Mechano-Signal Transduction Pathways of the Diaphragmatic Muscle and Role of Cytoskeleton
Abstract
1. Introduction
2. Diaphragm Mechanics
2.1. The Unique Structure of the Diaphragm Muscle
2.2. The Specific Role of the Diaphragm Muscle
2.3. In Vivo Biaxial Loading of the Diaphragm
3. Role of Cytoskeletal Proteins in Diaphragm Mechanics
3.1. Cytoskeletal Proteins
3.2. Desmin’s Role in the Mechanotransduction Pathways of the Diaphragm Muscle
3.3. Role of α-Sarcoglycan in Modulating the Mechanical Properties of the Diaphragm Muscle
3.4. Dystrophin and Its Role in the Mechanotransduction of the Diaphragm Muscle
3.5. Role of Integrins in the Mechanotransduction of the Diaphragm Muscle
3.6. Titin’s Role in Diaphragm Muscle Mechanotransduction
4. Anisotropic Mechanotransduction Pathways of the Diaphragmatic Muscle
4.1. Anisotropic Regulation of Signaling Pathways in Diaphragm Muscle
4.2. Anisotropic Regulation of Gene Expression in Diaphragm Muscle
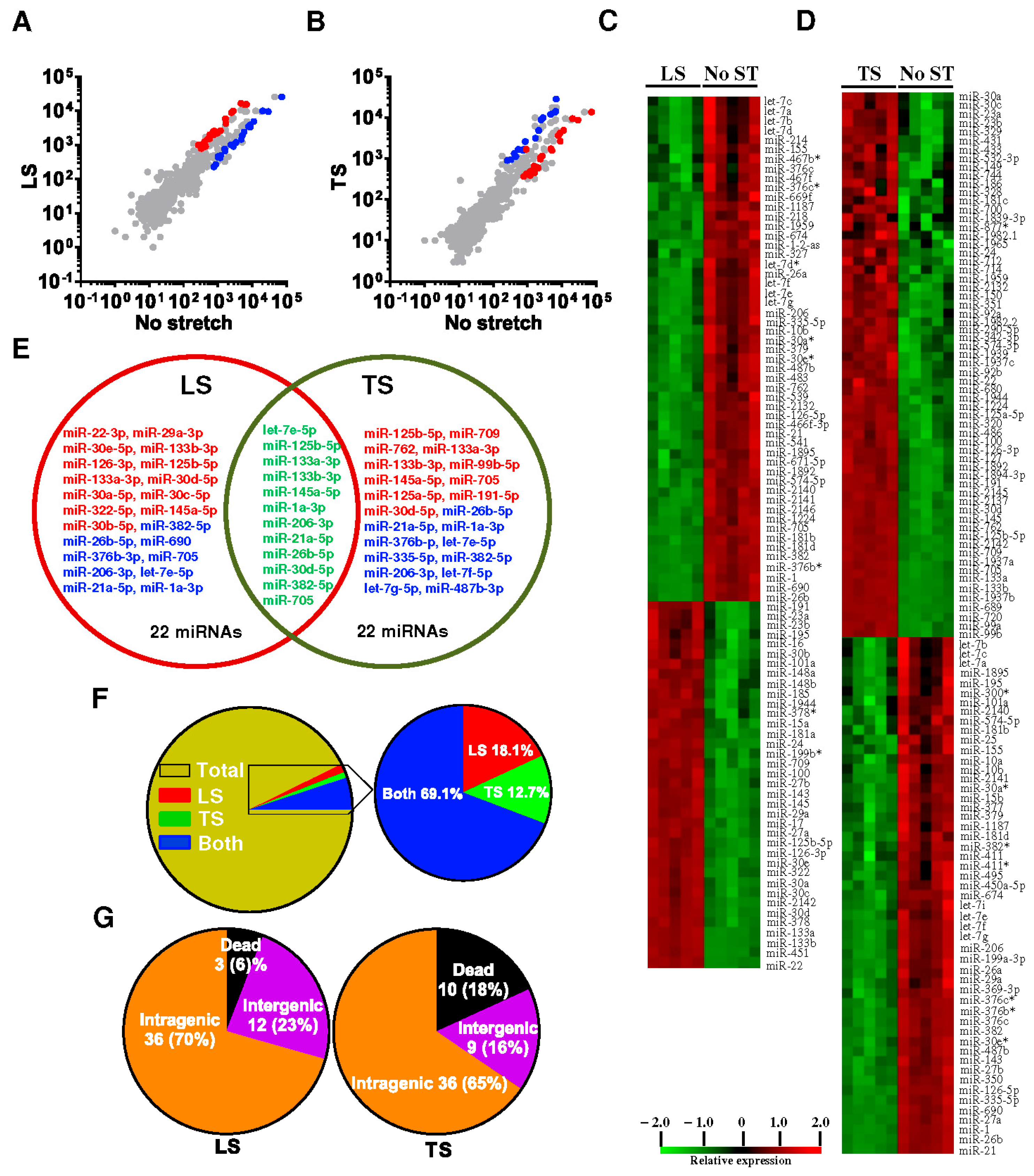
4.3. Anisotropic Regulation of mechanomiRs in Diaphragm Muscle
4.4. Mechanoregulation of SIRT1 Gene Expression in Diaphragm Muscle
4.5. Mechanical Dysfunction of the Diaphragm Muscle and Its Clinical Implications
5. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Jaalouk, D.E.; Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 2009, 10, 63–73. [Google Scholar] [CrossRef]
- Hahn, C.; Schwartz, M.A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 2009, 10, 53–62. [Google Scholar] [CrossRef]
- Miyasaka, K.; Kida, Y.; Ogura, T. Mechanotransduction in cardiovascular and skeletal muscle. Seikagaku 2009, 81, 494–501. [Google Scholar] [PubMed]
- Ottenheijm, C.A.; van Hees, H.W.; Heunks, L.M.; Granzier, H. Titin-based mechanosensing and signaling: Role in diaphragm atrophy during unloading? Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L161–L166. [Google Scholar] [CrossRef]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef]
- Bains, K.N.S.; Kashyap, S.; Lappin, S.L. Anatomy, Thorax: Diaphragm; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Boriek, A.M.; Wilson, T.A.; Rodarte, J.R. Displacements and Strains in the Costal Diaphragm of the Dog. J. Appl. Physiol. 1994, 76, 223–229. [Google Scholar] [CrossRef]
- Boriek, A.M.; Liu, S.; Rodarte, J.R. Costal diaphragm curvature in the dog. J. Appl. Physiol. 1993, 75, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Boriek, A.M.; Rodarte, J.R. Effects of transverse fiber stiffness and central tendon on displacement and shape of a simple diaphragm model. J. Appl. Physiol. 1997, 82, 1626–1636. [Google Scholar] [CrossRef]
- Amancharla, M.R.; Rodarte, J.R.; Boriek, A.M. Modeling the kinematics of the canine midcostal diaphragm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R588–R597. [Google Scholar] [CrossRef] [PubMed]
- Boriek, A.M.; Kelly, N.G.; Rodarte, J.R.; Wilson, T.A. Biaxial constitutive relations for the passive canine diaphragm. J. Appl. Physiol. 2000, 89, 2187–2190. [Google Scholar] [CrossRef]
- Boriek, A.M.; Rodarte, J.R.; Reid, M.B. Shape and tension distribution of the passive rat diaphragm. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R33–R41. [Google Scholar] [CrossRef]
- Boriek, A.M.; Capetanaki, Y.; Hwang, W.; Officer, T.; Badshah, M.; Rodarte, J.; Tidball, J.G. Desmin integrates the three-dimensional mechanical properties of muscles. Am. J. Physiol. Cell Physiol. 2001, 280, C46–C52. [Google Scholar] [CrossRef]
- Trinick, J. Elastic filaments and giant proteins in muscle. Curr. Opin. Cell Biol. 1991, 3, 112–119. [Google Scholar] [CrossRef]
- Tidball, J.G. Force transmission across muscle cell membranes. J. Biomech. 1991, 24, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.S.; Lei, G.T.; Farkas, G.A.; Rodarte, J.R. Finite-element analysis of stress in the canine diaphragm. J. Appl. Physiol. 1994, 76, 2070–2075. [Google Scholar] [CrossRef]
- Jannapureddy, S.R.; Patel, N.D.; Hwang, W.; Boriek, A.M. Merosin deficiency leads toalterations in passive and active skeletal muscle mechanics. Appl. Physiol. 2003, 94, 2524–2533. [Google Scholar] [CrossRef]
- Tokuyasu, K.T.; Dutton, A.H.; Singer, S.J. Immunoelectron Microscopic Studies of Desmin (Skeletin) Localization and Intermediate Filament Organization in Chicken Cardiac-Muscle. J. Cell Biol. 1983, 96, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Price, M.G.; Sanger, J.W. Intermediate Filaments in Striated Muscle. A Review of Structural Studies in Embryonic and Adult Skeletal and Cardiac Muscle. In Cell and Muscle Motility; Springer: Boston, MA, USA, 1983; Volume 3, pp. 1–40. [Google Scholar]
- Hack, A.A.; Groh, M.E.; McNally, E.M. Sarcoglycans in muscular dystrophy. Microsc. Res. Tech. 2000, 48, 167–180. [Google Scholar] [CrossRef]
- Duclos, F.; Straub, V.; Moore, S.A.; Venzke, D.P.; Hrstka, R.F.; Crosbie, R.H.; Durbeej, M.; Lebakken, C.S.; Ettinger, A.J.; van der Meulen, J.; et al. Progressive muscular dystrophy in alpha-sarcoglycan-deficient mice. J. Cell Biol. 1998, 142, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.A.; Engvall, E. Sarcoglycan isoforms in skeletal muscle. J. Biol. Chem. 1999, 274, 38171–38176. [Google Scholar] [CrossRef]
- Patel, N.D.; Jannapureddy, S.R.; Hwang, W.; Chaudhry, I.; Boriek, A.M. Altered muscle force and stiffness of skeletal muscles in alpha-sarcoglycan-deficient mice. Am. J. Physiol. Cell Physiol. 2003, 284, C962–C968. [Google Scholar] [CrossRef]
- Durbeej, M.; Campbell, K.P. Muscular dystrophies involving the dystrophin-glycoprotein complex: An overview of current mouse models. Curr. Opin. Genet. Dev. 2002, 12, 349–361. [Google Scholar] [CrossRef]
- Ehmsen, J.; Poon, E.; Davies, K. The dystrophin-associated protein complex. J. Cell Sci. 2002, 115, 2801–2803. [Google Scholar] [CrossRef]
- O’Brien, K.F.; Kunkel, L.M. Dystrophin and muscular dystrophy: Past, present, and future. Mol. Genet. Metab. 2001, 74, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Henry, M.D.; Campbell, K.P. Dystroglycan: An extracellular matrix receptor linked to the cytoskeleton. Curr. Opin. Cell Biol. 1996, 8, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Yang, B.; Meyer, J.; Chamberlain, J.S.; Campbell, K.P. Identification and Characterization of the Dystrophin Anchoring Site on Beta-Dystroglycan. J. Biol. Chem. 1995, 270, 27305–27310. [Google Scholar] [CrossRef]
- Rybakova, I.N.; Amann, K.J.; Ervasti, J.M. A new model for the interaction of dystrophin with F-actin. J. Cell Biol. 1996, 135, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin—the Protein Product of the Duchenne Muscular-Dystrophy Locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Monaco, A.P.; Feener, C.C.; Kunkel, L.M. Conservation of the Duchenne Muscular-Dystrophy Gene in Mice and Humans. Science 1987, 238, 347–350. [Google Scholar] [CrossRef]
- Kumar, A.; Khandelwal, N.; Malya, R.; Reid, M.B.; Boriek, A.M. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004, 18, 102–113. [Google Scholar] [CrossRef]
- Lopez, M.A.; Bontiff, S.; Adeyeye, M.; Shaibani, A.I.; Alexander, M.S.; Wynd, S.; Boriek, A.M. Mechanics of dystrophin deficient skeletal muscles in very young mice and effects of age. Am. J. Physiol. Cell Physiol. 2021, 321, C230–C246. [Google Scholar] [CrossRef]
- Pasternak, C.; Wong, S.; Elson, E.L. Mechanical Function of Dystrophin in Muscle-Cells. J. Cell Biol. 1995, 128, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.Z.; Lakonishok, M.; Kaufman, S.; Horwitz, A.F. Alpha-7-Beta-1 Integrin Is a Component of the Myotendinous Junction on Skeletal-Muscle. J. Cell Sci. 1993, 106, 579–590. [Google Scholar] [CrossRef]
- Miosge, N.; Klenczar, C.; Herken, R.; Willem, M.; Mayer, U. Organization of the myotendinous junction is dependent on the presence of alpha7beta1 integrin. Lab. Investig. 1999, 79, 1591–1599. [Google Scholar]
- Swasdison, S.; Mayne, R. Location of the Integrin Complex and Extracellular-Matrix Molecules at the Chicken Myotendinous Junction. Cell Tissue Res. 1989, 257, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.C.; Sheard, P.W.; Kaufman, S.J.; Duxson, M.J. Localization of alpha 7 integrins and dystrophin suggests potential for both lateral and longitudinal transmission of tension in large mammalian muscles. Cell Tissue Res. 2002, 308, 255–265. [Google Scholar] [CrossRef]
- Burkin, D.J.; Kaufman, S.J. The alpha 7 beta 1 integrin in muscle development and disease. Cell Tissue Res. 1999, 296, 183–190. [Google Scholar] [CrossRef]
- Song, W.K.; Wang, W.W.; Foster, R.F.; Bielser, D.A.; Kaufman, S.J. H36-Alpha-7 Is a Novel Integrin Alpha-Chain That Is Developmentally Regulated during Skeletal Myogenesis. J. Cell Biol. 1992, 117, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Giancotti, F.G.; Ruoslahti, E. Transduction-Integrin signaling. Science 1999, 285, 1028–1032. [Google Scholar] [CrossRef]
- Hayashi, Y.K.; Chou, F.L.; Engvall, E.; Ogawa, M.; Matsuda, C.; Hirabayashi, S.; Yokochi, K.; Ziober, B.L.; Kramer, R.H.; Kaufman, S.J.; et al. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat. Genet. 1998, 19, 94–97. [Google Scholar] [CrossRef]
- Cohn, R.D.; Mayer, U.; Saher, G.; Herrmann, R.; van der Flier, A.; Sonnenberg, A.; Sorokin, L.; Voit, T. Secondary reduction of alpha 7B integrin in laminin alpha 2 deficient congenital muscular dystrophy supports an additional transmembrane link in skeletal muscle. J. Neurol. Sci. 1999, 163, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Imanaka-Yoshida, K.; Enomoto-Iwamoto, M.; Yoshida, T.; Sakakura, T. Vinculin, Talin, Integrin alpha6beta1 and laminin can serve as components of attachment complex mediating contraction force transmission from cardiomyocytes to extracellular matrix. Cell Motil. Cytoskelet. 1999, 42, 1–11. [Google Scholar] [CrossRef]
- Lopez, M.A.; Mayer, U.; Hwang, W.; Taylor, T.; Hashmi, M.A.; Jannapureddy, S.R.; Boriek, A.M. Force transmission, compliance, and viscoelasticity are altered in the alpha7-integrin-null mouse diaphragm. Am. J. Physiol. Cell Physiol. 2005, 288, C282–C289. [Google Scholar] [CrossRef]
- Maruyama, K.; Matsubara, S.; Natori, R.; Nonomura, Y.; Kimura, S. Connectin, an elastic protein of muscle. Characterization and Function. J. Biochem. 1977, 82, 317–337. [Google Scholar] [CrossRef]
- Gautel, M.; Mues, A.; Young, P. Control of sarcomeric assembly: The flow of information on titin. Rev. Physiol. Biochem. Pharmacol. 1999, 138, 97–137. [Google Scholar]
- van der Ven, P.F.; Bartsch, J.W.; Gautel, M.; Jockusch, H.; Furst, D.O. A functional knock-out of titin results in defective myofibril assembly. J. Cell Sci. 2000, 113, 1405–1414. [Google Scholar] [CrossRef]
- Fukuda, N.; Granzier, H.L.; Ishiwata, S.; Kurihara, S. Physiological functions of the giant elastic protein titin in mammalian striated muscle. J. Physiol. Sci. 2008, 58, 151–159. [Google Scholar] [CrossRef]
- Wang, K.; McCarter, R.; Wright, J.; Beverly, J.; Ramirez-Mitchell, R. Viscoelasticity of the sarcomere matrix of skeletal muscles. The titin-myosin composite filament is a dual-stage molecular spring. Biophys. J. 1993, 64, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, C.; Ono, Y.; Doi, N.; Kitamura, F.; Tagami, M.; Mineki, R.; Arai, T.; Taguchi, H.; Yanagida, M.; Hirner, S.; et al. Multiple molecular interactions implicate the connectin/titin N2A region as a modulating scaffold for p94/calpain 3 activity in skeletal muscle. J. Biol. Chem. 2008, 283, 14801–14814. [Google Scholar] [CrossRef]
- Miller, M.K.; Bang, M.L.; Witt, C.C.; Labeit, D.; Trombitas, C.; Watanabe, K.; Granzier, H.; McElhinny, A.S.; Gregorio, C.C.; Labeit, S. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J. Mol. Biol. 2003, 333, 951–964. [Google Scholar] [CrossRef]
- Heimann, P. Muscle Pathology and Density of Satellite Cells in the Mouse Mutant Muscular-Dystrophy with Myositis (Mdm). J. Muscle Res. Cell Motil. 1993, 14, 259. [Google Scholar]
- Lopez, M.A.; Pardo, P.S.; Cox, G.A.; Boriek, A.M. Early mechanical dysfunction of the diaphragm in the muscular dystrophy with myositis (Ttnmdm) model. Am. J. Physiol. Cell Physiol. 2008, 295, C1092–C1102. [Google Scholar] [CrossRef]
- Kumar, A.; Chaudhry, I.; Reid, M.B.; Boriek, A.M. Distinct signaling pathways are activated in response to mechanical stress applied axially and transversely to skeletal muscle fibers. J. Biol. Chem. 2002, 277, 46493–46503. [Google Scholar] [CrossRef]
- Mohamed, J.S.; Lopez, M.A.; Cox, G.A.; Boriek, A.M. Anisotropic regulation of Ankrd2 gene expression in skeletal muscle by mechanical stretch. FASEB J. 2010, 24, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Bogoyevitch, M.A.; Ketterman, A.J.; Sugden, P.H. Cellular stresses differentially activate c-Jun N-terminal protein kinases and extracellular signal-regulated protein kinases in cultured ventricular myocytes. J. Biol. Chem. 1995, 270, 29710–29717. [Google Scholar] [CrossRef]
- Boppart, M.D.; Hirshman, M.F.; Sakamoto, K.; Fielding, R.A.; Goodyear, L.J. Static stretch increases c-Jun NH2-terminal kinase activity and p38 phosphorylation in rat skeletal muscle. Am. J. Physiol. Cell Physiol. 2001, 280, C352–C358. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Cellular basis of mechanotransduction. Biol. Bull. 1998, 194, 323–327. [Google Scholar] [CrossRef]
- Wretman, C.; Widegren, U.; Lionikas, A.; Westerblad, H.; Henriksson, J. Differential activation of mitogen-activated protein kinase signalling pathways by isometric contractions in isolated slow- and fast-twitch rat skeletal muscle. Acta Physiol. Scand. 2000, 170, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.S.; Hajira, A.; Lopez, M.A.; Boriek, A.M. Genome-Wide Mechanosensitive MicroRNA (MechanomiR) Screen Uncovers Dysregulation of Their Regulatory Networks in the mdm Mouse Model of Muscular Dystrophy. J. Biol. Chem. 2015, 290, 24986–25011. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef]
- Essers, M.A.; Weijzen, S.; de Vries-Smits, A.M.; Saarloos, I.; de Ruiter, N.D.; Bos, J.L.; Burgering, B.M. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004, 23, 4802–4812. [Google Scholar] [CrossRef] [PubMed]
- Pardo, P.S.; Mohamed, J.S.; Lopez, M.A.; Boriek, A.M. Induction of SIRT1 by mechanical stretch of skeletal muscle through the early response factor EGR1 triggers an antioxidative response. J. Biol. Chem. 2011, 286, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Van Gammeren, D.; Deruisseau, K.C.; Zergeroglu, A.M.; McKenzie, M.J.; Yarasheski, K.E.; Powers, S.K. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am. J. Respir. Crit. Care Med. 2004, 170, 994–999. [Google Scholar] [CrossRef] [PubMed]
- DeRuisseau, K.C.; Kavazis, A.N.; Deering, M.A.; Falk, D.J.; Van Gammeren, D.; Yimlamai, T.; Ordway, G.A.; Powers, S.K. Mechanical ventilation induces alterations of the ubiquitin-proteasome pathway in the diaphragm. J. Appl. Physiol. 2005, 98, 1314–1321. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, J.S.; Pardo, P.S.; Boriek, A.M. Mechano-Signal Transduction Pathways of the Diaphragmatic Muscle and Role of Cytoskeleton. Genes 2025, 16, 968. https://doi.org/10.3390/genes16080968
Mohamed JS, Pardo PS, Boriek AM. Mechano-Signal Transduction Pathways of the Diaphragmatic Muscle and Role of Cytoskeleton. Genes. 2025; 16(8):968. https://doi.org/10.3390/genes16080968
Chicago/Turabian StyleMohamed, Junaith S., Patricia S. Pardo, and Aladin M. Boriek. 2025. "Mechano-Signal Transduction Pathways of the Diaphragmatic Muscle and Role of Cytoskeleton" Genes 16, no. 8: 968. https://doi.org/10.3390/genes16080968
APA StyleMohamed, J. S., Pardo, P. S., & Boriek, A. M. (2025). Mechano-Signal Transduction Pathways of the Diaphragmatic Muscle and Role of Cytoskeleton. Genes, 16(8), 968. https://doi.org/10.3390/genes16080968






