Extinction of Contextual Fear Memory and Passive Avoidance Memory and Subsequent Anxiety-like and Depressive-like Behavior of A53T and A53T-L444P Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
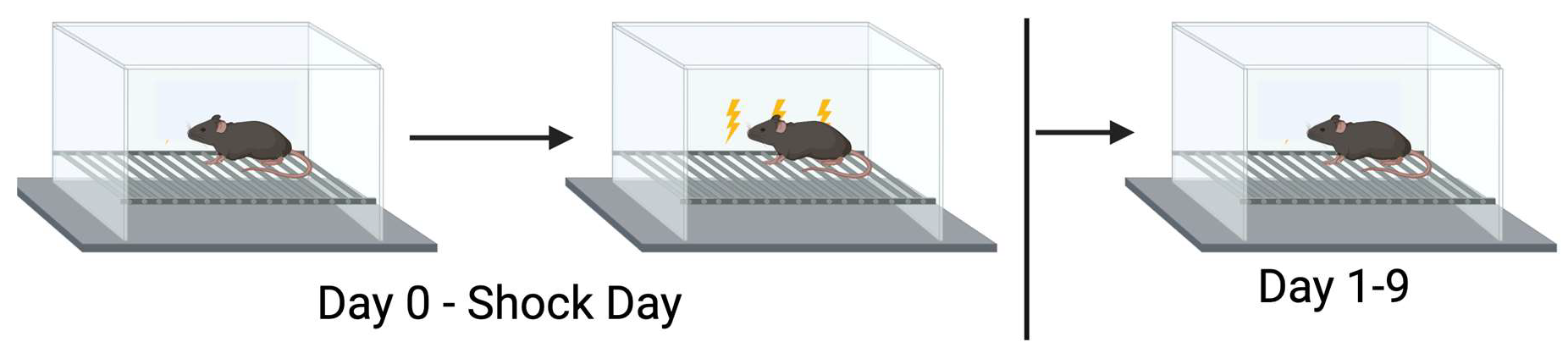
2.2. Extinction of Contextual Fear
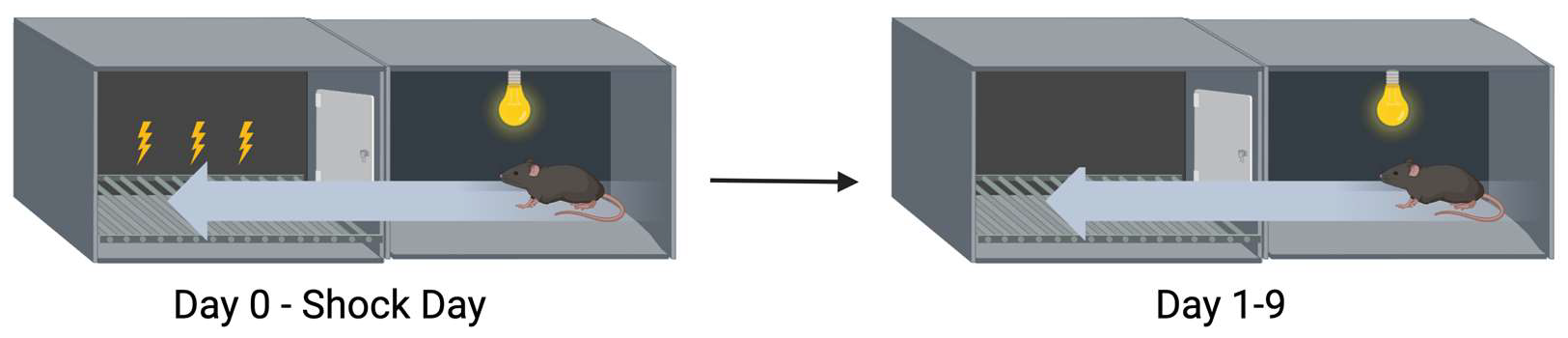
2.3. Extinction of Passive Avoidance Memory
2.4. Open Field Test
2.5. Forced Swim Test
2.6. Phospoyrylated Tau Levels
2.7. Statistical Analyses
3. Results
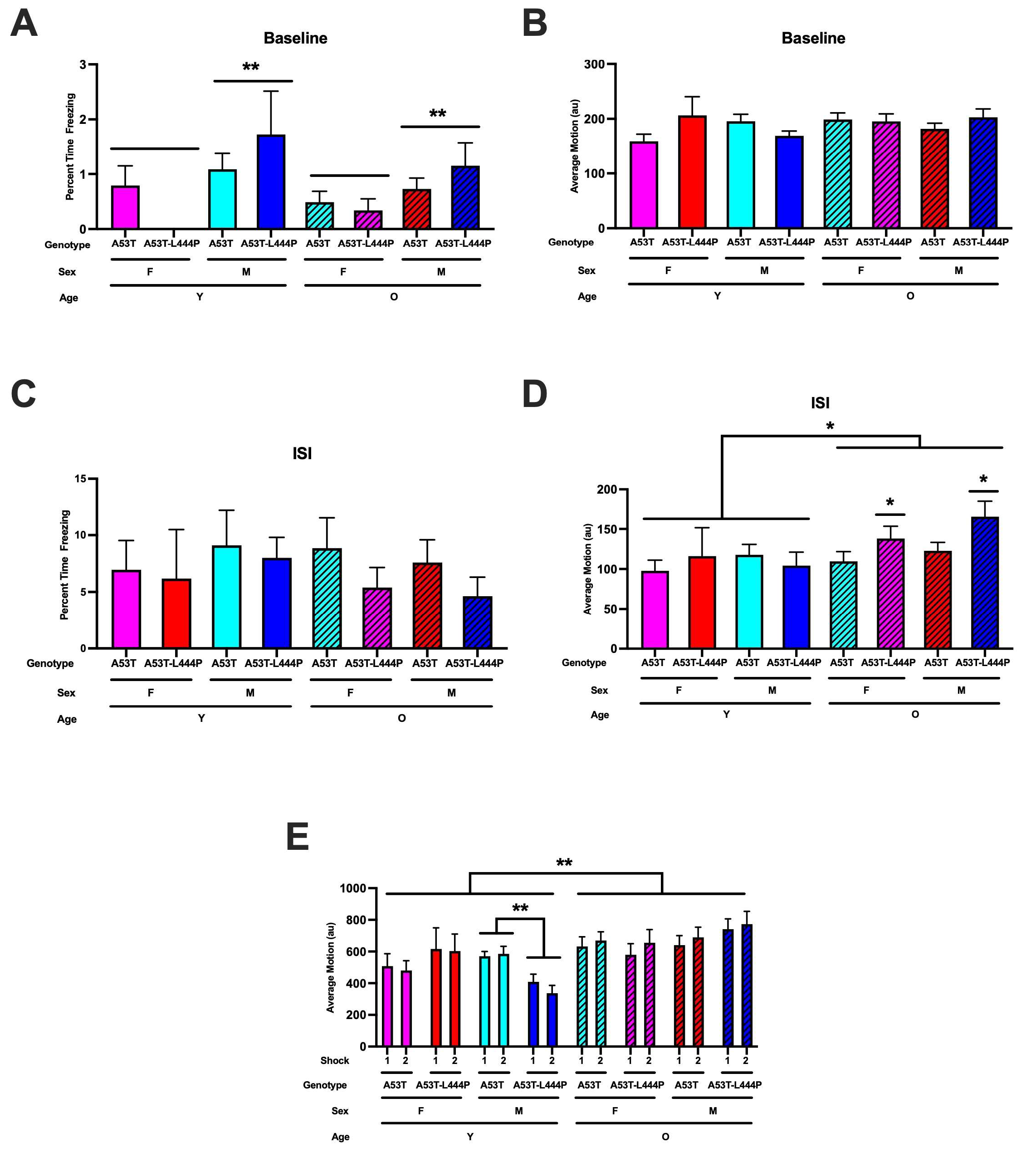
3.1. Contextual Fear Learning
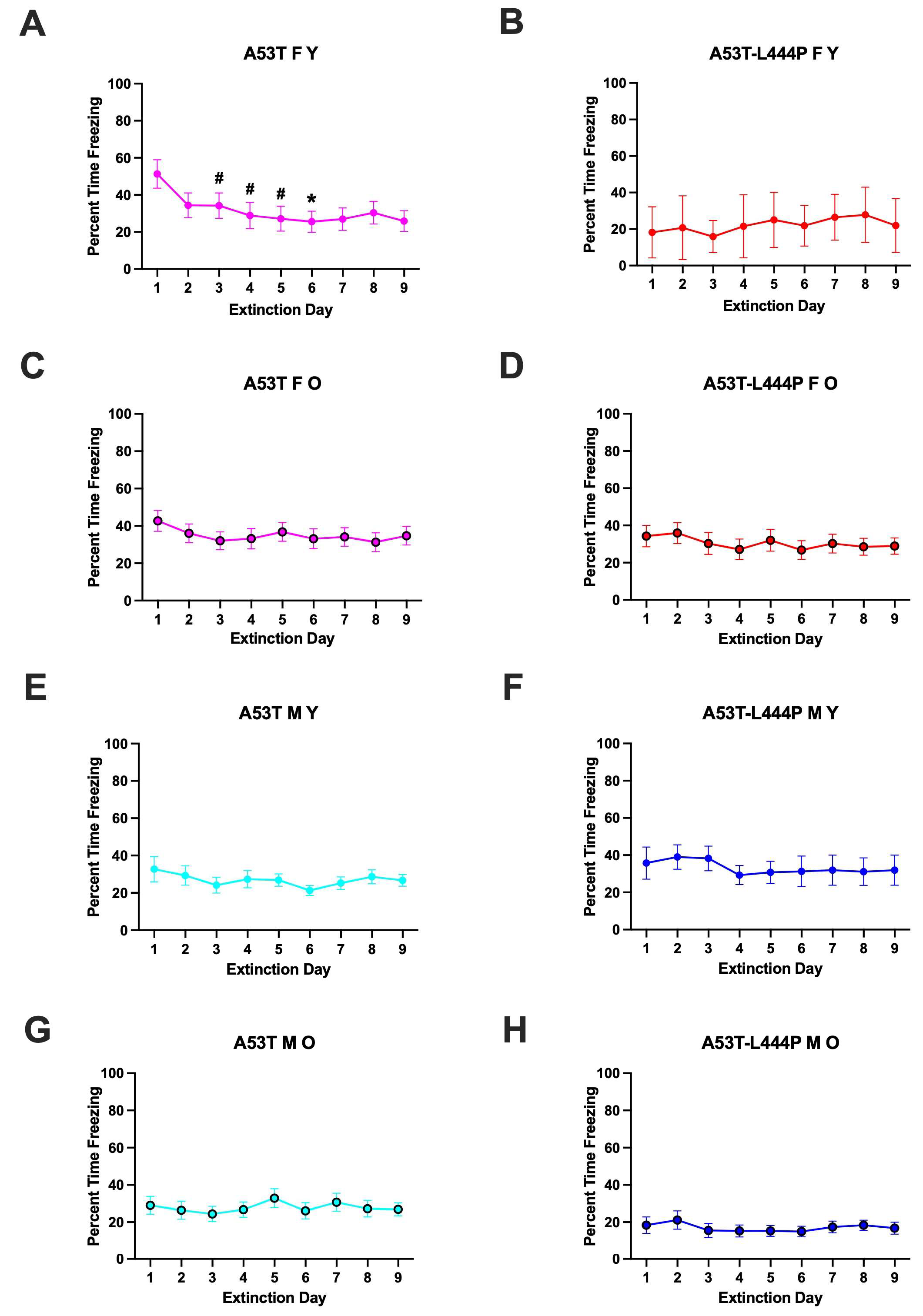
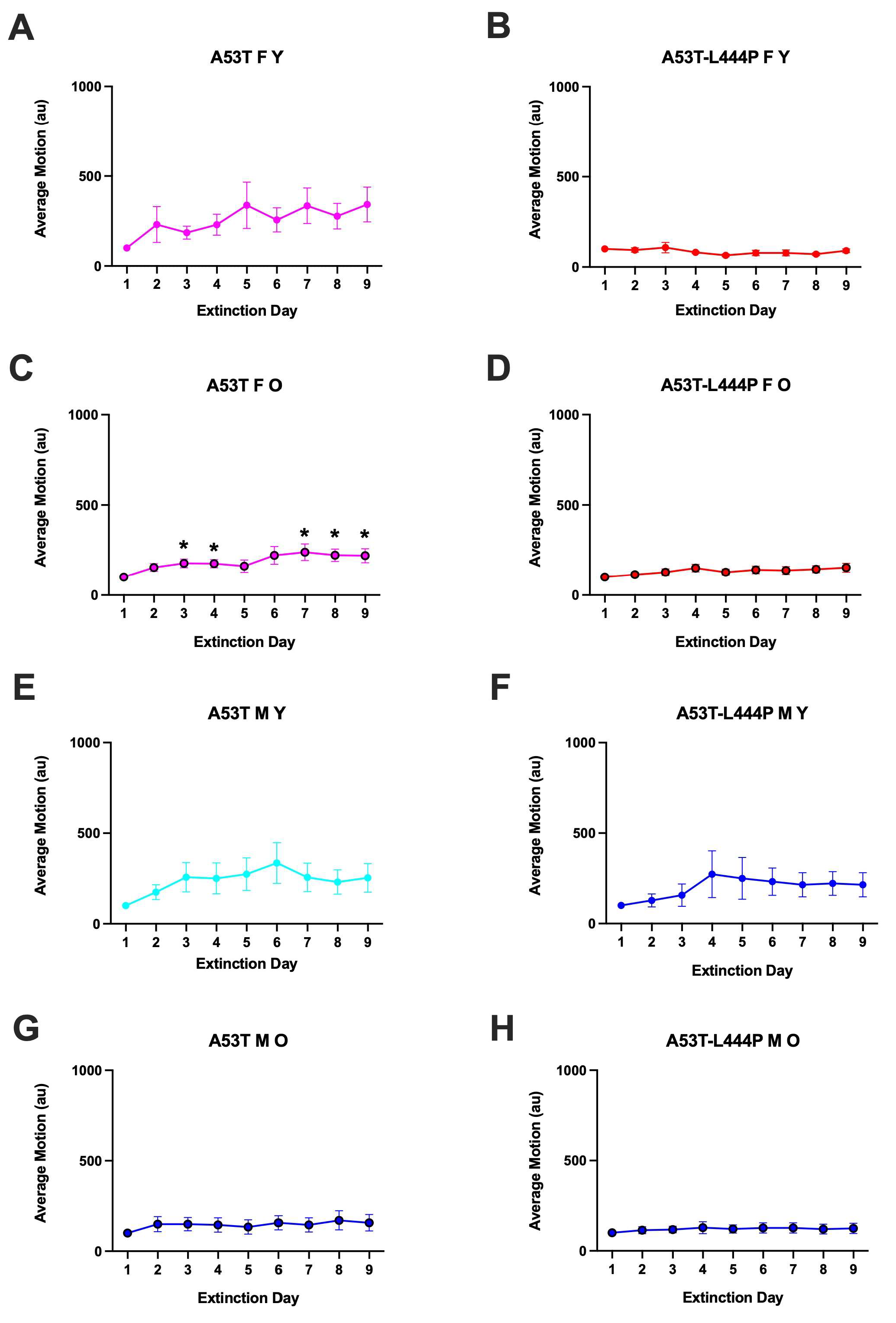
3.2. Extinction of Contextual Fear Memory
3.3. Passive Avoidance Learning
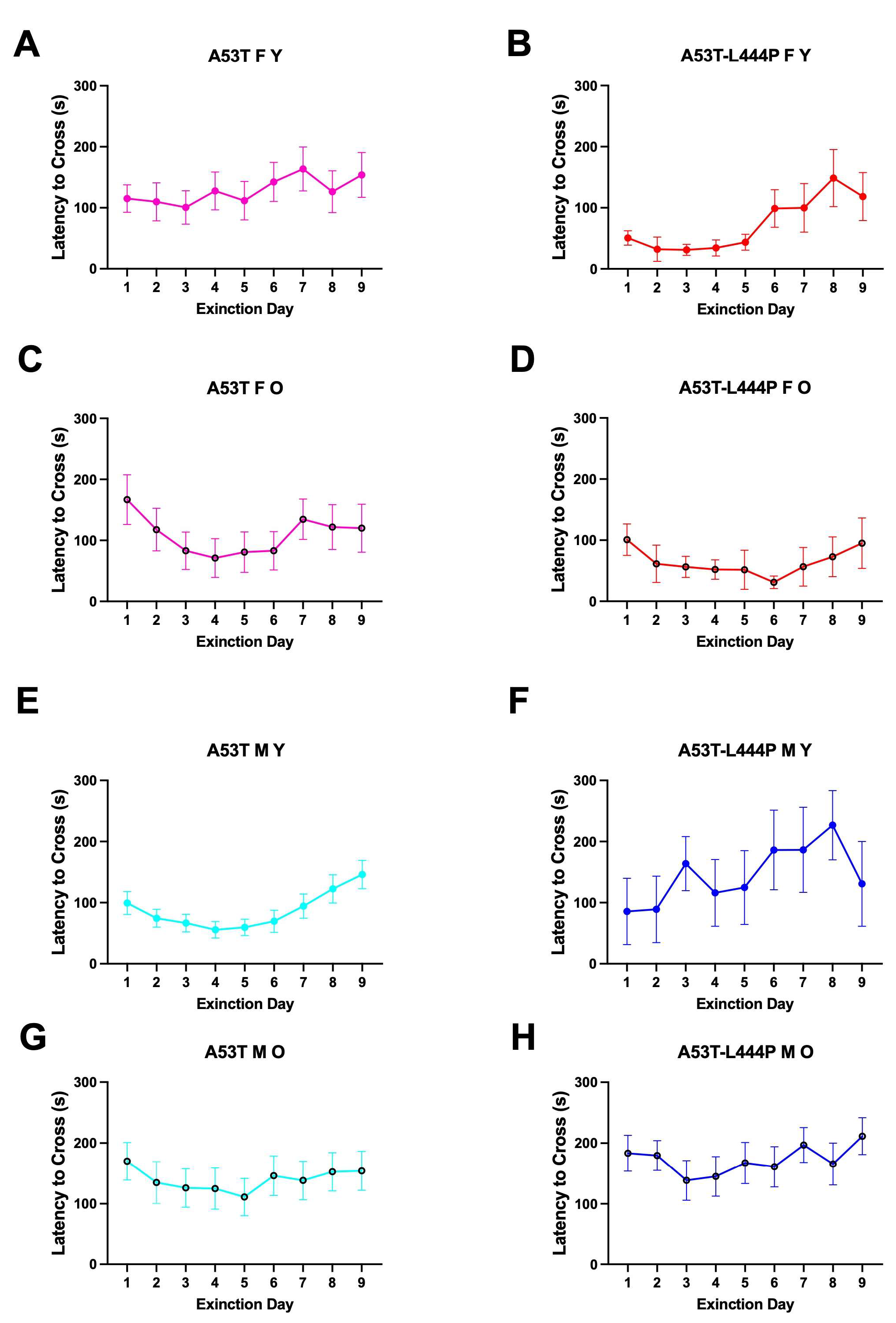
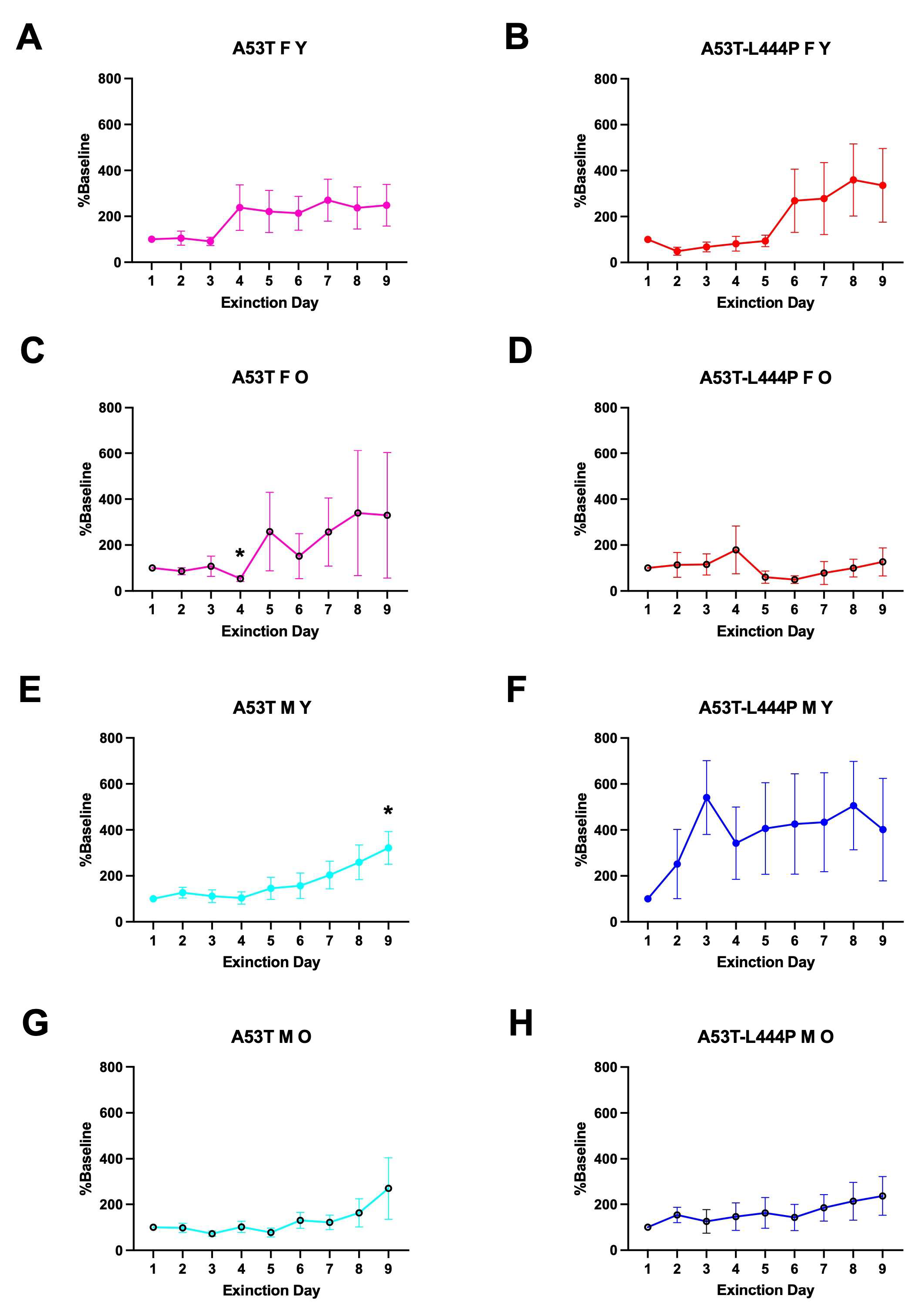
3.4. Extinction of Passive Avoidance Memory
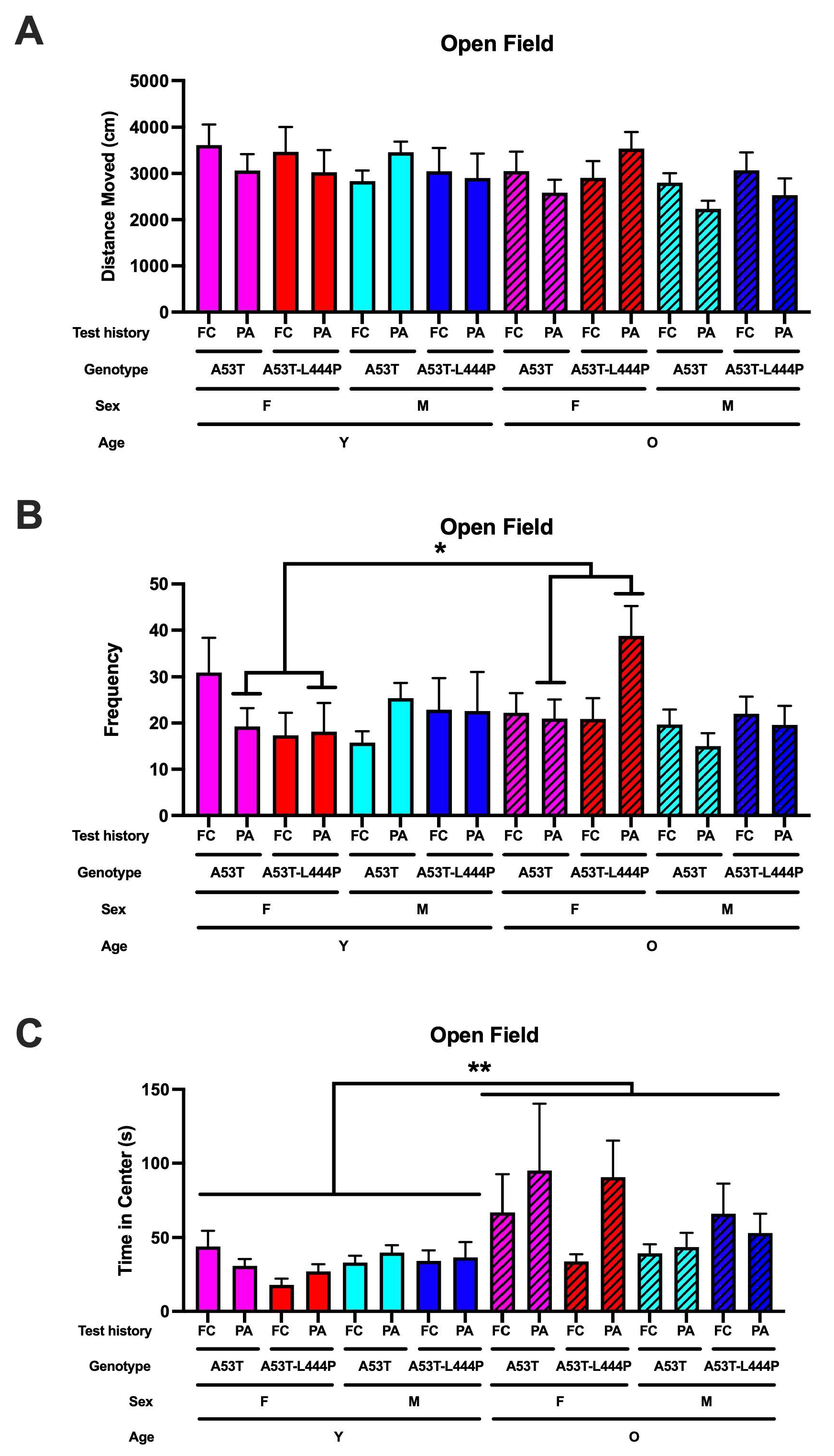
3.5. Performance in the Open Field
3.6. Performance in the Forced Swim Test
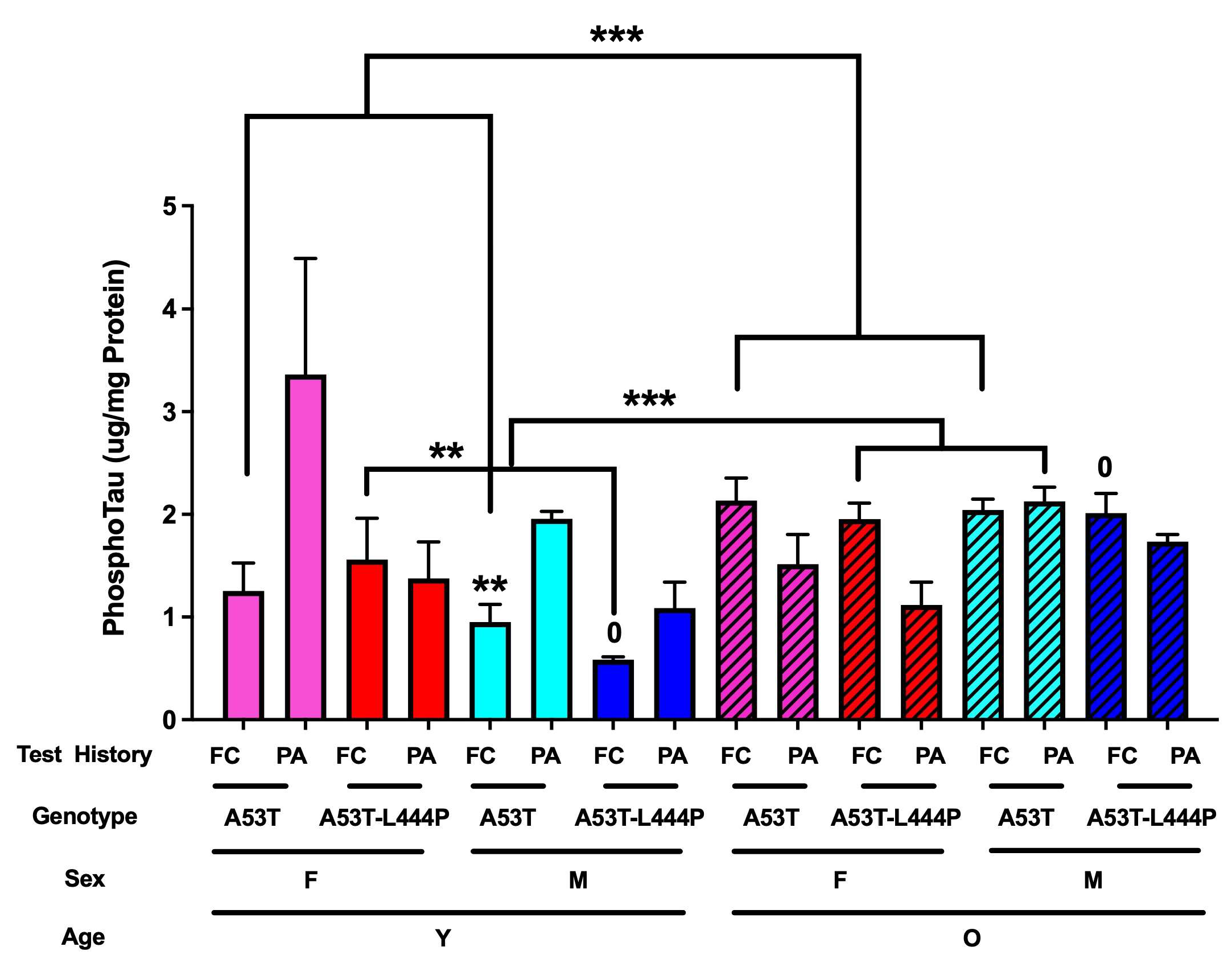
3.7. Phosphorylated Tau Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Caviness, J.N.; Driver-Dunckley, E.; Connor, D.J.; Sabbagh, M.N.; Hentz, J.G.; Noble, B.; Evidente, V.G.; Shill, H.A.; Adler, C.H. Defining mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2007, 22, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Palavra, N.C.; Naismith, S.L.; Lewis, S.J. Mild cognitive impairment in Parkinson’s disease: A review of current concepts. Neurol. Res. Int. 2013, 2013, 576091. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, I.; Taylor, S.F.; Amdur, R.; Jung, T.D.; Chamberlain, K.R.; Minoshima, S.; A Koeppe, R.; Fig, L.M. Brain activation in PTSD in response to trauma-related stimuli. Biol. Psychiatry 1999, 45, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, I.; Abelson, J.L. Abelson, Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Neuron 2016, 92, 14–30. [Google Scholar] [CrossRef]
- Hofmann, S.G. Cognitive processes during fear acquisition and extinction in animals and humans: Implications for exposure therapy of anxiety disorders. Clin. Psychol. Rev. 2008, 28, 199–210. [Google Scholar] [CrossRef]
- Lokshina, Y.; Sheynin, J.; Vogt, G.S.; Liberzon, I. Fear Extinction Learning in Posttraumatic Stress Disorder. In Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2023; Volume 64, pp. 257–272. [Google Scholar]
- Salkovskis, P.M.; Clark, D.M.; Hackmann, A.; Wells, A.; Gelder, M.G. An experimental investigation of the role of safety-seeking behaviours in the maintenance of panic disorder with agoraphobia. Behav. Res. Ther. 1999, 37, 559–574. [Google Scholar] [CrossRef]
- LeDoux, J.E.; Gorman, J.M. A call to action: Overcoming anxiety through active coping. Am. J. Psychiatry 2001, 158, 1953–1955. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Hay, A.C. Rethinking Avoidance: Toward a Balanced Approach to Avoidance in Treating Anxiety Disorders. J. Anxiety Disord. 2018, 55, 14–21. [Google Scholar] [CrossRef]
- Milosevic, I.; Radomsky, A. Keep your eye on the target: Safety behavior reduces targeted threat beliefs following a behavioral experiment. Cogn. Ther. Res. 2013, 37, 557–571. [Google Scholar] [CrossRef]
- Rachman, S.; Radomsky, A.S.; Shafran, R. Safety behaviour: A reconsideration. Behav. Res. Ther. 2008, 46, 163–173. [Google Scholar] [CrossRef]
- Taylor, C.T.; Alden, L.E. To see ourselves as others see us: An experimental integration of the intra and interpersonal consequences of self-protection in social anxiety disorder. J. Abnorm. Psychol. 2011, 120, 129–141. [Google Scholar] [CrossRef]
- Telch, M.; Lancaster, C. Exposure therapy: Rethinking the model, refining the method. In Is There Room for Safety Behaviors in Exposure Therapy for Anxiety Disorders; Neudeck, P., Wittchen, H.U., Eds.; Springer: New York, NY, USA, 2012; pp. 313–334. [Google Scholar]
- Barer, Y.; Chodick, G.; Chodick, N.G.; Gurevich, T. Risk of Parkinson Disease Among Adults With vs Without Posttraumatic Stress Disorder. JAMA Netw. Open 2022, 5, e2225445. [Google Scholar] [CrossRef]
- Chan, Y.-L.E.; Bai, Y.-M.; Hsu, J.-W.; Huang, K.-L.; Su, T.-P.; Li, C.-T.; Lin, W.-C.; Pan, T.-L.; Chen, T.-J.; Tsai, S.-J.; et al. Post-traumatic stress disorder and risk of Parkinson Disease: A nationwide longitudinal study. Am. J. Geriatr. Psychiatry 2017, 25, 917–923. [Google Scholar] [CrossRef]
- White, D.L.; Kunik, M.E.; Yu, H.; Lin, H.L.; Richardson, P.A.; Moore, S.; Sarwar, A.I.; Marsh, L.; Jorge, R.E. Post-Traumatic Stress Disorder is Associated with further Increased Parkinson’s Disease Risk in Veterans with Traumatic Brain Injury. Ann. Neurol. 2020, 88, 33–41. [Google Scholar] [CrossRef]
- Olff, M. Sex and gender differences in post-traumatic stress disorder: An update. Eur. J. Psychotraumatol. 2017, 8 (Suppl. S4), 1351204. [Google Scholar] [CrossRef]
- Cerri, S.; Mus, L.; Blandini, F. Blandinin, Parkinson’s Disease in Women and Men: What’s the Difference? J. Park. Dis. 2019, 9, 501–515. [Google Scholar]
- Nievergelt, C.M.; Maihofer, A.X.; Klengel, T.; Atkinson, E.G.; Chen, C.-Y.; Choi, K.W.; Coleman, J.R.I.; Dalvie, S.; Duncan, L.E.; Gelernter, J.; et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat. Commun. 2019, 10, 4558. [Google Scholar] [CrossRef] [PubMed]
- Malek, N.; Weil, R.S.; Bresner, C.; Lawton, M.A.; Grosset, K.A.; Tan, M.; Bajaj, N.; Barker, R.A.; Burn, D.J.; Foltynie, T.; et al. Features of GBA-associated Parkinson’s disease at presentation in the UK Tracking Parkinson’s study. J. Neurol. Neurosurg. Psychiatry 2018, 89, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Migdalska-Richards, A.; Schapira, A.H.V. The relationship between glucocerebrosidase mutations and Parkinson disease. J. Neurochem. 2016, 139 (Suppl. S1), 77–90. [Google Scholar] [CrossRef]
- Avenali, M.; Blandini, F.; Cerri, S. Cerri, Glucocerebrosidase defects as a major risk factor for Parkinson’s disease. Front. Aging Neurosci. 2020, 12, 97. [Google Scholar] [CrossRef]
- Yun, S.P.; Kim, D.; Kim, S.; Kim, S.; Karuppagounder, S.S.; Kwon, S.-H.; Lee, S.; Kam, T.-I.; Lee, S.; Ham, S.; et al. α-Synuclein accumulation and GBA deficiency due to L444P GBA mutation contributes to MPTP-induced parkinsonism. Mol. Neurodegener. 2018, 13, 1. [Google Scholar] [CrossRef]
- Fishbein, I.; Kuo, Y.-M.; Giasson, B.I.; Nussbaum, R.L. Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a Gaucher mutation. Brain 2014, 137, 3235–3247. [Google Scholar] [CrossRef]
- Chaklai, A.; O’neil, A.; Goel, S.; Margolies, N.; Krenik, D.; Perez, R.; Kessler, K.; Staltontall, E.; Yoon, H.K.; Pantoja, M.; et al. Effects of Paraquat, Dextran Sulfate Sodium, and Irradiation on Behavioral and Cognitive Performance and the Gut Microbiome in A53T and A53T-L444P Mice. Genes 2024, 15, 282. [Google Scholar] [CrossRef]
- Zuj, D.V.; Palmer, M.A.; Hsu, C.-M.K.; Nicholson, E.L.; Cushing, P.J.; Gray, K.E.; Felmingham, K.L. Impaired Fear Extinction Associated with Ptsd Increases with Hours-since-Waking. Depress. Anxiety 2016, 33, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Briscione, M.A.; Jovanovic, T.; Norrholm, S.D. Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Front. Psychiatry 2014, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, M.S.; Poulos, A.M. The neuroscience of mammalian associative learning. Annu. Rev. Psychol. 2005, 56, 207–234. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.A.; LeDoux, J.E. Contribution of ventrolateral prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Neurobiol. Learn. Mem. 1999, 72, 244–251. [Google Scholar] [CrossRef]
- Milad, M.R.; Orr, S.P.; Pitman, R.K.; Rauch, S.L. Context modulation of memory for fear extinction in humans. Psychophysiology 2005, 42, 456–464. [Google Scholar] [CrossRef]
- Milad, M.R.; Igoe, S.; Orr, S.P. Fear conditioning in rodents and humans. In Neuromethods: Animal Models of Behavioral Analysis; Humana Press: Totowa, NJ, USA, 2011; Volume 50, pp. 111–132. [Google Scholar]
- Milad, M.R.; Quirk, G.J. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu. Rev. Psychol. 2012, 63, 129–151. [Google Scholar] [CrossRef]
- Rosen, J.B.; Schulkin, J. From normal fear to pathological anxiety. Psychol. Rev. 1998, 105, 325–350. [Google Scholar] [CrossRef]
- Rauch, S.L.; Shin, L.M.; Phelps, E.A. Neurocircuitry models of posttraumatic stress disorder and extinction: Human neuroimaging research--past, present, and future. Biological. Psychiatry 2006, 60, 376–382. [Google Scholar] [CrossRef]
- Cheng, W.H.; Martens, K.M.; Bashir, A.; Cheung, H.; Stukas, S.; Gibbs, E.; Namjoshi, D.R.; Button, E.B.; Wilkinson, A.; Barron, C.J.; et al. CHIMERA repetitive mild traumatic brain injury induces chronic behavioural and neuropathological phenotypes in wild-type and APP/PS1 mice. Alzheimer’s Res. Ther. 2019, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Whitcomb, D.J.; Jo, J.; Regan, P.; Piers, T.; Heo, S.; Brown, C.; Hashikawa, T.; Murayama, M.; Seok, H.; et al. Microtubule-associated protein tau is essential for long-term depression in the hippocampus. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130144. [Google Scholar] [CrossRef] [PubMed]
- Digma, L.; Madsen, J.; Reas, E.; Dale, A.; Brewer, J.; Banks, S. Tau and atrophy: Domain-specific relationships with cognition. Alzheimer’s Res. Ther. 2019, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, S.; Meng, Z.; Ye, Y.; Shan, G.; Wang, X.; Zhao, X.; Jin, Y. Tau protein plays a role in the mechanism of cognitive disorders induced by anesthetic drugs. Front. Neurosci. 2023, 17, 1145318. [Google Scholar] [CrossRef]
- Taylor, T.; Caudle, W.; Shepherd, K.; Noorian, A.; Jackson, C.; Iuvone, P.M.; Weinschenker, D.; Greene, J.; Miller, G. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J. Neurosci. 2009, 29, 8103–8113. [Google Scholar] [CrossRef]
- Raheel, K.; Deegan, G.; Giulio, I.; Cash, D.J.; Ilic, K.; Gnoni, V.; Ch’audhuri, K.; Drakatos, P.; Moran, R.; Rosenzweig, I. Sex differences in alpha-synucleinopathies: A systematic review. Front. Neurol. 2023, 14, 1204104. [Google Scholar] [CrossRef]
- Caranci, G.; Piscopo, P.; Rivabene, R.; Traficante, A.; Riozzi, B.; Castellano, A.E.; Ruggieri, S.; Vanacore, N.; Confaloni, A. Gender differences in Parkinson’s disease: Focus on plasma α-synuclein. J. Neural Transm. 2013, 120, 1209–1215. [Google Scholar] [CrossRef]
- Saleem, T.H.; Hassan, M.H.; Ahmed, A.E.-A.; Sayed, A.A.; Mohamed, N.A.; Elsayh, K.I.; El-Ebidi, A.M.; Mohammed, N.B. Clinical and genetic assessment of pediatric patients with Gaucher’s disease in Upper Egypt. Egypt. J. Med. Hum. Genet. 2017, 18, 249–255. [Google Scholar] [CrossRef]
- Giraldo, P.; Pérez-López, J.; Núñez, R.; de la Puebla, R.F.; Luño, E.; Saura-Grau, S.; Bureo, J.C.; Plaza, S.; de la Serna, J. Patients with type 1 Gaucher disease in Spain: A cross-sectional evaluation of health status. Blood Cells Mol. Dis. 2016, 56, 23–30. [Google Scholar] [CrossRef]
- Olsen, R.H.J.; Agam, M.; Davis, M.J.; Raber, J. ApoE isoform-dependent deficits in extinction of contextual fear conditioning. Genes Brain Behav. 2012, 7, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Paylor, R.; Tracy, R.; Wehner, J.; Rudy, J.W. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav. Neurosci. 1994, 108, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Gerlai, R. Contextual learning and cue association in fear conditioning in mice: A strain comparison and a lesion study. Behav. Brain Res. 1998, 95, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Behavioral phenotyping of transgenic and knockout mice: Experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999, 835, 18–26. [Google Scholar] [CrossRef]
- Stiedl, O.; Radulovic, J.; Lohmann, R.; Birkenfeld, K.; Palve, M.; Kammermeier, J.; Sananbenesi, F.; Spiess, J. Strain and substrain differences in context- and tone-dependent fear conditioning of inbred mice. Behav. Brain Res. 1999, 104, 1–12. [Google Scholar] [CrossRef]
- Baarendse, P.J.; van Grootheest, G.; Jansen, R.F.; Pieneman, A.W.; Ögren, S.O.; Verhage, M.; Stiedl, O. Differential involvement of the dorsal hippocampus in passive avoidance in C57bl/6J and DBA/2J mice. Hippocampus 2007, 18, 11–19. [Google Scholar] [CrossRef]
- Chizhova, N.D.; Smirnova, K.V.; Dubrovina, N.I.; Lipina, T.V.; Amstislavskaya, T.G. Sex- and strain-related differences in the extinction of a conditioned passive avoidance reaction in DISC-L100P and C57BL/6 mice. Neurosci. Behav. Physiol. 2023, 53, 14781482. [Google Scholar] [CrossRef]
- Chikatimalla, R.; Dasaradhan, T.; Koneti, J.; Cherukuri, S.; Kalluru, R.; Gadde, S. Depression in Parkinson’s Disease: A Narrative Review. Cureus 2022, 14, e27750. [Google Scholar] [CrossRef]
- Branchi, I.; D’ANdrea, I.; Armida, M.; Cassano, T.; Pèzzola, A.; Potenza, R.L.; Morgese, M.G.; Popoli, P.; Alleva, E. Nonmotor symptoms in Parkinson’s disease: Investigating early-phase onset of behavioral dysfunction in the 6-hydroxydopamine-lesioned rat model. J. Neurosci. Res. 2008, 86, 2050–2061. [Google Scholar] [CrossRef]
- Gradinaru, V.; Mogri, M.; Thompson, K.R.; Henderson, J.M.; Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 2009, 324, 354–359. [Google Scholar] [CrossRef]
- Gotham, A.M.; Brown, R.G.; Marsden, C.D. Marsden, Depression in Parkinson’s disease: A quantitative and qualitative analysis. J. Neurol. Neurosurg. Psychiatry 1986, 49, 381–389. [Google Scholar] [CrossRef]
- Lado, W.; Ham, A.; Li, H.; Zhang, H.; Chang, A.Y.; Sardi, S.P.; Alcalay, R.N.; Arancio, O.; Przedborski, S.; Tang, G. Synaptic and cognitive impairment associated with L444P heterozygous glucocerebrosidase mutation. Brain 2025, 148, 1621–1638. [Google Scholar] [CrossRef]
- Granek, Z.; Barczuk, J.; Siwecka, N.; Rozpędek-Kamińska, W.; Kucharska, E.; Majsterek, I. GBA1 Gene Mutations in α-Synucleinopathies—Molecular Mechanisms Underlying Pathology and Their Clinical Significance. Int. J. Mol. Sci. 2023, 24, 2044. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunnell, E.; Saltonstall, E.; Pederson, A.; Baxter, C.; Ramicciotti, E.; Robinson, N.; Sandholm, P.; O′Niel, A.; Raber, J. Extinction of Contextual Fear Memory and Passive Avoidance Memory and Subsequent Anxiety-like and Depressive-like Behavior of A53T and A53T-L444P Mice. Genes 2025, 16, 1004. https://doi.org/10.3390/genes16091004
Bunnell E, Saltonstall E, Pederson A, Baxter C, Ramicciotti E, Robinson N, Sandholm P, O′Niel A, Raber J. Extinction of Contextual Fear Memory and Passive Avoidance Memory and Subsequent Anxiety-like and Depressive-like Behavior of A53T and A53T-L444P Mice. Genes. 2025; 16(9):1004. https://doi.org/10.3390/genes16091004
Chicago/Turabian StyleBunnell, Emily, Elizabeth Saltonstall, Alexandra Pederson, Charlie Baxter, Elia Ramicciotti, Naomi Robinson, Phoebe Sandholm, Abigail O′Niel, and Jacob Raber. 2025. "Extinction of Contextual Fear Memory and Passive Avoidance Memory and Subsequent Anxiety-like and Depressive-like Behavior of A53T and A53T-L444P Mice" Genes 16, no. 9: 1004. https://doi.org/10.3390/genes16091004
APA StyleBunnell, E., Saltonstall, E., Pederson, A., Baxter, C., Ramicciotti, E., Robinson, N., Sandholm, P., O′Niel, A., & Raber, J. (2025). Extinction of Contextual Fear Memory and Passive Avoidance Memory and Subsequent Anxiety-like and Depressive-like Behavior of A53T and A53T-L444P Mice. Genes, 16(9), 1004. https://doi.org/10.3390/genes16091004





