Beyond TLR4 and Its Alternative Lipopolysaccharide (LPS) Sensing Pathways in Zebrafish
Abstract
1. Introduction
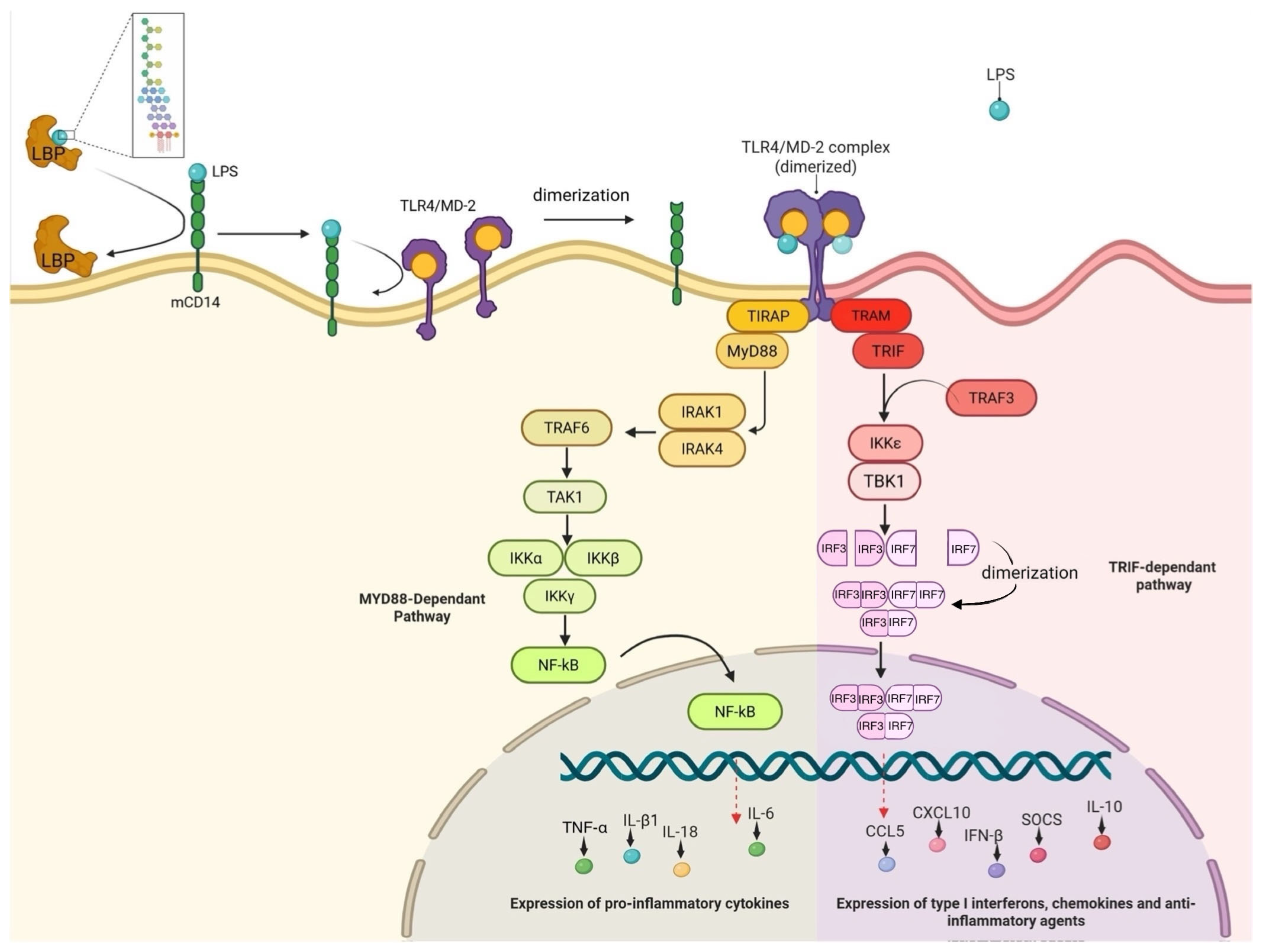
2. Established Pathways for LPS Recognition in Mammals
3. Early Studies and the Mystery of Certain Teleost Fish
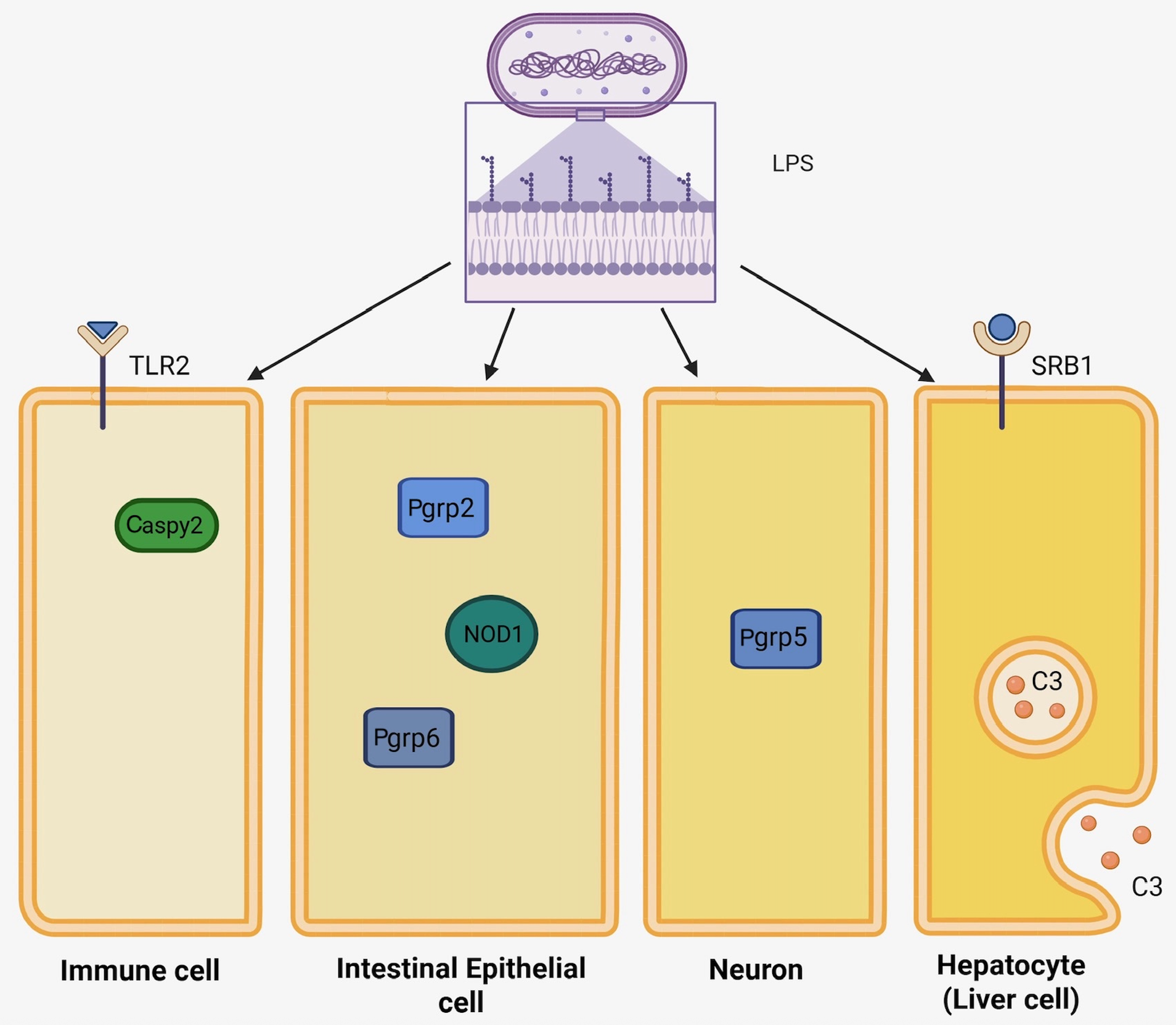
4. Alternative LPS Detection Mechanisms in Zebrafish
4.1. Caspases
4.2. Peptidoglycan Recognition Proteins (PRGPs)
4.3. Scavenger Receptors (SRs)
4.4. Nucleotide-Binding Oligomerization Domain (NOD) Genes
4.5. Toll-like Receptor 2 (TLR2)
4.6. Complement Protein C3
4.7. Updated Perspectives on Zf LPS Detection Mechanisms
5. Implications and Evolutionary Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Kang, R.; Tang, D. Lipopolysaccharide Delivery Systems in Innate Immunity. Trends Immunol. 2024, 45, 274–287. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of Lipopolysaccharide Pattern by TLR4 Complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Adhish, M.; Manjubala, I. Effectiveness of Zebrafish Models in Understanding Human Diseases-A Review of Models. Heliyon 2023, 9, e14557. [Google Scholar] [CrossRef] [PubMed]
- Irabin, A.F.; Ollewagen, T.; Smith, C.; Ahmed, R.; Reineke, J.; Reijnders, R.; Sampson, S.L.; Plessis, N.D.; Dube, A. Synthesis of Immunomodulatory Biomimetic Lipid Polymer Hybrid Nanoparticles and Application of Zebrafish Larvae in Immunomodulation Screening. Eur. J. Pharm. Sci. 2025, 207, 107037. [Google Scholar] [CrossRef]
- Bui, C.V.; Boswell, C.W.; Ciruna, B.; Rocheleau, J.V. Apollo-NADP+ Reveals in Vivo Adaptation of NADPH/NADP+ Metabolism in Electrically Activated Pancreatic β Cells. Sci. Adv. 2023, 9, eadi8317. [Google Scholar] [CrossRef]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent Adult Zebrafish as a Tool for In Vivo Transplantation Analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, C.; Li, Y.; Ng, G.H.B.; Liu, C.; Zhang, X.; Gong, Z. Generation of Tg(Cyp1a:Gfp) Transgenic Zebrafish for Development of a Convenient and Sensitive In Vivo Assay for Aryl Hydrocarbon Receptor Activity. Mar. Biotechnol. 2015, 17, 831–840. [Google Scholar] [CrossRef]
- Shahid, M.; Takamiya, M.; Stegmaier, J.; Middel, V.; Gradl, M.; Klüver, N.; Mikut, R.; Dickmeis, T.; Scholz, S.; Rastegar, S.; et al. Zebrafish Biosensor for Toxicant Induced Muscle Hyperactivity. Sci. Rep. 2016, 6, 23768. [Google Scholar] [CrossRef]
- Cano-Nicolau, J.; Vaillant, C.; Pellegrini, E.; Charlier, T.D.; Kah, O.; Coumailleau, P. Estrogenic Effects of Several BPA Analogs in the Developing Zebrafish Brain. Front. Neurosci. 2016, 10, 112. [Google Scholar] [CrossRef]
- Nadiga, A.P.R.; Suman; Krishna, K.L. A Novel Zebrafish Model of Alzheimer’s Disease by Aluminium Chloride; Involving Nitro-Oxidative Stress, Neuroinflammation and Cholinergic Pathway. Eur. J. Pharmacol. 2024, 965, 176332. [Google Scholar] [CrossRef]
- Muto, A.; Kawakami, K. Calcium Imaging of Neuronal Activity in Free-Swimming Larval Zebrafish. Methods Mol. Biol. 2016, 1451, 333–341. [Google Scholar] [CrossRef]
- Casano, A.M.; Albert, M.; Peri, F. Developmental Apoptosis Mediates Entry and Positioning of Microglia in the Zebrafish Brain. Cell Rep. 2016, 16, 897–906. [Google Scholar] [CrossRef]
- Sepulcre, M.P.; Alcaraz-Pérez, F.; López-Muñoz, A.; Roca, F.J.; Meseguer, J.; Cayuela, M.L.; Mulero, V. Evolution of Lipopolysaccharide (LPS) Recognition and Signaling: Fish TLR4 Does Not Recognize LPS and Negatively Regulates NF-kappaB Activation. J. Immunol. 2009, 182, 1836–1845. [Google Scholar] [CrossRef]
- Vaz, R.; Hofmeister, W.; Lindstrand, A. Zebrafish Models of Neurodevelopmental Disorders: Limitations and Benefits of Current Tools and Techniques. Int. J. Mol. Sci. 2019, 20, 1296. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 Trafficking and Its Influence on LPS-Induced pro-Inflammatory Signaling. Cell Mol. Life Sci. 2020, 78, 1233–1261. [Google Scholar] [CrossRef]
- Ahmed-Hassan, H.; Abdul-Cader, M.S.; Sabry, M.A.; Hamza, E.; Abdul-Careem, M.F. Toll-like Receptor (TLR)4 Signalling Induces Myeloid Differentiation Primary Response Gene (MYD) 88 Independent Pathway in Avian Species Leading to Type I Interferon Production and Antiviral Response. Virus Res. 2018, 256, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, P.; Prasad, P.; Pateria, A.; Deshmukh, S.D.; Gupta, S. A Single Step in Vitro Bioassay Mimicking TLR4-LPS Pathway and the Role of MD2 and CD14 Coreceptors. Front. Immunol. 2020, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cheng, Z.; Chu, H.; Wang, W.; Jin, Y.; Yang, L. TRIF-Dependent Signaling and Its Role in Liver Diseases. Front. Cell Dev. Biol. 2024, 12, 1370042. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.O.; Sweet, M.J.; Mansell, A.; Kellie, S.; Kobe, B. TRIF-Dependent TLR Signaling, Its Functions in Host Defense and Inflammation, and Its Potential as a Therapeutic Target. J. Leukoc. Biol. 2016, 100, 27–45. [Google Scholar] [CrossRef]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 Controls the LPS-Induced Endocytosis of Toll-like Receptor 4. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Yum, S.; Li, M.; Fang, Y.; Chen, Z.J. TBK1 Recruitment to STING Activates Both IRF3 and NF-κB That Mediate Immune Defense against Tumors and Viral Infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2100225118. [Google Scholar] [CrossRef] [PubMed]
- Qing, F.; Liu, Z. Interferon Regulatory Factor 7 in Inflammation, Cancer and Infection. Front. Immunol. 2023, 14, 1190841. [Google Scholar] [CrossRef]
- Pietretti, D.; Spaink, H.P.; Falco, A.; Forlenza, M.; Wiegertjes, G.F. Accessory Molecules for Toll-like Receptors in Teleost Fish. Identification of TLR4 Interactor with Leucine-Rich Repeats (TRIL). Mol. Immunol. 2013, 56, 745–756. [Google Scholar] [CrossRef]
- Palti, Y. Toll-like Receptors in Bony Fish: From Genomics to Function. Dev. Comp. Immunol. 2011, 35, 1263–1272. [Google Scholar] [CrossRef]
- Sullivan, C.; Charette, J.; Catchen, J.; Lage, C.R.; Giasson, G.; Postlethwait, J.H.; Millard, P.J.; Kim, C.H. The Gene History of Zebrafish Tlr4a and Tlr4b Is Predictive of Their Divergent Functions. J. Immunol. 2009, 183, 5896. [Google Scholar] [CrossRef]
- Novoa, B.; Bowman, T.V.; Zon, L.; Figueras, A. LPS Response and Tolerance in the Zebrafish (Danio Rerio). Fish Shellfish Immunol. 2009, 26, 326–331. [Google Scholar] [CrossRef]
- Wang, M.X.; Shandilya, U.K.; Wu, X.; Huyben, D.; Karrow, N.A. Assessing Larval Zebrafish Survival and Gene Expression Following Sodium Butyrate Exposure and Subsequent Lethal Bacterial Lipopolysaccharide (LPS) Endotoxin Challenge. Toxins 2023, 15, 588. [Google Scholar] [CrossRef]
- Robinson, C.D.; Klein, H.S.; Murphy, K.D.; Parthasarathy, R.; Guillemin, K.; Bohannan, B.J.M. Experimental Bacterial Adaptation to the Zebrafish Gut Reveals a Primary Role for Immigration. PLoS Biol. 2018, 16, e2006893. [Google Scholar] [CrossRef]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a Core Gut Microbiota in the Zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef]
- Nag, D.; Farr, D.A.; Walton, M.G.; Withey, J.H. Zebrafish Models for Pathogenic Vibrios. J. Bacteriol. 2020, 202, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Kayagaki, N.; Warming, S.; Lamkanfi, M.; Vande Walle, L.; Louie, S.; Dong, J.; Newton, K.; Qu, Y.; Liu, J.; Heldens, S.; et al. Non-Canonical Inflammasome Activation Targets Caspase-11. Nature 2011, 479, 117–121. [Google Scholar] [CrossRef]
- Park, H.H. Caspase Recruitment Domains for Protein Interactions in Cellular Signaling (Review). Int. J. Mol. Med. 2019, 43, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Agnew, A.; Nulty, C.; Creagh, E.M. Regulation, Activation and Function of Caspase-11 during Health and Disease. Int. J. Mol. Sci. 2021, 22, 1506. [Google Scholar] [CrossRef]
- Knodler, L.A.; Crowley, S.M.; Sham, H.P.; Yang, H.; Wrande, M.; Ma, C.; Ernst, R.K.; Steele-Mortimer, O.; Celli, J.; Vallance, B.A. Noncanonical Inflammasome Activation of Caspase-4/Caspase-11 Mediates Epithelial Defenses against Enteric Bacterial Pathogens. Cell Host Microbe 2014, 16, 249–256. [Google Scholar] [CrossRef]
- Masumoto, J.; Zhou, W.; Chen, F.F.; Su, F.; Kuwada, J.Y.; Hidaka, E.; Katsuyama, T.; Sagara, J.; Taniguchi, S.; Ngo-Hazelett, P.; et al. Caspy, a Zebrafish Caspase, Activated by ASC Oligomerization Is Required for Pharyngeal Arch Development. J. Biol. Chem. 2003, 278, 4268–4276. [Google Scholar] [CrossRef]
- Park, H.H.; Lo, Y.-C.; Lin, S.-C.; Wang, L.; Yang, J.K.; Wu, H. The Death Domain Superfamily in Intracellular Signaling of Apoptosis and Inflammation. Annu. Rev. Immunol. 2007, 25, 561–586. [Google Scholar] [CrossRef]
- Yang, D.; Zheng, X.; Chen, S.; Wang, Z.; Xu, W.; Tan, J.; Hu, T.; Hou, M.; Wang, W.; Gu, Z.; et al. Sensing of Cytosolic LPS through Caspy2 Pyrin Domain Mediates Noncanonical Inflammasome Activation in Zebrafish. Nat. Commun. 2018, 9, 3052. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, Z.; Hou, Q.; Chen, W.; Mu, D.; Zhang, Y.; Liu, Q.; Liu, Z.; Yang, D. Zebrafish GSDMEb Cleavage-Gated Pyroptosis Drives Septic Acute Kidney Injury In Vivo. J. Immunol. 2020, 204, 1929–1942. [Google Scholar] [CrossRef] [PubMed]
- Goodson, M.S.; Kojadinovic, M.; Troll, J.V.; Scheetz, T.E.; Casavant, T.L.; Soares, M.B.; McFall-Ngai, M.J. Identifying Components of the NF-κB Pathway in the Beneficial Euprymna Scolopes-Vibrio Fischeri Light Organ Symbiosis. Appl. Environ. Microbiol. 2005, 71, 6934–6946. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, S.; Qi, J.; Echtenkamp, S.F.; Chatterjee, R.; Wang, M.; Boons, G.-J.; Dziarski, R.; Gupta, D. Zebrafish Peptidoglycan Recognition Proteins Are Bactericidal Amidases Essential for Defense against Bacterial Infections. Immunity 2007, 27, 518–529. [Google Scholar] [CrossRef]
- Li, X.; Xiong, G.; Luo, M.; Mao, S.; Zhang, R.; Meng, Z.; Li, J.; Liao, X. Peptidoglycan Recognition Protein PGRP-5 Is Involved in Immune Defence and Neuro-Behavioral Disorders in Zebrafish Embryos. PLoS ONE 2025, 20, e0315714. [Google Scholar] [CrossRef] [PubMed]
- Montaño, A.M.; Tsujino, F.; Takahata, N.; Satta, Y. Evolutionary Origin of Peptidoglycan Recognition Proteins in Vertebrate Innate Immune System. BMC Evol. Biol. 2011, 11, 79. [Google Scholar] [CrossRef]
- Iatsenko, I.; Kondo, S.; Mengin-Lecreulx, D.; Lemaitre, B. PGRP-SD, an Extracellular Pattern-Recognition Receptor, Enhances Peptidoglycan-Mediated Activation of the Drosophila Imd Pathway. Immunity 2016, 45, 1013–1023. [Google Scholar] [CrossRef]
- Alquraini, A.; El Khoury, J. Scavenger Receptors. Curr. Biol. 2020, 30, R790–R795. [Google Scholar] [CrossRef]
- Chen, Y.; Wermeling, F.; Sundqvist, J.; Jonsson, A.-B.; Tryggvason, K.; Pikkarainen, T.; Karlsson, M.C.I. A Regulatory Role for Macrophage Class A Scavenger Receptors in TLR4-Mediated LPS Responses. Eur. J. Immunol. 2010, 40, 1451–1460. [Google Scholar] [CrossRef]
- Baranova, I.N.; Bocharov, A.V.; Vishnyakova, T.G.; Chen, Z.; Birukova, A.A.; Ke, Y.; Hu, X.; Yuen, P.S.T.; Star, R.A.; Birukov, K.G.; et al. Class B Scavenger Receptors BI and BII Protect against LPS-Induced Acute Lung Injury in Mice by Mediating LPS. Infect. Immun. 2021, 89, e0030121. [Google Scholar] [CrossRef]
- Gough, P.J.; Gordon, S. The Role of Scavenger Receptors in the Innate Immune System. Microbes Infect. 2000, 2, 305–311. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, X.; Guo, J.; Xiang, L.; Shao, J. Scavenger Receptor in Fish Is a Lipopolysaccharide Recognition Molecule Involved in Negative Regulation of NF-κB Activation by Competing with TNF Receptor-Associated Factor 2 Recruitment into the TNF-α Signaling Pathway. J. Immunol. 2012, 189, 4024–4039. [Google Scholar] [CrossRef]
- Moss, S.P.; Joyce, D.A.; Humphries, S.; Tindall, K.J.; Lunt, D.H. Comparative Analysis of Teleost Genome Sequences Reveals an Ancient Intron Size Expansion in the Zebrafish Lineage. Genome Biol. Evol. 2011, 3, 1187–1196. [Google Scholar] [CrossRef]
- Su, J.; Yang, C.; Xiong, F.; Wang, Y.; Zhu, Z. Toll-like Receptor 4 Signaling Pathway Can Be Triggered by Grass Carp Reovirus and Aeromonas Hydrophila Infection in Rare Minnow Gobiocypris Rarus. Fish Shellfish Immunol. 2009, 27, 33–39. [Google Scholar] [CrossRef]
- Li, C.; Zheng, X.; Chang, M.; Tian, Q.; He, Z.; Tang, Z.; Chen, X.; Liu, X.; Yang, D.; Yan, T. Toll-like Receptor-4 in the Fish Immune System. Dev. Comp. Immunol. 2025, 169, 105400. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, Y.; Wang, Y.; Wei, S.; Feng, D.; Huang, Q.; Zhang, S.; Liu, Z. Developmental Expression and Immune Role of the Class B Scavenger Receptor Cd36 in Zebrafish. Dev. Comp. Immunol. 2016, 60, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-Z.; Yang, M.-C.; Kang, X.-L.; Li, Y.-X.; Hong, P.-P.; Zhao, X.-F.; Vasta, G.R.; Wang, J.-X. Scavenger Receptor B2, a Type III Membrane Pattern Recognition Receptor, Senses LPS and Activates the IMD Pathway in Crustaceans. Proc. Natl. Acad. Sci. USA 2023, 120, e2216574120. [Google Scholar] [CrossRef] [PubMed]
- Myllymäki, H.; Valanne, S.; Rämet, M. The Drosophila Imd Signaling Pathway. J. Immunol. 2014, 192, 3455–3462. [Google Scholar] [CrossRef]
- Mukherjee, T.; Hovingh, E.S.; Foerster, E.G.; Abdel-Nour, M.; Philpott, D.J.; Girardin, S.E. NOD1 and NOD2 in Inflammation, Immunity and Disease. Arch. Biochem. Biophys. 2019, 670, 69–81. [Google Scholar] [CrossRef]
- Bi, D.; Wang, Y.; Gao, Y.; Li, X.; Chu, Q.; Cui, J.; Xu, T. Recognition of Lipopolysaccharide and Activation of NF-κB by Cytosolic Sensor NOD1 in Teleost Fish. Front. Immunol. 2018, 9, 1413. [Google Scholar] [CrossRef]
- Allen, I.C. A NOD to Zebrafish Models of Inflammatory Bowel Disease Pathogenesis. Dis. Model. Mech. 2011, 4, 711–712. [Google Scholar] [CrossRef]
- Schäfer, Y.; Palitzsch, K.; Leptin, M.; Whiteley, A.R.; Wiehe, T.; Suurväli, J. Copy Number Variation and Population-Specific Immune Genes in the Model Vertebrate Zebrafish. eLife 2024, 13, e98058. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Cao, X.; Jin, X.; Jin, T. Pattern Recognition Receptors in Zebrafish Provide Functional and Evolutionary Insight into Innate Immune Signaling Pathways. Cell Mol. Immunol. 2017, 14, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Sha, Z.; Abernathy, J.W.; Wang, S.; Li, P.; Kucuktas, H.; Liu, H.; Peatman, E.; Liu, Z. NOD-like Subfamily of the Nucleotide-Binding Domain and Leucine-Rich Repeat Containing Family Receptors and Their Expression in Channel Catfish. Dev. Comp. Immunol. 2009, 33, 991–999. [Google Scholar] [CrossRef]
- Laing, K.J.; Purcell, M.K.; Winton, J.R.; Hansen, J.D. A Genomic View of the NOD-like Receptor Family in Teleost Fish: Identification of a Novel NLR Subfamily in Zebrafish. BMC Evol. Biol. 2008, 8, 42. [Google Scholar] [CrossRef]
- Uehara, A.; Fujimoto, Y.; Kawasaki, A.; Kusumoto, S.; Fukase, K.; Takada, H. Meso-Diaminopimelic Acid and Meso-Lanthionine, Amino Acids Specific to Bacterial Peptidoglycans, Activate Human Epithelial Cells through NOD1. J. Immunol. 2006, 177, 1796–1804. [Google Scholar] [CrossRef]
- Bi, D.; Gao, Y.; Chu, Q.; Cui, J.; Xu, T. NOD1 Is the Innate Immune Receptor for iE-DAP and Can Activate NF-κB Pathway in Teleost Fish. Dev. Comp. Immunol. 2017, 76, 238–246. [Google Scholar] [CrossRef]
- Hu, Y.W.; Wu, X.M.; Ren, S.S.; Cao, L.; Nie, P.; Chang, M.X. NOD1 Deficiency Impairs CD44a/Lck as Well as PI3K/Akt Pathway. Sci. Rep. 2017, 7, 2979. [Google Scholar] [CrossRef]
- Colleselli, K.; Stierschneider, A.; Wiesner, C. An Update on Toll-like Receptor 2, Its Function and Dimerization in Pro- and Anti-Inflammatory Processes. Int. J. Mol. Sci. 2023, 24, 12464. [Google Scholar] [CrossRef]
- Mukherjee, S.; Karmakar, S.; Babu, S.P.S. TLR2 and TLR4 Mediated Host Immune Responses in Major Infectious Diseases: A Review. Braz. J. Infect. Dis. 2016, 20, 193–204. [Google Scholar] [CrossRef]
- Hu, W.; Yang, S.; Shimada, Y.; Münch, M.; Marín-Juez, R.; Meijer, A.H.; Spaink, H.P. Infection and RNA-Seq Analysis of a Zebrafish Tlr2 Mutant Shows a Broad Function of This Toll-like Receptor in Transcriptional and Metabolic Control and Defense to Mycobacterium Marinum Infection. BMC Genom. 2019, 20, 878. [Google Scholar] [CrossRef]
- Good, D.W.; George, T.; Watts, B.A. Toll-like Receptor 2 Is Required for LPS-Induced Toll-like Receptor 4 Signaling and Inhibition of Ion Transport in Renal Thick Ascending Limb. J. Biol. Chem. 2012, 287, 20208–20220. [Google Scholar] [CrossRef]
- Francisco, S.; Billod, J.-M.; Merino, J.; Punzón, C.; Gallego, A.; Arranz, A.; Martin-Santamaria, S.; Fresno, M. Induction of TLR4/TLR2 Interaction and Heterodimer Formation by Low Endotoxic Atypical LPS. Front. Immunol. 2021, 12, 748303. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Meijer, A.H.; Schaaf, M.J.M. Modeling Inflammation in Zebrafish for the Development of Anti-Inflammatory Drugs. Front. Cell Dev. Biol. 2020, 8, 620984. [Google Scholar] [CrossRef] [PubMed]
- Ricklin, D.; Hajishengallis, G.; Yang, K.; Lambris, J.D. Complement—A Key System for Immune Surveillance and Homeostasis. Nat. Immunol. 2010, 11, 785. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, J.; Wu, Y.; Shen, J.; Fu, X.; Liu, M.; Liang, S. New Insights into the Role of Complement System in Colorectal Cancer (Review). Mol. Med. Rep. 2025, 31, 68. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.C.; Tambourgi, D.V. Complement System Inhibitory Drugs in a Zebrafish (Danio Rerio) Model: Computational Modeling. Int. J. Mol. Sci. 2023, 24, 13895. [Google Scholar] [CrossRef]
- Ricklin, D.; Reis, E.S.; Mastellos, D.C.; Gros, P.; Lambris, J.D. Complement Component C3—The “Swiss Army Knife” of Innate Immunity and Host Defense. Immunol. Rev. 2016, 274, 33–58. [Google Scholar] [CrossRef]
- Forn-Cuní, G.; Reis, E.S.; Dios, S.; Posada, D.; Lambris, J.D.; Figueras, A.; Novoa, B. The Evolution and Appearance of C3 Duplications in Fish Originate an Exclusive Teleost C3 Gene Form with Anti-Inflammatory Activity. PLoS ONE 2014, 9, e99673. [Google Scholar] [CrossRef]
- Wu, M.; Jia, B.; Li, M. Complement C3 and Activated Fragment C3a Are Involved in Complement Activation and Anti-Bacterial Immunity. Front. Immunol. 2022, 13, 813173. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Wang, G. Response of Complement Expression to Challenge with Lipopolysaccharide in Embryos/Larvae of Zebrafish Danio Rerio: Acquisition of Immunocompetent Complement. Fish Shellfish Immunol. 2008, 25, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Loes, A.N.; Hinman, M.N.; Farnsworth, D.R.; Miller, A.C.; Guillemin, K.; Harms, M.J. Identification and Characterization of Zebrafish Tlr4 Coreceptor Md-2. J. Immunol. 2021, 206, 1046–1057. [Google Scholar] [CrossRef]
- Das, S.; Jain, D.; Chaudhary, P.; Quintela-Tizon, R.M.; Banerjee, A.; Kesavardhana, S. Bat Adaptations in Inflammation and Cell Death Regulation Contribute to Viral Tolerance. mBio 2025, 16, e03204-23. [Google Scholar] [CrossRef]
- Razali, K.; Mohd Nasir, M.H.; Kumar, J.; Mohamed, W.M.Y. Mitophagy: A Bridge Linking HMGB1 and Parkinson’s Disease Using Adult Zebrafish as a Model Organism. Brain Sci. 2023, 13, 1076. [Google Scholar] [CrossRef]
- Tam, J.S.Y.; Coller, J.K.; Hughes, P.A.; Prestidge, C.A.; Bowen, J.M. Toll-like Receptor 4 (TLR4) Antagonists as Potential Therapeutics for Intestinal Inflammation. Indian. J. Gastroenterol. 2021, 40, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Heine, H.; Zamyatina, A. Therapeutic Targeting of TLR4 for Inflammation, Infection, and Cancer: A Perspective for Disaccharide Lipid A Mimetics. Pharmaceuticals 2022, 16, 23. [Google Scholar] [CrossRef]
- Costa, B.; Estrada, M.F.; Gomes, A.; Fernandez, L.M.; Azevedo, J.M.; Póvoa, V.; Fontes, M.; Alves, A.; Galzerano, A.; Castillo-Martin, M.; et al. Zebrafish Avatar-Test Forecasts Clinical Response to Chemotherapy in Patients with Colorectal Cancer. Nat. Commun. 2024, 15, 4771. [Google Scholar] [CrossRef]
- Shwartz, A.; Goessling, W.; Yin, C. Macrophages in Zebrafish Models of Liver Diseases. Front. Immunol. 2019, 10, 2840. [Google Scholar] [CrossRef]
- Mathias, J.R.; Dodd, M.E.; Walters, K.B.; Yoo, S.K.; Ranheim, E.A.; Huttenlocher, A. Characterization of Zebrafish Larval Inflammatory Macrophages. Dev. Comp. Immunol. 2009, 33, 1212–1217. [Google Scholar] [CrossRef]
| Research Area | Findings and Contributions | Key Reference(s) |
|---|---|---|
| Immunology | Nanoparticles were tested in Zf embryos, which demonstrated increased innate immune activation. | [6] |
| Cellular Metabolism and Redox Biology | An Apollo-NADP+ biosensor was developed to track real-time NADPH/NADP+ dynamics in Zf embryos. | [7] |
| Cancer Research | Adult transparent Zf were used to study RAS melanoma cell xenotransplantation, tumor engraftment, proliferation, and metastases. | [8] |
| Toxicology | Genetically engineered Zf were used for detecting toxicant exposure. Their rapid life cycle allows for quick assessment of exposure effects. | [9,10,11] |
| Neuroscience and Neurodegeneration | Zf were used as an Alzheimer’s disease model due to genetic similarity, simple nervous system, and transparent embryos. | [12,13,14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grebennikova, D.V.; Shandilya, U.K.; Karrow, N.A. Beyond TLR4 and Its Alternative Lipopolysaccharide (LPS) Sensing Pathways in Zebrafish. Genes 2025, 16, 1014. https://doi.org/10.3390/genes16091014
Grebennikova DV, Shandilya UK, Karrow NA. Beyond TLR4 and Its Alternative Lipopolysaccharide (LPS) Sensing Pathways in Zebrafish. Genes. 2025; 16(9):1014. https://doi.org/10.3390/genes16091014
Chicago/Turabian StyleGrebennikova, Dara V., Umesh K. Shandilya, and Niel A. Karrow. 2025. "Beyond TLR4 and Its Alternative Lipopolysaccharide (LPS) Sensing Pathways in Zebrafish" Genes 16, no. 9: 1014. https://doi.org/10.3390/genes16091014
APA StyleGrebennikova, D. V., Shandilya, U. K., & Karrow, N. A. (2025). Beyond TLR4 and Its Alternative Lipopolysaccharide (LPS) Sensing Pathways in Zebrafish. Genes, 16(9), 1014. https://doi.org/10.3390/genes16091014







