhibitor |
| MRD | Measurable residual disease |
| BH3 | B-cell lymphoma 2 homology 3 |
| BAX | B-cell lymphoma 2-associated X proteins |
| BAK | B-cell lymphoma 2 antagonist/killer |
| BIM | B-cell lymphoma 2-interacting mediator |
| uMRD | Undetectable measurable residual disease |
| AML | Acute myeloid leukemia |
| BTK | Bruton’s tyrosine kinase |
| cBTKi | Covalent Bruton’s tyrosine kinase inhibitor |
| ncBTKi | Non-covalent Bruton’s tyrosine kinase inhibitor |
| R/R | Relapsed/refractory |
| PFS | Progression-free survival |
| OS | Overall survival |
| XLA | X-linked agammaglobulinemia |
| IGH | Immunoglobulin heavy chain |
| IVO | Ibrutinib, venetoclax, and abinutuzumab |
| AVO | Acalabrutinib, venetoclax, and obinutuzumab |
| PVO | Pirtobrutinib, venetoclax, and obinutuzumab |
| BM | Bone marrow |
| PB | Peripheral blood |
| PROTAC | Proteolysis-targeting chimera |
| POI | Protein of interest |
| VHL | Von Hippel–Lindau |
| CRBN | Cereblon |
| FC | Flow cytometry |
| IgH-PCR | Immunoglobulin heavy-chain polymerase chain reaction |
| NGS | Next-generation sequencing |
| ASO | Allele-specific oligonucleotide |
References
- Dighiero, G. CLL Biology and Prognosis. Hematology 2005, 2005, 278–284. [Google Scholar] [CrossRef]
- Rai, K.R.; Jain, P. Chronic lymphocytic leukemia (CLL)—Then and now. Am. J. Hematol. 2016, 91, 330–340. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Shadman, M. Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Review. JAMA 2023, 329, 918–932. [Google Scholar] [CrossRef]
- Abrisqueta, P.; Pereira, A.; Rozman, C.; Aymerich, M.; Giné, E.; Moreno, C.; Muntañola, A.; Rozman, M.; Villamor, N.; Hodgson, K.; et al. Improving survival in patients with chronic lymphocytic leukemia (1980–2008): The Hospital Clínic of Barcelona experience. Blood 2009, 114, 2044–2050. [Google Scholar] [CrossRef] [PubMed]
- Mauro, F.R.; Foa, R.; Giannarelli, D.; Cordone, I.; Crescenzi, S.; Pescarmona, E.; Sala, R.; Cerretti, R.; Mandelli, F. Clinical Characteristics and Outcome of Young Chronic Lymphocytic Leukemia Patients: A Single Institution Study of 204 Cases. Blood 1999, 94, 448–454. [Google Scholar]
- Rai, K.R.; Sawitsky, A.; Cronkite, E.P.; Chanana, A.D.; Levy, R.N.; Pasternack, B.S. Clinical Staging of Chronic Lymphocytic Leukemia. Blood 1975, 46, 219–234. [Google Scholar]
- Binet, J.L.; Auquier, A.; Dighiero, G.; Chastang, C.; Piguet, H.; Goasguen, J.; Vaugier, G.; Potron, G.; Colona, P.; Oberling, F.; et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981, 48, 198–206. [Google Scholar]
- Kay, N.E.; Hampel, P.J.; Van Dyke, D.L.; Parikh, S.A. CLL update 2022: A continuing evolution in care. Blood Rev. 2022, 54, 100930. [Google Scholar] [CrossRef]
- Karr, M.; Roeker, L. A History of Targeted Therapy Development and Progress in Novel–Novel Combinations for Chronic Lymphocytic Leukemia (CLL). Cancers 2023, 15, 1018. [Google Scholar] [CrossRef]
- Smolej, L.; Vodárek, P.; Écsiová, D.; Šimkovič, M. Chemoimmunotherapy in the First-Line Treatment of Chronic Lymphocytic Leukaemia: Dead Yet, or Alive and Kicking? Cancers 2021, 13, 3134. [Google Scholar] [CrossRef]
- Kluck, R.M.; Bossy-Wetzel, E.; Green, D.R.; Newmeyer, D.D. The Release of Cytochrome c from Mitochondria: A Primary Site for Bcl-2 Regulation of Apoptosis. Science 1997, 275, 1132–1136. [Google Scholar] [CrossRef]
- Kvansakul, M.; Yang, H.; Fairlie, W.D.; Czabotar, P.E.; Fischer, S.F.; Perugini, M.A.; Huang, D.C.S.; Colman, P.M. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008, 15, 1564–1571. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Fabbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 102, 13944–13949. [Google Scholar] [CrossRef] [PubMed]
- Stilgenbauer, S.; Eichhorst, B.; Schetelig, J.; Coutre, S.; Seymour, J.F.; Munir, T.; Puvvada, S.D.; Wendtner, C.-M.; Roberts, A.W.; Jurczak, W.; et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: A multicentre, open-label, phase 2 study. Lancet Oncol. 2016, 17, 768–778. [Google Scholar] [CrossRef]
- Bose, P.; Gandhi, V.; Konopleva, M. Pathways and mechanisms of venetoclax resistance. Leuk. Lymphoma 2017, 58, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, R.W.; Gong, J.-N.; Luo, C.S.; Lio, D.; White, C.A.; Anderson, M.A.; Blombery, P.; Lessene, G.; Majewski, I.J.; Thijssen, R.; et al. Structures of BCL-2 in complex with venetoclax reveal the molecular basis of resistance mutations. Nat. Commun. 2019, 10, 2385. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Deng, J.; Seymour, J.F.; Tam, C.; Kim, S.Y.; Fein, J.; Yu, L.; Brown, J.R.; Westerman, D.; Si, E.G.; et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood 2016, 127, 3215–3224. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Seymour, J.F.; Ma, S.; Brander, D.M.; Choi, M.Y.; Barrientos, J.; Davids, M.S.; Anderson, M.A.; Beaven, A.W.; Rosen, S.T.; Tam, C.S.; et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: A phase 1b study. Lancet Oncol. 2017, 18, 230–240. [Google Scholar] [CrossRef]
- Fischer, K.; Al-Sawaf, O.; Bahlo, J.; Fink, A.-M.; Tandon, M.; Dixon, M.; Robrecht, S.; Warburton, S.; Humphrey, K.; Samoylova, O.; et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019, 380, 2225–2236. [Google Scholar] [CrossRef]
- Flinn, I.W.; Gribben, J.G.; Dyer, M.J.S.; Wierda, W.; Maris, M.B.; Furman, R.R.; Hillmen, P.; Rogers, K.A.; Iyer, S.P.; Quillet-Mary, A.; et al. Phase 1b study of venetoclax-obinutuzumab in previously untreated and relapsed/refractory chronic lymphocytic leukemia. Blood 2019, 133, 2765–2775. [Google Scholar] [CrossRef]
- Kater, A.P.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.-D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Robak, T.; et al. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. NEJM Evid. 2022, 1. [Google Scholar] [CrossRef]
- Kersting, S.; Dubois, J.; Nasserinejad, K.; A Dobber, J.; Mellink, C.; van der Kevie-Kersemaekers, A.-M.F.; Evers, L.M.; de Boer, F.; Koene, H.R.; Schreurs, J.; et al. Venetoclax consolidation after fixed-duration venetoclax plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (HOVON 139/GiVe): Primary endpoint analysis of a multicentre, open-label, randomised, parallel-group, phase 2 trial. Lancet Haematol. 2022, 9, e190–e199. [Google Scholar] [CrossRef]
- Ryan, C.E.; Davids, M.S. BCL-2 Inhibitors, Present and Future. Cancer J. 2019, 25, 401–409. [Google Scholar] [CrossRef]
- Lee, H.H.; Dadgostar, H.; Cheng, Q.; Shu, J.; Cheng, G. NF-kappaB-mediated up-regulation of Bcl-x and Bfl-1/A1 is required for CD40 survival signaling in B lymphocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 9136–9141. [Google Scholar] [CrossRef]
- Tausch, E.; Close, W.; Dolnik, A.; Bloehdorn, J.; Chyla, B.; Bullinger, L.; Döhner, H.; Mertens, D.; Stilgenbauer, S. Venetoclax resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica 2019, 104, e434–e437. [Google Scholar] [CrossRef]
- Ramsey, H.E.; Fischer, M.A.; Lee, T.; Gorska, A.E.; Arrate, M.P.; Fuller, L.; Boyd, K.L.; Strickland, S.A.; Sensintaffar, J.; Hogdal, L.J.; et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018, 8, 1566–1581. [Google Scholar] [CrossRef] [PubMed]
- Leverson, J.D.; Zhang, H.; Chen, J.; Tahir, S.K.; Phillips, D.C.; Xue, J.; Nimmer, P.; Jin, S.; Smith, M.; Xiao, Y.; et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax). Cell Death Dis. 2015, 6, e1590. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Bian, Z.; Zhao, B.; Hogdal, L.J.; Sensintaffar, J.L.; Goodwin, C.M.; Belmar, J.; Shaw, S.; Tarr, J.C.; Veerasamy, N.; et al. Discovery and biological characterization of potent myeloid cell leukemia-1 inhibitors. FEBS Lett. 2017, 591, 240–251. [Google Scholar] [CrossRef]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.A.; Grant, B.; Sharman, J.P.; Coleman, M.; Wierda, W.G.; et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013, 369, 32–42. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef]
- Honigberg, L.A.; Smith, A.M.; Sirisawad, M.; Verner, E.; Loury, D.; Chang, B.; Li, S.; Pan, Z.; Thamm, D.H.; Miller, R.A.; et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080. [Google Scholar] [CrossRef] [PubMed]
- Mulder, T.A.; Peña-Pérez, L.; Berglöf, A.; Meinke, S.; Estupiñán, H.Y.; Heimersson, K.; Zain, R.; Månsson, R.; Smith, C.I.E.; Palma, M. Ibrutinib Has Time-dependent On- and Off-target Effects on Plasma Biomarkers and Immune Cells in Chronic Lymphocytic Leukemia. HemaSphere 2021, 5, e564. [Google Scholar] [CrossRef]
- Lipsky, A.H.; Farooqui, M.Z.; Tian, X.; Martyr, S.; Cullinane, A.M.; Nghiem, K.; Sun, C.; Valdez, J.; Niemann, C.U.; Herman, S.E.; et al. Incidence and risk factors of bleeding-related adverse events in patients with chronic lymphocytic leukemia treated with ibrutinib. Haematologica 2015, 100, 1571–1578. [Google Scholar] [CrossRef]
- Barf, T.; Covey, T.; Izumi, R.; van de Kar, B.; Gulrajani, M.; van Lith, B.; van Hoek, M.; de Zwart, E.; Mittag, D.; Demont, D.; et al. Acalabrutinib (ACP-196): A Covalent Bruton Tyrosine Kinase Inhibitor with a Differentiated Selectivity and In Vivo Potency Profile. J. Pharmacol. Exp. Ther. 2017, 363, 240–252. [Google Scholar] [CrossRef]
- Flinsenberg, T.W.H.; Tromedjo, C.C.; Hu, N.; Liu, Y.; Guo, Y.; Thia, K.Y.T.; Noori, T.; Song, X.; Aw Yeang, H.X.; Tantalo, D.G.; et al. Differential effects of BTK inhibitors ibrutinib and zanubrutinib on NK-cell effector function in patients with mantle cell lymphoma. Haematologica 2020, 105, e76–e79. [Google Scholar] [CrossRef] [PubMed]
- Montoya, S.; Thompson, M.C. Non-Covalent Bruton’s Tyrosine Kinase Inhibitors in the Treatment of Chronic Lymphocytic Leukemia. Cancers 2023, 15, 3648. [Google Scholar] [CrossRef]
- Montoya, S.; Bourcier, J.; Noviski, M.; Lu, H.; Thompson, M.C.; Chirino, A.; Jahn, J.; Sondhi, A.K.; Gajewski, S.; Tan, Y.S.; et al. Kinase-impaired BTK mutations are susceptible to clinical-stage BTK and IKZF1/3 degrader NX-2127. Science 2024, 383, eadi5798. [Google Scholar] [CrossRef]
- Gui, F.; Jiang, J.; He, Z.; Li, L.; Li, Y.; Deng, Z.; Lu, Y.; Wu, X.; Chen, G.; Su, J.; et al. A non-covalent inhibitor XMU-MP-3 overrides ibrutinib-resistant BtkC481S mutation in B-cell malignancies. Br. J. Pharmacol. 2019, 176, 4491–4509. [Google Scholar] [CrossRef]
- Woyach, J.A.; Furman, R.R.; Liu, T.M.; Ozer, H.G.; Zapatka, M.; Ruppert, A.S.; Xue, L.; Li, D.H.; Steggerda, S.M.; Versele, M.; et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 2014, 370, 2286–2294. [Google Scholar] [CrossRef]
- Zain, R.; Vihinen, M. Structure-Function Relationships of Covalent and Non-Covalent BTK Inhibitors. Front. Immunol. 2021, 12, 694853. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Mi, X.; Thompson, M.C.; Montoya, S.; Notti, R.Q.; Afaghani, J.; Durham, B.H.; Penson, A.; Witkowski, M.T.; Lu, S.X.; et al. Mechanisms of Resistance to Noncovalent Bruton’s Tyrosine Kinase Inhibitors. N. Engl. J. Med. 2022, 386, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, K.; Puła, B. A Review of Resistance Mechanisms to Bruton’s Kinase Inhibitors in Chronic Lymphocytic Leukemia. Int. J. Mol. Sci. 2024, 25, 5246. [Google Scholar] [CrossRef]
- Bender, A.T.; Gardberg, A.; Pereira, A.; Johnson, T.; Wu, Y.; Grenningloh, R.; Head, J.; Morandi, F.; Haselmayer, P.; Liu-Bujalski, L. Ability of Bruton’s Tyrosine Kinase Inhibitors to Sequester Y551 and Prevent Phosphorylation Determines Potency for Inhibition of Fc Receptor but not B-Cell Receptor Signaling. Mol. Pharmacol. 2017, 91, 208–219. [Google Scholar] [CrossRef]
- Blombery, P.; Thompson, E.R.; Lew, T.E.; Tiong, I.S.; Bennett, R.; Cheah, C.Y.; Lewis, K.L.; Handunnetti, S.M.; Tang, C.P.S.; Roberts, A.; et al. Enrichment of BTK Leu528Trp mutations in patients with CLL on zanubrutinib: Potential for pirtobrutinib cross-resistance. Blood Adv. 2022, 6, 5589–5592. [Google Scholar] [CrossRef]
- Brown, J.R.; Desikan, S.P.; Nguyen, B.; Won, H.; Tantawy, S.I.; McNeely, S.; Marella, N.; Ebata, K.; Woyach, J.A.; Patel, K.; et al. Genomic Evolution and Resistance during Pirtobrutinib Therapy in Covalent BTK-Inhibitor (cBTKi) Pre-Treated Chronic Lymphocytic Leukemia Patients: Updated Analysis from the BRUIN Study. Blood 2023, 142, 326. [Google Scholar] [CrossRef]
- Handunnetti, S.M.; Tang, C.P.S.; Nguyen, T.; Zhou, X.; Thompson, E.; Sun, H.; Xing, H.; Zhang, B.; Guo, Y.; Sutton, L.A.; et al. BTK Leu528Trp—A Potential Secondary Resistance Mechanism Specific for Patients with Chronic Lymphocytic Leukemia Treated with the Next Generation BTK Inhibitor Zanubrutinib. Blood 2019, 134, 170. [Google Scholar] [CrossRef]
- Xu, B.; Liang, L.; Jiang, Y.; Zhao, Z. Investigating the ibrutinib resistance mechanism of L528W mutation on Bruton’s tyrosine kinase via molecular dynamics simulations. J. Mol. Graph. Model. 2024, 126, 108623. [Google Scholar] [CrossRef]
- Veeraraghavan, S.; Viswanadha, S.; Thappali, S.; Govindarajulu, B.; Vakkalanka, S.; Rangasamy, M. Simultaneous quantification of lenalidomide, ibrutinib and its active metabolite PCI-45227 in rat plasma by LC–MS/MS: Application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2015, 107, 151–158. [Google Scholar] [CrossRef]
- Gomez, E.B.; Ebata, K.; Randeria, H.S.; Rosendahl, M.S.; Cedervall, E.P.; Morales, T.H.; Hanson, L.M.; Brown, N.E.; Gong, X.; Stephens, J.; et al. Preclinical characterization of pirtobrutinib, a highly selective, noncovalent (reversible) BTK inhibitor. Blood 2023, 142, 62–72. [Google Scholar]
- Nalawansha, D.A.; Crews, C.M. PROTACs: An Emerging Therapeutic Modality in Precision Medicine. Cell Chem. Biol. 2020, 27, 998–1014. [Google Scholar] [CrossRef]
- Paiva, S.-L.; Crews, C.M. Targeted protein degradation: Elements of PROTAC design. Curr. Opin. Chem. Biol. 2019, 50, 111–119. [Google Scholar] [CrossRef]
- Pettersson, M.; Crews, C.M. PROteolysis TArgeting Chimeras (PROTACs)—Past, present and future. Drug Discov. Today Technol. 2019, 31, 15–27. [Google Scholar] [CrossRef]
- Rutherford, K.A.; McManus, K.J. PROTACs: Current and Future Potential as a Precision Medicine Strategy to Combat Cancer. Mol. Cancer Ther. 2024, 23, 454–463. [Google Scholar] [CrossRef]
- Hyak, J.M.; Huang, Y.; Rogers, K.A.; Bhat, S.A.; Grever, M.R.; Byrd, J.C.; Kittai, A.S.; Jones, D.; Miller, C.R.; Woyach, J.A. Combined BCL2 and BTK inhibition in CLL demonstrates efficacy after monotherapy with both classes. Blood Adv. 2022, 6, 5124–5127. [Google Scholar]
- Wierda, W.G.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Opat, S.; Tedeschi, A.; Badoux, X.C.; Kuss, B.J.; Jackson, S.; Moreno, C.; et al. Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results from the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study. J. Clin. Oncol. 2021, 39, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- Roy Chowdhury, S.; Singh, A.; Nguyen, E.; Marshall, A.; Johnston, J.B.; Gibson, S.B.; Banerji, V. Ibrutinib in Combination with Venetoclax Decreases Mitochondrial Bioenergetics through the Impaired BTK, AKT and AMPK/SIRT/PGC-1α Signaling Pathway in CLL. Blood 2019, 134, 1282. [Google Scholar] [CrossRef]
- Tam, C.S.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.; Opat, S.; Barr, P.M.; Tedeschi, A.; Trentin, L.; Bannerji, R.; et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: Primary analysis of the CAPTIVATE FD cohort. Blood 2022, 139, 3278–3289. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Keating, M.J.; Thompson, P.A.; Ferrajoli, A.; Burger, J.A.; Borthakur, G.; Takahashi, K.; Estrov, Z.E.; Sasaki, K.; Fowler, N.H.; et al. Combined Ibrutinib and Venetoclax for First-Line Treatment for Patients with Chronic Lymphocytic Leukemia (CLL): Focus on MRD Results. Blood 2020, 136, 42–43. [Google Scholar] [CrossRef]
- Hillmen, P.; Rawstron, A.C.; Brock, K.; Muñoz-Vicente, S.; Yates, F.J.; Bishop, R.; Boucher, R.; MacDonald, D.; Fegan, C.; McCaig, A.; et al. Ibrutinib Plus Venetoclax in Relapsed/Refractory Chronic Lymphocytic Leukemia: The CLARITY Study. J. Clin. Oncol. 2019, 37, 2722–2729. [Google Scholar] [CrossRef]
- Rogers, K.A.; Woyach, J.A. The evolving frontline management of CLL: Are triplets better than doublets? How will we find out? Hematol. Am. Soc. Hematol. Educ. Program. 2024, 2024, 467–473. [Google Scholar] [CrossRef]
- Rogers, K.A.; Huang, Y.; Ruppert, A.S.; Abruzzo, L.V.; Andersen, B.L.; Awan, F.T.; Bhat, S.A.; Dean, A.; Lucas, M.; Banks, C.; et al. Phase II Study of Combination Obinutuzumab, Ibrutinib, and Venetoclax in Treatment-Naïve and Relapsed or Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 3626–3637. [Google Scholar] [CrossRef]
- Woyach, J.A.; Yin, J.; Brown, J.R.; Dinner, S.; Lozanski, G.; Little, R.F.; Miller, C.; Damarla, V.K.; Coutre, S.E.; Ding, W.; et al. Results of a phase 3 study of IVO vs IO for previously untreated older patients (pts) with chronic lymphocytic leukemia (CLL) and impact of COVID-19 (Alliance). J. Clin. Oncol. 2023, 41 (Suppl. S16), 7500. [Google Scholar] [CrossRef]
- Davids, M.S.; Lampson, B.L.; Tyekucheva, S.; Wang, Z.; Lowney, J.C.; Pazienza, S.; Montegaard, J.; Patterson, V.; Weinstock, M.; Crombie, J.L.; et al. Acalabrutinib, venetoclax, and obinutuzumab as frontline treatment for chronic lymphocytic leukaemia: A single-arm, open-label, phase 2 study. Lancet Oncol. 2021, 22, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Ferrajoli, A.; Swaminathan, M.; Reville, P.K.; Burger, J.A.; Bharathi, V.; Atluri, H.; Cherng, H.-J.J.; Bataller, A.; Jabbour, E.; et al. Combined Pirtobrutinib, Venetoclax, and Obinutuzumab As First-Line Treatment of Patients with Chronic Lymphocytic Leukemia (CLL). Blood 2024, 144, 1011. [Google Scholar] [CrossRef]
- Buhimschi, A.D.; Armstrong, H.A.; Toure, M.; Jaime-Figueroa, S.; Chen, T.L.; Lehman, A.M.; Woyach, J.A.; Johnson, A.J.; Byrd, J.C.; Crews, C.M. Targeting the C481S Ibrutinib-Resistance Mutation in Bruton’s Tyrosine Kinase Using PROTAC-Mediated Degradation. Biochemistry 2018, 57, 3564–3575. [Google Scholar] [CrossRef] [PubMed]
- Salvaris, R.T.; Brennan, J.; Lewis, K.L. BTK Is the Target That Keeps on Giving: A Review of BTK-Degrader Drug Development, Clinical Data, and Future Directions in CLL. Cancers 2025, 17, 557. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.; Rodriguez-Rodriguez, S.; Roleder, C.; Whelan, S.; Tan, M.; Lee, E.; Munson, P.; Danilov, A.V. Nx-2127 and Nx-5948, Two Clinical Stage Cereblon-Recruiting BTK Degraders, Facilitate T Cell Functionality in Chronic Lymphocytic Leukemia. Blood 2024, 144, 77. [Google Scholar] [CrossRef]
- Robbins, D.W.; Kelly, A.; Tan, M.; McIntosh, J.; Wu, J.; Konst, Z.; Kato, D.; Peng, G.; Mihalic, J.; Weiss, D.; et al. Nx-2127, a Degrader of BTK and IMiD Neosubstrates, for the Treatment of B-Cell Malignancies. Blood 2020, 136, 34. [Google Scholar] [CrossRef]
- Fiskus, W.C.; Das, K.; Mill, C.P.; Birdwell, C.E.; Davis, J.A.; Alhamadani, N.; Philip, K.; Tan, M.; Brown, R.A.; Green, M.R.; et al. Bruton’s Tyrosine Kinase (BTK) Degrader Nx-2127 Exhibits Lethal Activity and Synergy with Venetoclax and BET Protein Inhibitor Against MCL Cells Sensitive or Resistant to Covalent BTK Inhibitors. Blood 2022, 140 (Suppl. S1), 5980–5981. [Google Scholar] [CrossRef]
- Wong, R.L.; Choi, M.Y.; Wang, H.-Y.; Kipps, T.J. Mutation in Bruton Tyrosine Kinase (BTK) A428D confers resistance To BTK-degrader therapy in chronic lymphocytic leukemia. Leukemia 2024, 38, 1818–1821. [Google Scholar] [CrossRef]
- Nawaratne, V.; Sondhi, A.K.; Abdel-Wahab, O.; Taylor, J. New Means and Challenges in the Targeting of BTK. Clin. Cancer Res. 2024, 30, 2333–2341. [Google Scholar] [CrossRef]
- Bonfiglio, S.; Sutton, L.-A.; Ljungström, V.; Capasso, A.; Pandzic, T.; Weström, S.; Foroughi-Asl, H.; Skaftason, A.; Gellerbring, A.; Lyander, A.; et al. BTK and PLCG2 remain unmutated in one-third of patients with CLL relapsing on ibrutinib. Blood Adv. 2023, 7, 2794–2806. [Google Scholar] [CrossRef]
- Brown, J.R.; Li, J.; Eichhorst, B.F.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Ramakrishnan, V.; Huang, R.; Shi, Y.; et al. Acquired Mutations in Patients (Pts) with Relapsed/Refractory (R/R) Chronic Lymphocytic Leukemia (CLL) That Progressed in the ALPINE Study. Blood 2023, 142, 1890. [Google Scholar] [CrossRef]
- Woyach, J.; Huang, Y.; Rogers, K.; Bhat, S.A.; Grever, M.R.; Lozanski, A.; Doong, T.-J.; Blachly, J.S.; Lozanski, G.; Jones, D.; et al. Resistance to Acalabrutinib in CLL Is Mediated Primarily By BTK Mutations. Blood 2019, 134, 504. [Google Scholar] [CrossRef]
- Palma, M.; Mulder, T.A.; Österborg, A. BTK Inhibitors in Chronic Lymphocytic Leukemia: Biological Activity and Immune Effects. Front. Immunol. 2021, 12, 686768. [Google Scholar] [CrossRef]
- Lévy, V.; Delmer, A.; Cymbalista, F. Frontline treatment in CLL: The case for time-limited treatment. Hematology 2021, 2021, 59–67. [Google Scholar] [CrossRef]
- Rhodes, J.M.; Lopez, C.A.; Barrientos, J.C. MRD-directed therapy in CLL: Ready for prime time? Hematology 2023, 2023, 413–420. [Google Scholar] [CrossRef]
- Benintende, G.; Pozzo, F.; Innocenti, I.; Autore, F.; Fresa, A.; D’Arena, G.; Gattei, V.; Lurenti, L. Measurable residual disease in chronic lymphocytic leukemia. Front. Oncol. 2023, 13, 1112616. [Google Scholar] [CrossRef]
- Rogers, K.A.; Woyach, J.A. A CAPTIVATE-ing new regimen for CLL. Blood 2022, 139, 3229–3230. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, S.; Ritgen, M.; Pott, C.; Brüggemann, M.; Raff, T.; Stilgenbauer, S.; Döhner, H.; Dreger, P.; Kneba, M. Comparative analysis of minimal residual disease detection using four-color flow cytometry, consensus IgH-PCR, and quantitative IgH PCR in CLL after allogeneic and autologous stem cell transplantation. Leukemia 2004, 18, 1637–1645. [Google Scholar] [CrossRef] [PubMed]
- Raponi, S.; Della Starza, I.; De Propris, M.S.; Del Giudice, I.; Mauro, F.R.; Marinelli, M.; Di Maio, V.; Piciocchi, A.; Foà, R.; Guarini, A. Minimal residual disease monitoring in chronic lymphocytic leukaemia patients. A comparative analysis of flow cytometry and ASO IgH RQ-PCR. Br. J. Haematol. 2014, 166, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Dogliotti, I.; Drandi, D.; Genuardi, E.; Ferrero, S. New Molecular Technologies for Minimal Residual Disease Evaluation in B-Cell Lymphoid Malignancies. J. Clin. Med. 2018, 7, 288. [Google Scholar] [CrossRef] [PubMed]
- Rawstron, A.C.; Kreuzer, K.-A.; Soosapilla, A.; Spacek, M.; Stehlikova, O.; Gambell, P.; McIver-Brown, N.; Villamor, N.; Psarra, K.; Arroz, M.; et al. Reproducible diagnosis of chronic lymphocytic leukemia by flow cytometry: An European Research Initiative on CLL (ERIC) & European Society for Clinical Cell Analysis (ESCCA) Harmonisation project. Cytom. Part B Clin. Cytom. 2018, 94, 121–128. [Google Scholar]
- Winter, A.M.; Landever, O.; Mendries, H.; Van Heeckeren, W.; Fu, C.-L.; Dean, R.M.; Brooks, T.R.; Jagadeesh, D.; Caimi, P.F.; Hill, B.T. Real World Experience with Time Limited Venetoclax and Obinutuzumab (VO) for Frontline Treatment of CLL/SLL with MRD Determination By Clonoseq®. Blood 2024, 144, 3247. [Google Scholar] [CrossRef]
- Chandhok, N.S.; Sekeres, M.A. Measurable residual disease in hematologic malignancies: A biomarker in search of a standard. eClinicalMedicine 2025, 86, 103348. [Google Scholar] [CrossRef] [PubMed]
| Mutation | Therapeutic Resistance | Mechanism of Resistance | Therapy that Overcomes Resistance | References |
|---|---|---|---|---|
| C481S | Ibrutinib/Zanubrutinib/Acalabrutinib | Gatekeeper; disrupts irreversible binding of cBTKi through alteration of 481 residue | Pirtobrutinib/BTK Degraders | [46,75] |
| L528W | Zanubrutinib/Pirtobrutinib | Kinase-impaired; blocks activity of BTK while continuing downstream signaling of the BCR pathway | BTK Degraders | [44,76] |
| T474I | Acalabrutinib/Pirtobrutinib | Gatekeeper; alters 474 residues to prevent binding | BTK Degraders | [43,44,46,76,77] |
| A428D | Ibrutinib/Zanubrutinib | A428D mutation has been associated with resistance, but the mechanism has not been determined or characterized | [76,77] | |
| Pirtobrutinib | Kinase-impaired; blocks activity of BTK while continuing downstream signaling of the BCR pathway | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekeres, S.; Lamkin, E.N.; Bravo, E., Jr.; Cool, A.; Taylor, J. Resistance Mutations in CLL: Genetic Mechanisms Shaping the Future of Targeted Therapy. Genes 2025, 16, 1064. https://doi.org/10.3390/genes16091064
Sekeres S, Lamkin EN, Bravo E Jr., Cool A, Taylor J. Resistance Mutations in CLL: Genetic Mechanisms Shaping the Future of Targeted Therapy. Genes. 2025; 16(9):1064. https://doi.org/10.3390/genes16091064
Chicago/Turabian StyleSekeres, Samantha, Erica N. Lamkin, Eduardo Bravo, Jr., Allison Cool, and Justin Taylor. 2025. "Resistance Mutations in CLL: Genetic Mechanisms Shaping the Future of Targeted Therapy" Genes 16, no. 9: 1064. https://doi.org/10.3390/genes16091064
APA StyleSekeres, S., Lamkin, E. N., Bravo, E., Jr., Cool, A., & Taylor, J. (2025). Resistance Mutations in CLL: Genetic Mechanisms Shaping the Future of Targeted Therapy. Genes, 16(9), 1064. https://doi.org/10.3390/genes16091064






