Focus on Clinical and Genetic Aspects of PKAN Through the Description of New Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Examination
2.2. Whole-Exome Sequencing (WES)
2.3. cDNA Analysis
3. Results
3.1. Patient’s Clinical History
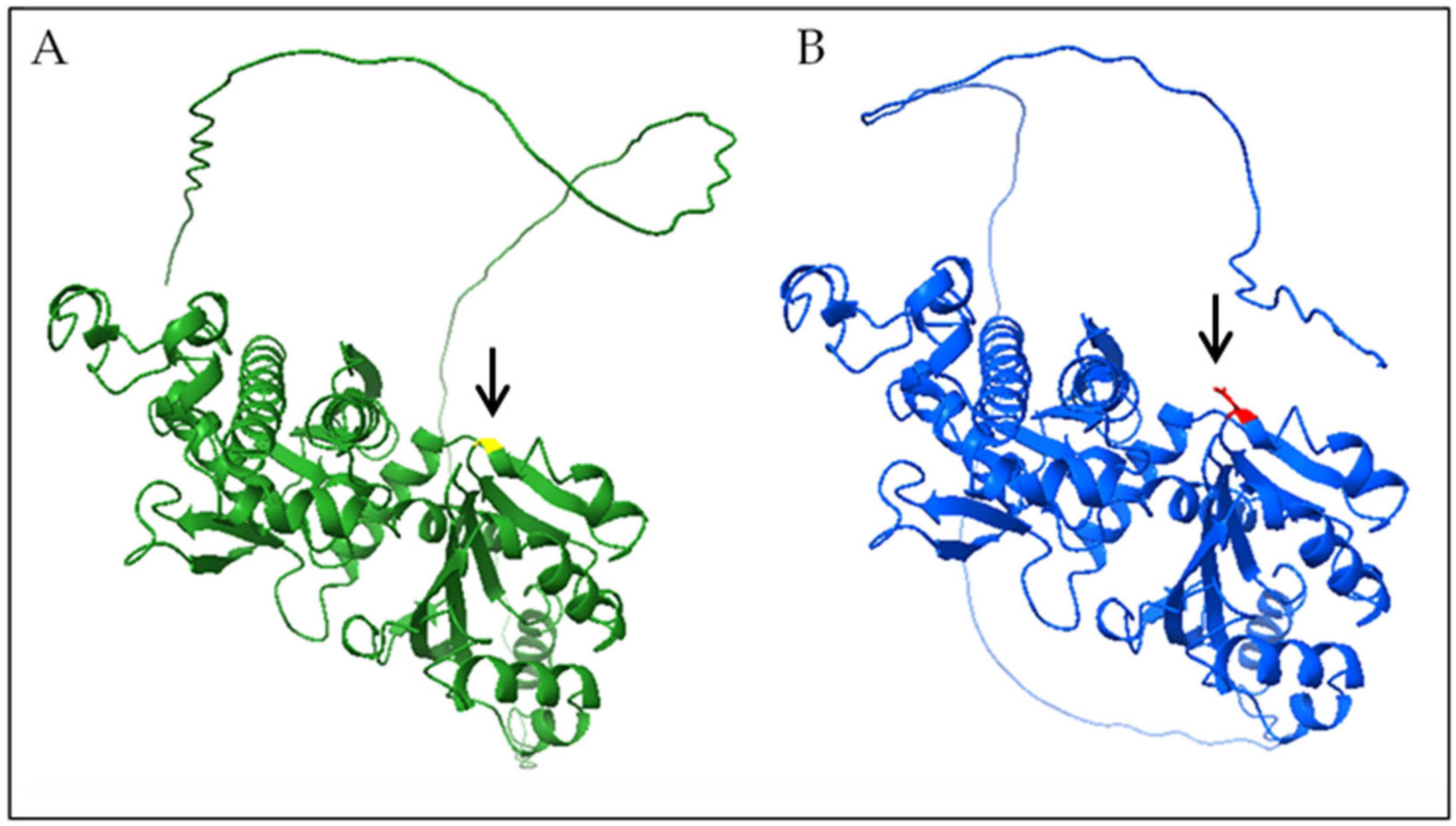
3.2. Genetic and Functional Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NBIA | Neurodegeneration with brain iron accumulation |
| MRI | Magnetic resonance imaging |
| PKAN | Pantothenate kinase-associated neurodegeneration |
| CoA | Coenzyme A |
| MPAN | Mitochondrial membrane protein-associated neurodegeneration |
| CoPAN | Coenzyme A synthase protein-associated neurodegeneration |
| EEG | Electroencephalography |
| EMG | Electromyography |
| WES | Whole-exome sequencing |
| ID | Intellectual disability |
| ACMG | American College of Medical Genetics |
References
- Wydrych, A.; Pakuła, B.; Janikiewicz, J.; Dobosz, A.M.; Jakubek-Olszewska, P.; Skowrońska, M.; Kurkowska-Jastrzębska, I.; Cwyl, M.; Popielarz, M.; Pinton, P.; et al. Metabolic Impairments in Neurodegeneration with Brain Iron Accumulation. Biochim. Biophys. Acta (BBA)-Bioenerg. 2025, 1866, 149517. [Google Scholar] [CrossRef] [PubMed]
- Iankova, V.; Karin, I.; Klopstock, T.; Schneider, S.A. Emerging Disease-Modifying Therapies in Neurodegeneration with Brain Iron Accumulation (NBIA) Disorders. Front. Neurol. 2021, 12, 629414. [Google Scholar] [CrossRef] [PubMed]
- Hajati, R.; Emamikhah, M.; Danaee Fard, F.; Rohani, M.; Alavi, A. Neurodegeneration with Brain Iron Accumulation and a Brief Report of the Disease in Iran. Can. J. Neurol. Sci. 2022, 49, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Aoun, M.; Corsetto, P.A.; Nugue, G.; Montorfano, G.; Ciusani, E.; Crouzier, D.; Hogarth, P.; Gregory, A.; Hayflick, S.; Zorzi, G.; et al. Changes in Red Blood Cell Membrane Lipid Composition: A New Perspective into the Pathogenesis of PKAN. Mol. Genet. Metab. 2017, 121, 180–189. [Google Scholar] [CrossRef]
- Marupudi, N.; Xiong, M.P. Genetic Targets and Applications of Iron Chelators for Neurodegeneration with Brain Iron Accumulation. ACS Bio Med. Chem. Au 2024, 4, 119–130. [Google Scholar] [CrossRef]
- Alfonso-Pecchio, A.; Garcia, M.; Leonardi, R.; Jackowski, S. Compartmentalization of Mammalian Pantothenate Kinases. PLoS ONE 2012, 7, e49509. [Google Scholar] [CrossRef] [PubMed]
- Kwinta, R.; Kopcik, K.; Koberling, A. Pathology and Treatment Methods in Pantothenate Kinase-Associated Neurodegeneration. Postep. Psychiatr. Neurol. 2024, 33, 163–171. [Google Scholar] [CrossRef]
- Gregory, A.; Hayflick, S.J. Pantothenate Kinase-Associated Neurodegeneration. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington-Seattle Campus: Seattle, WA, USA, 1993. [Google Scholar]
- Chang, X.; Zhang, J.; Jiang, Y.; Wang, J.; Wu, Y. Natural History and Genotype-Phenotype Correlation of Pantothenate Kinase-Associated Neurodegeneration. CNS Neurosci. Ther. 2020, 26, 754–761. [Google Scholar] [CrossRef]
- Santambrogio, P.; Ripamonti, M.; Cozzi, A.; Raimondi, M.; Cavestro, C.; Di Meo, I.; Rubio, A.; Taverna, S.; Tiranti, V.; Levi, S. Massive Iron Accumulation in PKAN-Derived Neurons and Astrocytes: Light on the Human Pathological Phenotype. Cell Death Dis. 2022, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Dehghan Manshadi, M.; Rohani, M.; Rezaei, A.; Aryani, O. A Case of MPAN with “Eye of the Tiger Sign,” Mimicking PKAN. Mov. Disord. Clin. Pr. 2022, 9, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Evers, C.; Seitz, A.; Assmann, B.; Opladen, T.; Karch, S.; Hinderhofer, K.; Granzow, M.; Paramasivam, N.; Eils, R.; Diessl, N.; et al. Diagnosis of CoPAN by Whole Exome Sequencing: Waking up a Sleeping Tiger’s Eye. Am. J. Med. Genet. A 2017, 173, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Litwin, T.; Karlinski, M.; Skowrońska, M.; Dziezyc, K.; Gołębiowski, M.; Członkowska, A. MR Image Mimicking the “Eye of the Tiger” Sign in Wilson’s Disease. J. Neurol. 2014, 261, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, S.; Malhotra, H.S.; Garg, R.K. Is the “Eye of Tiger” Really Emblematic of Pantothenate Kinase-Associated Neurodegeneration Type 1? An Uncommon MR Image in Wilson’s Disease. Neurol. India 2024, 72, 221–222. [Google Scholar] [CrossRef]
- Hayflick, S.; Westaway, S. Pantothenate Kinase 2 Mutation without “eye-of-the-Tiger” Sign. Pediatr. Radiol. 2006, 36, 1329. [Google Scholar] [CrossRef] [PubMed]
- Zolkipli, Z.; Dahmoush, H.; Saunders, D.E.; Chong, W.K.K.; Surtees, R. Pantothenate Kinase 2 Mutation with Classic Pantothenate-Kinase-Associated Neurodegeneration without “eye-of-the-Tiger” Sign on MRI in a Pair of Siblings. Pediatr. Radiol. 2006, 36, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, F.A.M.; Auer, D.P.; Hörtnagel, K.; Freisinger, P.; Meitinger, T. The Eye-of-the-Tiger Sign Is Not a Reliable Disease Marker for Hallervorden-Spatz Syndrome. Neuropediatrics 2005, 36, 221–222. [Google Scholar] [CrossRef]
- Zhou, B.; Westaway, S.K.; Levinson, B.; Johnson, M.A.; Gitschier, J.; Hayflick, S.J. A Novel Pantothenate Kinase Gene (PANK2) Is Defective in Hallervorden-Spatz Syndrome. Nat. Genet. 2001, 28, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.A.; Hayflick, S.J. Pantothenate Kinase-Associated Neurodegeneration (PKAN) and PLA2G6-Associated Neurodegeneration (PLAN): Review of Two Major Neurodegeneration with Brain Iron Accumulation (NBIA) Phenotypes. Int. Rev. Neurobiol. 2013, 110, 49–71. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; You, L.; Zhang, J.; Chang, Y.-Z.; Yu, P. Brain Iron Metabolism, Redox Balance and Neurological Diseases. Antioxidants 2023, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Delgado, R.F.; Sanchez, P.R.; Speckter, H.; Then, E.P.; Jimenez, R.; Oviedo, J.; Dellani, P.R.; Foerster, B.; Stoeter, P. Missense PANK2 Mutation without “Eye of the Tiger” Sign: MR Findings in a Large Group of Patients with Pantothenate Kinase-Associated Neurodegeneration (PKAN). J. Magn. Reson. Imaging 2012, 35, 788–794. [Google Scholar] [CrossRef] [PubMed]


| c.1169A > T (p.N390I) | ||
|---|---|---|
| Criteria for Classifying Variants | Category Code | Description |
| Pathogenic Moderate: | PM1 | Non-truncating non-synonymous variant is located in a mutational hot spot and/or critical and well-established functional domain |
| Pathogenic Supporting: | PP2 | Missense variant in a gene with low rate of benign missense mutations and for which missense mutation is a common mechanism of a disease |
| Pathogenic Moderate: | PM2 | Extremely low frequency in gnomAD population databases |
| Pathogenic Moderate: | PP3 | For a missense or a splicing region variant, computational prediction tools unanimously support a deleterious effect on the gene |
| Pathogenic Supporting: | PP5 | Reputable source recently reports variant as pathogenic, but the evidence is not available to the laboratory to perform an independent evaluation |
| ACMG variant classification | Likely Pathogenic | |
| c.1231G > A (p.G411R) | ||
|---|---|---|
| Criteria for Classifying Variants | Category Code | Description |
| Pathogenic Very Strong: | PM3 | For recessive disorders, detected in trans with a pathogenic variant, or in a homozygous or compound heterozygous state in affected cases |
| Pathogenic Supporting: | PP1 | Cosegregation with disease in multiple affected family members in a gene definitively known to cause the disease |
| Pathogenic Supporting: | PS3 | Well-established functional studies show damaging effect on the gene or gene product |
| Pathogenic Strong: | PP3 | For a missense or a splicing region variant, computational prediction tools unanimously support a deleterious effect on the gene |
| Pathogenic Moderate: | PM2 | Extremely low frequency in gnomAD population databases |
| Pathogenic Supporting: | PP2 | Missense variant in a gene with low rate of benign missense mutations and for which missense mutation is a common mechanism of a disease |
| ACMG variant classification | Pathogenic | |
| c.906-1G > A | ||
|---|---|---|
| Criteria for Classifying Variants | Category Code | Description |
| Pathogenic Very Strong: | PM3 | For recessive disorders, detected in trans with a pathogenic variant, or in a homozygous or compound heterozygous state in affected cases |
| Pathogenic Strong: | PVS1 | Null variant in a gene where loss of function is a known mechanism of disease |
| Pathogenic Moderate: | PM2 | Extremely low frequency in gnomAD population databases |
| ACMG variant classification | Pathogenic | |
| c.617G > A (p.G206D) | ||
|---|---|---|
| Criteria for Classifying Variants | Category Code | Description |
| Pathogenic Moderate: | PM1 | Non-truncating non-synonymous variant is located in a mutational hot spot and/or critical and well-established functional domain |
| Pathogenic Supporting: | PP2 | Missense variant in a gene with low rate of benign missense mutations and for which missense mutation is a common mechanism of a disease |
| Pathogenic Moderate: | PM2 | Extremely low frequency in gnomAD population databases |
| Pathogenic Strong: | PP3 | For a missense or a splicing region variant, computational prediction tools unanimously support a deleterious effect on the gene |
| ACMG variant classification | Likely Pathogenic | |
| Case | Sex | Age/y | Age/mo of Onset | PANK2 Variants | Cognitive Levels | Language Disorders | Spastic Paraplegia | Retinitis Pigmentosa | Deambulation | Dystonia | Imaging Features on MRI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 13 | 16 | c.1169A > T (p.N390I) | Mild ID | + | + | + | Non-ambulatory | + | At 3 years: bilateral hyperintensity of the globus pallidus and white matter abnormalities At 9 years: eye-of-the-tiger sign |
| 2 | M | 7 | 13 | c.1561G > A (p.G521R) c.906-1G > A | Mild ID | + | − | − | Ataxic gait | + | Eye-of-the-tiger sign |
| 3 | F | 33 | 36 | c.617G > A p.(G206D) c.906-1G > A | Mild ID | + | − | + | Ataxic gait | + | Eye-of-the-tiger sign |
| 4 | M | 38 | 18 | c.1169A > T (p.N390I) | Dementia | + | + | + | Non-ambulatory | + | Bilateral hypointensity of the globus pallidus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giuliano, M.; Borgione, E.; Lo Giudice, M.; Di Blasi, F.D.; Santa Paola, S.; Vitello, G.A.; Elia, M.; Russo, R.; Romano, C.; Scuderi, C. Focus on Clinical and Genetic Aspects of PKAN Through the Description of New Patients. Genes 2025, 16, 1008. https://doi.org/10.3390/genes16091008
Giuliano M, Borgione E, Lo Giudice M, Di Blasi FD, Santa Paola S, Vitello GA, Elia M, Russo R, Romano C, Scuderi C. Focus on Clinical and Genetic Aspects of PKAN Through the Description of New Patients. Genes. 2025; 16(9):1008. https://doi.org/10.3390/genes16091008
Chicago/Turabian StyleGiuliano, Marika, Eugenia Borgione, Mariangela Lo Giudice, Francesco Domenico Di Blasi, Sandro Santa Paola, Girolamo Aurelio Vitello, Maurizio Elia, Roberto Russo, Corrado Romano, and Carmela Scuderi. 2025. "Focus on Clinical and Genetic Aspects of PKAN Through the Description of New Patients" Genes 16, no. 9: 1008. https://doi.org/10.3390/genes16091008
APA StyleGiuliano, M., Borgione, E., Lo Giudice, M., Di Blasi, F. D., Santa Paola, S., Vitello, G. A., Elia, M., Russo, R., Romano, C., & Scuderi, C. (2025). Focus on Clinical and Genetic Aspects of PKAN Through the Description of New Patients. Genes, 16(9), 1008. https://doi.org/10.3390/genes16091008










