Angiogenic microRNAs in Systemic Sclerosis: Insights into Microvascular Dysfunction and Therapeutic Implications
Abstract
1. Introduction
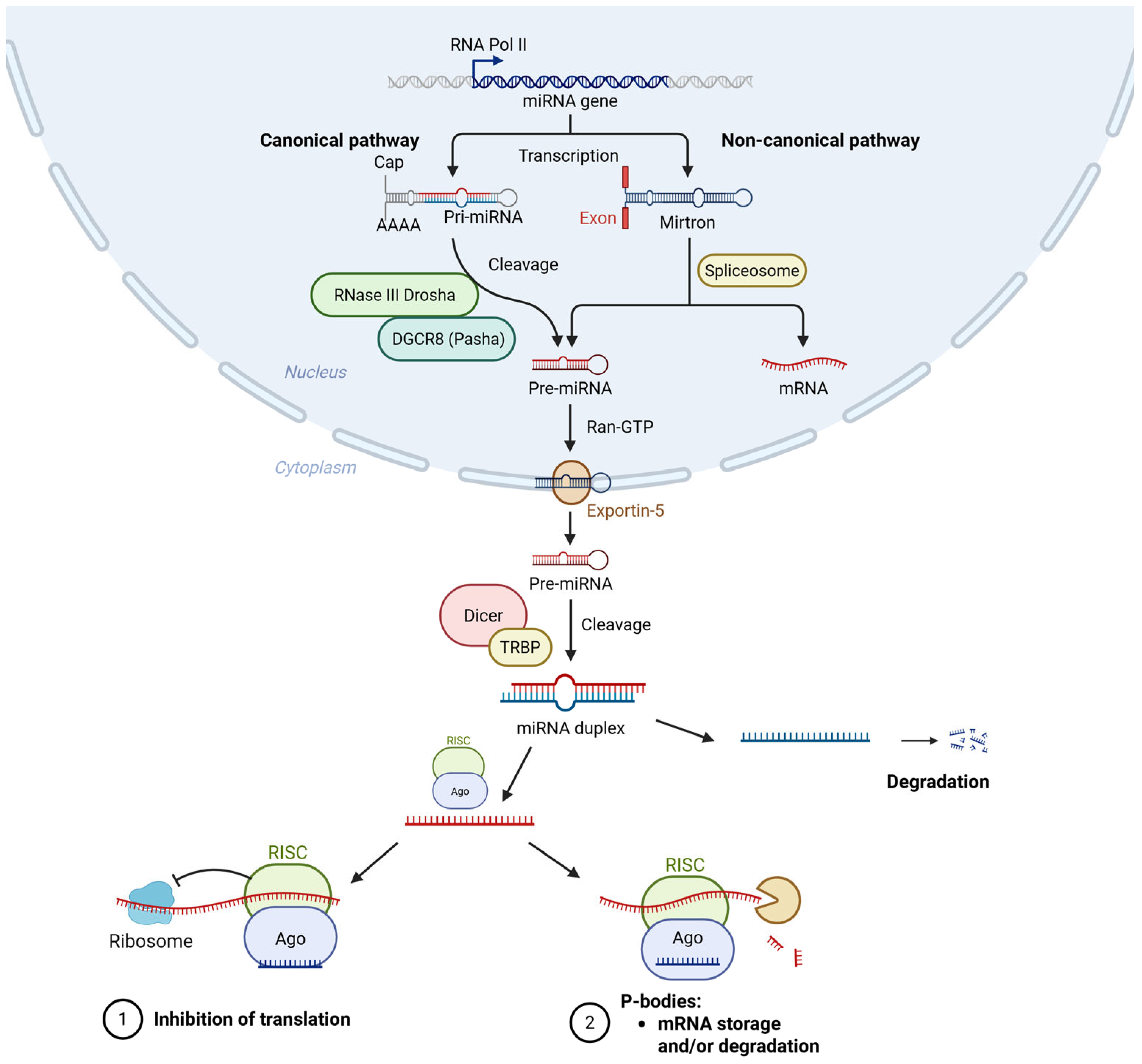
2. microRNAs Biogenesis and Function
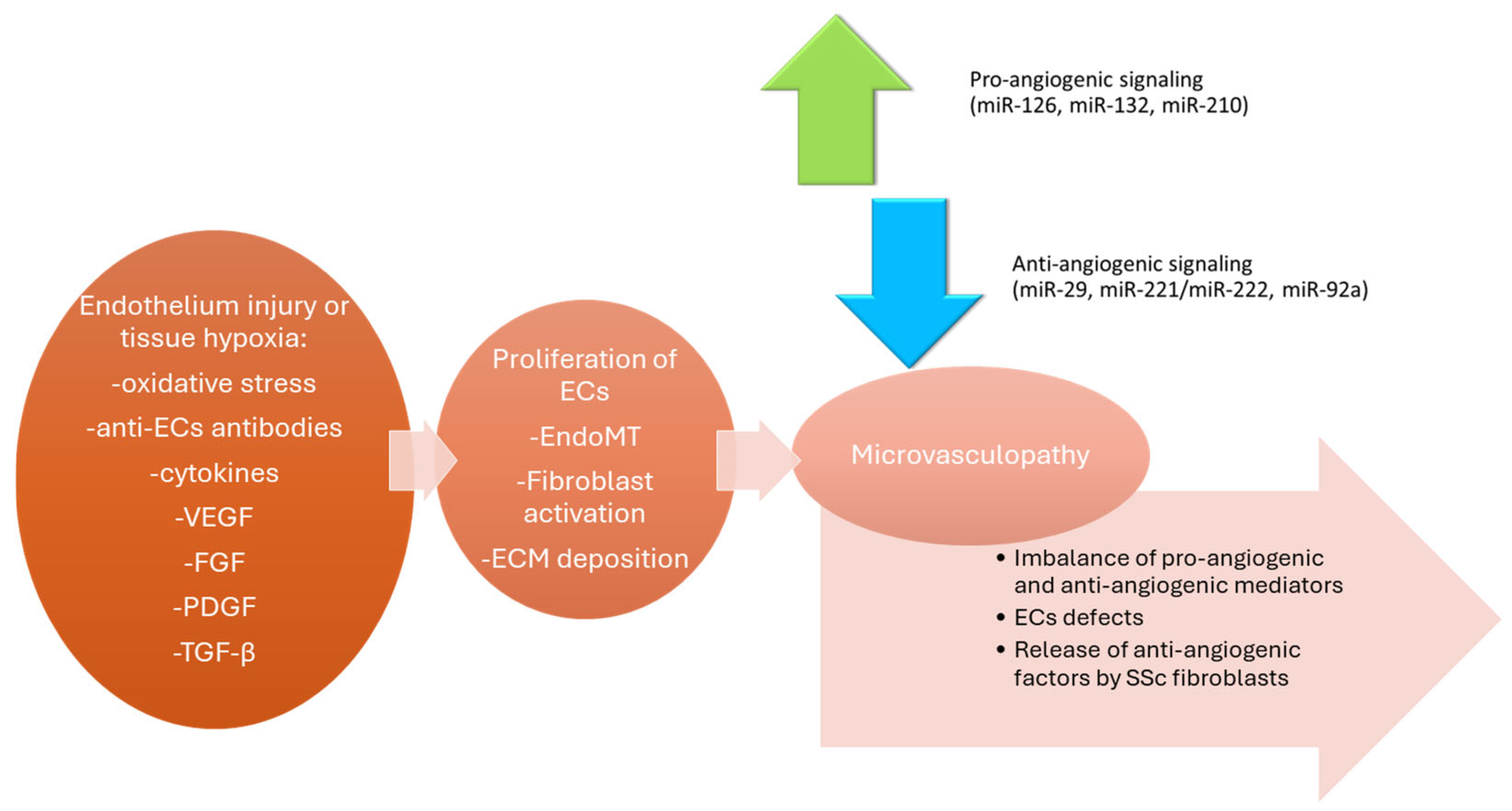
3. Endothelial Dysfunction and Microvascular Damage in SSc
4. Angiogenic and Anti-Angiogenic miRNAs in SSc
4.1. Pro-Angiogenic miRNAs
4.1.1. miR-126
4.1.2. miR-130a
4.1.3. miR-210
4.1.4. miR-296
4.1.5. miR-148b
4.1.6. miR-155
4.1.7. let-7f and miR-27b
4.1.8. miR-152
4.1.9. miR-193b
4.1.10. miR-20a
4.1.11. miR-146a
4.1.12. miR-125a
4.2. Anti-Angiogenic miRNAs
4.2.1. miR-221/222
4.2.2. miR-92a
4.2.3. miR-15b, miR-16, miR-20a/b
4.2.4. miR-22
4.2.5. miR-200c
4.2.6. miR-34a
4.2.7. miR-214
4.2.8. miR-217
4.2.9. miR-328
4.3. miRNAs Regulating EndoMT and EC Apoptosis
5. miRNAs as Diagnostic and Prognostic Biomarkers Results
6. Therapeutic Potential of Targeting miRNAs
6.1. Experimental miRNA-Based Therapies in SSc
6.2. Challenges in Delivery and Specificity
6.3. miRNA–lncRNA–circRNA Interactions
6.4. Clinical Translation: Current Status
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AECAs | Anti-endothelial cell antibodies |
| Ago2 | Argonaut 2 |
| AT1R | Angiotensin II type 1 receptor |
| CD133 | Prominin-1 |
| ceRNAs | Competing endogenous RNAs |
| CEUS | Contrast-enhanced ultrasound |
| circRNAs | Circular RNAs |
| c-Kit (CD117) | Stem cell factor receptor |
| COL1A1 | Collagen type 1, alpha 1 |
| CTGF | Connective tissue growth factor |
| CUL2 | Cullin-2 |
| DNMT1 | DNA methyltransferase 1 |
| ECM | Extracellular Matrix |
| ECs | Endothelial cells |
| EFNA3 | Ephrin-A3 |
| EndoMT | Endothelial-to-mesenchymal transition |
| eNOS | Endothelial nitric oxide synthase |
| EPCs | Endothelial progenitor cells |
| ET-1 | Endothelin-1 |
| FBs | Fibroblasts |
| FGF | Fibroblast growth factor |
| Fli1 | Friend leukemia integration 1 transcription factor |
| FOXO | Forkhead family of transcription factors |
| Fra2 | Fos-related antigen 2 |
| FUS-1 | Nuclear fusion protein |
| GAX | Growth arrest-specific homeobox gene |
| HGS | Hepatocyte growth factor-regulated tyrosine kinase substrate |
| HIF1α | Hypoxia-inducible factor 1alfa |
| HOX-A5 | Homeobox protein HOX-A5 |
| HUVECs | Human umbilical vein endothelial cells |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IGF-1 | Insulin-like growth factor 1 |
| IL-1β | Interleukin 1beta |
| ITG-α5 | Integrin alfa5 |
| KLF-4, 5 | Kruppel-like factor 4, 5 |
| LNA | Locked nucleic acid |
| lncRNAs | Long non-coding RNAs |
| MAP3K7 | Mitogen-activated protein kinase 7 |
| MiRNAs | MicroRNAs |
| MKK3 | Mitogen-activated protein kinase 3 |
| MMP1 | Matrix metalloproteinase 1 |
| MVECs | Microvascular endothelial cells |
| NFκβ | Nuclear factor kappa beta |
| NO | Nitric oxide |
| NOX4 | NADPH oxidase 4 |
| NPM1 | Nucleophosmin 1 |
| NPTX1 | Neuronal pentraxin 1 |
| NVC | Nailfold videocapillaroscopy |
| OCTA | Optical coherence tomography angiography |
| p53 | Protein p53 |
| PAH | Pulmonary hypertension |
| PAK1 | p21-Activated kinase 1 |
| PDGF | Platelet derived growth factor |
| PDGFRB | Platelet derived growth factor receptor beta |
| PFKFB3 | 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 |
| PHD1,2 | Prolyl hydroxylase |
| PIK3R2 | Phosphoinositide-3-kinase regulatory subunit 2 |
| PLAU | Plasminogen activator, urokinase |
| Pre-miRNAs | Precursor miRNAs |
| Pri-miRNAs | Primary miRNAs |
| PTEN | Phosphatase and tensin homolog |
| RAS/ERK pathway | Rat Sarcoma/Extracellular Signal-Regulated Kinase |
| RISC | RNA-induced silencing complex |
| ROBO1 | Roundabout guidance receptor 1 |
| ROS | Reactive oxygen species |
| SEMA6A | Semaphoring 6A |
| siRNAs | Silent interfering RNAs |
| SIRT1 | Sirtuin 1 |
| SMAD3/7 | Mothers against decapentaplegic homologue 3/7 |
| SPRED | Sprouty-related, EVH1 do-main-containing protein 1 |
| SSc | Systemic sclerosis |
| STAT5a | Signal transducer and activator of transcription 5a |
| SUFU | Suppressor of fused homolog |
| TAK1 | Transforming growth factor beta-activated kinase 1 |
| TGF-β1 | Transforming growth factor beta-1 |
| TIMP-1 | Tissue inhibitor of metalloproteinases |
| TNF-α | Tumor necrosis factor alfa |
| TSP-1 | Thrombospondin 1 |
| TSR | Methyl-accepting chemotaxis protein |
| UTR | Untranslated region |
| VASH1 | Vasohibin 1 |
| VCAM-1 | Vascular cell adhesion protein 1 |
| VE-cadherin | Vascular endothelia cadherin |
| VEGF | Vascular endothelial growth factor |
| VEGFR-2 | Vascular endothelial growth factor receptor 2 |
| VSMCs | Vascular smooth muscle cells |
| XPO5 | Exportin 5 |
| ZEB1 | Zinc finger E-box-binding homeobox 1 |
| α-SMA | alfa-smooth muscle actin |
| β-TRC | beta-transducin repeat-containing gene |
References
- Trojanowska, M. Cellular and Molecular Aspects of Vascular Dysfunction in Systemic Sclerosis. Nat. Rev. Rheumatol. 2010, 6, 453. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.N.; Schwartz, S.M. The Pathology of Scleroderma Vascular Disease. Rheum. Dis. Clin. N. Am. 2008, 34, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Abraham, D. Systemic Sclerosis: A Prototypic Multisystem Fibrotic Disorder. J. Clin. Investig. 2007, 117, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Shumnalieva, R.; Monov, S.; Velikova, T. MicroRNAs in Systemic Sclerosis: Involvement in Disease Pathogenesis and Potential Use as Diagnostic Biomarkers and Therapeutic Targets. Biomedicines 2025, 13, 1216. [Google Scholar] [CrossRef]
- Jimenez, S.A.; Mendoza, F.A.; Piera-Velazquez, S. A Review of Recent Studies on the Pathogenesis of Systemic Sclerosis: Focus on Fibrosis Pathways. Front. Immunol. 2025, 16, 1551911. [Google Scholar] [CrossRef]
- Flower, V.A.; Barratt, S.L.; Ward, S.; Pauling, J.D. The Role of Vascular Endothelial Growth Factor in Systemic Sclerosis. Curr. Rheumatol. Rev. 2018, 15, 99–109. [Google Scholar] [CrossRef]
- Maurer, B.; Distler, A.; Suliman, Y.A.; Gay, R.E.; Michel, B.A.; Gay, S.; Distler, J.H.W.; Distler, O. Vascular Endothelial Growth Factor Aggravates Fibrosis and Vasculopathy in Experimental Models of Systemic Sclerosis. Ann. Rheum. Dis. 2014, 73, 1880–1887. [Google Scholar] [CrossRef]
- Campitiello, R.; Soldano, S.; Gotelli, E.; Hysa, E.; Montagna, P.; Casabella, A.; Paolino, S.; Pizzorni, C.; Sulli, A.; Smith, V.; et al. The Intervention of Macrophages in Progressive Fibrosis Characterizing Systemic Sclerosis: A Systematic Review. Autoimmun. Rev. 2024, 23, 103637. [Google Scholar] [CrossRef]
- Dudley, A.C.; Griffioen, A.W. Pathological Angiogenesis: Mechanisms and Therapeutic Strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wei, J.; Varga, J. Understanding Fibrosis in Systemic Sclerosis: Shifting Paradigms, Emerging Opportunities. Nat. Rev. Rheumatol. 2012, 8, 42–54. [Google Scholar] [CrossRef]
- Al-Dhaher, F.F.; Pope, J.E.; Ouimet, J.M. Determinants of Morbidity and Mortality of Systemic Sclerosis in Canada. Semin. Arthritis Rheum. 2010, 39, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Simeon-Aznar, C.P.; Fonollosa-Pla, V.; Tolosa-Vilella, C.; Espinosa-Garriga, G.; Campillo-Grau, M.; Ramos-Casals, M.; Garcia-Hernandez, F.J.; Castillo-Palma, M.J.; Sanchez-Roman, J.; Callejas-Rubio, J.L.; et al. Registry of the Spanish Network for Systemic Sclerosis: Survival, Prognostic Factors, and Causes of Death. Medicine 2015, 94, e1728. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lunardi, C.; Bason, C.; Navone, R.; Millo, E.; Damonte, G.; Corrocher, R.; Puccetti, A. Systemic Sclerosis Immunoglobulin G Autoantibodies Bind the Human Cytomegalovirus Late Protein UL94 and Induce Apoptosis in Human Endothelial Cells. Nat. Med. 2000, 6, 1183–1186. [Google Scholar] [CrossRef]
- Lunardi, C.; Dolcino, M.; Peterlana, D.; Bason, C.; Navone, R.; Tamassia, N.; Beri, R.; Corrocher, R.; Puccetti, A. Antibodies against Human Cytomegalovirus in the Pathogenesis of Systemic Sclerosis: A Gene Array Approach. PLoS Med. 2005, 3, e2. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Wang, Q.; Xiao, R.; Lu, Q. Systemic Sclerosis: Genetics and Epigenetics. J. Autoimmun. 2013, 41, 161–167. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Y.; Shu, Y.; Lu, Q.; Xiao, R. Epigenetic Mechanisms: An Emerging Role in Pathogenesis and Its Therapeutic Potential in Systemic Sclerosis. Int. J. Biochem. Cell Biol. 2015, 67, 92–100. [Google Scholar] [CrossRef]
- Dolcino, M.; Pelosi, A.; Fiore, P.F.; Patuzzo, G.; Tinazzi, E.; Lunardi, C.; Puccetti, A. Gene Profiling in Patients with Systemic Sclerosis Reveals the Presence of Oncogenic Gene Signatures. Front. Immunol. 2018, 9, 449. [Google Scholar] [CrossRef]
- Altorok, N.; Almeshal, N.; Wang, Y.; Kahaleh, B. Epigenetics, the Holy Grail in the Pathogenesis of Systemic Sclerosis. Rheumatology 2015, 54, 1759–1770. [Google Scholar] [CrossRef]
- Makino, T.; Jinnin, M. Genetic and Epigenetic Abnormalities in Systemic Sclerosis. J. Dermatol. 2016, 43, 10–18. [Google Scholar] [CrossRef]
- Fioretto, B.S.; Rosa, I.; Matucci-Cerinic, M.; Romano, E.; Manetti, M. Current Trends in Vascular Biomarkers for Systemic Sclerosis: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 4097. [Google Scholar] [CrossRef]
- Matucci-Cerinic, M.; Manetti, M.; Bruni, C.; Chora, I.; Bellando-Randone, S.; Lepri, G.; De Paulis, A.; Guiducci, S. The “Myth” of Loss of Angiogenesis in Systemic Sclerosis: A Pivotal Early Pathogenetic Process or Just a Late Unavoidable Event? Arthritis Res. Ther. 2017, 19, 162. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Rosa, I.; Fioretto, B.S.; Manetti, M. Recent Insights into Cellular and Molecular Mechanisms of Defective Angiogenesis in Systemic Sclerosis. Biomedicines 2024, 12, 1331. [Google Scholar] [CrossRef] [PubMed]
- Broen, J.C.A.; Radstake, T.R.D.J.; Rossato, M. The Role of Genetics and Epigenetics in the Pathogenesis of Systemic Sclerosis. Nat. Rev. Rheumatol. 2014, 10, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, H.; Zuo, X. MicroRNAs: Their Involvement in Fibrosis Pathogenesis and Use as Diagnostic Biomarkers in Scleroderma. Exp. Mol. Med. 2013, 45, e41. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Singhvi, G.; Manchanda, P.; Krishna Rapalli, V.; Kumar Dubey, S.; Gupta, G.; Dua, K. MicroRNAs as Biological Regulators in Skin Disorders. Biomed. Pharmacother. 2018, 108, 996–1004. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting Effective MicroRNA Target Sites in Mammalian MRNAs. elife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Duttagupta, R.; Jiang, R.; Gollub, J.; Getts, R.C.; Jones, K.W. Impact of Cellular MiRNAs on Circulating MiRNA Biomarker Signatures. PLoS ONE 2011, 6, e20769. [Google Scholar] [CrossRef]
- Deng, X.; Su, Y.; Wu, H.; Wu, R.; Zhang, P.; Dai, Y.; Chan, T.M.; Zhao, M.; Lu, Q. The Role of MicroRNAs in Autoimmune Diseases with Skin Involvement. Scand. J. Immunol. 2015, 81, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ruan, K. MicroRNA Detection by Microarray. Anal. Bioanal. Chem. 2009, 394, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, C.C.; Cheng, H.H.; Tewari, M. MicroRNA Profiling: Approaches and Considerations. Nat. Rev. Genet. 2012, 13, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Kirschner, M.B.; van Zandwijk, N. Circulating MicroRNAs: Association with Disease and Potential Use as Biomarkers. Crit. Rev. Oncol. Hematol. 2011, 80, 193–208. [Google Scholar] [CrossRef]
- Andreasen, D.; Fog, J.U.; Biggs, W.; Salomon, J.; Dahslveen, I.K.; Baker, A.; Mouritzen, P. Improved MicroRNA Quantification in Total RNA from Clinical Samples. Methods 2010, 50, S6–S9. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Rao, D.S.; Baltimore, D. MicroRNA Regulation of Inflammatory Responses. Annu. Rev. Immunol. 2012, 30, 295–312. [Google Scholar] [CrossRef]
- Quiat, D.; Olson, E.N. MicroRNAs in Cardiovascular Disease: From Pathogenesis to Prevention and Treatment. J. Clin. Investig. 2013, 123, 11–18. [Google Scholar] [CrossRef]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of Extracellular Circulating MicroRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef]
- Murakami, Y.; Tanahashi, T. Analysis of Circulating MicroRNA by Microarray in Liver Disease. Methods Mol. Biol. 2013, 1024, 173–182. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Wu, W.; Shan, P.; Chen, Y.; Meng, J.; Xing, L.; Yun, J.; Hao, L.; Wang, X.; et al. Mechanisms of CircRNA/LncRNA-MiRNA Interactions and Applications in Disease and Drug Research. Biomed. Pharmacother. 2023, 162, 114672. [Google Scholar] [CrossRef]
- Denzler, R.; Agarwal, V.; Stefano, J.; Bartel, D.P.; Stoffel, M. Assessing the CeRNA Hypothesis with Quantitative Measurements of MiRNA and Target Abundance. Mol. Cell 2014, 54, 766–776. [Google Scholar] [CrossRef]
- Gao, Y.; Takenaka, K.; Xu, S.-M.; Cheng, Y.; Janitz, M. Recent Advances in Investigation of CircRNA/LncRNA-MiRNA-MRNA Networks Through RNA Sequencing Data Analysis. Brief. Funct. Genom. 2025, 24, elaf005. [Google Scholar] [CrossRef]
- Wu, F.; Yang, Z.; Li, G. Role of Specific MicroRNAs for Endothelial Function and Angiogenesis. Biochem. Biophys. Res. Commun. 2009, 386, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Zaccone, V.; Falsetti, L.; Contegiacomo, S.; Cataldi, S.; Benfaremo, D.; Moroncini, G. Systemic Sclerosis: A Key Model of Endothelial Dysfunction. Biomedicines 2025, 13, 1771. [Google Scholar] [CrossRef] [PubMed]
- Kazerounian, S.; Lawler, J. Integration of Pro- and Anti-Angiogenic Signals by Endothelial Cells. J. Cell Commun. Signal. 2018, 12, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Ihn, H.; Yamane, K.; Jinnin, M.; Tamaki, K. Increased Expression of Integrin Avβ5 Induces the Myofibroblastic Differentiation of Dermal Fibroblasts. Am. J. Pathol. 2006, 168, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Lawler, P.R.; Lawler, J. Molecular Basis for the Regulation of Angiogenesis by Thrombospondin-1 and -2. Cold Spring Harb. Perspect. Med. 2012, 2, a006627. [Google Scholar] [CrossRef]
- Altorok, N.; Wang, Y.; Kahaleh, B. Endothelial Dysfunction in Systemic Sclerosis. Curr. Opin. Rheumatol. 2014, 26, 615–620. [Google Scholar] [CrossRef]
- Bonella, F.; Patuzzo, G.; Lunardi, C. Biomarker Discovery in Systemic Sclerosis: State of the Art. Curr. Biomark. Find. 2015, 5, 47–68. [Google Scholar] [CrossRef]
- Kuwana, M.; Okazaki, Y.; Yasuoka, H.; Kawakami, Y.; Ikeda, Y. Defective Vasculogenesis in Systemic Sclerosis. Lancet 2004, 364, 603–610. [Google Scholar] [CrossRef]
- Whitfield, M.L.; Finlay, D.R.; Murray, J.I.; Troyanskaya, O.G.; Chi, J.-T.; Pergamenschikov, A.; McCalmont, T.H.; Brown, P.O.; Botstein, D.; Connolly, M.K. Systemic and Cell Type-Specific Gene Expression Patterns in Scleroderma Skin. Proc. Natl. Acad. Sci. USA 2003, 100, 12319–12324. [Google Scholar] [CrossRef]
- Matucci-Cerinic, M.; Kahaleh, B.; Wigley, F.M. Review: Evidence That Systemic Sclerosis Is a Vascular Disease. Arthritis Rheum. 2013, 65, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Romano, E.; Rosa, I.; Guiducci, S.; Bellando-Randone, S.; De Paulis, A.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Endothelial-to-Mesenchymal Transition Contributes to Endothelial Dysfunction and Dermal Fibrosis in Systemic Sclerosis. Ann. Rheum. Dis. 2017, 76, 924–934. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A.J.; Rowell, N.; Wollheim, F. Scleroderma (Systemic Sclerosis): Classification, Subsets and Pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Rabquer, B.J.; Koch, A.E. Angiogenesis and Vasculopathy in Systemic Sclerosis: Evolving Concepts. Curr. Rheumatol. Rep. 2012, 14, 56–63. [Google Scholar] [CrossRef]
- Tsou, P.-S.; Rabquer, B.J.; Ohara, R.A.; Stinson, W.A.; Campbell, P.L.; Amin, M.A.; Balogh, B.; Zakhem, G.; Renauer, P.A.; Lozier, A.; et al. Scleroderma Dermal Microvascular Endothelial Cells Exhibit Defective Response to Pro-Angiogenic Chemokines. Rheumatology 2016, 55, 745–754. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis. Annu. Rev. Med. 2006, 57, 1–18. [Google Scholar] [CrossRef]
- Manetti, M.; Guiducci, S.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Mechanisms in the Loss of Capillaries in Systemic Sclerosis: Angiogenesis versus Vasculogenesis. J. Cell. Mol. Med. 2010, 14, 1241–1254. [Google Scholar] [CrossRef]
- Liakouli, V.; Cipriani, P.; Marrelli, A.; Alvaro, S.; Ruscitti, P.; Giacomelli, R. Angiogenic Cytokines and Growth Factors in Systemic Sclerosis. Autoimmun. Rev. 2011, 10, 590–594. [Google Scholar] [CrossRef]
- Distler, O.; Del Rosso, A.; Giacomelli, R.; Cipriani, P.; Conforti, M.L.; Guiducci, S.; Gay, R.E.; Michel, B.A.; Bruhlmann, P.; Muller-Ladner, U.; et al. Angiogenic and Angiostatic Factors in Systemic Sclerosis: Increased Levels of Vascular Endothelial Growth Factor Are a Feature of the Earliest Disease Stages and Are Associated with the Absence of Fingertip Ulcers. Arthritis Res. 2002, 4, R11. [Google Scholar] [CrossRef]
- Faller, D. V Endothelial Cell Responses to Hypoxic Stress. Clin. Exp. Pharmacol. Physiol. 1999, 26, 74–84. [Google Scholar] [CrossRef]
- Toyama, T.; Asano, Y.; Miyagawa, T.; Nakamura, K.; Hirabayashi, M.; Yamashita, T.; Saigusa, R.; Miura, S.; Ichimura, Y.; Takahashi, T.; et al. The Impact of Transcription Factor Fli1 Deficiency on the Regulation of Angiogenesis. Exp. Dermatol. 2017, 26, 912–918. [Google Scholar] [CrossRef]
- Noda, S.; Asano, Y.; Nishimura, S.; Taniguchi, T.; Fujiu, K.; Manabe, I.; Nakamura, K.; Yamashita, T.; Saigusa, R.; Akamata, K.; et al. Simultaneous Downregulation of KLF5 and Fli1 Is a Key Feature Underlying Systemic Sclerosis. Nat. Commun. 2014, 5, 5797. [Google Scholar] [CrossRef]
- Corallo, C.; Franci, B.; Lucani, B.; Montella, A.; Chirico, C.; Gonnelli, S.; Nuti, R.; Giordano, N. From Microvasculature to Fibroblasts: Contribution of Anti-Endothelial Cell Antibodies in Systemic Sclerosis. Int. J. Immunopathol. Pharmacol. 2015, 28, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ingegnoli, F.; Gualtierotti, R.; Lubatti, C.; Bertolazzi, C.; Gutierrez, M.; Boracchi, P.; Fornili, M.; De Angelis, R. Nailfold Capillary Patterns in Healthy Subjects: A Real Issue in Capillaroscopy. Microvasc. Res. 2013, 90, 90–95. [Google Scholar] [CrossRef]
- Avouac, J.; Vallucci, M.; Smith, V.; Senet, P.; Ruiz, B.; Sulli, A.; Pizzorni, C.; Frances, C.; Chiocchia, G.; Cutolo, M.; et al. Correlations between Angiogenic Factors and Capillaroscopic Patterns in Systemic Sclerosis. Arthritis Res. Ther. 2013, 15, R55. [Google Scholar] [CrossRef]
- Smith, V.; Riccieri, V.; Pizzorni, C.; Decuman, S.; Deschepper, E.; Bonroy, C.; Sulli, A.; Piette, Y.; De Keyser, F.; Cutolo, M. Nailfold Capillaroscopy for Prediction of Novel Future Severe Organ Involvement in Systemic Sclerosis. J. Rheumatol. 2013, 40, 2023–2028. [Google Scholar] [CrossRef]
- Cutolo, M.; Cerinic, M.M. Nailfold Videocapillaroscopy for the Early Diagnosis of Systemic Sclerosis in Raynaud’s Phenomenon. Future Rheumatol. 2006, 1, 41–51. [Google Scholar] [CrossRef]
- Anghel, D.; Prioteasă, O.-G.; Nicolau, I.-N.; Bucurică, S.; Belinski, D.-O.; Popescu, G.-G.; Ghinescu, M.C.; Bobircă, A.; Groșeanu, M.-L.; Bojincă, V.-C. The Role of Nailfold Videocapillaroscopy in the Diagnosis and Monitoring of Interstitial Lung Disease Associated with Rheumatic Autoimmune Diseases. Diagnostics 2025, 15, 362. [Google Scholar] [CrossRef]
- Abou Ali, A.N.; Fittipaldi, A.; Rocha-Neves, J.; Ruaro, B.; Benedetto, F.; Al Ghadban, Z.; Simon, G.; Lepidi, S.; D’Oria, M. Clinical Applications of Contrast-Enhanced Ultrasound in Vascular Surgery: State-of-the-Art Narrative and Pictorial Review. JVS-Vasc. Insights 2025, 3, 100254. [Google Scholar] [CrossRef]
- Schinkel, A.F.L.; Kaspar, M.; Staub, D. Contrast-Enhanced Ultrasound: Clinical Applications in Patients with Atherosclerosis. Int. J. Cardiovasc. Imaging 2016, 32, 35–48. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K.; Sadda, S.R.; Staurenghi, G. Optical Coherence Tomography Angiography. Prog. Retin. Eye Res. 2018, 64, 1–55. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The Endothelial-Specific MicroRNA MiR-126 Governs Vascular Integrity and Angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of MicroRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Nalbant, E.; Akkaya-Ulum, Y.Z. Exploring Regulatory Mechanisms on MiRNAs and Their Implications in Inflammation-Related Diseases. Clin. Exp. Med. 2024, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Romero, I.A.; Guerra-Calderas, L.; Salgado-Albarrán, M.; Maldonado-Huerta, T.; Soto-Reyes, E. The Regulatory Roles of Non-Coding RNAs in Angiogenesis and Neovascularization from an Epigenetic Perspective. Front. Oncol. 2019, 9, 1091. [Google Scholar] [CrossRef]
- Fahs, F.; Bi, X.; Yu, F.S.; Zhou, L.; Mi, Q.S. New Insights into MicroRNAs in Skin Wound Healing. IUBMB Life 2015, 67, 889–896. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Guo, M.; Zuo, X. MicroRNAs Regulating Signaling Pathways: Potential Biomarkers in Systemic Sclerosis. Genom. Proteom. Bioinform. 2015, 13, 234–241. [Google Scholar] [CrossRef]
- Suarez, Y.; Fernandez-Hernando, C.; Yu, J.; Gerber, S.A.; Harrison, K.D.; Pober, J.S.; Iruela-Arispe, M.L.; Merkenschlager, M.; Sessa, W.C. Dicer-Dependent Endothelial MicroRNAs Are Necessary for Postnatal Angiogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 14082–14087. [Google Scholar] [CrossRef]
- Kuehbacher, A.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Role of Dicer and Drosha for Endothelial MicroRNA Expression and Angiogenesis. Circ. Res. 2007, 101, 59–68. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.-F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.R.; Srivastava, D. MiR-126 Regulates Angiogenic Signaling and Vascular Integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gorski, D.H. Regulation of Angiogenesis Through a MicroRNA (MiR-130a) That down-Regulates Antiangiogenic Homeobox Genes GAX and HOXA5. Blood 2008, 111, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Fasanaro, P.; D’Alessandra, Y.; Di Stefano, V.; Melchionna, R.; Romani, S.; Pompilio, G.; Capogrossi, M.C.; Martelli, F. MicroRNA-210 Modulates Endothelial Cell Response to Hypoxia and Inhibits the Receptor Tyrosine Kinase Ligand Ephrin-A3. J. Biol. Chem. 2008, 283, 15878–15883. [Google Scholar] [CrossRef]
- Wurdinger, T.; Tannous, B.A.; Saydam, O.; Skog, J.; Grau, S.; Soutschek, J.; Weissleder, R.; Breakefield, X.O.; Krichevsky, A.M. MiR-296 Regulates Growth Factor Receptor Overexpression in Angiogenic Endothelial Cells. Cancer Cell 2008, 14, 382–393. [Google Scholar] [CrossRef]
- Lee, D.Y.; Deng, Z.; Wang, C.-H.; Yang, B.B. MicroRNA-378 Promotes Cell Survival, Tumor Growth, and Angiogenesis by Targeting SuFu and Fus-1 Expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20350–20355. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K.; et al. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef]
- Doebele, C.; Bonauer, A.; Fischer, A.; Scholz, A.; Reiss, Y.; Urbich, C.; Hofmann, W.-K.; Zeiher, A.M.; Dimmeler, S. Members of the MicroRNA-17-92 Cluster Exhibit a Cell-Intrinsic Antiangiogenic Function in Endothelial Cells. Blood 2010, 115, 4944–4950. [Google Scholar] [CrossRef]
- Hua, Z.; Lv, Q.; Ye, W.; Wong, C.-K.A.; Cai, G.; Gu, D.; Ji, Y.; Zhao, C.; Wang, J.; Yang, B.B.; et al. MiRNA-Directed Regulation of VEGF and Other Angiogenic Factors under Hypoxia. PLoS ONE 2006, 1, e116. [Google Scholar] [CrossRef]
- Wang, X.H.; Qian, R.Z.; Zhang, W.; Chen, S.F.; Jin, H.M.; Hu, R.M. MicroRNA-320 Expression in Myocardial Microvascular Endothelial Cells and Its Relationship with Insulin-like Growth Factor-1 in Type 2 Diabetic Rats. Clin. Exp. Pharmacol. Physiol. 2009, 36, 181–188. [Google Scholar] [CrossRef]
- Poliseno, L.; Tuccoli, A.; Mariani, L.; Evangelista, M.; Citti, L.; Woods, K.; Mercatanti, A.; Hammond, S.; Rainaldi, G. MicroRNAs Modulate the Angiogenic Properties of HUVECs. Blood 2006, 108, 3068–3071. [Google Scholar] [CrossRef]
- Landskroner-Eiger, S.; Moneke, I.; Sessa, W.C. MiRNAs as Modulators of Angiogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a006643. [Google Scholar] [CrossRef] [PubMed]
- Suárez, Y.; Sessa, W.C. MicroRNAs as Novel Regulators of Angiogenesis. Circ. Res. 2009, 104, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Staszel, T.; Zapala, B.; Polus, A.; Sadakierska-Chudy, A.; Kiec-Wilk, B.; Stepien, E.; Wybranska, I.; Chojnacka, M.; Dembinska-Kiec, A. Role of MicroRNAs in Endothelial Cell Pathophysiology. Pol. Arch. Med. Wewn. 2011, 121, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Zheng, M.; Hayashi, M.; Lee, J.-D.; Yoshino, O.; Lin, S.; Han, J. Impaired MicroRNA Processing Causes Corpus Luteum Insufficiency and Infertility in Mice. J. Clin. Investig. 2008, 118, 1944–1954. [Google Scholar] [CrossRef]
- Xie, M.; Dart, D.A.; Guo, T.; Xing, X.-F.; Cheng, X.-J.; Du, H.; Jiang, W.G.; Wen, X.-Z.; Ji, J.-F. MicroRNA-1 Acts as a Tumor Suppressor MicroRNA by Inhibiting Angiogenesis-Related Growth Factors in Human Gastric Cancer. Gastric Cancer 2018, 21, 41–54. [Google Scholar] [CrossRef]
- Fang, Y.; Shi, C.; Manduchi, E.; Civelek, M.; Davies, P.F. MicroRNA-10a Regulation of Proinflammatory Phenotype in Athero-Susceptible Endothelium in Vivo and in Vitro. Proc. Natl. Acad. Sci. USA 2010, 107, 13450–13455. [Google Scholar] [CrossRef]
- Nikolic, I.; Plate, K.-H.; Schmidt, M.H. EGFL7 Meets MiRNA-126: An Angiogenesis Alliance. J. Angiogenes Res. 2010, 2, 9. [Google Scholar] [CrossRef]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 Regulates Endothelial Expression of Vascular Cell Adhesion Molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef]
- Zomorrod, M.S.; Kouhkan, F.; Soleimani, M.; Aliyan, A.; Tasharrofi, N. Overexpression of MiR-133 Decrease Primary Endothelial Cells Proliferation and Migration via FGFR1 Targeting. Exp. Cell Res. 2018, 369, 11–16. [Google Scholar] [CrossRef]
- Yu, B.T.; Yu, N.; Wang, Y.; Zhang, H.; Wan, K.; Sun, X.; Zhang, C.S. Role of MiR-133a in Regulating TGF-Β1 Signaling Pathway in Myocardial Fibrosis after Acute Myocardial Infarction in Rats. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8588–8597. [Google Scholar]
- Vasa-Nicotera, M.; Chen, H.; Tucci, P.; Yang, A.L.; Saintigny, G.; Menghini, R.; Mahe, C.; Agostini, M.; Knight, R.A.; Melino, G.; et al. MiR-146a Is Modulated in Human Endothelial Cell with Aging. Atherosclerosis 2011, 217, 326–330. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 Is Induced During the Macrophage Inflammatory Response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Christmann, R.B.; Wooten, A.; Sampaio-Barros, P.; Borges, C.L.; Carvalho, C.R.R.; Kairalla, R.A.; Feghali-Bostwick, C.; Ziemek, J.; Mei, Y.; Goummih, S.; et al. MiR-155 in the Progression of Lung Fibrosis in Systemic Sclerosis. Arthritis Res. Ther. 2016, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Xiao-qin; Hu, H.; Tian, S.; Lu, Z.; Zhang, T.; Bai, Y. Down-Regulation of MicroRNA-155 Attenuates Retinal Neovascularization via the PI3K/Akt Pathway. Mol. Vis. 2015, 21, 1173–1184. [Google Scholar]
- Meng, Y.-C.; Ding, Z.-Y.; Wang, H.-Q.; Ning, L.-P.; Wang, C. Effect of MicroRNA-155 on Angiogenesis after Cerebral Infarction of Rats Through AT1R/VEGFR2 Pathway. Asian Pac. J. Trop. Med. 2015, 8, 829–835. [Google Scholar] [CrossRef]
- Shan, S.W.; Lee, D.Y.; Deng, Z.; Shatseva, T.; Jeyapalan, Z.; Du, W.W.; Zhang, Y.; Xuan, J.W.; Yee, S.-P.; Siragam, V.; et al. MicroRNA MiR-17 Retards Tissue Growth and Represses Fibronectin Expression. Nat. Cell Biol. 2009, 11, 1031–1038. [Google Scholar] [CrossRef]
- Yin, R.; Wang, R.; Guo, L.; Zhang, W.; Lu, Y. MiR-17-3p Inhibits Angiogenesis by Downregulating Flk-1 in the Cell Growth Signal Pathway. J. Vasc. Res. 2013, 50, 157–166. [Google Scholar] [CrossRef]
- Fontana, L.; Pelosi, E.; Greco, P.; Racanicchi, S.; Testa, U.; Liuzzi, F.; Croce, C.M.; Brunetti, E.; Grignani, F.; Peschle, C. MicroRNAs 17-5p-20a-106a Control Monocytopoiesis Through AML1 Targeting and M-CSF Receptor Upregulation. Nat. Cell Biol. 2007, 9, 775–787. [Google Scholar] [CrossRef]
- Kuhnert, F.; Kuo, C.J. MiR-17-92 Angiogenesis Micromanagement. Blood 2010, 115, 4631–4633. [Google Scholar] [CrossRef]
- Guan, J.-T.; Li, X.-X.; Peng, D.-W.; Zhang, W.-M.; Qu, J.; Lu, F.; D’Amato, R.J.; Chi, Z.-L. MicroRNA-18a-5p Administration Suppresses Retinal Neovascularization by Targeting FGF1 and HIF1A. Front. Pharmacol. 2020, 11, 276. [Google Scholar] [CrossRef]
- Qin, X.; Wang, X.; Wang, Y.; Tang, Z.; Cui, Q.; Xi, J.; Li, Y.-S.J.; Chien, S.; Wang, N. MicroRNA-19a Mediates the Suppressive Effect of Laminar Flow on Cyclin D1 Expression in Human Umbilical Vein Endothelial Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 3240–3244. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.C.P.; Moonen, J.-R.A.J.; Brinker, M.G.L.; Krenning, G. FGF2 Inhibits Endothelial–Mesenchymal Transition Through MicroRNA-20a-Mediated Repression of Canonical TGF-β Signaling. J. Cell Sci. 2016, 129, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Luo, H.; Li, Y.; Zhou, Y.; Jiang, Y.; Chai, J.; Xiao, X.; You, Y.; Zuo, X. MicroRNA-21 in Scleroderma Fibrosis and Its Function in TGF-β- Regulated Fibrosis-Related Genes Expression. J. Clin. Immunol. 2013, 33, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.-L.; Guo, F.; Liu, F.; Gao, F.-L.; Zhang, P.-Q.; Niu, X.; Guo, S.-C.; Yin, J.-H.; Wang, Y.; Deng, Z.-F. MiR-210 Activates Notch Signaling Pathway in Angiogenesis Induced by Cerebral Ischemia. Mol. Cell. Biochem. 2012, 370, 45–51. [Google Scholar] [CrossRef]
- Menghini, R.; Casagrande, V.; Cardellini, M.; Martelli, E.; Terrinoni, A.; Amati, F.; Vasa-Nicotera, M.; Ippoliti, A.; Novelli, G.; Melino, G.; et al. MicroRNA 217 Modulates Endothelial Cell Senescence via Silent Information Regulator 1. Circulation 2009, 120, 1524–1532. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; He, Y.; Zhao, M.; Liu, Z.; Wang, N.; Jiang, M.; Zhang, Z.; Liu, G.; Liu, H.; et al. MiR-218 Inhibited Tumor Angiogenesis by Targeting ROBO1 in Gastric Cancer. Gene 2017, 615, 42–49. [Google Scholar] [CrossRef]
- Yamakuchi, M.; Yagi, S.; Ito, T.; Lowenstein, C.J. MicroRNA-22 Regulates Hypoxia Signaling in Colon Cancer Cells. PLoS ONE 2011, 6, e20291. [Google Scholar] [CrossRef]
- Celic, T.; Metzinger-Le Meuth, V.; Six, I.; Massy, Z.A.; Metzinger, L. The Mir-221/222 Cluster Is a Key Player in Vascular Biology via the Fine-Tuning of Endothelial Cell Physiology. Curr. Vasc. Pharmacol. 2017, 15, 40–46. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. A Necessary Role of MiR-221 and MiR-222 in Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia. Circ. Res. 2009, 104, 476–487. [Google Scholar] [CrossRef]
- Dentelli, P.; Rosso, A.; Orso, F.; Olgasi, C.; Taverna, D.; Brizzi, M.F. MicroRNA-222 Controls Neovascularization by Regulating Signal Transducer and Activator of Transcription 5A Expression. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1562–1568. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Hung, J.-Y.; Chang, W.-A.; Lin, Y.-S.; Pan, Y.-C.; Tsai, P.-H.; Wu, C.-Y.; Kuo, P.-L. Hypoxic Lung Cancer-Secreted Exosomal MiR-23a Increased Angiogenesis and Vascular Permeability by Targeting Prolyl Hydroxylase and Tight Junction Protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef]
- Urbich, C.; Kaluza, D.; Frömel, T.; Knau, A.; Bennewitz, K.; Boon, R.A.; Bonauer, A.; Doebele, C.; Boeckel, J.-N.; Hergenreider, E.; et al. MicroRNA-27a/b Controls Endothelial Cell Repulsion and Angiogenesis by Targeting Semaphorin 6A. Blood 2012, 119, 1607–1616. [Google Scholar] [CrossRef]
- Zhong, L.; Simard, M.J.; Huot, J. Endothelial MicroRNAs Regulating the NF-ΚB Pathway and Cell Adhesion Molecules During Inflammation. FASEB J. 2018, 32, 4070–4084. [Google Scholar] [CrossRef]
- Ito, T.; Yagi, S.; Yamakuchi, M. MicroRNA-34a Regulation of Endothelial Senescence. Biochem. Biophys. Res. Commun. 2010, 398, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. MiR-34a Repression of SIRT1 Regulates Apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13421–13426. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, T.; Satoh, M.; Itoh, T.; Nakamura, M. MicroRNA-34a Regulates the Longevity-Associated Protein SIRT1 in Coronary Artery Disease: Effect of Statins on SIRT1 and MicroRNA-34a Expression. Clin. Sci. 2012, 123, 161–171. [Google Scholar] [CrossRef]
- Fan, W.; Fang, R.; Wu, X.; Liu, J.; Feng, M.; Dai, G.; Chen, G.; Wu, G. Shear-Sensitive MicroRNA-34a Modulates Flow-Dependent Regulation of Endothelial Inflammation. J. Cell Sci. 2015, 128, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xu, W.W.; Han, L.; Chan, K.T.; Tsao, S.W.; Lee, N.P.Y.; Law, S.; Xu, L.Y.; Li, E.M.; Chan, K.W.; et al. MicroRNA-377 Suppresses Initiation and Progression of Esophageal Cancer by Inhibiting CD133 and VEGF. Oncogene 2017, 36, 3986–4000. [Google Scholar] [CrossRef]
- Ghosh, G.; Subramanian, I.V.; Adhikari, N.; Zhang, X.; Joshi, H.P.; Basi, D.; Chandrashekhar, Y.S.; Hall, J.L.; Roy, S.; Zeng, Y.; et al. Hypoxia-Induced MicroRNA-424 Expression in Human Endothelial Cells Regulates HIF-α Isoforms and Promotes Angiogenesis. J. Clin. Investig. 2010, 120, 4141–4154. [Google Scholar] [CrossRef]
- Zhang, T.; Jing, L.; Li, H.; Ding, L.; Ai, D.; Lyu, J.; Zhong, L. MicroRNA-4530 Promotes Angiogenesis by Targeting VASH1 in Breast Carcinoma Cells. Oncol. Lett. 2017, 14, 111–118. [Google Scholar] [CrossRef]
- Sing, T.; Jinnin, M.; Yamane, K.; Honda, N.; Makino, K.; Kajihara, I.; Makino, T.; Sakai, K.; Masuguchi, S.; Fukushima, S.; et al. MicroRNA-92a Expression in the Sera and Dermal Fibroblasts Increases in Patients with Scleroderma. Rheumatology 2012, 51, 1550–1556. [Google Scholar] [CrossRef]
- Daniel, J.-M.; Penzkofer, D.; Teske, R.; Dutzmann, J.; Koch, A.; Bielenberg, W.; Bonauer, A.; Boon, R.A.; Fischer, A.; Bauersachs, J.; et al. Inhibition of MiR-92a Improves Re-Endothelialization and Prevents Neointima Formation Following Vascular Injury. Cardiovasc. Res. 2014, 103, 564–572. [Google Scholar] [CrossRef]
- Fang, Y.; Davies, P.F. Site-Specific MicroRNA-92a Regulation of Kruppel-like Factors 4 and 2 in Atherosusceptible Endothelium. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Hong, Q.; Wang, Y.; Hou, K.; Wang, L.; Zhang, Y.; Fu, B.; Zhou, Y.; Zheng, W.; Chen, X.; et al. High Concentrations of Uric Acid Inhibit Angiogenesis via Regulation of the Krüppel-like Factor 2-Vascular Endothelial Growth Factor-A Axis by MiR-92a. Circ. J. 2015, 79, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Wade, S.M.; Ohnesorge, N.; McLoughlin, H.; Biniecka, M.; Carter, S.P.; Trenkman, M.; Cunningham, C.C.; McGarry, T.; Canavan, M.; Kennedy, B.N.; et al. Dysregulated MiR-125a Promotes Angiogenesis through Enhanced Glycolysis. EBioMedicine 2019, 47, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Nazari-Jahantigh, M.; Neth, P.; Weber, C.; Schober, A. MicroRNA-126, -145, and -155: A Therapeutic Triad in Atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2013, 33, 449–454. [Google Scholar] [CrossRef]
- Guenther, S.P.W.; Schrepfer, S. MiR-126: A Potential New Key Player in Hypoxia and Reperfusion? Ann. Transl. Med. 2016, 4, 377. [Google Scholar] [CrossRef]
- van Solingen, C.; Bijkerk, R.; de Boer, H.C.; Rabelink, T.J.; van Zonneveld, A.J. The Role of MicroRNA-126 in Vascular Homeostasis. Curr. Vasc. Pharmacol. 2015, 13, 341–351. [Google Scholar] [CrossRef]
- Iwamoto, N.; Vettori, S.; Maurer, B.; Brock, M.; Pachera, E.; Jüngel, A.; Calcagni, M.; Gay, R.E.; Whitfield, M.L.; Distler, J.H.W.; et al. Downregulation of MiR-193b in Systemic Sclerosis Regulates the Proliferative Vasculopathy by Urokinase-Type Plasminogen Activator Expression. Ann. Rheum. Dis. 2015, 75, 303–310. [Google Scholar] [CrossRef]
- Yang, C.; Tahiri, H.; Cai, C.; Gu, M.; Gagnon, C.; Hardy, P. MicroRNA-181a Inhibits Ocular Neovascularization by Interfering with Vascular Endothelial Growth Factor Expression. Cardiovasc. Ther. 2018, 36, e12329. [Google Scholar] [CrossRef]
- Li, Y.; Kuscu, C.; Banach, A.; Zhang, Q.; Pulkoski-Gross, A.; Kim, D.; Liu, J.; Roth, E.; Li, E.; Shroyer, K.R.; et al. MiR-181a-5p Inhibits Cancer Cell Migration and Angiogenesis via Downregulation of Matrix Metalloproteinase-14. Cancer Res. 2015, 75, 2674–2685. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Li, J.; You, C.; Lu, P.; Feng, H.; Kong, Y.; Zhang, H.; Liu, Y.; Jiao, R.; et al. MicroRNA-181a Promotes Angiogenesis in Colorectal Cancer by Targeting SRCIN1 to Promote the SRC/VEGF Signaling Pathway. Cell Death Dis. 2018, 9, 438. [Google Scholar] [CrossRef]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. MiR-200c Is Upregulated by Oxidative Stress and Induces Endothelial Cell Apoptosis and Senescence via ZEB1 Inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Guo, Y.; Sun, S. Down-Regulated MicroRNA-152 Induces Aberrant DNA Methylation in Hepatitis B Virus-Related Hepatocellular Carcinoma by Targeting DNA Methyltransferase 1. Hepatology 2010, 52, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Suto, A.; Ikeda, K.; Sanayama, Y.; Nakagomi, D.; Iwamoto, T.; Suzuki, K.; Kambe, N.; Matsue, H.; Matsumura, R.; et al. Alteration of Circulating MiRNAs in SSc: MiR-30b Regulates the Expression of PDGF Receptor Beta. Rheumatology 2013, 52, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Kazda, K.; Addison, C.L. MicroRNA-30b Controls Endothelial Cell Capillary Morphogenesis Through Regulation of Transforming Growth Factor Beta 2. PLoS ONE 2017, 12, e0185619. [Google Scholar] [CrossRef] [PubMed]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of MicroRNAs in Vascular Diseases, Inflammation, and Angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef]
- Woźniak, O.; Mierzejewski, B.; Brzoska, E. MicroRNA-126: A Key Regulator of Angiogenesis, Inflammation, and Tumorigenesis—Exploring Its Multifaceted Functions in Vascular Health and Cancer. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2025, 1871, 167984. [Google Scholar] [CrossRef]
- Zhou, Q.; Anderson, C.; Hanus, J.; Zhao, F.; Ma, J.; Yoshimura, A.; Wang, S. Strand and Cell Type-Specific Function of MicroRNA-126 in Angiogenesis. Mol. Ther. 2016, 24, 1823–1835. [Google Scholar] [CrossRef]
- Wang, J.-M.; Xu, W.-D.; Yuan, Z.-C.; Wu, Q.; Zhou, J.; Huang, A.-F. Serum Levels and Gene Polymorphisms of Angiopoietin 2 in Systemic Lupus Erythematosus Patients. Sci. Rep. 2021, 11, 10. [Google Scholar] [CrossRef]
- Enteshari-Moghadam, A.; Fouladi, N.; Pordel, S.; Jeddi, F.; Asghariazar, V.; Eterafi, M.; Safarzadeh, E. Evaluation of the MiRNA-126 and VCAM-1 in Scleroderma Patients and Its Association with Clinical Characteristics. Am. J. Med. Sci. 2025, 369, 339–345. [Google Scholar] [CrossRef]
- Wajda, A.; Walczyk, M.; Dudek, E.; Stypińska, B.; Lewandowska, A.; Romanowska-Próchnicka, K.; Chojnowski, M.; Olesińska, M.; Paradowska-Gorycka, A. Serum MicroRNAs in Systemic Sclerosis, Associations with Digital Vasculopathy and Lung Involvement. Int. J. Mol. Sci. 2022, 23, 10731. [Google Scholar] [CrossRef]
- Liakouli, V.; Cipriani, P.; Di Benedetto, P.; Panzera, N.; Ruscitti, P.; Pantano, I.; Berardicurti, O.; Carubbi, F.; Esteves, F.; Mavria, G.; et al. Epidermal Growth Factor Like-Domain 7 and MiR-126 Are Abnormally Expressed in Diffuse Systemic Sclerosis Fibroblasts. Sci. Rep. 2019, 9, 4589. [Google Scholar] [CrossRef]
- Guo, B.; Gu, J.; Zhuang, T.; Zhang, J.; Fan, C.; Li, Y.; Zhao, M.; Chen, R.; Wang, R.; Kong, Y.; et al. MicroRNA-126: From Biology to Therapeutics. Biomed. Pharmacother. 2025, 185, 117953. [Google Scholar] [CrossRef] [PubMed]
- Miscianinov, V.; Martello, A.; Rose, L.; Parish, E.; Cathcart, B.; Mitic, T.; Gray, G.A.; Meloni, M.; Al Haj Zen, A.; Caporali, A. MicroRNA-148b Targets the TGF-Beta Pathway to Regulate Angiogenesis and Endothelial-to-Mesenchymal Transition During Skin Wound Healing. Mol. Ther. 2018, 26, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Chen, S.; Liu, X.; Lin, L.; Huang, X.; Guo, Z.; Liu, J.; Wang, Y.; Yuan, W.; et al. Endothelial Enriched MicroRNAs Regulate Angiotensin II-Induced Endothelial Inflammation and Migration. Atherosclerosis. 2011, 215, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Strapazzon, G.; Ragazzo, F.; Bratti, P.; Fabricio, A.S.C.; Squarcina, E.; Gion, M.; Palatini, P.; et al. Interplay Between MiR-155, AT1R A1166C Polymorphism, and AT1R Expression in Young Untreated Hypertensives. Am. J. Hypertens. 2011, 24, 241–246. [Google Scholar] [CrossRef]
- Tkachuk, V.A.; Plekhanova, O.S.; Parfyonova, Y. V Regulation of Arterial Remodeling and Angiogenesis by Urokinase-Type Plasminogen Activator. Can. J. Physiol. Pharmacol. 2009, 87, 231–251. [Google Scholar] [CrossRef]
- Manetti, M.; Rosa, I.; Milia, A.F.; Guiducci, S.; Carmeliet, P.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Inactivation of Urokinase-Type Plasminogen Activator Receptor (UPAR) Gene Induces Dermal and Pulmonary Fibrosis and Peripheral Microvasculopathy in Mice: A New Model of Experimental Scleroderma? Ann. Rheum. Dis. 2014, 73, 1700–1709. [Google Scholar] [CrossRef]
- van Mil, A.; Grundmann, S.; Goumans, M.-J.; Lei, Z.; Oerlemans, M.I.; Jaksani, S.; Doevendans, P.A.; Sluijter, J.P.G. MicroRNA-214 Inhibits Angiogenesis by Targeting Quaking and Reducing Angiogenic Growth Factor Release. Cardiovasc. Res. 2012, 93, 655–665. [Google Scholar] [CrossRef]
- Suarez, Y.; Fernandez-Hernando, C.; Pober, J.S.; Sessa, W.C. Dicer Dependent MicroRNAs Regulate Gene Expression and Functions in Human Endothelial Cells. Circ. Res. 2007, 100, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Sobenin, I.A.; Orekhov, A.N.; Bobryshev, Y. V Human MiR-221/222 in Physiological and Atherosclerotic Vascular Remodeling. BioMed Res. Int. 2015, 2015, 354517. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Yanagisawa, K.; Tanaka, M.; Cao, K.; Matsuyama, Y.; Goto, H.; Takahashi, T. Identification of Hypoxia-Inducible Factor-1 Alpha as a Novel Target for MiR-17-92 MicroRNA Cluster. Cancer Res. 2008, 68, 5540–5545. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Lee, D.Y.; Deng, Z.; Jeyapalan, Z.; Lee, S.-C.; Kahai, S.; Lu, W.-Y.; Zhang, Y.; Yang, B.B. MicroRNA MiR-328 Regulates Zonation Morphogenesis by Targeting CD44 Expression. PLoS ONE 2008, 3, e2420. [Google Scholar] [CrossRef]
- Raitoharju, E.; Lyytikäinen, L.-P.; Levula, M.; Oksala, N.; Mennander, A.; Tarkka, M.; Klopp, N.; Illig, T.; Kähönen, M.; Karhunen, P.J.; et al. MiR-21, MiR-210, MiR-34a, and MiR-146a/b Are up-Regulated in Human Atherosclerotic Plaques in the Tampere Vascular Study. Atherosclerosis 2011, 219, 211–217. [Google Scholar] [CrossRef]
- Ge, X.; Huang, S.; Gao, H.; Han, Z.; Chen, F.; Zhang, S.; Wang, Z.; Kang, C.; Jiang, R.; Yue, S.; et al. MiR-21-5p Alleviates Leakage of Injured Brain Microvascular Endothelial Barrier in Vitro Through Suppressing Inflammation and Apoptosis. Brain Res. 2016, 1650, 31–40. [Google Scholar] [CrossRef]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of MicroRNAs in Serum: A Novel Class of Biomarkers for Diagnosis of Cancer and Other Diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.-B.; Kumar, R.; et al. Extracellular Vesicles as Tools and Targets in Therapy for Diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef]
- Lei, X.; Ring, S.; Jin, S.; Singh, S.; Mahnke, K. Extracellular Vesicles and Their Role in Skin Inflammatory Diseases: From Pathogenesis to Therapy. Int. J. Mol. Sci. 2025, 26, 3827. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Makino, K.; Jinnin, M.; Kajihara, I.; Honda, N.; Sakai, K.; Masuguchi, S.; Fukushima, S.; Inoue, Y.; Ihn, H. Circulating MiR-142-3p Levels in Patients with Systemic Sclerosis. Clin. Exp. Dermatol. 2012, 37, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. The Role of MiR-126 in Embryonic Angiogenesis, Adult Vascular Homeostasis, and Vascular Repair and Its Alterations in Atherosclerotic Disease. J. Mol. Cell. Cardiol. 2016, 97, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, J.R.; Flores-García, M.; García-Flores, E.; Cazarín-Santos, B.G.; Peña-Duque, M.A.; Sánchez-Muñoz, F.; Ballinas-Verdugo, M.A.; Delgadillo-Rodríguez, H.; Martínez-Ríos, M.A.; Angles-Cano, E.; et al. Circulating Microvesicles Enriched in MiR–126–5p and MiR–223–3p: Potential Biomarkers in Acute Coronary Syndrome. Biomedicines 2025, 13, 510. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, X.; Liu, M.; Yun, L.; Cong, B. Diagnostic Value of Cardiac MiR-126-5p, MiR-134-5p, and MiR-499a-5p in Coronary Artery Disease-Induced Sudden Cardiac Death. Front. Cardiovasc. Med. 2022, 9, 944317. [Google Scholar] [CrossRef]
- Szabo, I.; Muntean, L.; Crisan, T.; Rednic, V.; Sirbe, C.; Rednic, S. Novel Concepts in Systemic Sclerosis Pathogenesis: Role for MiRNAs. Biomedicines 2021, 9, 1471. [Google Scholar] [CrossRef]
- Chirshev, E.; Oberg, K.C.; Ioffe, Y.J.; Unternaehrer, J.J. Let-7 as Biomarker, Prognostic Indicator, and Therapy for Precision Medicine in Cancer. Clin. Transl. Med. 2019, 8, e24. [Google Scholar] [CrossRef]
- Mahesh, G.; Biswas, R. MicroRNA-155: A Master Regulator of Inflammation. J. Interferon Cytokine Res. 2019, 39, 321–330. [Google Scholar] [CrossRef]
- Zapata-Martínez, L.; Águila, S.; de los Reyes-García, A.M.; Carrillo-Tornel, S.; Lozano, M.L.; González-Conejero, R.; Martínez, C. Inflammatory MicroRNAs in Cardiovascular Pathology: Another Brick in the Wall. Front. Immunol. 2023, 14, 1196104. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, M.; Qin, G.; Weintraub, N.L.; Tang, Y. MiR-92a Regulates Viability and Angiogenesis of Endothelial Cells under Oxidative Stress. Biochem. Biophys. Res. Commun. 2014, 446, 952–958. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Li, Y.-S.; Wu, C.-C.; Wang, K.-C.; Huang, T.-C.; Chen, Z.; Chien, S. Extracellular MicroRNA-92a Mediates Endothelial Cell–Macrophage Communication. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2492–2504. [Google Scholar] [CrossRef]
- Wronska, A. The Role of MicroRNA in the Development, Diagnosis, and Treatment of Cardiovascular Disease: Recent Developments. J. Pharmacol. Exp. Ther. 2023, 384, 123–132. [Google Scholar] [CrossRef]
- Al-Gburi, S.; Moinzadeh, P.; Krieg, T. Pathophysiology in Systemic Sclerosis: Current Insights and Future Perspectives. Sclerosis 2025, 3, 17. [Google Scholar] [CrossRef]
- Santos, C.S.; Galdo, F. Del New Horizons in Systemic Sclerosis Treatment: Advances and Emerging Therapies in 2025. RMD Open 2025, 11, e005776. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, Y.; Jinnn, M.; Kimura, Y.; Wang, Z.; Onoue, Y.; Hanatani, S.; Araki, S.; Ihn, H.; Ogawa, H. Expression of Let-7 Family MicroRNAs in Skin Correlates Negatively with Severity of Pulmonary Hypertension in Patients with Systemic Scleroderma. IJC Heart Vasc. 2015, 8, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.; Zhao, M.; Lu, Q. Meta-Analysis of Differentially Expressed MicroRNAs in Systemic Sclerosis. Int. J. Rheum. Dis. 2020, 23, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.T.D.; Tagliaferri, P.; Tassone, P. MicroRNA in Cancer Therapy: Breakthroughs and Challenges in Early Clinical Applications. J. Exp. Clin. Cancer Res. 2025, 44, 126. [Google Scholar] [CrossRef]
- Seyhan, A.A. Trials and Tribulations of MicroRNA Therapeutics. Int. J. Mol. Sci. 2024, 25, 1469. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, J.; Huang, Z. Recent Progress in MicroRNA-Based Delivery Systems for the Treatment of Human Disease. ExRNA 2019, 1, 24. [Google Scholar] [CrossRef]
- Makino, K.; Jinnin, M.; Hirano, A.; Yamane, K.; Eto, M.; Kusano, T.; Honda, N.; Kajihara, I.; Makino, T.; Sakai, K.; et al. The Downregulation of MicroRNA Let-7a Contributes to the Excessive Expression of Type I Collagen in Systemic and Localized Scleroderma. J. Immunol. 2013, 190, 3905–3915. [Google Scholar] [CrossRef]
- Li, Q.; Yao, Y.; Shi, S.; Zhou, M.; Zhou, Y.; Wang, M.; Chiu, J.-J.; Huang, Z.; Zhang, W.; Liu, M.; et al. Inhibition of MiR-21 Alleviated Cardiac Perivascular Fibrosis via Repressing EndMT in T1DM. J. Cell. Mol. Med. 2020, 24, 910–920. [Google Scholar] [CrossRef]
- Man, S.; Sanchez Duffhues, G.; ten Dijke, P.; Baker, D. The Therapeutic Potential of Targeting the Endothelial-to-Mesenchymal Transition. Angiogenesis 2019, 22, 3–13. [Google Scholar] [CrossRef]
- Khalaji, A.; Mehrtabar, S.; Jabraeilipour, A.; Doustar, N.; Youshanlouei, H.R.; Tahavvori, A.; Fattahi, P.; Alavi, S.M.A.; Taha, S.R.; Fazlollahpour-Naghibi, A.; et al. Inhibitory Effect of MicroRNA-21 on Pathways and Mechanisms Involved in Cardiac Fibrosis Development. Ther. Adv. Cardiovasc. Dis. 2024, 18, 17539447241253134. [Google Scholar] [CrossRef]
- Pagoni, M.; Cava, C.; Sideris, D.C.; Avgeris, M.; Zoumpourlis, V.; Michalopoulos, I.; Drakoulis, N. MiRNA-Based Technologies in Cancer Therapy. J. Pers. Med. 2023, 13, 1586. [Google Scholar] [CrossRef]
- Mc Cormack, B.A.; González-Cantó, E.; Agababyan, C.; Espinoza-Sánchez, N.A.; Tomás-Pérez, S.; Llueca, A.; Marí-Alexandre, J.; Götte, M.; Gilabert-Estellés, J. MiRNAs in the Era of Personalized Medicine: From Biomarkers to Therapeutics. Int. J. Mol. Sci. 2021, 22, 8154. [Google Scholar] [CrossRef]
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of MiRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Castañón-Cortés, L.G.; Bravo-Vázquez, L.A.; Santoyo-Valencia, G.; Medina-Feria, S.; Sahare, P.; Duttaroy, A.K.; Paul, S. Current Advances in the Development of MicroRNA-Integrated Tissue Engineering Strategies: A Cornerstone of Regenerative Medicine. Front. Bioeng. Biotechnol. 2024, 12, 1484151. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.-H.; Xu, X.-W.; Fu, X.-Y.; Zhou, L.-D.; Liu, S.-P.; Tan, D.-M. Long Non-Coding RNA MALAT1 Promotes Angiogenesis and Immunosuppressive Properties of HCC Cells by Sponging MiR-140. Am. J. Physiol.-Cell Physiol. 2019, 318, C649–C663. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Wang, S. Angio-LncRs: LncRNAs That Regulate Angiogenesis and Vascular Disease. Theranostics 2018, 8, 3654–3675. [Google Scholar] [CrossRef]
- Ran, L.; Pan, W.; Feng, J.; Tang, L. Long Non-Coding RNA MALAT1: A Crucial Factor in Fibrotic Diseases. Mol. Ther. Nucleic Acids 2025, 36, 102630. [Google Scholar] [CrossRef]
- Chen, X.; Yang, T.; Wang, W.; Xi, W.; Zhang, T.; Li, Q.; Yang, A.; Wang, T. Circular RNAs in Immune Responses and Immune Diseases. Theranostics 2019, 9, 588–607. [Google Scholar] [CrossRef]
- Zhao, M.; Lin, M.; Zhang, Z.; Ye, L. Research Progress of Circular RNA FOXO3 in Diseases (Review). Glob. Med. Genet. 2025, 12, 100003. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Piper, J.; Dickinson, B.A.; Dalby, C.M.; Pestano, L.A.; Jackson, A.L. A Synthetic MicroRNA-92a Inhibitor (MRG-110) Accelerates Angiogenesis and Wound Healing in Diabetic and Nondiabetic Wounds. Wound Repair Regen. 2018, 26, 311–323. [Google Scholar] [CrossRef]
- Abplanalp, W.T.; Fischer, A.; John, D.; Zeiher, A.M.; Gosgnach, W.; Darville, H.; Montgomery, R.; Pestano, L.; Allée, G.; Paty, I.; et al. Efficiency and Target Derepression of Anti-MiR-92a: Results of a First in Human Study. Nucleic Acid. Ther. 2020, 30, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Seto, A.G.; Beatty, X.; Hermreck, M.; Gilles, M.-E.; Stroopinsky, D.; Pinter-Brown, L.C.; Pestano, L.; Marchese, C.; Avigan, D.; et al. Cobomarsen, an Oligonucleotide Inhibitor of MiR-155, Slows DLBCL Tumor Cell Growth In Vitro and In Vivo. Clin. Cancer Res. 2021, 27, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Seto, A.G.; Beatty, X.; Lynch, J.M.; Hermreck, M.; Tetzlaff, M.; Duvic, M.; Jackson, A.L. Cobomarsen, an Oligonucleotide Inhibitor of MiR-155, Co-Ordinately Regulates Multiple Survival Pathways to Reduce Cellular Proliferation and Survival in Cutaneous T-Cell Lymphoma. Br. J. Haematol. 2018, 183, 428–444. [Google Scholar] [CrossRef]
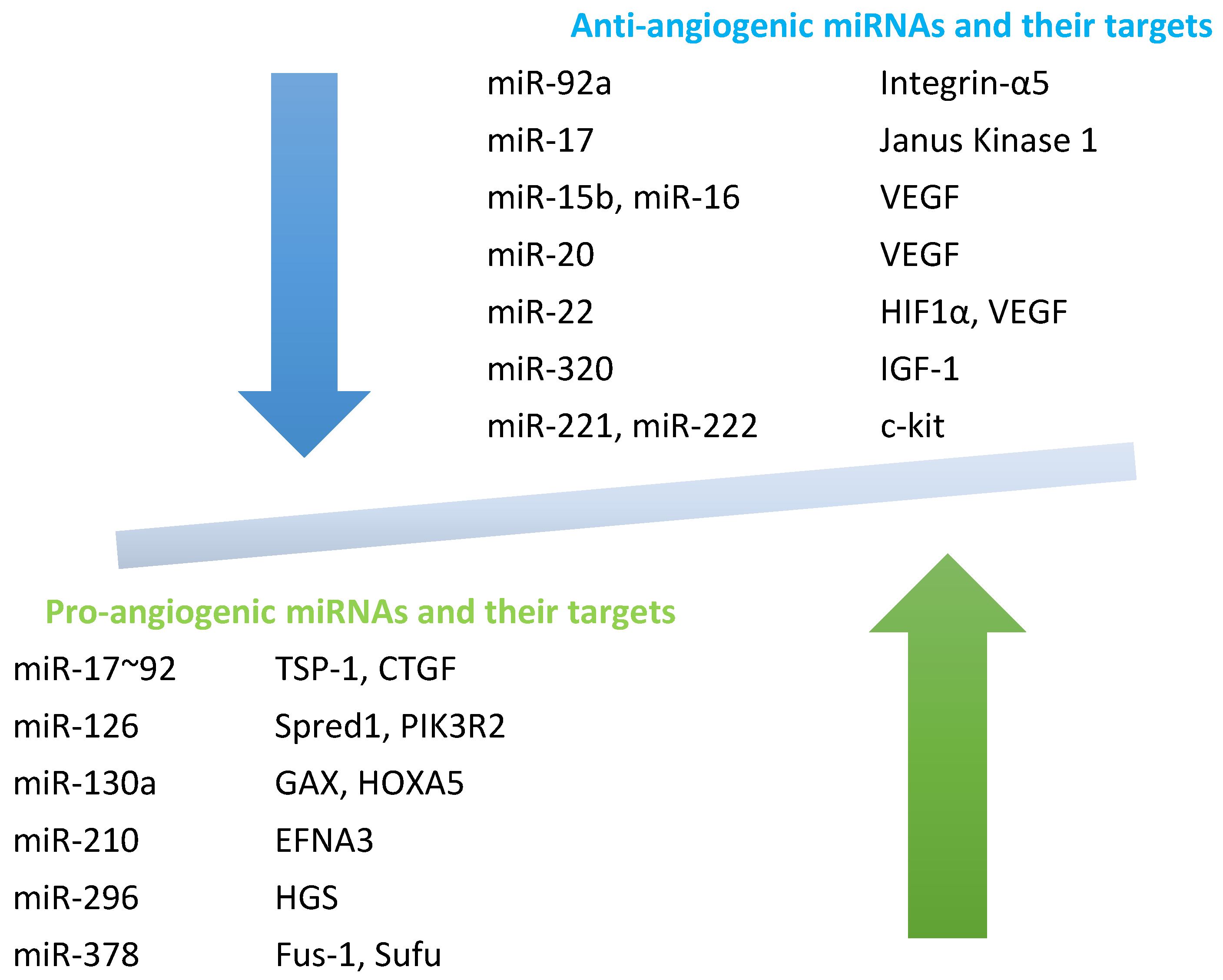
| miRNAs | Level | Targets | Effects | Ref |
|---|---|---|---|---|
| Angiogenesis | ||||
| let-7f | ↓ | TIMP-1 | Migration and proliferation of ECs, sprout formation | [94] |
| miR-1 | ↓ | VEGF-A | Tube formation and proliferation of ECs | [95] |
| miR-10a | ↑ | MAP3K7, TAK1, βTRC | Modulation of proinflammatory EC phenotypes in atherosusceptible regions in vivo | [96] |
| miR-126 | ↑ | VCAM-1 SPRED, PIK3R2, VEGFR-2, p85-β | Vascular integrity, cell adhesion and migration Proliferation of ECs Angiogenesis in vivo | [81,97,98] |
| miR-130a | ↑ | GAX, HOX-A5 | Inhibition of ECs migration and proliferation | [82] |
| miR-133a/b | ↑ | TGF-β1 | ECs’ proliferation and branch formation | [99,100] |
| miR-146a | ↑ | VEGF, PAK-1 | Formation of new blood vessels | [101] |
| miR-155 | ↑ | AT1R, VEGFR-2 | Migration and proliferation of ECs Angiogenesis in the region of ischemia | [102,103,104,105] |
| miR-17 | ↓ | ICAM-1, Janus Kinase 1 | EC’s adhesion and migration | [106,107] |
| miR-17-5p | ↑ | TSP-1/CTGF, TIMP1 | Migration and growth of ECs | [108] |
| miR-17~92 | ↑ | TSP-1/CTGF | Migration and growth of ECs | [87,109] |
| miR-18a | ↑ | TSR/VEGFR-2 | Migration and growth of ECs | [110] |
| miR-19a | ↑ | TSR/VEGFR-2 | Migration and growth of ECs | [111] |
| miR-20a | ↓ ↑ | VEGF MKK3 | Migration and growth of ECs ECs’ migration and angiogenesis | [112] |
| miR-21 | ↓ | PTEN, SMAD7 | ECs’ migration and proliferation | [113] |
| miR-210 | ↑ | Ephrin-A3 NPTX1 | ECs’ tube formation, proliferation, and migration ECs-mediated angiogenesis | [83,114] |
| miR-217 | ↑ | FOXO, eNOS, SIRT1 | Vessel formation, maturation | [115] |
| miR-218 | ↓ | ROBO-1 | Neovascularization Dysregulated endothelial migration Impaired retinal vasculature | [116] |
| miR-22 | ↑ | HIF1α, VEGF | Inhibition of VEGF secretion | [117] |
| miR-221 | ↓ | c-Kit, eNOS | Migration and proliferation of ECs Vessel permeability Tube formation, migration, and impaired wound healing | [118,119] |
| miR-222 | ↓ | c-Kit, eNOS STAT5a | Migration and proliferation of ECs Vessel permeability Inflammation-mediated vascular remodeling Tube formation, migration, and impaired wound healing | [119,120] |
| miR-23a | ↑ | PHD1,2 | Vascular permeability and cellular migration | [121] |
| miR-27b | ↑ | SEMA6A | Sprout formation | [122] |
| miR-296 | ↑ | HGS | Tube formation and migration in vitro, angiogenesis in vivo | [84] |
| miR-31 | ↓ | E-selectin | Immune cell infiltration at sites of inflammation | [123] |
| miR-34a | ↑ | SIRT1, p53 | Angiogenesis blockade in ECs | [124,125,126,127] |
| miR-320 | ↓ | IGF-1 | Angiogenesis in diabetic ECs | [89] |
| miR-377 | ↓ | CD133, VEGF | Angiogenesis | [128] |
| miR-378 | ↑ | FUS-1, SUFU | Angiogenesis | [85] |
| miR-424 | ↑ | CUL-2, HIF-1α | Cell proliferation, chemotaxis, angiogenesis, vascular remodeling | [129] |
| miR-4530 | ↑ | VASH-1 | Angiogenesis | [130] |
| miR-92a | ↓ | ITG-α5 KLF-4 KLF-5 | Angiogenesis and vessel formation Proliferation Cell adhesion and cell interactions | [86,131,132,133,134] |
| Vascular inflammation | ||||
| miR-125a | ↓ | PFKFB3 | ECs metabolism | [135] |
| miR-126 | ↑ | SPRED-1 VCAM-1 ITG-α5 | Inflammatory response Vascular integrity and homeostasis Angiogenesis | [73,81,98,136,137,138] |
| miR-193b | ↓ | PLAU | uPA signaling in MVECs | [139] |
| Cellular senescence | ||||
| miR-146a | ↑ | NOX4, KLF-4 | Cell growth | [101] |
| miR-181a | ↑ | NOX4 | Cell growth | [140,141,142] |
| miR-200c | ↑ | ZEB1 | Cell growth | [143] |
| miR-217 | ↑ | SIRT1, FOXO | Stress resistance | [115] |
| miR-34a | ↓ | SIRT1, p53 | Stress resistance | [124,125] |
| miR-152 | ↓ | DNMT1 | Hypermethylation in MVECs | [144] |
| miR-30b | ↓ | PDGFRB | PDGF signaling | [145,146] |
| miRNA | Direction of Change | Validated Targets or Pathways | Functional Role | Associated Vascular Complication | Diagnostic or Prognostic Potential |
|---|---|---|---|---|---|
| miR-126 | ↓ | SPRED1, PIK3R2 (VEGF signaling) | Pro-angiogenic (lost in SSc) | Capillary loss, impaired angiogenesis | Circulating biomarker of SSc vasculopathy |
| miR-130a | ↓ | HOXA5, GAX | Pro-angiogenic (downregulated) | Defective neovascularization | Potential marker of angiogenesis defects |
| miR-210 | ↑ | EFNA3, PHDs | Hypoxia-induced angiogenesis | Hypoxia-driven angiogenesis | Associated with hypoxia severity; candidate circulating biomarker |
| miR-132 | ↑ | RAS/ERK pathway regulators | Pro-angiogenic, endothelial proliferation | Enhanced vascular repair | Exploratory biomarker (needs validation) |
| miR-92a | ↑ | ITGα5, KLF4/5 | Anti-angiogenic | Inhibited angiogenesis, vascular rarefaction | Linked to digital ulcers; candidate biomarker |
| miR-155 | ↑ | AT1R, VCAM-1 | Pro-inflammatory, vascular injury | Inflammation-driven vasculopathy, PAH | Associated with PAH and inflammation severity |
| miR-29 | ↓ | Collagens, ECM genes | Anti-fibrotic (loss promotes fibrosis) | Fibrosis, vascular remodeling | Circulating biomarker of fibrosis severity |
| miR-221 | ↑ | c-Kit, endothelial proliferation | Anti-angiogenic, impairs endothelial repair | Endothelial dysfunction, impaired repair | Preliminary evidence in SSc; exploratory biomarker |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusek, M. Angiogenic microRNAs in Systemic Sclerosis: Insights into Microvascular Dysfunction and Therapeutic Implications. Genes 2025, 16, 1057. https://doi.org/10.3390/genes16091057
Rusek M. Angiogenic microRNAs in Systemic Sclerosis: Insights into Microvascular Dysfunction and Therapeutic Implications. Genes. 2025; 16(9):1057. https://doi.org/10.3390/genes16091057
Chicago/Turabian StyleRusek, Marta. 2025. "Angiogenic microRNAs in Systemic Sclerosis: Insights into Microvascular Dysfunction and Therapeutic Implications" Genes 16, no. 9: 1057. https://doi.org/10.3390/genes16091057
APA StyleRusek, M. (2025). Angiogenic microRNAs in Systemic Sclerosis: Insights into Microvascular Dysfunction and Therapeutic Implications. Genes, 16(9), 1057. https://doi.org/10.3390/genes16091057






