Expression of miRNA in the Semitendinosus Muscle of Cattle Breeds with Varying Intramuscular Fat Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Muscle Samples Collection
2.3. RNA Isolation and Validation
2.4. miRNA Microarray Analysis
2.5. Real-Time qPCR Procedure
2.6. Target Gene Prediction Protocol
2.7. Functional Analysis
2.8. Quantitative Trait Loci Annotation of Identified Genes
2.9. Statistical Analysis
3. Results
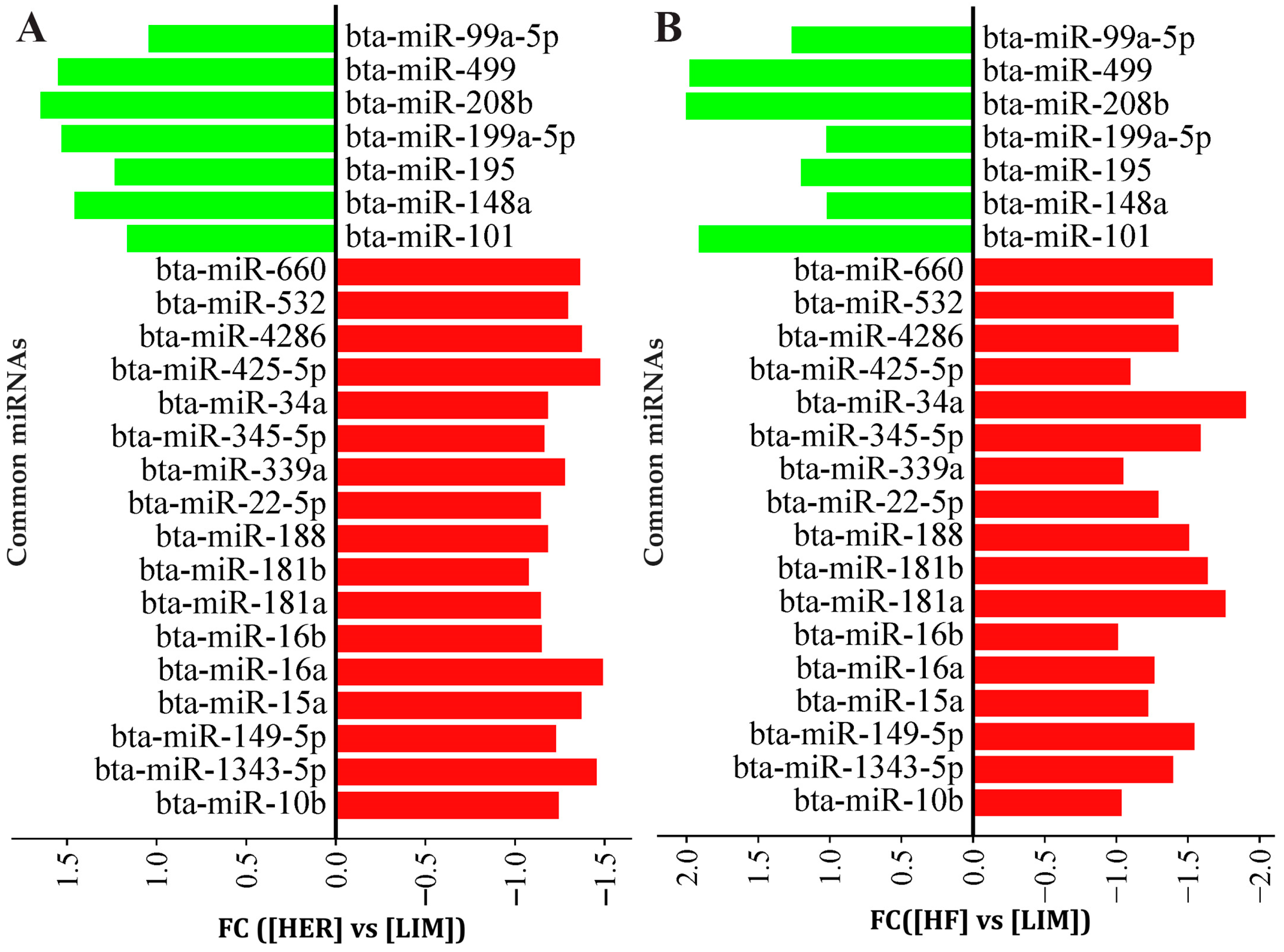
3.1. Microarray Analysis
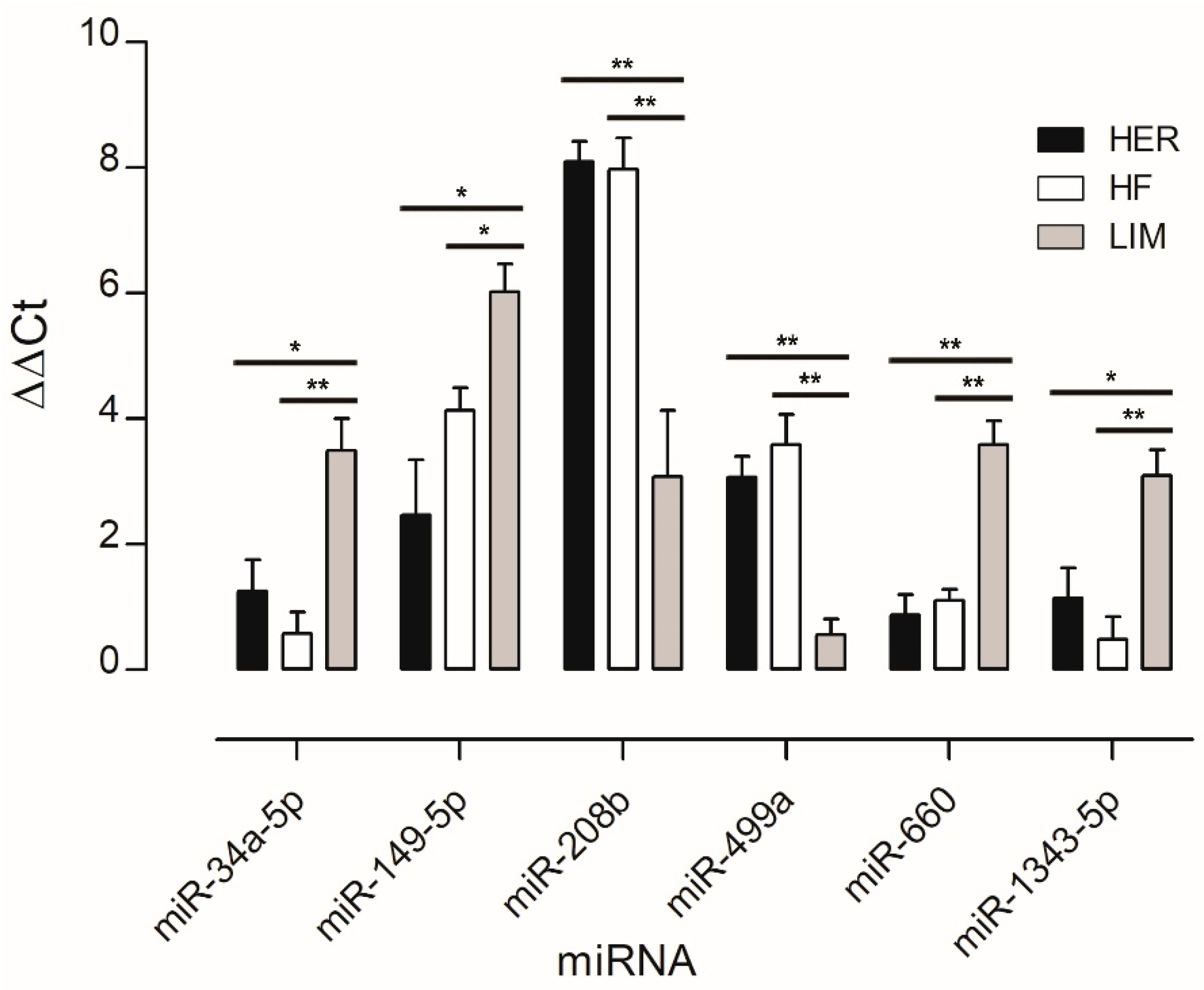
3.2. Real-Time qPCR Validation
3.3. Target Genes Predicted for Identified miRNAs
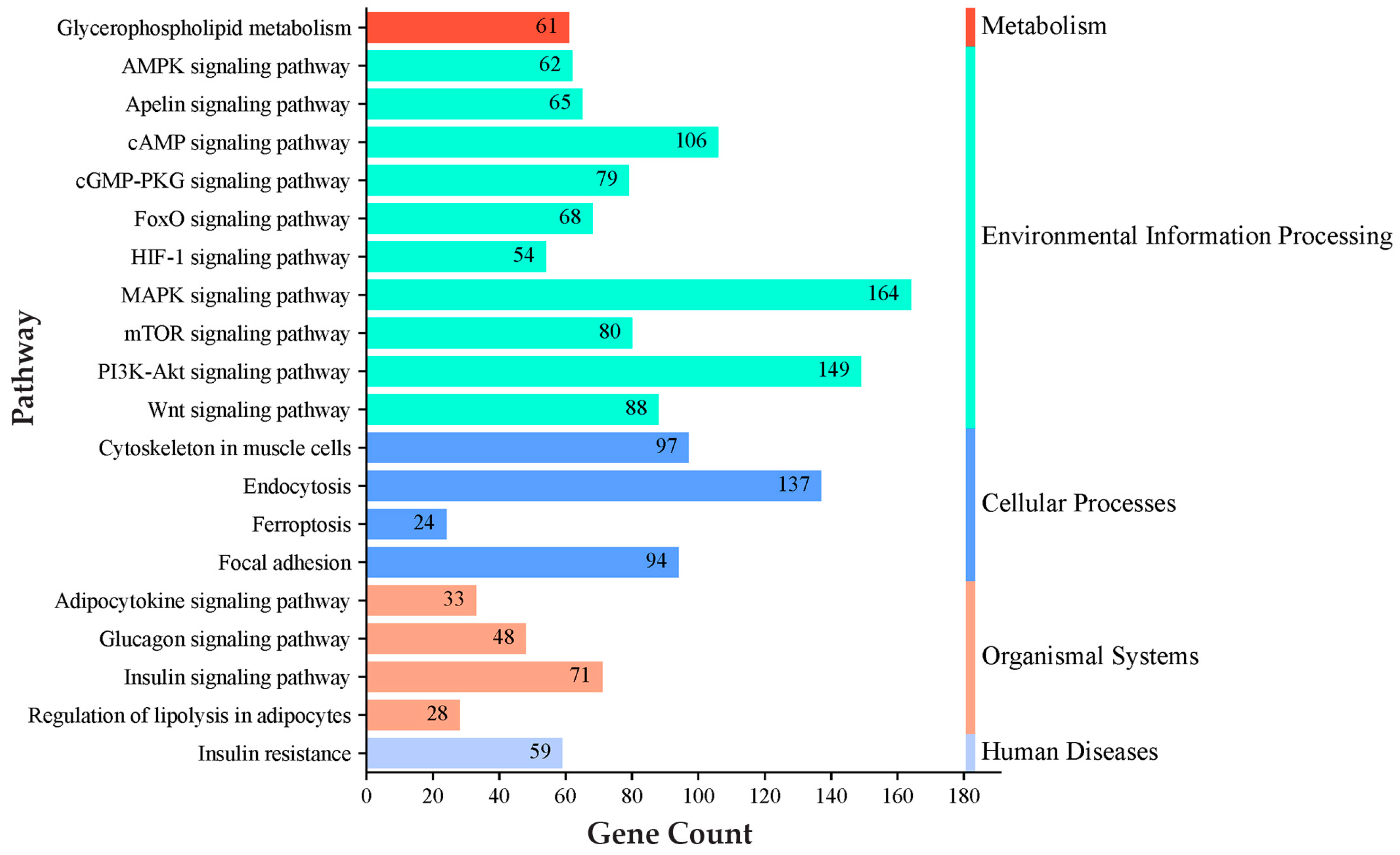
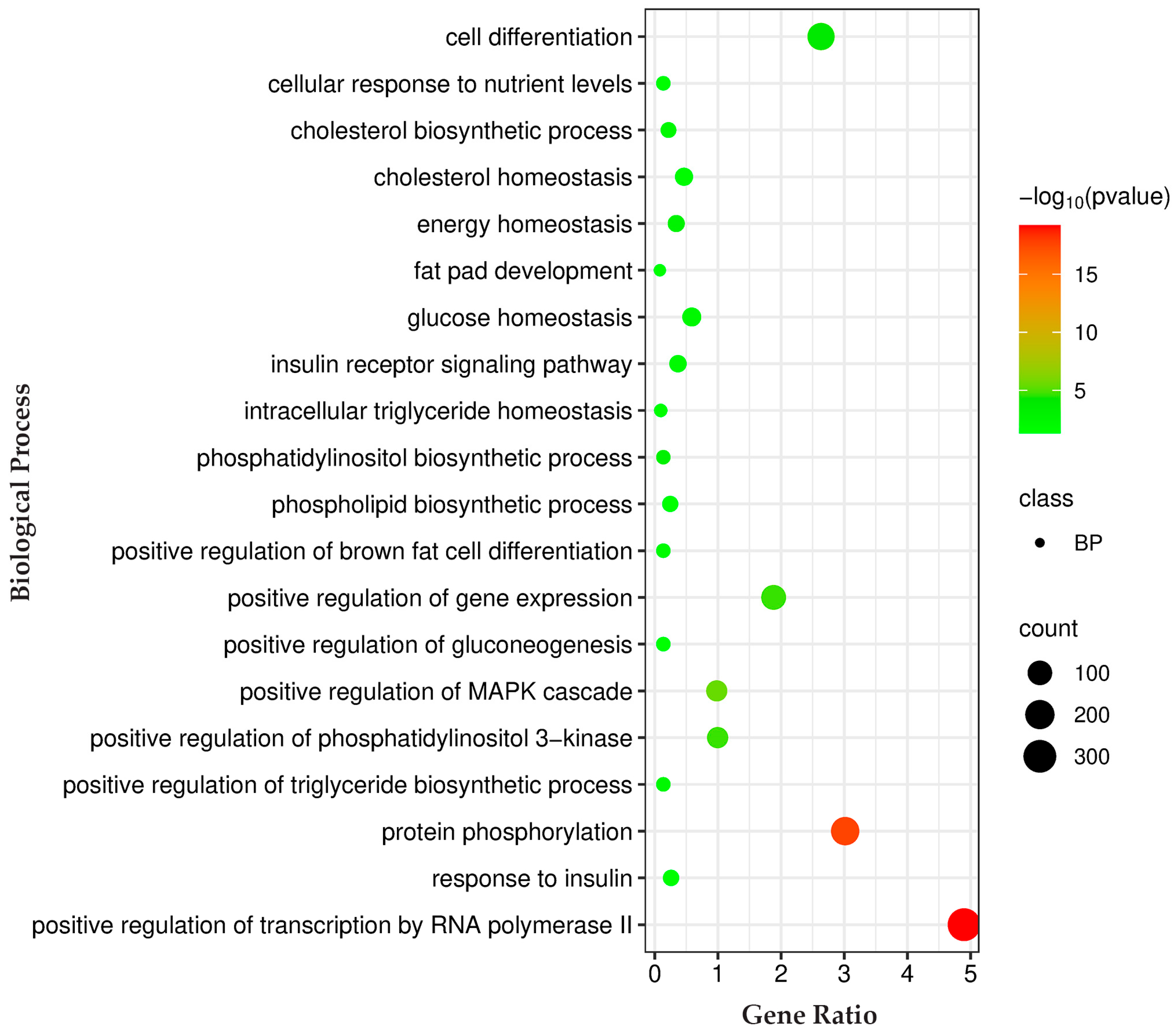
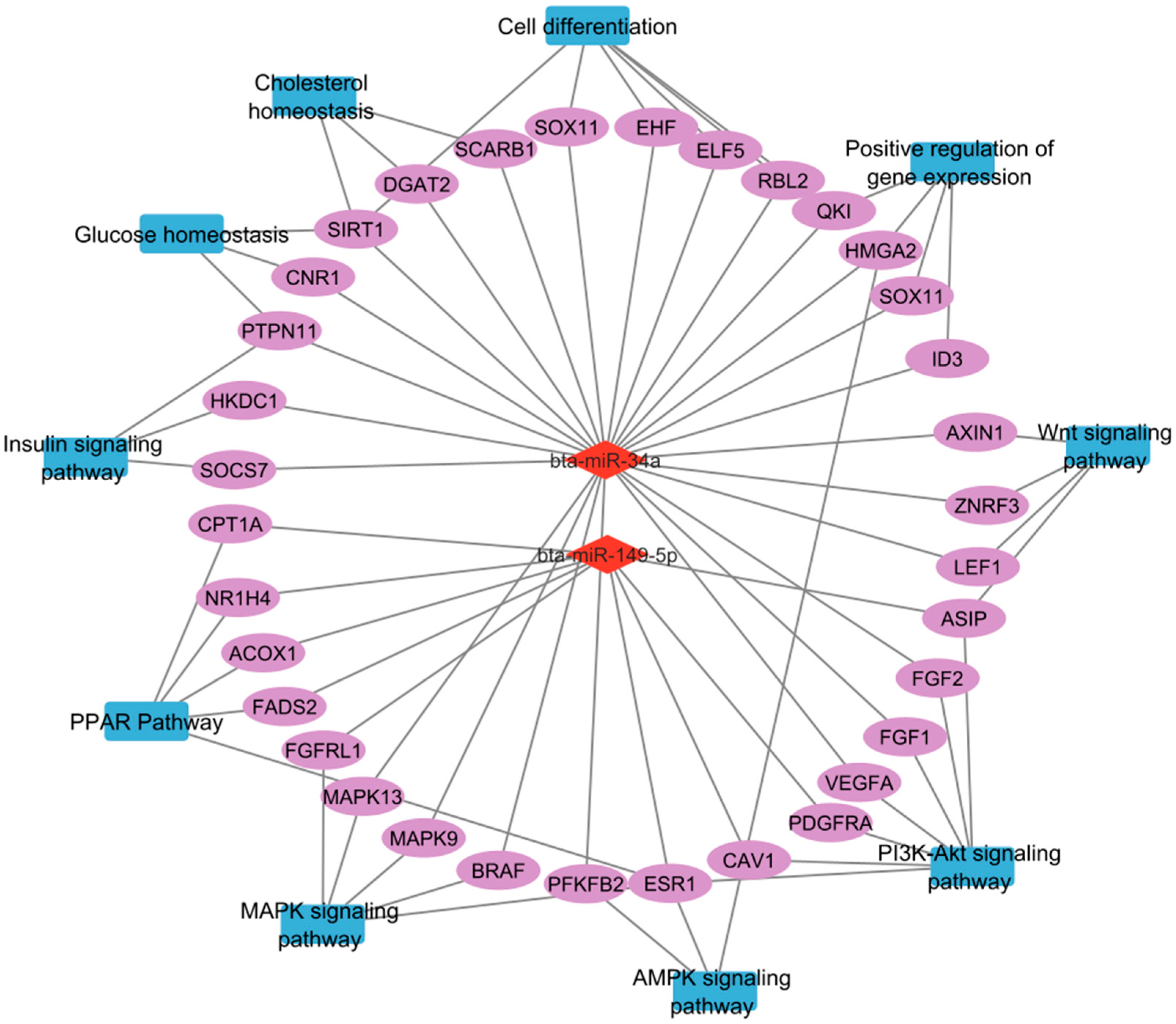
3.4. KEGG Pathway GO Enrichment Analysis
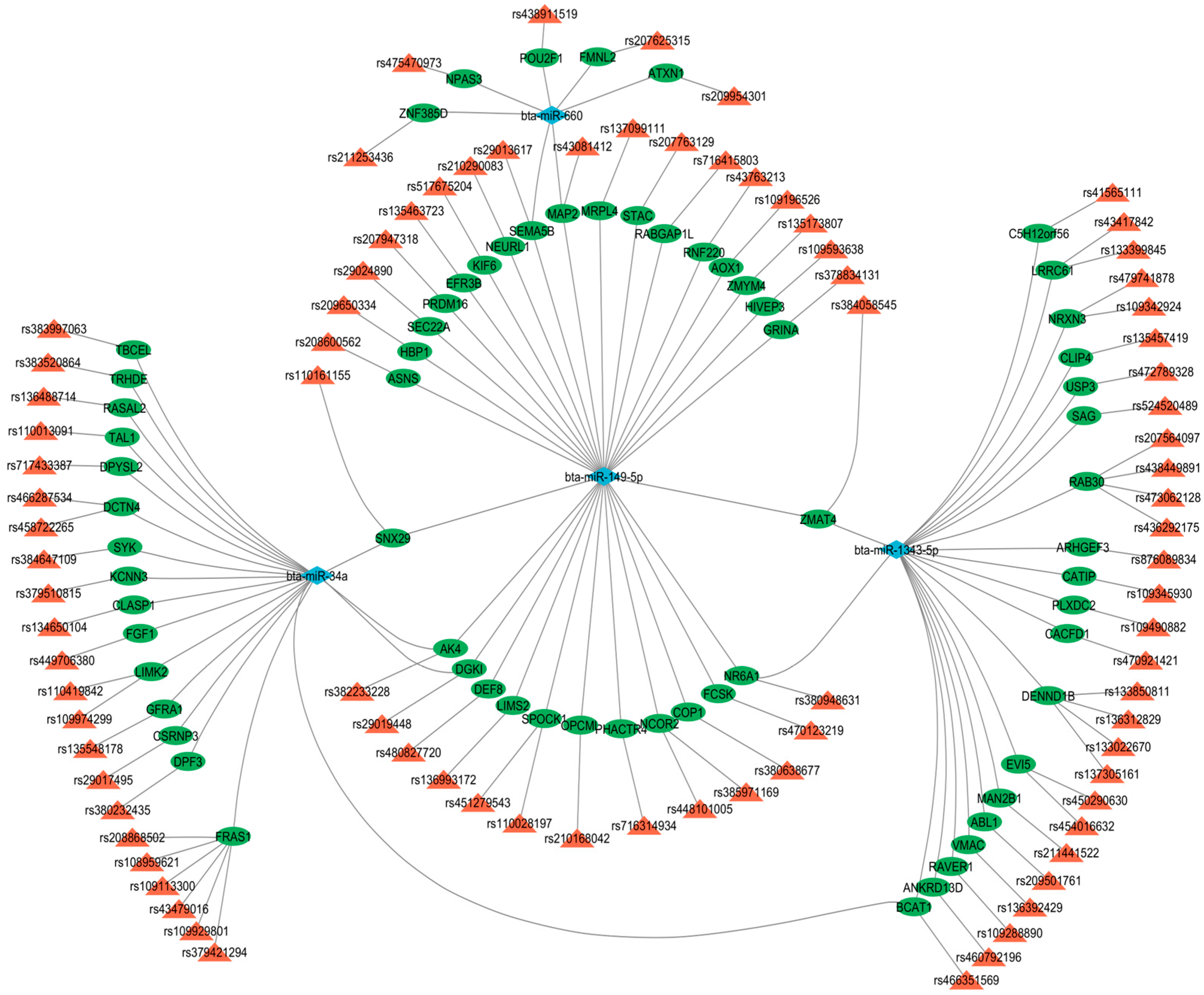
3.5. miRNA–Gene–QTL
4. Discussion
4.1. miR-34a and miR-149-5p as Key Regulators of IMF Deposition
4.2. Regulatory Roles of Other miRNAs in Adipogenesis and Muscle Remodeling
4.3. Integrative Analysis of miRNAs, Target Genes, and Trait-Associated QTLs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| ANOVA | Analysis of variance |
| ASIP | Agouti signaling protein |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase |
| CDNA | Complementary deoxyribonucleic acid |
| CY3 | Cyanine 3-pCp |
| ECM | Extracellular matrix |
| ESR1 | Estrogen receptor 1 |
| EU | European Union |
| FABP4 | Fatty acid-binding protein 4 |
| FC | Fold change |
| FDR | False Discovery Rate |
| FGF1 | Fibroblast growth factor 1 |
| FGF2 | Fibroblast growth factor 2 |
| FUS1 | Fused in sarcoma |
| GEO | Gene Expression Omnibus |
| GO | Gene Ontology |
| HER | Hereford |
| HF | Holstein-Friesian |
| HMGA2 | High-mobility group AT-hook 2 |
| IMF | Intramuscular fat |
| LIM | Limousin |
| MAPK | Mitogen-activated protein kinase |
| miRNAs | microRNAs |
| NCBI | National Center for Biotechnology Information |
| PI3K-AKT | Phosphoinositide 3-kinase-Akt |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| PRDM16 | PR/SET domain 16 |
| PTPN11 | Protein tyrosine phosphatase non-receptor type 11 |
| QPCR | Quantitative Polymerase Chain Reaction |
| QTL | Quantitative Trait Loci |
| RIN | RNA Integrity Number |
| SCD | Stearoyl-CoA desaturase |
| SEM | Standard error of the mean |
| SETDB1 | SET domain bifurcated histone lysine methyltransferase 1 |
| SIRT1 | Sirtuin 1 |
| SLC29A2 | Solute carrier family 29 member 2 |
| SNR-NA | Small nucleolar RNA |
| SUFU | Suppressor of fused homolog |
| SYBR | SYBR Green |
| TMR | Total mixed ration |
| TRIM32 | Tripartite motif containing 32 |
| VEGFA | Vascular endothelial growth factor A |
References
- Roudbari, Z.; Coort, S.L.; Kutmon, M.; Eijssen, L.; Melius, J.; Sadkowski, T.; Evelo, C.T. Identification of Biological Pathways Contributing to Marbling in Skeletal Muscle to Improve Beef Cattle Breeding. Front. Genet. 2019, 10, 1370. [Google Scholar] [CrossRef]
- Wandita, T.G.; Joshi, N.; Kim, H.H.; An, S.J.; Hwang, S.G. Pre-Adipocyte Determination and Adipocyte Differentiation of Stromal Vascular Cells Isolated From Intramuscular Tissue of Hanwoo Beef Cattle Treated by Acetate and Propionate. Trop. Anim. Sci. J. 2018, 41, 207–214. [Google Scholar] [CrossRef]
- Albrecht, E.; Teuscher, F.; Ender, K.; Wegner, J. Growth- and Breed-Related Changes of Marbling Characteristics in Cattle1. J. Anim. Sci. 2006, 84, 1067–1075. [Google Scholar] [CrossRef]
- Gotoh, T.; Albrecht, E.; Teuscher, F.; Kawabata, K.; Sakashita, K.; Iwamoto, H.; Wegner, J. Differences in Muscle and Fat Accretion in Japanese Black and European Cattle. Meat Sci. 2009, 82, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, A.; Birk, R. Adipose Tissue Hyperplasia and Hypertrophy in Common and Syndromic Obesity-The Case of BBS Obesity. Nutrients 2023, 15, 3445. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.X.; Yang, Z.P.; Shi, X.K.; Li, J.Y.; Ji, D.J.; Mao, Y.J.; Chang, L.L.; Gao, H.J. Association of SCD1 and DGAT1 SNPs with the Intramuscular Fat Traits in Chinese Simmental Cattle and Their Distribution in Eight Chinese Cattle Breeds. Mol. Biol. Rep. 2012, 39, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Sadkowski, T.; Ciecierska, A.; Majewska, A.; Oprządek, J.; Dasiewicz, K.; Ollik, M.; Wicik, Z.; Motyl, T. Transcriptional Background of Beef Marbling—Novel Genes Implicated in Intramuscular Fat Deposition. Meat Sci. 2014, 97, 32–41. [Google Scholar] [CrossRef]
- Fujimori, K.; Yoneda, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Maruyama, T.; Mizuno, H.; Sugiura, Y.; Morita, M. Detection of Salivary miRNAs Reflecting Chronic Periodontitis: A Pilot Study. Molecules 2019, 24, 1034. [Google Scholar] [CrossRef]
- Downie Ruiz Velasco, A.; Parsons, A.L.; Heatley, M.C.; Martin, A.R.G.; Smart, A.D.; Shah, N.; Jopling, C.L. MicroRNA Biogenesis Is Broadly Disrupted by Inhibition of the Splicing Factor SF3B1. Nucleic Acids Res. 2024, 52, 9210–9229. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Xia, J.; Jin, Y. Domestication of Transposable Elements into MicroRNA Genes in Plants. PLoS ONE 2011, 6, e19212. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Vernooy, S.Y.; Guo, M.; Hay, B.A. The Drosophila microRNA Mir-14 Suppresses Cell Death and Is Required for Normal Fat Metabolism. Curr. Biol. CB 2003, 13, 790–795. [Google Scholar] [CrossRef]
- Xie, H.; Lim, B.; Lodish, H.F. MicroRNAs Induced During Adipogenesis That Accelerate Fat Cell Development Are Downregulated in Obesity. Diabetes 2009, 58, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Ma, K.; Zhang, Z.; Zhao, T.; Zhang, X.; Tang, B.; Li, Z. miR-17, miR-21, and miR-143 Enhance Adipogenic Differentiation from Porcine Bone Marrow-Derived Mesenchymal Stem Cells. DNA Cell Biol. 2016, 35, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, B.; Fatica, A.; Sorci, M.; Sorrentino, A.; Signore, M.; Cerio, A.; Felicetti, F.; Feo, A.D.; Pelosi, E.; Caré, A.; et al. Effect of miR-204&211 and RUNX2 Control on the Fate of Human Mesenchymal Stromal Cells. Regen. Med. Res. 2017, 5, 2. [Google Scholar] [CrossRef]
- Jin, W.; Dodson, M.V.; Moore, S.S.; Basarab, J.A.; Guan, L.L. Characterization of microRNA Expression in Bovine Adipose Tissues: A Potential Regulatory Mechanism of Subcutaneous Adipose Tissue Development. BMC Mol. Biol. 2010, 11, 29. [Google Scholar] [CrossRef]
- Gerin, I.; Bommer, G.T.; McCoin, C.S.; Sousa, K.M.; Krishnan, V.; MacDougald, O.A. Roles for miRNA-378/378* in Adipocyte Gene Expression and Lipogenesis. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E198–E206. [Google Scholar] [CrossRef]
- Lee, D.Y.; Deng, Z.; Wang, C.-H.; Yang, B.B. MicroRNA-378 Promotes Cell Survival, Tumor Growth, and Angiogenesis by Targeting SuFu and Fus-1 Expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20350–20355. [Google Scholar] [CrossRef]
- Romao, J.M.; Jin, W.; He, M.; McAllister, T.; Guan, L.L. Altered MicroRNA Expression in Bovine Subcutaneous and Visceral Adipose Tissues from Cattle under Different Diet. PLoS ONE 2012, 7, e40605. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Y.; Wang, G.; Li, H. Identification of microRNA and Bioinformatics Target Gene Analysis in Beef Cattle Intramuscular Fat and Subcutaneous Fat. Mol. Biosyst. 2013, 9, 2154–2162. [Google Scholar] [CrossRef]
- Mir, B.A.; Reyer, H.; Komolka, K.; Ponsuksili, S.; Kühn, C.; Maak, S. Differentially Expressed miRNA-Gene Targets Related to Intramuscular Fat in Musculus Longissimus Dorsi of Charolais × Holstein F2-Crossbred Bulls. Genes 2020, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.-F.; Ellies-Oury, M.-P.; Lherm, M.; Pineau, C.; Deblitz, C.; Farmer, L. Current Situation and Future Prospects for Beef Production in Europe—A Review. Asian-Australas. J. Anim. Sci. 2018, 31, 1017. [Google Scholar] [CrossRef] [PubMed]
- Sadkowski, T.; Jank, M.; Zwierzchowski, L.; Oprzadek, J.; Motyl, T. Transcriptomic Index of Skeletal Muscle of Beef Breeds Bulls. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2009, 60 (Suppl. 1), 15–28. [Google Scholar]
- Sadkowski, T.; Ciecierska, A.; Oprządek, J.; Balcerek, E. Breed-Dependent microRNA Expression in the Primary Culture of Skeletal Muscle Cells Subjected to Myogenic Differentiation. BMC Genomics 2018, 19, 109. [Google Scholar] [CrossRef]
- Ntumi, S. Reporting and Interpreting One-Way Analysis of Variance (ANOVA) Using a Data-Driven Example: A Practical Guide for Social Science Researchers. J. Res. Educ. Sci. 2021, 12, 38–47. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, S.; Cao, S.; Arhatte, M.; Li, D.; Shi, Y.; Kurz, S.; Hu, J.; Wang, L.; Shao, J.; Atzberger, A.; et al. Adipocyte Piezo1 Mediates Obesogenic Adipogenesis through the FGF1/FGFR1 Signaling Pathway in Mice. Nat. Commun. 2020, 11, 2303. [Google Scholar] [CrossRef]
- Tan, Z.; Jiang, H. Molecular and Cellular Mechanisms of Intramuscular Fat Development and Growth in Cattle. Int. J. Mol. Sci. 2024, 25, 2520. [Google Scholar] [CrossRef]
- Duan, K.; Ge, Y.-C.; Zhang, X.-P.; Wu, S.-Y.; Feng, J.-S.; Chen, S.-L.; Zhang, L.I.; Yuan, Z.-H.; Fu, C.-H. miR-34a Inhibits Cell Proliferation in Prostate Cancer by Downregulation of SIRT1 Expression. Oncol. Lett. 2015, 10, 3223–3227. [Google Scholar] [CrossRef]
- Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. miR-34a Repression of SIRT1 Regulates Apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13421–13426. [Google Scholar] [CrossRef] [PubMed]
- Moisá, S.J.; Shike, D.W.; Shoup, L.; Loor, J.J. Maternal Plane of Nutrition During Late-Gestation and Weaning Age Alter Steer Calf Longissimus Muscle Adipogenic MicroRNA and Target Gene Expression. Lipids 2016, 51, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.B.; Gionbelli, M.P.; Rodrigues, R.T.S.; Bonilha, S.F.M.; Newbold, C.J.; Guimarães, S.E.F.; Silva, W.; Verardo, L.L.; Silva, F.F.; Detmann, E.; et al. Differentially Expressed mRNAs, Proteins and miRNAs Associated to Energy Metabolism in Skeletal Muscle of Beef Cattle Identified for Low and High Residual Feed Intake. BMC Genomics 2019, 20, 501. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Tabassum, S.; Ahmad, A. MicroRNA-34a: A Versatile Regulator of Myriads of Targets in Different Cancers. Int. J. Mol. Sci. 2017, 18, 2089. [Google Scholar] [CrossRef]
- Lal, A.; Thomas, M.P.; Altschuler, G.; Navarro, F.; O’Day, E.; Li, X.L.; Concepcion, C.; Han, Y.-C.; Thiery, J.; Rajani, D.K.; et al. Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling. PLoS Genet. 2011, 7, e1002363. [Google Scholar] [CrossRef]
- Ghandadi, M.; Sahebkar, A. MicroRNA-34a and Its Target Genes: Key Factors in Cancer Multidrug Resistance. Curr. Pharm. Des. 2016, 22, 933–939. [Google Scholar] [CrossRef]
- Hu, G.; Do, D.N.; Davoudi, P.; Miar, Y. Emerging Roles of Non-Coding RNAs in the Feed Efficiency of Livestock Species. Genes 2022, 13, 297. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Ding, N.; Teng, J.; Zhang, S.; Zhang, Q.; Tang, H. miR-34a Regulates Adipogenesis in Porcine Intramuscular Adipocytes by Targeting ACSL4. BMC Genet. 2020, 21, 33. [Google Scholar] [CrossRef]
- Zhao, T.; Li, J.; Chen, A.F. MicroRNA-34a Induces Endothelial Progenitor Cell Senescence and Impedes Its Angiogenesis via Suppressing Silent Information Regulator 1. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E110–E116. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Tang, K.; Wang, Y.; Zan, L.; Yang, W. Bta-miR-34b Regulates Milk Fat Biosynthesis by Targeting mRNA Decapping Enzyme 1A (DCP1A) in Cultured Bovine Mammary Epithelial Cells1. J. Anim. Sci. 2019, 97, 3823–3831. [Google Scholar] [CrossRef]
- Guo, H.; Khan, R.; Abbas Raza, S.H.; Suhail, S.M.; Khan, H.; Khan, S.B.; Abd El-Aziz, A.H.; Zan, L. RNA-Seq Reveals Function of Bta-miR-149-5p in the Regulation of Bovine Adipocyte Differentiation. Animals 2021, 11, 1207. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, I.; Lee, C.; Kim, D.; Kim, S.; Lee, Y. SNPs in microRNA Seed Region and Impact of miR-375 in Concurrent Regulation of Multiple Lipid Accumulation-Related Genes. Sci. Rep. 2024, 14, 10924. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Y.; Dou, Y.; Li, C.; Song, C.; Zhang, Z.; Qi, K.; Li, X.; Qiao, R.; Wang, K.; et al. Effect of miR-149-5p on Intramuscular Fat Deposition in Pigs Based on Metabolomics and Transcriptomics. BMC Genomics 2023, 24, 293. [Google Scholar] [CrossRef]
- Shao, J.; Pan, T.; Wang, J.; Tang, T.; Li, Y.; Jia, X.; Lai, S. MiR-208b Regulates Rabbit Preadipocyte Proliferation and Differentiation. Genes 2021, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Liu, T.; Yousuf, S.; Zhang, X.; Huang, W.; Li, A.; Xie, L.; Miao, X. Identification of Potential miRNA-mRNA Regulatory Network and the Key miRNAs in Intramuscular and Subcutaneous Adipose. Front. Vet. Sci. 2022, 9, 976603. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Zhang, M.; Shan, Y.-J.; Pang, L.-C.; Ji, G.-G.; Ju, X.-J.; Tu, Y.-J.; Shi, S.-Y.; Bai, H.; Zou, J.-M. Transcriptome Sequencing Analysis of the Role of miR-499-5p and SOX6 in Chicken Skeletal Myofiber Specification. Front. Genet. 2022, 13, 1008649. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, X.; Zhou, D.; Lai, L.; Xiao, L.; Liu, L.; Fu, T.; Kong, Y.; Zhou, Q.; Vega, R.B.; et al. Coupling of Mitochondrial Function and Skeletal Muscle Fiber Type by a miR-499/Fnip1/ AMPK Circuit. EMBO Mol. Med. 2016, 8, 1212–1228. [Google Scholar] [CrossRef]
- Xie, Y.; Li, J.; Kang, R.; Tang, D. Interplay between Lipid Metabolism and Autophagy. Front. Cell Dev. Biol. 2020, 8, 431. [Google Scholar] [CrossRef]
- Guo, Y.; Ding, X.; Dai, C.; Wang, W.; Chen, J.; Chen, S.; Yang, L.; Chen, G. miR-1343-3p Inhibits Autophagy by Directly Targeting ATG7 in Multiple Myeloma Cells. Biomed. Rep. 2024, 21, 185. [Google Scholar] [CrossRef]
- Chen, C.; Deng, B.; Qiao, M.; Zheng, R.; Chai, J.; Ding, Y.; Peng, J.; Jiang, S. Solexa Sequencing Identification of Conserved and Novel microRNAs in Backfat of Large White and Chinese Meishan Pigs. PLoS ONE 2012, 7, e31426. [Google Scholar] [CrossRef]
- De Oliveira, P.S.N.; Coutinho, L.L.; Tizioto, P.C.; Cesar, A.S.M.; de Oliveira, G.B.; Diniz, W.J.d.S.; De Lima, A.O.; Reecy, J.M.; Mourão, G.B.; Zerlotini, A.; et al. An Integrative Transcriptome Analysis Indicates Regulatory mRNA-miRNA Networks for Residual Feed Intake in Nelore Cattle. Sci. Rep. 2018, 8, 17072. [Google Scholar] [CrossRef]
- Fang, L.; Sørensen, P.; Sahana, G.; Panitz, F.; Su, G.; Zhang, S.; Yu, Y.; Li, B.; Ma, L.; Liu, G.; et al. MicroRNA-Guided Prioritization of Genome-Wide Association Signals Reveals the Importance of microRNA-Target Gene Networks for Complex Traits in Cattle. Sci. Rep. 2018, 8, 9345. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Li, A.; Raza, S.H.A. Editorial: Genetic Regulation of Meat Quality Traits in Livestock Species. Front. Genet. 2022, 13, 1092562. [Google Scholar] [CrossRef]
- Huang, J.; Wang, S.; Feng, X.; Liu, X.; Zhao, J.; Zheng, Q.; Wei, X.; Ma, Y. miRNA Transcriptome Comparison between Muscle and Adipose Tissues Indicates Potential miRNAs Associated with Intramuscular Fat in Chinese Swamp Buffalo. Genome 2019, 62, 729–738. [Google Scholar] [CrossRef]
- Chengcheng, L.; Raza, S.H.A.; Zhimei, Y.; Sihu, W.; Shengchen, Y.; Aloufi, B.H.; Bingzhi, L.; Zan, L. Bta-miR-181d and Bta-miR-196a Mediated Proliferation, Differentiation, and Apoptosis in Bovine Myogenic Cells. J. Anim. Sci. 2024, 102, skae142. [Google Scholar] [CrossRef]
- Yu, X.; Fang, X.; Gao, M.; Mi, J.; Zhang, X.; Xia, L.; Zhao, Z.; Albrecht, E.; Maak, S.; Yang, R. Isolation and Identification of Bovine Preadipocytes and Screening of MicroRNAs Associated with Adipogenesis. Animals 2020, 10, 818. [Google Scholar] [CrossRef]
- Zeng, H.; Li, S.; Chang, H.; Zhai, Y.; Wang, H.; Weng, H.; Han, Z. Circ_002033 Regulates Proliferation, Apoptosis, and Oxidative Damage of Bovine Mammary Epithelial Cells via the miR-199a-5p-MAP3K11 Axis in Heat Stress. J. Agric. Food Chem. 2024, 72, 14386–14401. [Google Scholar] [CrossRef]
- Dehghanian Reyhan, V.; Ghafouri, F.; Sadeghi, M.; Miraei-Ashtiani, S.R.; Kastelic, J.P.; Barkema, H.W.; Shirali, M. Integrated Comparative Transcriptome and circRNA-lncRNA-miRNA-mRNA ceRNA Regulatory Network Analyses Identify Molecular Mechanisms Associated with Intramuscular Fat Content in Beef Cattle. Animals 2023, 13, 2598. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, R.; Lu, X.; Liu, Y.; He, W.; Li, Y.; Yu, H.; Qin, L.; Cao, Y.; Zhao, Z.; et al. RNA-Seq Analysis Identifies Differentially Expressed Genes in the Longissimus Dorsi of Wagyu and Chinese Red Steppe Cattle. Int. J. Mol. Sci. 2022, 24, 387. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, E.; Kang, Z.; Bi, Y.; Liu, H.; Xu, H.; Wang, Z.; Lei, C.; Chen, H.; Lan, X. CircRNA Profiling Reveals an Abundant circBDP1 That Regulates Bovine Fat Development by Sponging miR-181b/miR-204 Targeting Sirt1/TRARG1. J. Agric. Food Chem. 2022, 70, 14312–14328. [Google Scholar] [CrossRef]
- Jilo, D.D.; Abebe, B.K.; Wang, J.; Guo, J.; Li, A.; Zan, L. Long Non-Coding RNA (LncRNA) and Epigenetic Factors: Their Role in Regulating the Adipocytes in Bovine. Front. Genet. 2024, 15, 1405588. [Google Scholar] [CrossRef] [PubMed]
- Leal-Gutiérrez, J.D.; Rezende, F.M.; Reecy, J.M.; Kramer, L.M.; Peñagaricano, F.; Mateescu, R.G. Whole Genome Sequence Data Provides Novel Insights Into the Genetic Architecture of Meat Quality Traits in Beef. Front. Genet. 2020, 11, 538640. [Google Scholar] [CrossRef]
- Cui, S.; Li, X.; Li, R.; Zhang, H.; Wang, Y.; Li, Y.; Zhu, J.; Li, Z.; Lin, Y. FGF1 Promotes the Differentiation of Goat Intramuscular and Subcutaneous Preadipocytes. Anim. Biotechnol. 2023, 34, 1196–1208. [Google Scholar] [CrossRef]
- Liu, R.; Fang, X.; Lu, X.; Liu, Y.; Li, Y.; Bai, X.; Ding, X.; Yang, R. Polymorphisms of the SCD1 Gene and Its Association Analysis with Carcass, Meat Quality, Adipogenic Traits, Fatty Acid Composition, and Milk Production Traits in Cattle. Animals 2024, 14, 1759. [Google Scholar] [CrossRef] [PubMed]
- Wuensch, T.; Wizenty, J.; Quint, J.; Spitz, W.; Bosma, M.; Becker, O.; Adler, A.; Veltzke-Schlieker, W.; Stockmann, M.; Weiss, S.; et al. Expression Analysis of Fibronectin Type III Domain-Containing (FNDC) Genes in Inflammatory Bowel Disease and Colorectal Cancer. Gastroenterol. Res. Pract. 2019, 2019, 3784172. [Google Scholar] [CrossRef]
- Lim, K.-S.; Kim, J.-M.; Lee, E.-A.; Choe, J.-H.; Hong, K.-C. A Candidate Single Nucleotide Polymorphism in the 3’ Untranslated Region of Stearoyl-CoA Desaturase Gene for Fatness Quality and the Gene Expression in Berkshire Pigs. Asian-Australas. J. Anim. Sci. 2015, 28, 151–157. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, U.; Tury, S.; Nicolas, G.; Wilson, M.S.; Jurici, S.; Ayrignac, X.; Courgnaud, V.; Saiardi, A.; Sitbon, M.; Battini, J.-L. Interplay between Primary Familial Brain Calcification-Associated SLC20A2 and XPR1 Phosphate Transporters Requires Inositol Polyphosphates for Control of Cellular Phosphate Homeostasis. J. Biol. Chem. 2020, 295, 9366–9378. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, L.; Dai, H.; Hu, P.; Wang, B.; Han, Q.; Xu, Y.; Lv, S.; Zhu, Y.; Gan, M.; et al. The Role of Vesicular Overexpressed in Cancer Pro-Survival Protein 1 in Hepatocellular Carcinoma Proliferation. Cancer Biomark. Sect. Dis. Markers 2020, 28, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, R.G.; Garrick, D.J.; Reecy, J.M. Network Analysis Reveals Putative Genes Affecting Meat Quality in Angus Cattle. Front. Genet. 2017, 8, 171. [Google Scholar] [CrossRef]
- Malgwi, I.H.; Halas, V.; Grünvald, P.; Schiavon, S.; Jócsák, I. Genes Related to Fat Metabolism in Pigs and Intramuscular Fat Content of Pork: A Focus on Nutrigenetics and Nutrigenomics. Animals 2022, 12, 150. [Google Scholar] [CrossRef]

| Primer | Target Sequence | Accession |
|---|---|---|
| bta-miR-34a LNA™ PCR primer set, UniRT | UGGCAGUGUCUUAGCUGGUUGU | MIMAT0004340 |
| bta-miR-149-5p LNA™ PCR primer set, UniRT | UCUGGCUCCGUGUCUUCACUCCC | MIMAT0024570 |
| bta-miR-208b LNA™ PCR primer set, UniRT | AUAAGACGAACAAAAGGUUUGU | MIMAT0009262 |
| bta-miR-499 LNA™ PCR primer set, UniRT | UUAAGACUUGCAGUGAUGUUU | MIMAT0003536 |
| bta-miR-660 LNA™ PCR primer set, UniRT | UACCCAUUGCAUAUCGGAGCUG | MIMAT0004344 |
| bta-miR-1343-5p LNA™ PCR primer set, UniRT | UGGGGAGCGGCCCCCGGGCGGG | MIMAT0011839 |
| U6 snRNA LNA™ PCR primer set, UniRT (reference) | GUGCUCGCUUCGGCAGCACAUAU ACUAAAAUUGGAACGAUACAGAG AAGAUUAGCAUGGCCCCUGCGCA AGGAUGACACGCAAAUUCGUGAA GCGUUCCAUAUUUUU | #203907 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciecierska, A.; Gorji, A.E.; Majewska, A.; Sadkowski, T. Expression of miRNA in the Semitendinosus Muscle of Cattle Breeds with Varying Intramuscular Fat Deposition. Genes 2025, 16, 969. https://doi.org/10.3390/genes16080969
Ciecierska A, Gorji AE, Majewska A, Sadkowski T. Expression of miRNA in the Semitendinosus Muscle of Cattle Breeds with Varying Intramuscular Fat Deposition. Genes. 2025; 16(8):969. https://doi.org/10.3390/genes16080969
Chicago/Turabian StyleCiecierska, Anna, Abdolvahab Ebrahimpour Gorji, Alicja Majewska, and Tomasz Sadkowski. 2025. "Expression of miRNA in the Semitendinosus Muscle of Cattle Breeds with Varying Intramuscular Fat Deposition" Genes 16, no. 8: 969. https://doi.org/10.3390/genes16080969
APA StyleCiecierska, A., Gorji, A. E., Majewska, A., & Sadkowski, T. (2025). Expression of miRNA in the Semitendinosus Muscle of Cattle Breeds with Varying Intramuscular Fat Deposition. Genes, 16(8), 969. https://doi.org/10.3390/genes16080969






