1. Introduction
L-2-hydroxyglutaric aciduria (OMIM #236792) is a rare metabolic disorder accompanied by increased levels of L-2-hydroxyglutarate in bodily fluids and L-2-hydroxyglutarate accumulation in several organs, i.e., the brain, resulting in neurological impairment. First identified in 1980 [
1], there have been fewer than 500 (Leiden Open Variation Database) documented cases globally. Orphanet classifies the disease as having a prevalence of less than 1 in 1,000,000, (Orphanet ORPHA: 79314). The condition follows an autosomal recessive pattern of inheritance, with the
L2HGDH gene responsible for producing the enzyme L-2-hydroxyglutarate dehydrogenase. Deficiencies in this enzyme arise from variants in
L2HGDH, disrupting the function of L2HGDH, the typical breakdown of L-2-hydroxyglutarate into α-ketoglutarate [
2]. As a result, L-2-hydroxyglutarate builds up to excessive levels in the plasma, urine, and cerebrospinal fluid of affected individuals [
3], which is believed to have neurotoxic effects that can lead to brain damage.
Clinically, L2HGA usually manifests in early childhood through developmental delays or regressions, seizures, cerebellar ataxia, and extrapyramidal symptoms. Intellectual disability typically becomes evident, and many patients experience macrocephaly and speech challenges [
4]. Brain magnetic resonance imaging (MRI) shows distinct abnormalities, particularly bilateral and symmetric subcortical white matter hyperintensities, often affecting the basal ganglia and dentate nuclei [
5,
6]. These neuroimaging characteristics are highly indicative of L2HGA when considered alongside the clinical context. Elevated levels of L-2-hydroxyglutarate, as confirmed by organic acid analysis, support the biochemical diagnosis [
2].
The
L2HGDH gene was identified in 2004 through studies that mapped L2HGA to chromosome 14q22.1 and demonstrated that variants in
L2HGDH abrogate L-2-hydroxyglutarate dehydrogenase activity [
7]. L2HGDH is a nuclear-encoded, FAD-dependent enzyme that localizes to mitochondria and catalyzes the oxidation of L-2-hydroxyglutarate to α-ketoglutarate [
7]. Pathogenic variants in
L2HGDH generally result in loss of enzyme function and failure to metabolize L-2-hydroxyglutarate. As of May 2025, the Leiden Open Variation Database (LOVD) has reported 111 variants associated with
L2HGDH deficiency. These include missense substitutions like p.(Gly60Arg), as well as frameshift variants (c.180delG) [
8], or nonsense variants (c.751C>T) that produce a truncated, nonfunctional enzyme. Despite the variety of variants, nearly all characterized cases show a common outcome of accumulation of toxic metabolite.
Here, we describe two siblings from a consanguineous family diagnosed with L2HGA, in whom we identified a homozygous missense variant in L2HGDH: c.905C>T, resulting in a p.(Pro302Leu) substitution. We performed a series of molecular experiments to characterize the effect of this variant on the L2HGDH protein. In particular, we examined whether the p.(Pro302Leu) change affects the enzyme’s expression, mitochondrial localization, and catalytic activity. Our results provide insight into the pathogenic mechanism of this variant and expand the understanding of genotype–phenotype correlations in L2HGA.
2. Materials and Methods
2.1. Sanger Sequencing for Variant Confirmation
The variant was previously identified using next-generation sequencing (NGS) for the older brother, and it was confirmed here by Sanger sequencing in the younger brother and parents. For this, genomic DNA was extracted from peripheral blood samples collected from the two affected siblings and their parents using standard extraction procedures. Approximately 3–5 mL of whole blood was collected in EDTA-containing tubes and processed within 24 h. The L2HGDH (transcript: ENST00000267436.8) gene was amplified by polymerase chain reaction (PCR) using primers flanking exon 7, where the c.905C>T p.(Pro302Leu) variant is located (forward primer: GCTGCCACCTAAATGCTAAAG; reverse primer: AACCACAAGAGAAACCGATG). PCR products were purified using the ExoSAP-IT™ PCR Product Cleanup reagent (Thermo Fisher Scientific, Waltham, MA, USA) and subjected to Sanger sequencing using the ABI 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Sanger sequences were analyzed using JSI SequencePilot software 4.2 (JSI medical systems, Ettenheim, Germany) and aligned to the reference genome (GRCh37/hg19) to detect variants.
2.2. Measurement of L-2-Hydroxyglutarate Levels
Plasma samples (50 µL each) from two heterozygous parents, two affected children and unrelated healthy controls were analyzed for L-2-hydroxyglutarate levels. Metabolite extraction and measurements were performed using anion chromatography coupled with high resolution mass spectrometry (AIC-HR-MS) [
9]. A commercial L-2-hydroxyglutarate standard (Sigma Aldrich, Taufkirchen, Germany) was used to confirm the identity of the AIC-HR-MS detected compound from serum samples. In the analyzed serum samples, two distinct peaks were detected at 3.21 min and 3.50 min, corresponding putatively to L and D-2-hydroxyglutarate, respectively.
2.3. Mitochondrial Isolation
Mitochondria were isolated from HEK293 cells 48 h post-transfection. Cells were collected on ice by gentle scraping and pelleted by centrifugation at 2500× g for 3 min at 4 °C. The cell pellet was resuspended in ice-cold mitochondrial isolation buffer (MIB), consisting of 6.846 g sucrose, 0.121 g Tris (100 mM), and 1 mL of 0.1 M EGTA dissolved in 80 mL of distilled water, with the pH adjusted to 7.4. The cell suspension was transferred to a pre-chilled Dounce homogenizer and homogenized with 25 strokes on ice. The homogenate was centrifuged at 600× g for 10 min at 4 °C to remove unbroken cells and nuclei. The supernatant was carefully collected and further centrifuged at 10,000× g for 10 min at 4 °C. The resulting pellet, containing crude mitochondria, was resuspended in appropriate buffer and used for downstream analyses.
2.4. Cloning of L2HGDH and Mutated L2HGDH
The coding sequence for human L2HGDH (kindly provided by Prof. Dr. Sunil Sudarshan, Department of Urology, University of Birmingham, Alabama) was cloned into a pAAV expression vector (Addgene #59462, Cambridge, MA, USA). For this, L2HGDH was PCR-amplified, flanked with restriction sites for BamHI (5′) and EcoRI (3′) and C-terminally tagged with an HA-tag. Fragments were loaded onto a 1% agarose gel and gel electrophoresis was conducted. The desired bands were cut out and purified using the NucleoSpin™ Gel & PCR Clean up Kit (Macherey & Nagel, Düren, Germany) according to the manufacturer’s protocol. DNA concentration was measured spectrophotometrically (NanoDrop™, Thermo Fisher Scientific, Waltham, MA, USA) and purified L2HGDH was cloned into a pAAV subvector using the CloneJET PCR Cloning Kit (TFS). For plasmid amplification, pAAV_L2HGDH was transformed into chemocompetent bacteria (Escherichia coli Top10™, Waltham, MA, USA) which were cultivated for 16–18 h at 37 °C on agar plates with antibiotics. Colonies were picked and grown in nutrient medium containing antibiotics and plasmids were isolated and purified for sequencing and propagation using the PureYield™ Plasmid Miniprep and Midiprep kit (ProMega, Madison, WI, USA).
To introduce the disease-associated point variant c.905C>T, pAAV_L2HGDH was PCR-amplified using a primer pair that binds to the variant site and carries the variant. Application of the Q5® Site-Directed Mutagenesis kit (New England Biolabs, Ipswich, MA, USA) according to the manufacturer’s protocol yielded a pAAV_mL2HGDH plasmid that was amplified as described above.
2.5. Transfection of HEK 293T Cells
HEK 293T cells were cultivated in DMEM plus GlutaMAX™ medium (Gibco, Waltham, MA, USA) with 10% FBS at 37 °C and 5% CO2 and maintained weekly. For transfection, HEK cells were either seeded for Western blot in 6-well plates with 300,000 cells/well in 2 mL medium, for immunostainings in 24-well plates on poly-d-lysine (PDL)-coated coverslips with 50,000 cells/well in 0.5 mL medium, or for enzyme activity assay in 10 cm dishes with 2,000,000 cells/dish in 10 mL medium. A total of 500–2000 ng of plasmid was mixed with 3 µL of polyethylenimine per 1 µg of DNA and adjusted to 200 µL with OptiMEM (Gibco) for Western blot, to 50 µL for immunostainings, and to 1 mL for the activity assay. The transfection mixture was incubated at room temperature (RT) for 20 min and added dropwise to the cells. Cells were incubated at 37 °C and 5% CO2 overnight.
2.6. Western Blot
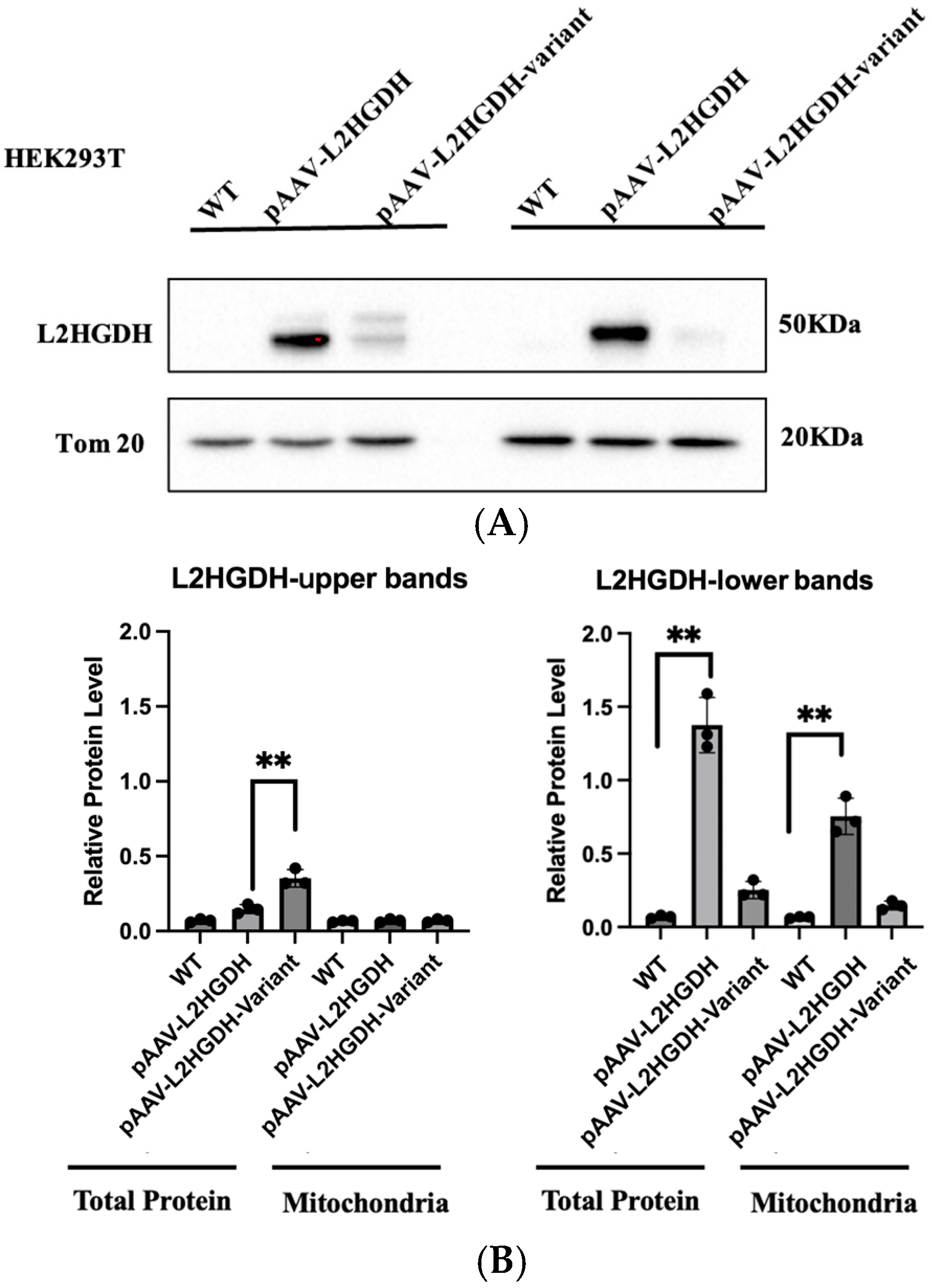
Western blot was conducted to validate protein overexpression and thus plasmid functionality. Transfected cells were harvested in ice cold PBS, centrifuged, and the pellet was resuspended in RIPA cell lysis buffer supplemented with phosphatase and protease inhibitor. The mixture was incubated for 20 min at 4 °C with shaking at 600 rpm, then centrifuged for 20 min at 4 °C and 16,000× g. The supernatant was collected and used for a BCA protein assay applying the Pierce™ BCA Protein Assay Kit (TFS) according to the manufacturer’s protocol and measuring using a Safire2 microplate reader (Tecan, Männedorf, Switzerland). SDS-Page was conducted in a 10% acrylamide gel containing 0.5% (v/v) trichlorethanol (TCE) for normalization against total protein using a Mini-PROTEAN Tetra Cell Kit (Bio-Rad, Hercules, CA, USA). A total of 10 µg of protein samples were diluted with 3× Laemmli buffer, boiled at 95 °C for 5 min and the gel was run at 80 V for 20 min and another subsequent 100 min at 120 V. Total protein was visualized by exposure to UV light for 1 min and transferred on a polyvinylidene fluoride (PVDF) membrane using the Trans-Blot Turbo Transfer System (Bio-Rad). The membrane was blocked with 5% milk in TBS-T at RT for 1 h and incubated with α-L2HGDH primary antibody oN at 4 °C with constant rotation. After incubation with the secondary antibody, either Pierce™ ECL Western blotting Substrate or SuperSignal™ West Femto Maximum Sensitivity Substrate was added on top of the membrane. Analysis was carried out using the Chemicdoc MP Imaging System (Bio-Rad), and pictures were processed using Image Lab 5.2.1.
2.7. Immunofluorescence Staining
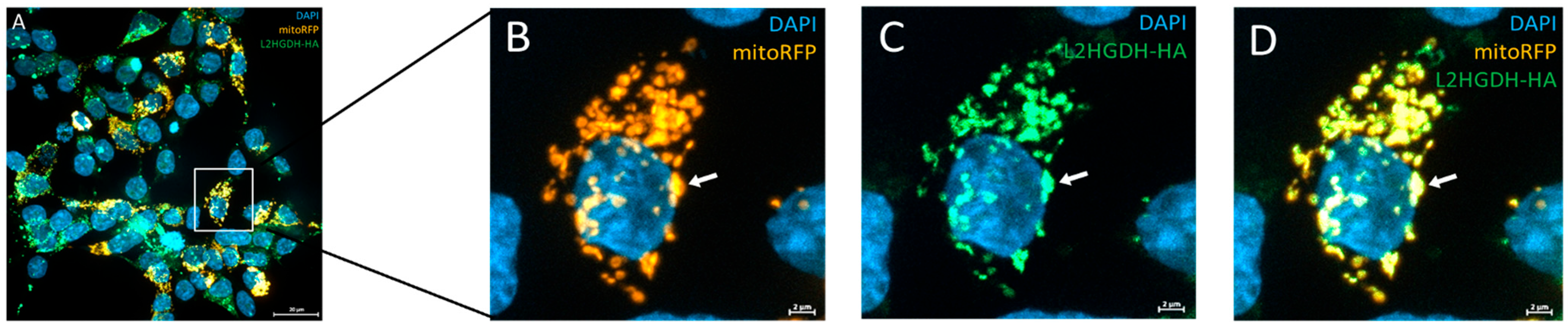
Protein overexpression and mitochondrial localization were assessed via immunofluorescence staining. Transfected cells grown on PDL-coated coverslips were fixed with 3.7% formaldehyde in PBS. After a washing step, cells were blocked and permeabilized with 5% bovine serum albumin (BSA) and 0.2% Triton X-100 in PBS for 10 min. Cells were washed and incubated with primary antibody at 4 °C. Then, cells were washed again and incubated with secondary antibody for 2 h at RT. Nuclei were stained using the NucBlue™ Live Cell reagent (Invitrogen, Waltham, MA, USA), before the coverslips were mounted on slides and left to dry for 24 h at RT. Immunofluorescence was analyzed using the Axio Imager.2M with Apotome.2 and Axiocam 705 mono (Zeiss, Oberkochen, Germany).
2.8. Enzyme Activity Assay
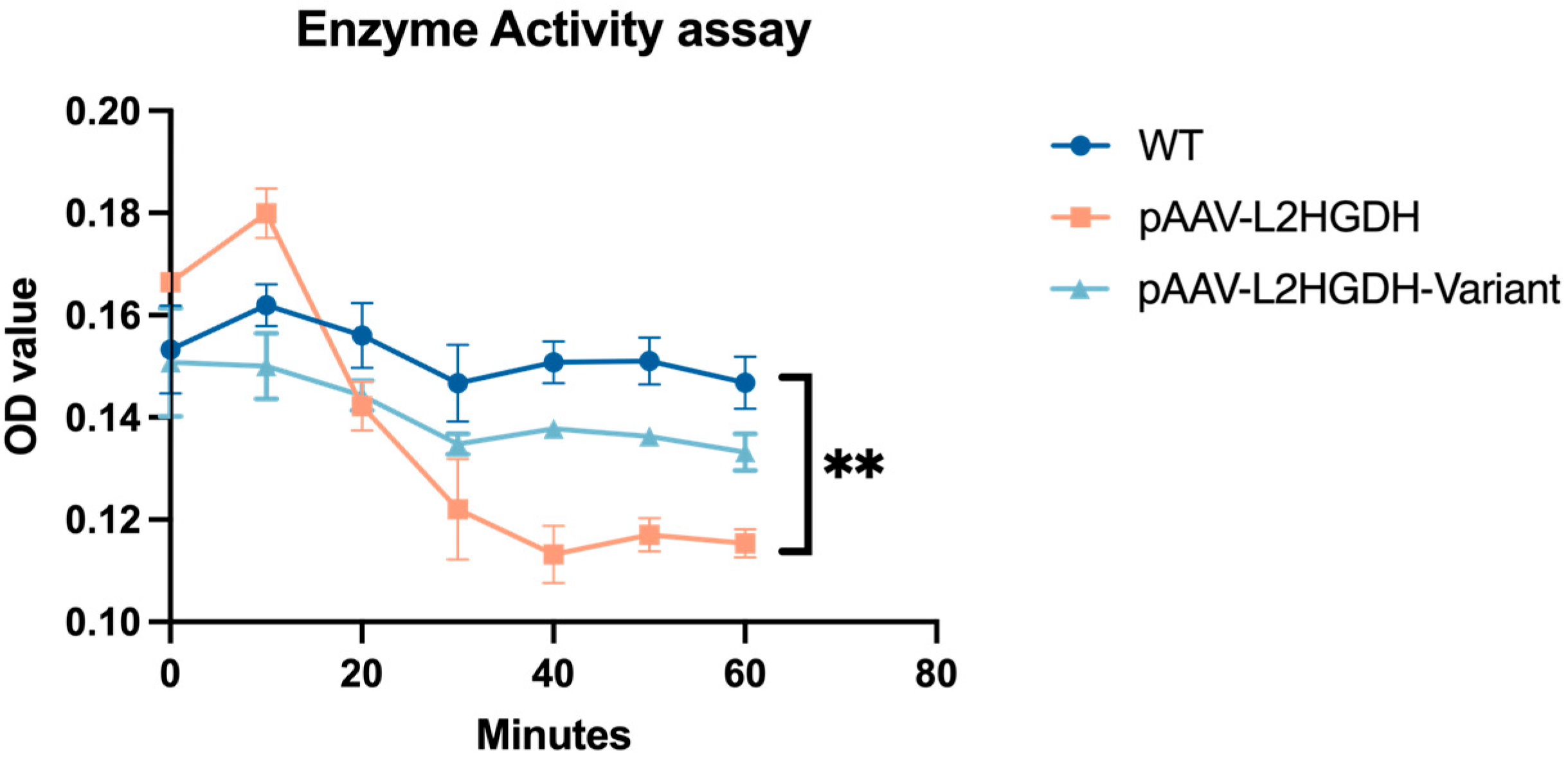
Protein functionality was verified using a colorimetric activity assay by monitoring the reduction in 2,6-dichloroindophenol (DCIP) spectrophometrically at 600 nm for 60 min. Protein concentration in the mitochondria fraction was determined using a Qubit™ Protein Assay Kit (Invitrogen) according to the manufacturer’s protocol. The activity assay was performed in a 96-well plate and reduction was measured using a Safire2 microplate reader (Tecan). Each 100 µL reaction mixture contained 5 µL of 2 mM PMS, 5 µL of 1 mM DCIP, 10 µL of 1.5 mM L2HG, and 20 µg of mitochondrial protein in HEPES-based buffer. Absorbance at 600 nm was measured every minute over 60 min using a microplate reader at RT. For each condition, a substrate-free control (without L2HG) and a blank control (without substrate and without mitochondria) were included.
2.9. Statistical Analysis
Quantitative data are presented as mean ± standard deviation (SD) from at least three independent experiments. Statistical analyses were performed using GraphPad Prism version 9.0 (GraphPad Software). Comparisons between two groups were performed using unpaired two-tailed Student’s t-tests. A p-value < 0.05 was considered statistically significant.
4. Discussion
We have identified a homozygous L2HGDH variant, c.905C>T p.(Pro302Leu), in two siblings affected by L-2-hydroxyglutaric aciduria and demonstrated through functional assays that this variant disrupts the L2HGDH enzyme’s localization and activity. The clinical presentation of the two patients—developmental delay, seizures, hypotonia, and characteristic MRI white matter changes is fully consistent with the known phenotype of L2HGA. By combining clinical and molecular analyses, our study provides strong evidence that the p.(Pro302Leu) variant is pathogenic and explains the patients’ symptoms. Using a combination of differential centrifugation and Western blot, as well as immunofluorescence microscopy and a specific enzymatic assay, we postulate that this rare variant does not primarily affect enzymatic activity but impairs mitochondrial targeting and protein stability, ultimately resulting in loss of function.
Most
L2HGDH variants reported to date cause a complete or near-complete loss of L2HGDH enzyme function [
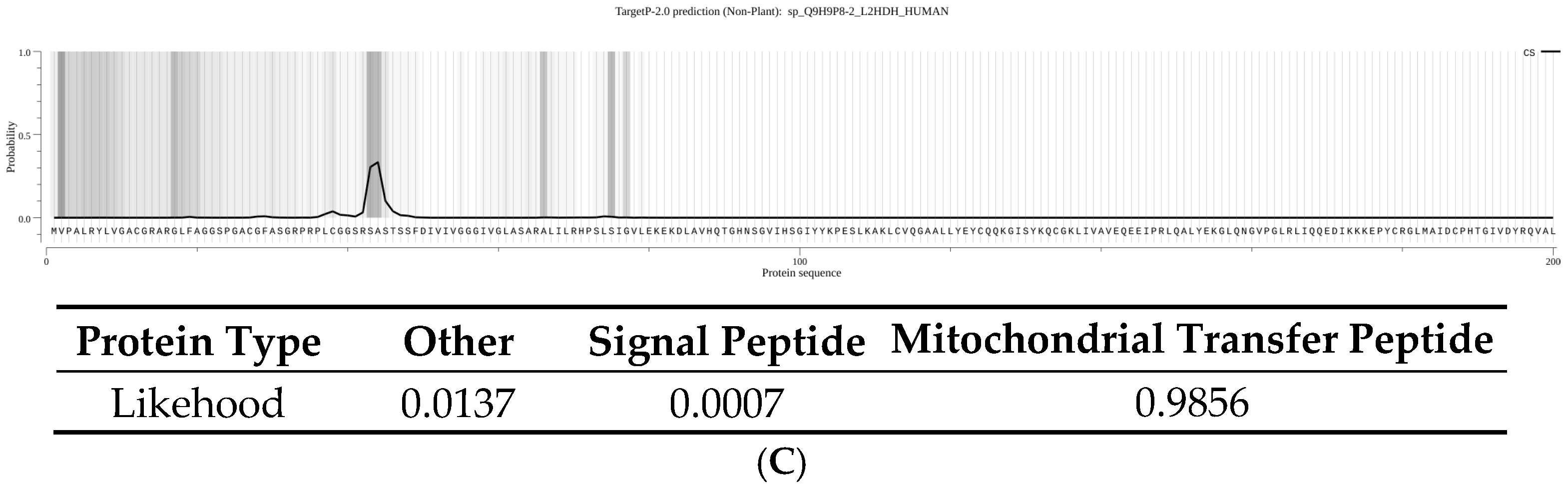
11], and p.Pro302Leu appears to follow this paradigm. The affected proline at position 302 is evolutionarily conserved, suggesting it plays an important role in the enzyme’s structure. Our results showed that the p.Pro302Leu mutant protein is present predominantly as an unprocessed precursor that fails to reach the mitochondria. Our data support that L2HGDH is a nuclear-encoded mitochondrial enzyme, synthesized in the cytosol with an N-terminal mitochondrial targeting sequence and then imported into the mitochondrial matrix or inner membrane. During import, the targeting presequence (44 amino acids) is cleaved off by mitochondrial processing peptidases, yielding the mature enzyme. In cells expressing the p.Pro302Leu mutant, we observed an accumulation of the full-length precursor form and a lack of the cleaved mature form, indicating a defect in the mitochondrial import of the mutant protein. Notably, the Pro302Leu substitution lies well beyond the N-terminal targeting signal, in the middle of the protein sequence, and therefore does not directly disrupt the signal peptide.
We propose that the p.Pro302Leu variant impairs mitochondrial import of L2HGDH by disrupting proper protein folding or structural stability. This hypothesis is supported by AlphaFold3 predicted models and structural comparison using PyMOL, which revealed a distinct local conformational alteration in the loop surrounding residue 302, despite the overall structural similarity between the wild-type and mutant proteins. Proline is among the most conformationally restricted amino acids and is frequently found at the termini of α-helices or within β-turns. In L2HGDH, Pro302 is located at the junction of a β-sheet and a loop, contributing to the stabilization of a structural turn. Substitution at this site is predicted to release backbone strain, induce local distortion, and potentially displace adjacent structural elements.
The Pro302Leu variant replaces this proline with leucine, a bulkier and more flexible hydrophobic residue. This alteration may disrupt the native turn architecture, leading to local misfolding. Although Pro302 is not situated within the predicted catalytic core (substrate-binding residues are located at positions 89, 195, and 402–404), the resulting conformational changes may compromise folding efficiency, mitochondrial recognition, or protein stability. In silico stability assessment using DynaMut2 predicted a mildly destabilizing effect, with a ΔΔG of −0.45 kcal/mol, further supporting a model in which reduced structural stability contributes to pathogenicity. Misfolded L2HGDH proteins are unlikely to be recognized by the mitochondrial import machinery, thereby failing to undergo translocation and subsequently being targeted for cytosolic degradation. Similar mitochondrial import deficiencies have been reported for other mitochondrial proteins, such as mutant COA7, which fails to enter mitochondria due to misfolding and is degraded in the cytosol [
12], suggesting that misfolded L2HGDH may follow a similar fate. This interpretation is consistent with our immunoblot results, which showed that the Pro302Leu variant accumulates predominantly in its precursor form and is present at reduced steady-state levels compared to the wild-type protein. Collectively, these findings suggest that the pathogenicity of the p.Pro302Leu variant arises primarily from folding defects and import failure, rather than direct impairment of catalytic activity.
From a clinical perspective, our report underscores the value of combining genetic diagnosis with functional studies in rare metabolic disorders. The identification of L2HGDH p.Pro302Leu in these patients enabled precise genetic counseling for the family and opened the possibility of prenatal testing in future pregnancies. Notably, a ~50% reduction in enzyme activity, as may occur in heterozygous carriers such as the parents or siblings, does not lead to a clinical phenotype, suggesting that the mutant protein does not exert a dominant-negative effect or aggregate with wild-type protein in vivo.
At present, there is no definitive cure for L2HGA. Current management is primarily supportive and includes antiepileptic medication, L-carnitine supplementation (recommended dose: 50–100 mg/kg/day), physical therapy, rehabilitation, and specialized educational interventions. Only a few case reports [
13,
14,
15] have suggested potential clinical benefits from riboflavin alone or in combination with L-carnitine. However, systematic clinical evidence remains lacking. In the present study, the p.Pro302Leu variant identified in our patients resulted in (partial) loss of L2HGDH enzymatic activity, which is likely due to impaired mitochondrial import and impaired protein stability rather than directly affecting catalysis. This raises uncertainty regarding the therapeutic efficacy of conventional metabolic supplementation in the context of such variants. Given the observed mislocalization and presumed misfolding of the mutant protein, therapeutic strategies based on molecular chaperones may warrant further investigation. For instance, the cystic fibrosis drug Trikafta [
16], which combines two correctors to promote proper folding and trafficking of the F508del CFTR mutant and a potentiator to enhance channel activity, has significantly improved outcomes in patients with folding-defective variants. Whether similar strategies could be applicable to misfolded
L2HGDH variants remains to be explored. In addition, gene therapy represents a promising future avenue for L2HGA and may offer a viable treatment option as the field advances.