Epilepsy Associated Gene, Pcdh7, Is Dispensable for Brain Development in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. CRISPR Gene Editing
2.3. Genotyping
2.4. RNA Isolation and RT-PCR
2.5. Histology and Immunohistochemistry
2.6. Flurothyl Seizure Induction
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
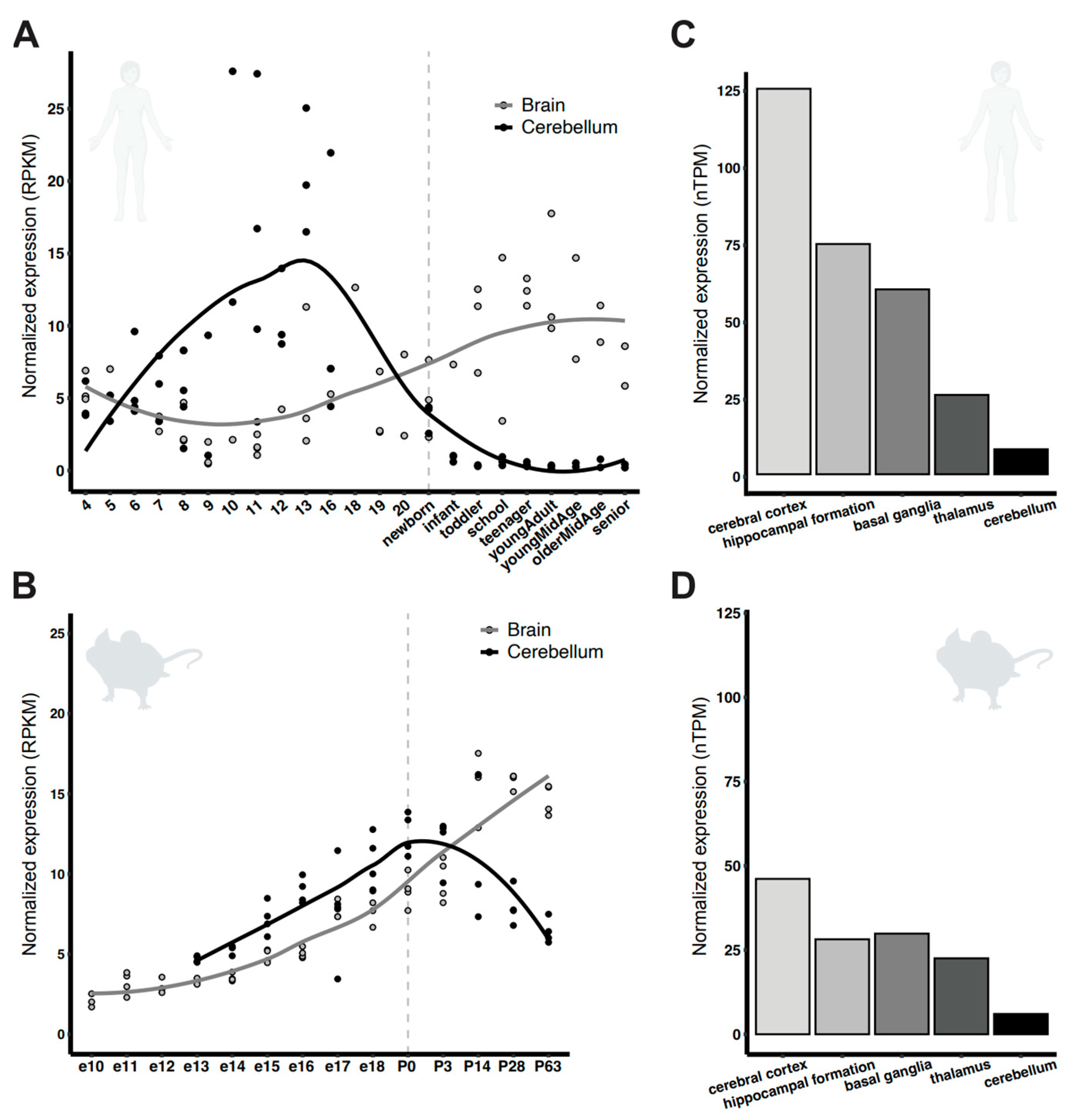
3.1. Pcdh7 Is Widely Expressed in Mice and Human Brain During Development
3.2. Generation and Validation of a Pcdh7 Null Mouse
3.3. Pcdh7+/− but Not Pcdh7−/− Mice Present with Increased Seizure Latencies
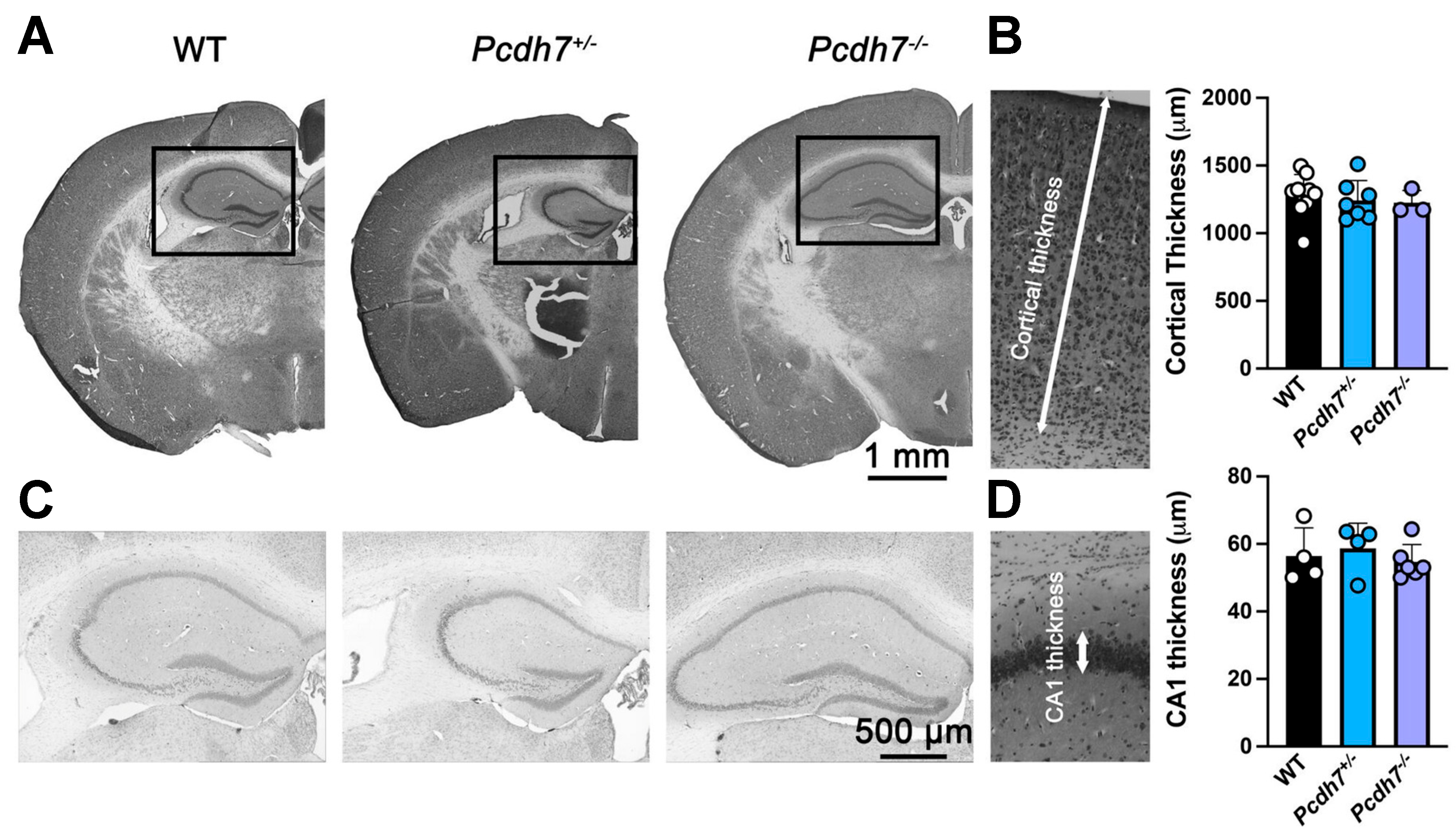
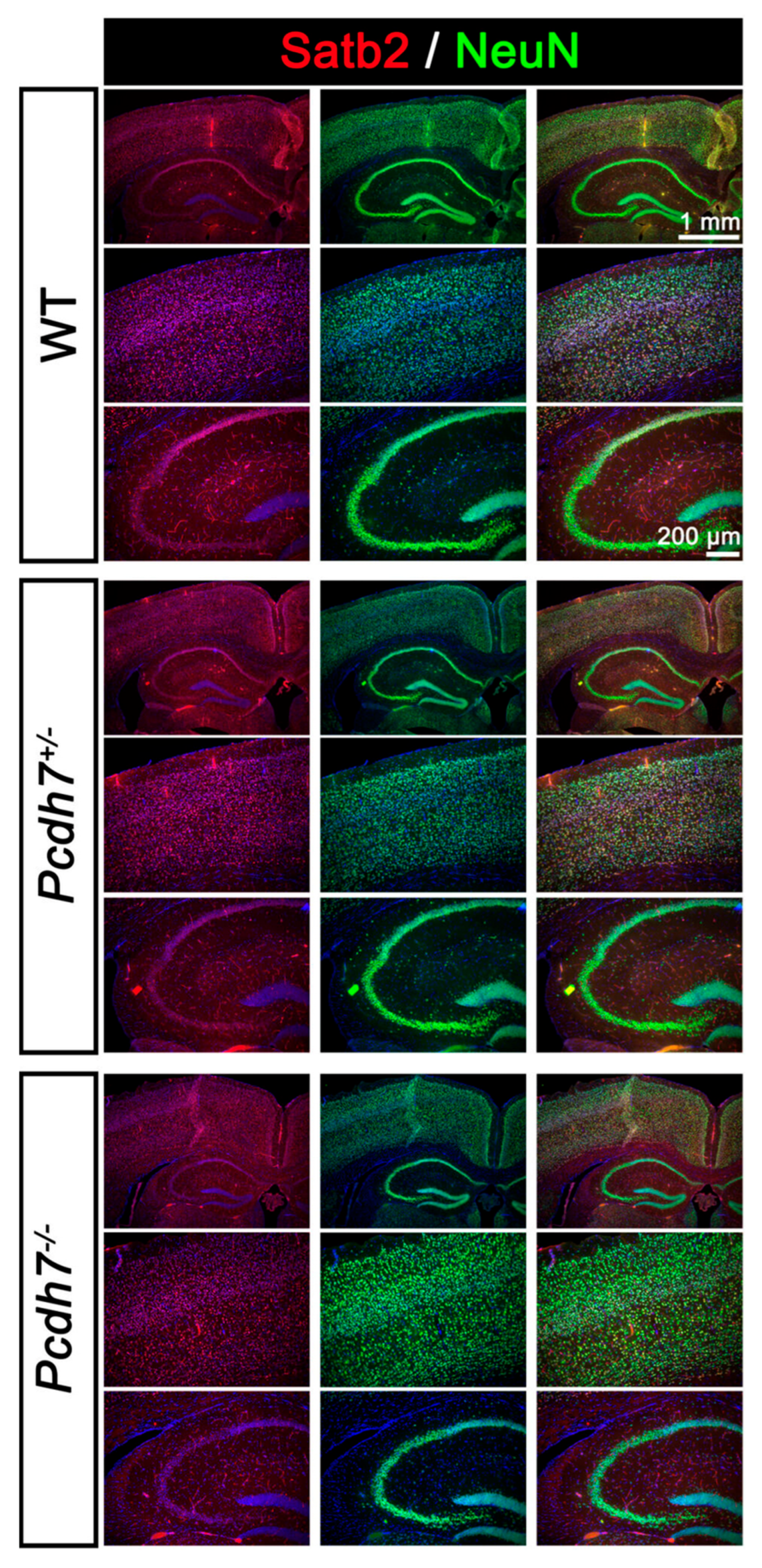
3.4. Pcdh7+/− and Pcdh7−/− Mice Exhibit No Gross Brain Abnormalities
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCDH7 | Protocadherin 7 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| HET | Heterozygous |
| KO | Knock-out |
| MJ | Myoclonic jerk |
| GTCS | Generalized tonic-clonic seizure |
| GS | Generalized seizure |
| gRNA | Guide RNA |
References
- Kim, S.Y.; Mo, J.W.; Han, S.; Choi, S.Y.; Han, S.B.; Moon, B.H.; Rhyu, I.J.; Sun, W.; Kim, H. The expression of non-clustered protocadherins in adult rat hippocampal formation and the connecting brain regions. Neuroscience 2010, 170, 189–199. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yasuda, S.; Tanaka, H.; Yamagata, K.; Kim, H. Non-clustered protocadherin. Cell Adhes. Migr. 2011, 5, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Morishita, H.; Yagi, T. Protocadherin family: Diversity, structure, and function. Curr. Opin. Cell Biol. 2007, 19, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Sung, J.Y.; Ceglia, I.; Lee, K.W.; Ahn, J.H.; Halford, J.M.; Kim, A.M.; Kwak, S.P.; Park, J.B.; Ho Ryu, S.; et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 2006, 442, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Uemura, M.; Nakao, S.; Suzuki, S.T.; Takeichi, M.; Hirano, S. OL-Protocadherin is essential for growth of striatal axons and thalamocortical projections. Nat. Neurosci. 2007, 10, 1151–1159. [Google Scholar] [CrossRef]
- Oishi, K.; Nakagawa, N.; Tachikawa, K.; Sasaki, S.; Aramaki, M.; Hirano, S.; Yamamoto, N.; Yoshimura, Y.; Nakajima, K. Identity of neocortical layer 4 neurons is specified through correct positioning into the cortex. eLife 2016, 5. [Google Scholar] [CrossRef]
- Hayashi, S.; Inoue, Y.; Kiyonari, H.; Abe, T.; Misaki, K.; Moriguchi, H.; Tanaka, Y.; Takeichi, M. Protocadherin-17 mediates collective axon extension by recruiting actin regulator complexes to interaxonal contacts. Dev. Cell 2014, 30, 673–687. [Google Scholar] [CrossRef]
- Biswas, S.; Emond, M.R.; Duy, P.Q.; Hao le, T.; Beattie, C.E.; Jontes, J.D. Protocadherin-18b interacts with Nap1 to control motor axon growth and arborization in zebrafish. Mol. Biol. Cell 2014, 25, 633–642. [Google Scholar] [CrossRef]
- Guemez-Gamboa, A.; Caglayan, A.O.; Stanley, V.; Gregor, A.; Zaki, M.S.; Saleem, S.N.; Musaev, D.; McEvoy-Venneri, J.; Belandres, D.; Akizu, N.; et al. Loss of Protocadherin-12 Leads to Diencephalic-Mesencephalic Junction Dysplasia Syndrome. Ann. Neurol. 2018, 84, 638–647. [Google Scholar] [CrossRef]
- Hoshina, N.; Tanimura, A.; Yamasaki, M.; Inoue, T.; Fukabori, R.; Kuroda, T.; Yokoyama, K.; Tezuka, T.; Sagara, H.; Hirano, S.; et al. Protocadherin 17 regulates presynaptic assembly in topographic corticobasal Ganglia circuits. Neuron 2013, 78, 839–854. [Google Scholar] [CrossRef]
- Leung, L.C.; Harris, W.A.; Holt, C.E.; Piper, M. NF-Protocadherin Regulates Retinal Ganglion Cell Axon Behaviour in the Developing Visual System. PLoS ONE 2015, 10, e0141290. [Google Scholar] [CrossRef]
- Piper, M.; Dwivedy, A.; Leung, L.; Bradley, R.S.; Holt, C.E. NF-protocadherin and TAF1 regulate retinal axon initiation and elongation in vivo. J. Neurosci. 2008, 28, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Keeler, A.B.; Molumby, M.J.; Weiner, J.A. Protocadherins branch out: Multiple roles in dendrite development. Cell Adhes. Migr. 2015, 9, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kerrisk Campbell, M.; Tom, I.; Foreman, O.; Hanson, J.E.; Sheng, M. PCDH7 interacts with GluN1 and regulates dendritic spine morphology and synaptic function. Sci. Rep. 2020, 10, 10951. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Tanaka, H.; Sugiura, H.; Okamura, K.; Sakaguchi, T.; Tran, U.; Takemiya, T.; Mizoguchi, A.; Yagita, Y.; Sakurai, T.; et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron 2007, 56, 456–471. [Google Scholar] [CrossRef]
- Tsai, N.P.; Wilkerson, J.R.; Guo, W.; Maksimova, M.A.; DeMartino, G.N.; Cowan, C.W.; Huber, K.M. Multiple autism-linked genes mediate synapse elimination via proteasomal degradation of a synaptic scaffold PSD-95. Cell 2012, 151, 1581–1594. [Google Scholar] [CrossRef]
- Pederick, D.T.; Richards, K.L.; Piltz, S.G.; Kumar, R.; Mincheva-Tasheva, S.; Mandelstam, S.A.; Dale, R.C.; Scheffer, I.E.; Gecz, J.; Petrou, S.; et al. Abnormal Cell Sorting Underlies the Unique X-Linked Inheritance of PCDH19 Epilepsy. Neuron 2018, 97, 59–66.e5. [Google Scholar] [CrossRef]
- Rakotomamonjy, J.; Rylaarsdam, L.; Fares-Taie, L.; McDermott, S.; Davies, D.; Yang, G.; Fagbemi, F.; Epstein, M.; Fairbanks-Santana, M.; Rozet, J.M.; et al. PCDH12 loss results in premature neuronal differentiation and impeded migration in a cortical organoid model. Cell Rep. 2023, 42, 112845. [Google Scholar] [CrossRef]
- Niu, W.; Deng, L.; Mojica-Perez, S.P.; Tidball, A.M.; Sudyk, R.; Stokes, K.; Parent, J.M. Abnormal cell sorting and altered early neurogenesis in a human cortical organoid model of Protocadherin-19 clustering epilepsy. Front. Cell Neurosci. 2024, 18, 1339345. [Google Scholar] [CrossRef]
- Trivisano, M.; Pietrafusa, N.; Terracciano, A.; Marini, C.; Mei, D.; Darra, F.; Accorsi, P.; Battaglia, D.; Caffi, L.; Canevini, M.P.; et al. Defining the electroclinical phenotype and outcome of PCDH19-related epilepsy: A multicenter study. Epilepsia 2018, 59, 2260–2271. [Google Scholar] [CrossRef]
- Dibbens, L.M.; Tarpey, P.S.; Hynes, K.; Bayly, M.A.; Scheffer, I.E.; Smith, R.; Bomar, J.; Sutton, E.; Vandeleur, L.; Shoubridge, C.; et al. X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat. Genet. 2008, 40, 776–781. [Google Scholar] [CrossRef]
- Gregorio, S.P.; Sallet, P.C.; Do, K.A.; Lin, E.; Gattaz, W.F.; Dias-Neto, E. Polymorphisms in genes involved in neurodevelopment may be associated with altered brain morphology in schizophrenia: Preliminary evidence. Psychiatry Res. 2009, 165, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aran, A.; Rosenfeld, N.; Jaron, R.; Renbaum, P.; Zuckerman, S.; Fridman, H.; Zeligson, S.; Segel, R.; Kohn, Y.; Kamal, L.; et al. Loss of function of PCDH12 underlies recessive microcephaly mimicking intrauterine infection. Neurology 2016, 86, 2016–2024. [Google Scholar] [CrossRef]
- Nicolas, G.; Sanchez-Contreras, M.; Ramos, E.M.; Lemos, R.R.; Ferreira, J.; Moura, D.; Sobrido, M.J.; Richard, A.C.; Lopez, A.R.; Legati, A.; et al. Brain calcifications and PCDH12 variants. Neurol. Genet. 2017, 3, e166. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Muromoto, S.; Wakusawa, K.; Miyabayashi, T.; Sato, R.; Okubo, Y.; Endo, W.; Inui, T.; Togashi, N.; Kato, A.; Oba, H.; et al. A case of new PCDH12 gene variants presented as dyskinetic cerebral palsy with epilepsy. J. Hum. Genet. 2018, 63, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Vineeth, V.S.; Das Bhowmik, A.; Balakrishnan, S.; Dalal, A.; Aggarwal, S. Homozygous PCDH12 variants result in phenotype of cerebellar ataxia, dystonia, retinopathy, and dysmorphism. J. Hum. Genet. 2019, 64, 183–189. [Google Scholar] [CrossRef]
- Morrow, E.M.; Yoo, S.Y.; Flavell, S.W.; Kim, T.K.; Lin, Y.; Hill, R.S.; Mukaddes, N.M.; Balkhy, S.; Gascon, G.; Hashmi, A.; et al. Identifying autism loci and genes by tracing recent shared ancestry. Science 2008, 321, 218–223. [Google Scholar] [CrossRef]
- Butler, M.G.; Rafi, S.K.; Hossain, W.; Stephan, D.A.; Manzardo, A.M. Whole exome sequencing in females with autism implicates novel and candidate genes. Int. J. Mol. Sci. 2015, 16, 1312–1335. [Google Scholar] [CrossRef]
- Chang, H.; Hoshina, N.; Zhang, C.; Ma, Y.; Cao, H.; Wang, Y.; Wu, D.D.; Bergen, S.E.; Landen, M.; Hultman, C.M.; et al. The protocadherin 17 gene affects cognition, personality, amygdala structure and function, synapse development and risk of major mood disorders. Mol. Psychiatry 2018, 23, 400–412. [Google Scholar] [CrossRef]
- Redies, C.; Vanhalst, K.; Roy, F. delta-Protocadherins: Unique structures and functions. Cell Mol. Life Sci. 2005, 62, 2840–2852. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Guo, H.; Du, K.; Chen, D. Integrative analysis illustrates the role of PCDH7 in lung cancer development, cisplatin resistance, and immunotherapy resistance: An underlying target. Front. Pharmacol. 2023, 14, 1217213. [Google Scholar] [CrossRef]
- International League Against Epilepsy Consortium on Complex Epilepsies. Genetic determinants of common epilepsies: A meta-analysis of genome-wide association studies. Lancet Neurol. 2014, 13, 893–903. [Google Scholar] [CrossRef] [PubMed]
- International League Against Epilepsy Consortium on Complex Epilepsies. GWAS meta-analysis of over 29,000 people with epilepsy identifies 26 risk loci and subtype-specific genetic architecture. Nat. Genet. 2023, 55, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Koch, A.E.; Garrett, M.E.; Gibson, J.; Liu, Y.; Dennis, M.F.; Kimbrel, N.A.; Veterans Affairs Mid-Atlantic Mental Illness Research, Education, and Clinical Center Workgroup; Beckham, J.C.; Hauser, M.A. Genome-wide association study of posttraumatic stress disorder in a cohort of Iraq-Afghanistan era veterans. J. Affect. Disord. 2015, 184, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ip, H.F.; van der Laan, C.M.; Krapohl, E.M.L.; Brikell, I.; Sanchez-Mora, C.; Nolte, I.M.; St Pourcain, B.; Bolhuis, K.; Palviainen, T.; Zafarmand, H.; et al. Genetic association study of childhood aggression across raters, instruments, and age. Transl. Psychiatry 2021, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Hirasawa, T.; Soutome, M.; Itoh, M.; Goto, Y.; Endoh, K.; Takahashi, K.; Kudo, S.; Nakagawa, T.; Yokoi, S.; et al. The protocadherins, PCDHB1 and PCDH7, are regulated by MeCP2 in neuronal cells and brain tissues: Implication for pathogenesis of Rett syndrome. BMC Neurosci. 2011, 12, 81. [Google Scholar] [CrossRef]
- Di, J.; Yenwongfai, L.; Rieger, H.T.; Zhang, S.; Wei, S. Familial 4p Interstitial Deletion Provides New Insights and Candidate Genes Underlying This Rare Condition. Genes 2023, 14, 635. [Google Scholar] [CrossRef]
- Loh, K.H.; Stawski, P.S.; Draycott, A.S.; Udeshi, N.D.; Lehrman, E.K.; Wilton, D.K.; Svinkina, T.; Deerinck, T.J.; Ellisman, M.H.; Stevens, B.; et al. Proteomic Analysis of Unbounded Cellular Compartments: Synaptic Clefts. Cell 2016, 166, 1295–1307 e1221. [Google Scholar] [CrossRef]
- Rashid, D.; Newell, K.; Shama, L.; Bradley, R. A requirement for NF-protocadherin and TAF1/Set in cell adhesion and neural tube formation. Dev. Biol. 2006, 291, 170–181. [Google Scholar] [CrossRef]
- Heggem, M.A.; Bradley, R.S. The cytoplasmic domain of Xenopus NF-protocadherin interacts with TAF1/set. Dev. Cell 2003, 4, 419–429. [Google Scholar] [CrossRef]
- Xiao, Y.; Hu, M.; Lin, Q.; Zhang, T.; Li, S.; Shu, L.; Song, X.; Xu, X.; Meng, W.; Li, X.; et al. Dopey2 and Pcdh7 orchestrate the development of embryonic neural stem cells/ progenitors in zebrafish. iScience 2023, 26, 106273. [Google Scholar] [CrossRef]
- Leung, L.C.; Urbancic, V.; Baudet, M.L.; Dwivedy, A.; Bayley, T.G.; Lee, A.C.; Harris, W.A.; Holt, C.E. Coupling of NF-protocadherin signaling to axon guidance by cue-induced translation. Nat. Neurosci. 2013, 16, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Concordet, J.P.; Haeussler, M. CRISPOR: Intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018, 46, W242–W245. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.S.; Tang, B.; Papale, L.A.; Yu, F.H.; Catterall, W.A.; Escayg, A. The voltage-gated sodium channel Scn8a is a genetic modifier of severe myoclonic epilepsy of infancy. Hum. Mol. Genet. 2007, 16, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Rakotomamonjy, J.; Sabetfakhri, N.P.; McDermott, S.L.; Guemez-Gamboa, A. Characterization of seizure susceptibility in Pcdh19 mice. Epilepsia 2020, 61, 2313–2320. [Google Scholar] [CrossRef]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascencao, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene expression across mammalian organ development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef]
- Sjostedt, E.; Zhong, W.; Fagerberg, L.; Karlsson, M.; Mitsios, N.; Adori, C.; Oksvold, P.; Edfors, F.; Limiszewska, A.; Hikmet, F.; et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 2020, 367, eaay5947. [Google Scholar] [CrossRef]
- Kim, S.Y.; Chung, H.S.; Sun, W.; Kim, H. Spatiotemporal expression pattern of non-clustered protocadherin family members in the developing rat brain. Neuroscience 2007, 147, 996–1021. [Google Scholar] [CrossRef]
- Yu, H.; Yan, H.; Wang, L.; Li, J.; Tan, L.; Deng, W.; Chen, Q.; Yang, G.; Zhang, F.; Lu, T.; et al. Five novel loci associated with antipsychotic treatment response in patients with schizophrenia: A genome-wide association study. Lancet Psychiatry 2018, 5, 327–338. [Google Scholar] [CrossRef]
- Yoshida, K.; Yoshitomo-Nakagawa, K.; Seki, N.; Sasaki, M.; Sugano, S. Cloning, expression analysis, and chromosomal localization of BH-protocadherin (PCDH7), a novel member of the cadherin superfamily. Genomics 1998, 49, 458–461. [Google Scholar] [CrossRef]
- Garrett, A.M.; Schreiner, D.; Lobas, M.A.; Weiner, J.A. gamma-protocadherins control cortical dendrite arborization by regulating the activity of a FAK/PKC/MARCKS signaling pathway. Neuron 2012, 74, 269–276. [Google Scholar] [CrossRef]
- Lefebvre, J.L.; Kostadinov, D.; Chen, W.V.; Maniatis, T.; Sanes, J.R. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature 2012, 488, 517–521. [Google Scholar] [CrossRef]
- Garrett, A.M.; Weiner, J.A. Control of CNS synapse development by gamma-protocadherin-mediated astrocyte-neuron contact. J. Neurosci. 2009, 29, 11723–11731. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Kontarakis, Z.; Gerri, C.; Nolte, H.; Holper, S.; Kruger, M.; Stainier, D.Y. Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 2015, 524, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.O.; Shin, M.; Ni, C.W.; Gupta, A.; Grosse, A.S.; van Impel, A.; Kirchmaier, B.C.; Peterson-Maduro, J.; Kourkoulis, G.; Male, I.; et al. Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Dev. Cell 2015, 32, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Pederick, D.T.; Homan, C.C.; Jaehne, E.J.; Piltz, S.G.; Haines, B.P.; Baune, B.T.; Jolly, L.A.; Hughes, J.N.; Gecz, J.; Thomas, P.Q. Pcdh19 Loss-of-Function Increases Neuronal Migration In Vitro but is Dispensable for Brain Development in Mice. Sci. Rep. 2016, 6, 26765. [Google Scholar] [CrossRef]
- Rampon, C.; Prandini, M.H.; Bouillot, S.; Pointu, H.; Tillet, E.; Frank, R.; Vernet, M.; Huber, P. Protocadherin 12 (VE-cadherin 2) is expressed in endothelial, trophoblast, and mesangial cells. Exp. Cell Res. 2005, 302, 48–60. [Google Scholar] [CrossRef]
- Esumi, S.; Kakazu, N.; Taguchi, Y.; Hirayama, T.; Sasaki, A.; Hirabayashi, T.; Koide, T.; Kitsukawa, T.; Hamada, S.; Yagi, T. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat. Genet. 2005, 37, 171–176. [Google Scholar] [CrossRef]


| Females | Males | Total | |||
| Numbers | 33 | 40 | 73 | ||
| % | 45 | 55 | 100 | ||
| WT | Pcdh7+/− | Pcdh7−/− | Total | ||
| Numbers | 15 | 38 | 17 | 70 | |
| % | 21 | 54 | 24 | 100 | |
| WT | Pcdh7+/− | Pcdh7−/− | Total | ||
| Numbers | Females | 7 | 15 | 8 | 30 |
| Males | 8 | 23 | 9 | 40 | |
| % | Females | 23 | 50 | 27 | 100 |
| Males | 20 | 58 | 23 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakotomamonjy, J.; Davies, D.; Valencia, X.; Son, O.; Gomez-Maqueo, X.; Guemez-Gamboa, A. Epilepsy Associated Gene, Pcdh7, Is Dispensable for Brain Development in Mice. Genes 2025, 16, 985. https://doi.org/10.3390/genes16080985
Rakotomamonjy J, Davies D, Valencia X, Son O, Gomez-Maqueo X, Guemez-Gamboa A. Epilepsy Associated Gene, Pcdh7, Is Dispensable for Brain Development in Mice. Genes. 2025; 16(8):985. https://doi.org/10.3390/genes16080985
Chicago/Turabian StyleRakotomamonjy, Jennifer, Devin Davies, Xavier Valencia, Olivia Son, Ximena Gomez-Maqueo, and Alicia Guemez-Gamboa. 2025. "Epilepsy Associated Gene, Pcdh7, Is Dispensable for Brain Development in Mice" Genes 16, no. 8: 985. https://doi.org/10.3390/genes16080985
APA StyleRakotomamonjy, J., Davies, D., Valencia, X., Son, O., Gomez-Maqueo, X., & Guemez-Gamboa, A. (2025). Epilepsy Associated Gene, Pcdh7, Is Dispensable for Brain Development in Mice. Genes, 16(8), 985. https://doi.org/10.3390/genes16080985






