Transcriptional Regulation of CYP2E1: Promoter Methylation in In Vitro Models and Human Liver Disease Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. CYP2E1 DNA Methylation in Gastrointestinal Tract and Liver
2.2. CYP2E1 DNA Methylation in Caco-2 and HepG2 Cells
2.3. CYP2E1 DNA Methylation in Liver Disease
3. Results
3.1. CYP2E1 DNA Methylation in Gastrointestinal Tract
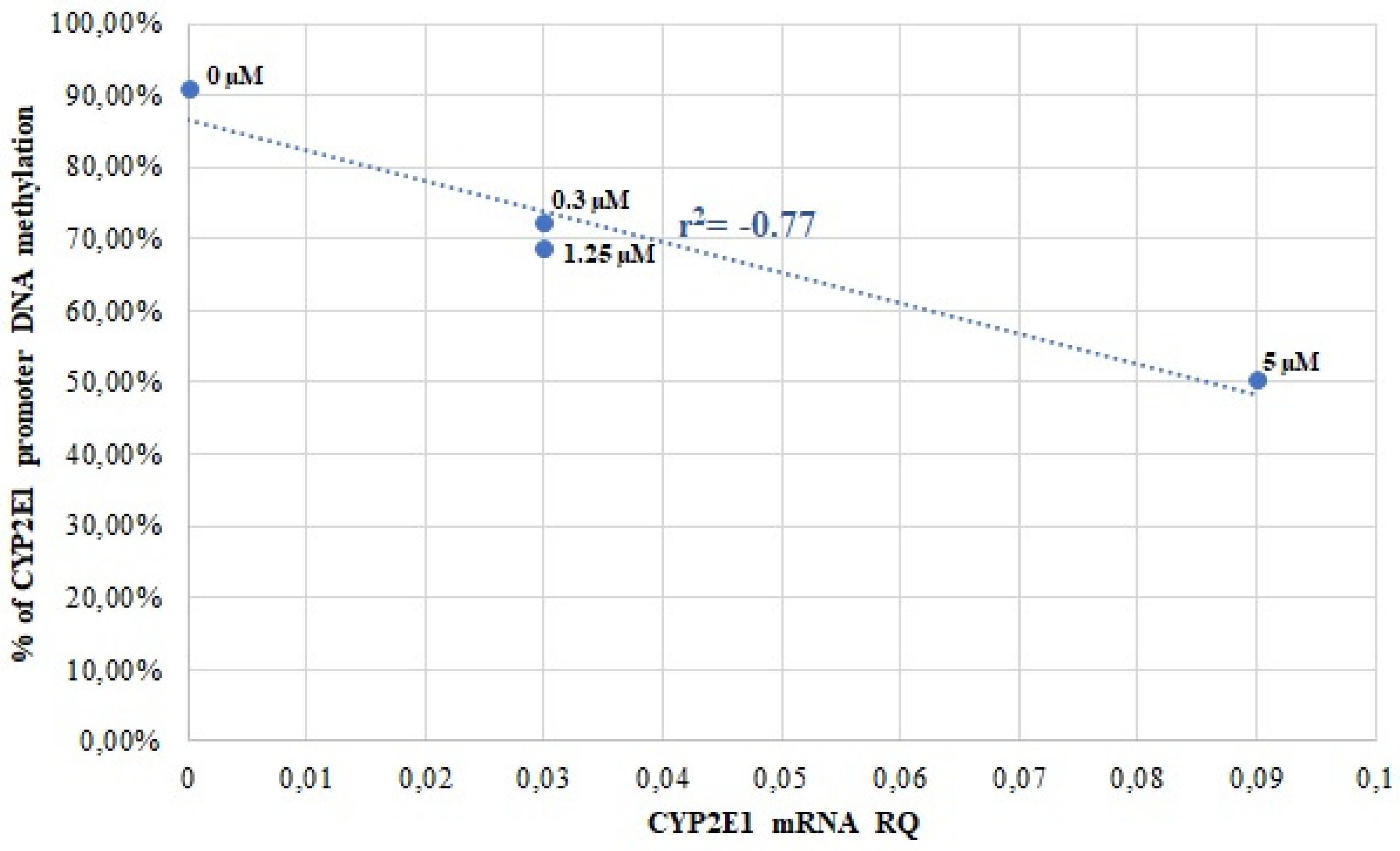
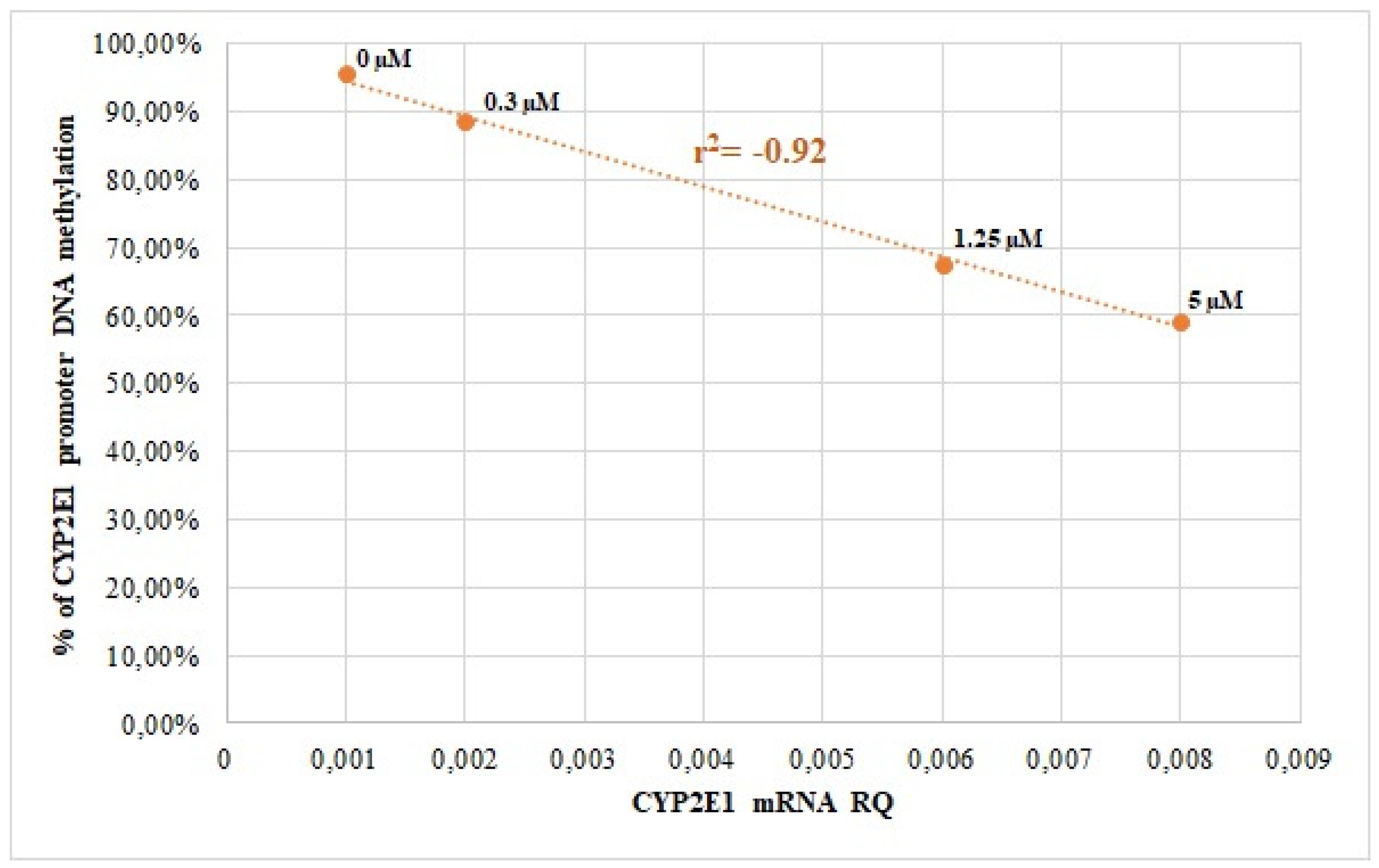
3.2. CYP2E1 DNA Methylation in Caco-2 and HepG2 Cells
3.3. CYP2E1 DNA Methylation in Liver Disease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCV | Hepatitis C Virus |
| PBC | Primary Biliary Cholangitis |
| PSC | Primary Sclerosing Cholangitis |
| ALD | Alcoholic Liver Disease |
| WD | Wilson’s Disease |
| AIH | Autoimmune Hepatitis |
| HCC | Hepatocellular Carcinoma |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
References
- Bommarito, P.A.; Fry, R.C. Chapter 2-1—The Role of DNA Methylation in Gene Regulation. In Toxicoepigenetics; McCullough, S.D., Dolinoy, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 127–151. [Google Scholar] [CrossRef]
- Riggs, A.D. X inactivation, differentiation, and DNA methylation. Cytogenet. Cell Genet. 2008, 14, 9–25. [Google Scholar] [CrossRef]
- Holliday, R.; Pugh, J.E. DNA Modification Mechanisms and Gene Activity During Development: Developmental clocks may depend on the enzymic modification of specific bases in repeated DNA sequences. Science 1975, 187, 226–232. [Google Scholar] [CrossRef]
- Suzuki, M.M.; Bird, A. DNA methylation landscapes: Provocative insights from epigenomics. Nat. Rev. Genet. 2008, 9, 465–476. [Google Scholar] [CrossRef]
- Saxonov, S.; Berg, P.; Brutlag, D.L. A genome-wide analysis of CpG dinucleotides in the human genome distinguishes two distinct classes of promoters. Proc. Natl. Acad. Sci. USA 2006, 103, 1412–1417. [Google Scholar] [CrossRef]
- Tate, P.H.; Bird, A.P. Effects of DNA methylation on DNA-binding proteins and gene expression. Curr. Opin. Genet. Dev. 1993, 3, 226–231. [Google Scholar] [CrossRef]
- Stols-Gonçalves, D.; Meijnikman, A.S.; Tristão, L.S.; Santos, C.L.D.; Denswil, N.P.; Verheij, J.; Bernardo, W.M.; Nieuwdorp, M. Metabolic Dysfunction-Associated Steatotic Liver Disease and Alcohol-Associated Liver Disease: Liver DNA Methylation Analysis—A Systematic Review. Cells 2024, 13, 1893. [Google Scholar] [CrossRef] [PubMed]
- Hlady, R.A.; Zhao, X.; El Khoury, L.Y.; Wagner, R.T.; Luna, A.; Pham, K.; Pyrosopoulos, N.T.; Jain, D.; Wang, L.; Liu, C.; et al. Epigenetic heterogeneity hotspots in human liver disease progression. Hepatology 2025, 81, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Heerboth, S.; Lapinska, K.; Snyder, N.; Leary, M.; Rollinson, S.; Sarkar, S. Use of epigenetic drugs in disease: An overview. Genet. Epigenetics 2014, 1, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Reactions and significance of cytochrome P-450 enzymes. J. Biol. Chem. 1991, 266, 10019–10022. [Google Scholar] [CrossRef]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free. Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S. Microsomal Ethanol-Oxidizing System (MEOS): The First 30 Years (1968–1998)–A Review. Alcohol. Clin. Exp. Res. 1999, 23, 991–1007. [Google Scholar] [CrossRef]
- Botto, F.; Seree, E.; El Khyari, S.; de Sousa, G.; Massacrier, A.; Placidi, M.; Cau, P.; Pellet, W.; Rahmani, R.; Barra, Y. Tissue-specific expression and methylation of the human CYP2E1 gene. Biochem. Pharmacol. 1994, 48, 1095–1103. [Google Scholar] [CrossRef]
- Neafsey, P.; Ginsberg, G.; Hattis, D.; Johns, D.O.; Guyton, K.Z.; Sonawane, B. Genetic Polymorphism in CYP2E1: Population Distribution of CYP2E1 Activity. J. Toxicol. Environ. Health Part B 2009, 12, 362–388. [Google Scholar] [CrossRef] [PubMed]
- Naselli, F.; Catanzaro, I.; Bellavia, D.; Perez, A.; Sposito, L.; Caradonna, F. Role and importance of polymorphisms with respect to DNA methylation for the expression of CYP2E1 enzyme. Gene 2014, 536, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Xiao, Y.; Du, J.; Zhang, X.; Chen, M.; Wu, B. The nuclear receptor REV-ERBα regulates CYP2E1 expression and acetaminophen hepatotoxicity. Xenobiotica 2022, 52, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Cederbaum, A.I. Cytochrome P450 2E1-dependent oxidant stress and upregulation of anti-oxidant defense in liver cells. J. Gastroenterol. Hepatol. 2006, 21, S22–S25. [Google Scholar] [CrossRef]
- Bansal, S.; Anandatheerthavarada, H.K.; Prabu, G.K.; Milne, G.L.; Martin, M.V.; Guengerich, F.P.; Avadhani, N.G. Human Cytochrome P450 2E1 Mutations That Alter Mitochondrial Targeting Efficiency and Susceptibility to Ethanol-induced Toxicity in Cellular Models. J. Biol. Chem. 2013, 288, 12627–12644. [Google Scholar] [CrossRef]
- Kronfol, M.M.; Jahr, F.M.; Dozmorov, M.G.; Phansalkar, P.S.; Xie, L.Y.; Aberg, K.A.; McRae, M.; Price, E.T.; Slattum, P.W.; Gerk, P.M.; et al. DNA methylation and histone acetylation changes to cytochrome P450 2E1 regulation in normal aging and impact on rates of drug metabolism in the liver. GeroScience 2020, 42, 819–832. [Google Scholar] [CrossRef]
- Dey, A. Cytochrome P450 2E1: Its Clinical Aspects and a Brief Perspective on the Current Research Scenario. In Cytochrome P450 2E1: Its Role in Disease and Drug Metabolism; Dey, A., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 1–104. [Google Scholar] [CrossRef]
- Franz, C.C.; Hildbrand, C.; Born, C.; Egger, S.; Rätz Bravo, A.E.; Krähenbühl, S. Dose adjustment in patients with liver cirrhosis: Impact on adverse drug reactions and hospitalizations. Eur. J. Clin. Pharmacol. 2013, 69, 1565–1573. [Google Scholar] [CrossRef][Green Version]
- Drozdzik, M.; Lapczuk-Romanska, J.; Wenzel, C.; Szelag-Pieniek, S.; Post, M.; Skalski, Ł.; Kurzawski, M.; Oswald, S. Gene Expression and Protein Abundance of Hepatic Drug Metabolizing Enzymes in Liver Pathology. Pharmaceutics 2021, 13, 1334. [Google Scholar] [CrossRef]
- Drozdzik, M.; Lapczuk-Romanska, J.; Wenzel, C.; Skalski, L.; Szeląg-Pieniek, S.; Post, M.; Parus, A.; Syczewska, M.; Kurzawski, M.; Oswald, S. Protein Abundance of Drug Metabolizing Enzymes in Human Hepatitis C Livers. Int. J. Mol. Sci. 2023, 24, 4543. [Google Scholar] [CrossRef] [PubMed]
- Szeląg-Pieniek, S.; Oswald, S.; Post, M.; Łapczuk-Romańska, J.; Droździk, M.; Kurzawski, M. Hepatic drug-metabolizing enzymes and drug transporters in Wilson’s disease patients with liver failure. Pharmacol. Rep. 2021, 73, 1427–1438. [Google Scholar] [CrossRef]
- Drozdzik, M.; Busch, D.; Lapczuk, J.; Müller, J.; Ostrowski, M.; Kurzawski, M.; Oswald, S. Protein Abundance of Clinically Relevant Drug-Metabolizing Enzymes in the Human Liver and Intestine: A Comparative Analysis in Paired Tissue Specimens. Clin. Pharmacol. Ther. 2018, 104, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Bonder, M.J.; Kasela, S.; Kals, M.; Tamm, R.; Lokk, K.; Barragan, I.; Buurman, W.A.; Deelen, P.; Greve, J.W.; Ivanov, M.; et al. Genetic and epigenetic regulation of gene expression in fetal and adult human livers. BMC Genom. 2014, 15, 860. [Google Scholar] [CrossRef]
- Park, H.J.; Choi, Y.J.; Kim, J.W.; Chun, H.S.; Im, I.; Yoon, S.; Han, Y.M.; Song, C.W.; Kim, H. Differences in the Epigenetic Regulation of Cytochrome P450 Genes between Human Embryonic Stem Cell-Derived Hepatocytes and Primary Hepatocytes. PLoS ONE 2015, 10, e0132992. [Google Scholar] [CrossRef]
- Vieira, I.; Sonnier, M.; Cresteil, T. Developmental Expression of CYP2E1 in the Human Liver. Eur. J. Biochem. 1996, 238, 476–483. [Google Scholar] [CrossRef]
- Gao, W.; Kondo, Y.; Shen, L.; Shimizu, Y.; Sano, T.; Yamao, K.; Natsume, A.; Goto, Y.; Ito, M.; Murakami, H.; et al. Variable DNA methylation patterns associated with progression of disease in hepatocellular carcinomas. Carcinogenesis 2008, 29, 1901–1910. [Google Scholar] [CrossRef]
- Zeybel, M.; Hardy, T.; Robinson, S.M.; Fox, C.; Anstee, Q.M.; Ness, T.; Masson, S.; Mathers, J.C.; French, J.; White, S.; et al. Differential DNA methylation of genes involved in fibrosis progression in non-alcoholic fatty liver disease and alcoholic liver disease. Clin. Epigenet. 2015, 7, 25. [Google Scholar] [CrossRef]
- Murphy, S.K.; Yang, H.; Moylan, C.A.; Pang, H.; Dellinger, A.; Abdelmalek, M.F.; Garrett, M.E.; Ashley–Koch, A.; Suzuki, A.; Tillmann, H.L.; et al. Relationship Between Methylome and Transcriptome in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2013, 145, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
| ALD n = 21 | HCV n = 58 | PBC n = 12 | PSC n = 7 | WD n = 8 | AIH n = 17 | CTRL n = 29 | |
|---|---|---|---|---|---|---|---|
| Sex (M/F) * | 11M, 10F | 30M, 28F | 1M, 11F | 6M, 1F | 5M, 3F | 7M, 10F | 17M, 11F |
| Age (mean ± SD) | 51 ± 7 | 56 ± 8 | 58 ± 7 | 42 ± 12 | 36 ± 12 | 47 ± 17 | 60 ± 14 |
| Child–Pugh scale | A1, B8, C12 | A29, B21, C8 | A4, B3, C5 | A5, B2, C0 | A1, B2, C5 | A5, B5, C7 | N/A |
| Total bilirubin [mg/dL] (mean ± SD) | 4.5 ± 4.2 | 1.7 ± 1.2 | 5.1 ± 5.8 | 4.3 ± 5.6 | 12.0 ± 14.2 | 3.6 ± 3.8 | 0.6 ± 0.3 |
| Albumin [g/dL] (mean ± SD) | 3.0 ± 0.6 | 3.4 ± 0.6 | 3.3 ± 0.6 | 3.7 ± 0.5 | 3.2 ± 1.3 | 3.4 ± 0.4 | 3.6 ± 0.7 |
| PT [s] (mean ± SD) | 16.1 ± 2.4 | 14.0 ± 4.0 | 13.3 ± 1.9 | 12.3 ± 3.0 | 35.9 ± 15.8 | 14.4 ± 5.3 | 13.0 ± 3.2 |
| INR (mean ± SD) | 1.5 ± 0.2 | 1.3 ± 0.3 | 1.2 ± 0.2 | 1.2 ± 0.3 | 2.9 ± 1.9 | 1.4 ± 0.6 | 1.2 ± 0.2 |
| CYP2E1 | Methylated DNA (%) | mRNA (Relative Quantity) |
|---|---|---|
| liver | 24.50 ± 7.89 | 58.27 ± 19.50 |
| duodenum | 65.17 ± 7.71 * | 0.02 ± 0.02 * |
| jejunum | 74.30 ± 5.33 * | 0.01 ± 0.01 * |
| colon | 78.23 ± 1.53 * | 0.00 ± 0.00 * |
| Methylated Cytosine | ALD | HCV | PBC | PSC | WD | AIH | ALL | CTRL | ALL + CTRL |
|---|---|---|---|---|---|---|---|---|---|
| C1 | 0.09 | −0.03 | 0.03 | −0.46 | 0.71 | −0.09 | −0.01 | −0.24 | −0.07 |
| C2 | −0.24 | −0.02 | 0.01 | −0.57 | 0.00 | −0.25 | −0.09 | −0.26 | −0.13 |
| C3 | −0.02 | −0.11 | 0.08 | −0.57 | 0.37 | −0.18 | −0.10 | −0.36 | −0.17 * |
| C4 | −0.04 | −0.02 | −0.11 | −0.57 | −0.14 | −0.22 | −0.04 | −0.15 | −0.12 |
| C5 | −0.01 | −0.01 | 0.07 | −0.57 | −0.03 | −0.35 | −0.06 | −0.19 | −0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komaniecka, N.; Kurzawski, M.; Szeląg-Pieniek, S.; Łapczuk-Romańska, J.; Post, M.; Adamiak-Giera, U.; Droździk, M. Transcriptional Regulation of CYP2E1: Promoter Methylation in In Vitro Models and Human Liver Disease Samples. Genes 2025, 16, 990. https://doi.org/10.3390/genes16080990
Komaniecka N, Kurzawski M, Szeląg-Pieniek S, Łapczuk-Romańska J, Post M, Adamiak-Giera U, Droździk M. Transcriptional Regulation of CYP2E1: Promoter Methylation in In Vitro Models and Human Liver Disease Samples. Genes. 2025; 16(8):990. https://doi.org/10.3390/genes16080990
Chicago/Turabian StyleKomaniecka, Nina, Mateusz Kurzawski, Sylwia Szeląg-Pieniek, Joanna Łapczuk-Romańska, Mariola Post, Urszula Adamiak-Giera, and Marek Droździk. 2025. "Transcriptional Regulation of CYP2E1: Promoter Methylation in In Vitro Models and Human Liver Disease Samples" Genes 16, no. 8: 990. https://doi.org/10.3390/genes16080990
APA StyleKomaniecka, N., Kurzawski, M., Szeląg-Pieniek, S., Łapczuk-Romańska, J., Post, M., Adamiak-Giera, U., & Droździk, M. (2025). Transcriptional Regulation of CYP2E1: Promoter Methylation in In Vitro Models and Human Liver Disease Samples. Genes, 16(8), 990. https://doi.org/10.3390/genes16080990








