Molecular Screening for Cervical Cancer
Abstract
1. Introduction
- A total of 90% of girls up to 15 years old must have been vaccinated.
- A total of 70% of women >25 years old must have been screened at least twice, at the ages of 35 and 45, using a high-performance test (NAAT).
- A total of 90% of women with precancer and cancer must have been appropriately treated.
2. Sensitivity or Specificity: The Permanent Diagnostic Dilemma
3. Molecular Screening
- o
- Tests should preferentially target only the 12 genotypes classified as carcinogenic (HPVs 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59).
- o
- A sensitivity ≥ 95% for CIN2+ and specificity ≥ 98% for ≤CIN1.
- o
- The sensitivity and specificity values stated above must be derived from at least three validation studies comparing the test to one of the first-generation assays fulfilling the VALGENT criteria.
4. Triage Alternatives
4.1. HPV Genotyping
4.2. DNA Methylation
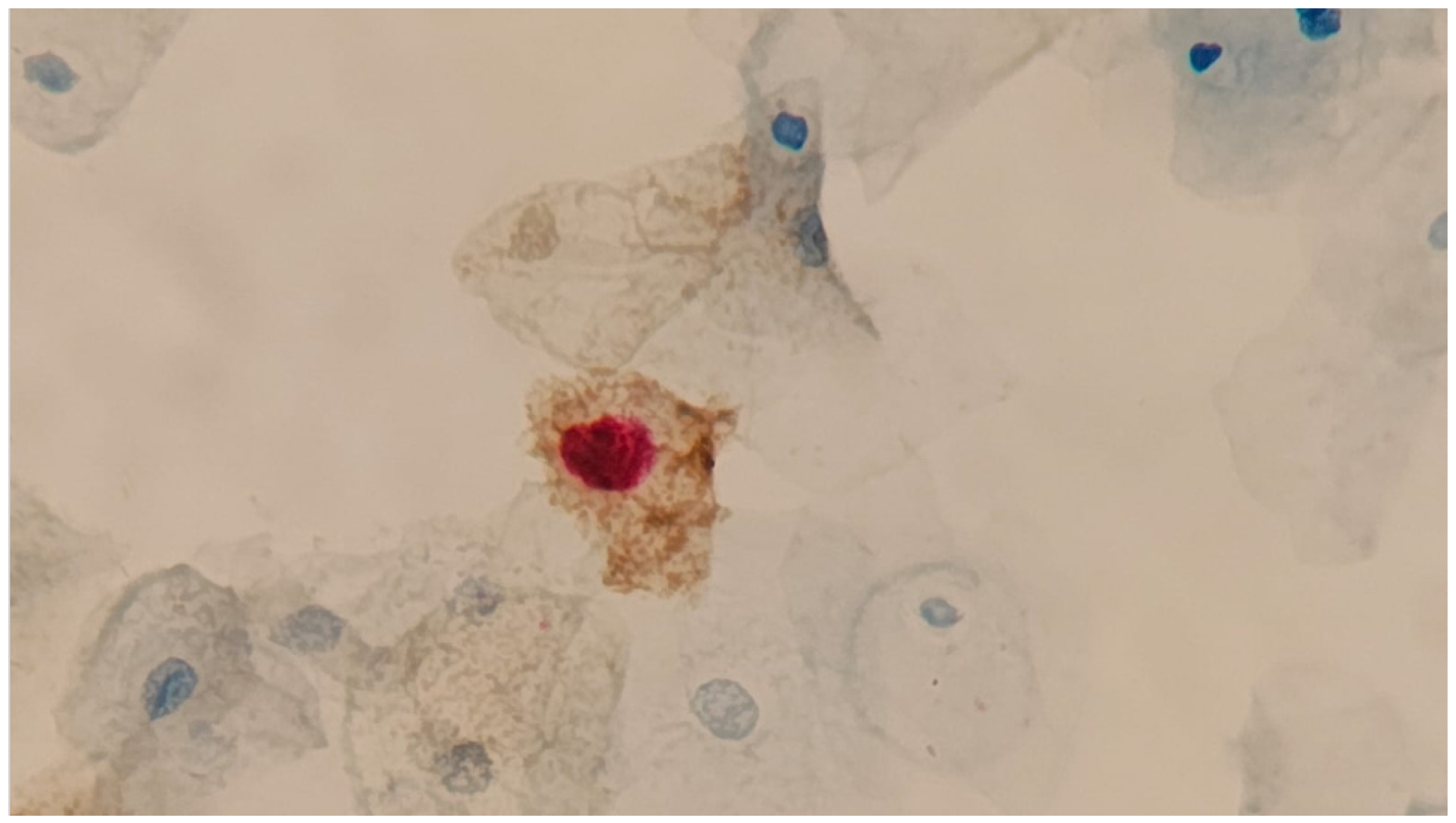
4.3. Dual Stain
4.4. Point-of-Care (POC) and New Molecular Assays
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Singh, D.; Vignat, J.; Lorenzoni, V.; Eslahi, M.; Ginsburg, O.; Lauby-Secretan, B.; Arbyn, M.; Basu, P.; Bray, F.; Vaccarella, S. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob. Health 2023, 11, e197–e206. [Google Scholar] [CrossRef]
- Schiffman, M.; Doorbar, J.; Wentzensen, N.; de Sanjosé, S.; Fakhry, C.; Monk, B.J.; Stanley, M.A.; Franceschi, S. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers 2016, 2, 16086. [Google Scholar] [CrossRef]
- Franco, E.L.; Villa, L.L.; Sobrinho, J.P.; Prado, J.M.; Rousseau, M.C.; Désy, M.; Rohan, T.E. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J. Infect. Dis. 1999, 180, 1415–1423. [Google Scholar] [CrossRef]
- Demarco, M.; Hyun, N.; Carter-Pokras, O.; Raine-Bennett, T.R.; Cheung, L.; Chen, X.; Hammer, A.; Campos, N.; Kinney, W.; Gage, J.C.; et al. A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs. EClinicalMedicine 2020, 22, 100293. [Google Scholar] [CrossRef]
- Vilos, G.A. The history of the Papanicolaou smear and the odyssey of George and Andromache Papanicolaou. Obstet. Gynecol. 1998, 91, 479–483. [Google Scholar] [CrossRef]
- Gustafsson, L.; Pontén, J.; Zack, M.; Adami, H.-O. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997, 8, 755–763. [Google Scholar] [CrossRef]
- Malagón, T.; Franco, E.L.; Tejada, R.; Vaccarella, S. Epidemiology of HPV-associated cancers past, present and future: Towards prevention and elimination. Nat. Rev. Clin. Oncol. 2024, 21, 522–538. [Google Scholar] [CrossRef] [PubMed]
- IARC. Cervical cancer screening. IARC Handb. Cancer Prev. 2022, 18, 1–456. Available online: https://publications.iarc.fr/604 (accessed on 1 July 2025).
- Arroyo, M.L.S.; Gini, A.; Yilmaz, E.; Hassan, S.S.; Lagheden, C.; Hultin, E.; Garcia Serrano, A.; Ure, A.E.; Andersson, H.; Merino, R.; et al. Concomitant human papillomavirus (HPV) vaccination and screening for elimination of HPV and cervical cancer. Nat. Commun. 2024, 15, 3679. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.T.; Castellsagué, X.; Garland, S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012, 30 (Suppl. S5), F123–F138. [Google Scholar] [CrossRef]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2022, 12, 805695. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cervical Cancer Elimination Initiative. Available online: https://www.who.int/initiatives/cervical-cancer-elimination-initiative (accessed on 24 June 2025).
- Bruni, L.; Diaz, M.; Castellsagué, X.; Ferrer, E.; Bosch, F.X.; de Sanjosé, S. Cervical human papillomavirus prevalence in 5 continents: Meta-analysis of 1 million women with normal cytological findings. J. Infect. Dis. 2010, 202, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Target Product Profiles for Human Papillomavirus Screening Tests to Detect Cervical Precancer and Cancer; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Benard, V.B.; Eheman, C.R.; Lawson, H.W.; Blackman, D.K.; Anderson, C.; Helsel, W.; Thames, S.F.; Lee, N.C. Cervical screening in the National Breast and Cervical Cancer Early Detection Program, 1995–2001. Obstet. Gynecol. 2004, 103, 564–571. [Google Scholar] [CrossRef]
- Lindquist, S.; Kjær, S.K.; Frederiksen, K.; Ørnskov, D.; Munk, C.; Waldstrøm, M. Comparative analysis of HPV testing versus cytology in Danish cervical cancer screening: Insights from a large-scale implementation study. Gynecol. Oncol. 2024, 191, 45–55. [Google Scholar] [CrossRef]
- Costa, R.F.A.; Longatto-Filho, A.; Pinheiro, C.; Zeferino, L.C.; Fregnani, J.H. Historical Analysis of the Brazilian Cervical Cancer Screening Program from 2006 to 2013: A Time for Reflection. PLoS ONE 2015, 10, e0138945. [Google Scholar] [CrossRef]
- Ramírez, A.T.; Valls, J.; Baena, A.; Rojas, F.D.; Ramírez, K.; Álvarez, R.; Cristaldo, C.; Henríquez, O.; Moreno, A.; Reynaga, D.C.; et al. Performance of cervical cytology and HPV testing for primary cervical cancer screening in Latin America: An analysis within the ESTAMPA study. Lancet Reg. Health Am. 2023, 26, 10059. [Google Scholar] [CrossRef]
- Martins, T.R.; Longatto-Filho, A.; Cohen, D.; Viscondi, J.Y.K.; Fuza, L.M.; Cury, L.; Villa, L.L.; Levi, J.E.; Eluf-Neto, J. Influence of Prior Knowledge of Human Papillomavirus Status on the Performance of Cytology Screening. Am. J. Clin. Pathol. 2018, 149, 316–323. [Google Scholar] [CrossRef]
- Cuzick, J.; Cadman, L.; Mesher, D.; Austin, J.; Ashdown-Barr, L.; Ho, L.; Terry, G.; Liddle, S.; Wright, C.; Lyons, D.; et al. Comparing the performance of six human papillomavirus tests in a screening population. Br. J. Cancer 2013, 108, 908–913. [Google Scholar] [CrossRef]
- Bruni, L.; Serrano, B.; Roura, E.; Alemany, L.; Cowan, M.; Herrero, R.; Poljak, M.; Murillo, R.; Broutet, N.; Riley, L.M.; et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: A review and synthetic analysis. Lancet Glob. Health 2022, 10, e1115-27. [Google Scholar] [CrossRef]
- Simms, K.T.; Keane, A.; Nguyen, D.T.N.; Caruana, M.; Hall, M.T.; Lui, G.; Gauvreau, C.; Demke, O.; Arbyn, M.; Basu, P.; et al. Benefits, harms and cost-effectiveness of cervical screening, triage and treatment strategies for women in the general population. Nat. Med. 2023, 29, 3050–3058. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.; Naber, S.K.; Aitken, C.A.; de Koning, H.J.; van Ballegooijen, M.; de Kok, I. Cost-effectiveness of HPV-based cervical screening based on first year results in the Netherlands: A modelling study. BJOG 2021, 128, 573–582. [Google Scholar] [CrossRef]
- Poljak, M.; Oštrbenk, V.A.; Cuschieri, K.; Bohinc, K.B.; Arbyn, M. 2023 global inventory of commercial molecular tests for human papillomaviruses (HPV). J. Clin. Virol. 2024, 172, 105671. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Depuydt, C.; Benoy, I.; Bogers, J.; Cuschieri, K.; Schmitt, M.; Pawlita, M.; Geraets, D.; Heard, I.; Gheit, T.; et al. VALGENT: A protocol for clinical validation of human papillomavirus assays. J. Clin. Virol. 2016, 76 (Suppl. S1), S14–S21. [Google Scholar] [CrossRef]
- Arbyn, M.; Cuschieri, K.; Bonde, J.; Schuurman, R.; Cocuzza, C.; Broeck, D.V.; Zhao, F.H.; Rezhake, R.; Gultekin, M.; de Sanjosé, S.; et al. Criteria for second generation comparator tests in validation of novel HPV DNA tests for use in cervical cancer screening. J. Med. Virol. 2024, 96, e29881. [Google Scholar] [CrossRef]
- Perkins, R.B.; Wentzensen, N.; Guido, R.S.; Schiffman, M. Cervical Cancer Screening: A Review. JAMA 2023, 330, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Elfström, K.M.; Dillner, J. The Impact of Cervical cancer Screening for Different HPV Genotypes. 2024, 264. Available online: https://www.HPVWorld.com (accessed on 28 August 2025).
- Massad, L.S.; Clarke, M.A.; Perkins, R.B.; Garcia, F.; Chelmow, D.; Cheung, L.C.; Darragh, T.M.; Egemen, D.; Lorey, T.S.; Nayar, R.; et al. Applying Results of Extended Genotyping to Management of Positive Cervicovaginal Human Papillomavirus Test Results: Enduring Guidelines. J. Low. Genit. Tract Dis. 2025, 29, 134–143. [Google Scholar] [CrossRef]
- Zhang, L.; Tan, W.; Yang, H.; Zhang, S.; Dai, Y. Detection of Host Cell Gene/HPV DNA Methylation Markers: A Promising Triage Approach for Cervical Cancer. Front. Oncol. 2022, 12, 831949. [Google Scholar] [CrossRef]
- Schreiberhuber, L.; Barrett, J.E.; Wang, J.; Redl, E.; Herzog, C.; Vavourakis, C.D.; Sundström, K.; Dillner, J.; Widschwendter, M. Cervical cancer screening using DNA methylation triage in a real-world population. Nat. Med. 2024, 30, 2251–2257. [Google Scholar] [CrossRef]
- Burdier, F.R.; Waheed, D.E.; Nedjai, B.; Steenbergen, R.D.M.; Poljak, M.; Baay, M.; Vorsters, A.; Van Keer, S. DNA methylation as a triage tool for cervical cancer screening—A meeting report. Prev. Med. Rep. 2024, 41, 102678. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, K.; Zhao, F.; Downham, L.; Baena, A.; Basu, P. Molecular triaging options for women testing HPV positive with self-collected samples. Front. Oncol. 2023, 13, 1243888. [Google Scholar] [CrossRef]
- Mittal, S.; Banks, L. Molecular mechanisms underlying human papillomavirus E6 and E7 oncoprotein-induced cell transformation. Mutat. Res. Rev. Mutat. Res. 2017, 772, 23–35. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Doeberitz, M.V.K. p16INK4a/Ki67 Dual-Staining Immunocytochemistry to Refer Women Infected by High-Risk Human Papillomavirus for Colposcopy. Acta Cytol. 2025, 69, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/news/item/14-06-2023-who-prequalifies-additional-hpv-test-expanding-options-as-countries-pursue-cervical-cancer-elimination? (accessed on 18 August 2025).
- Barra, M.J.; Wilkinson, A.F.; Ma, A.E.; Goli, K.; Atif, H.; Osman, N.M.R.B.; Lorenzoni, C.; Tivir, G.; Lathrop, E.H.; Castle, P.E.; et al. One-hour extraction-free loop-mediated isothermal amplification HPV DNA assay for point-of-care testing in Maputo, Mozambique. Nat. Commun. 2025, 16, 7295. [Google Scholar] [CrossRef]
- Desai, K.T.; Ajenifuja, K.O.; Adepiti, C.A.; Inturrisi, F.; Dagnall, C.; Hoffman, A.C.; Egemen, D.; Gage, J.C.; Wentzensen, N.; de Sanjose, S.; et al. Validation of a simplified HPV genotyping assay designed for cervical screening in low-resource settings. J. Clin. Microbiol. 2025, 63, e0163924. [Google Scholar] [CrossRef]
- Lamsisi, M.; Benlghazi, A.; Kouach, J.; Laraqui, A.; Ennaji, M.M.; Chauleur, C.; Bourlet, T.; Li, G. Isothermal Nucleic Acid Amplification for Point-of-Care Primary Cervical Cancer Screening. Viruses 2024, 16, 1852. [Google Scholar] [CrossRef]
- Lowy, D.R. Harald zur Hausen (1936 to 2023): Discoverer of human papillomavirus infection as the main cause of cervical cancer. Proc. Natl. Acad. Sci. USA 2024, 121, e2400517121. [Google Scholar] [CrossRef]

| Assay | Manufacturer | Target | Genotyping Coverage |
|---|---|---|---|
| RealTime High-Risk HPV Test | Abbott | HPV L1 | HPV types 16 and 18, identified individually, and 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68, aggregated as other HR-HPVs |
| Onclarity HPV Assay | BD Diagnostics | HPV E6/7 | 5 types identified individually (HPV16, HPV18, HPV31, HPV45, HPV52) and 3 groups of types aggregated (HPV33/58, 35/39/68 and 56/59/66) |
| Cobas-x800 HPV Test | Roche Molecular Systems | HPV L1 | HPV types 16 and 18, identified individually, and 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68, aggregated with other HR-HPVs |
| Anyplex II HPV HR Detection | Seegene | HPV L1 | HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68, individually identified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, T.R.; Levi, J.E. Molecular Screening for Cervical Cancer. Genes 2025, 16, 1041. https://doi.org/10.3390/genes16091041
Martins TR, Levi JE. Molecular Screening for Cervical Cancer. Genes. 2025; 16(9):1041. https://doi.org/10.3390/genes16091041
Chicago/Turabian StyleMartins, Toni Ricardo, and José Eduardo Levi. 2025. "Molecular Screening for Cervical Cancer" Genes 16, no. 9: 1041. https://doi.org/10.3390/genes16091041
APA StyleMartins, T. R., & Levi, J. E. (2025). Molecular Screening for Cervical Cancer. Genes, 16(9), 1041. https://doi.org/10.3390/genes16091041






