Beyond the Tissue: Unlocking NSCLC Treatment Potential Through Liquid Biopsy
Abstract
1. Introduction
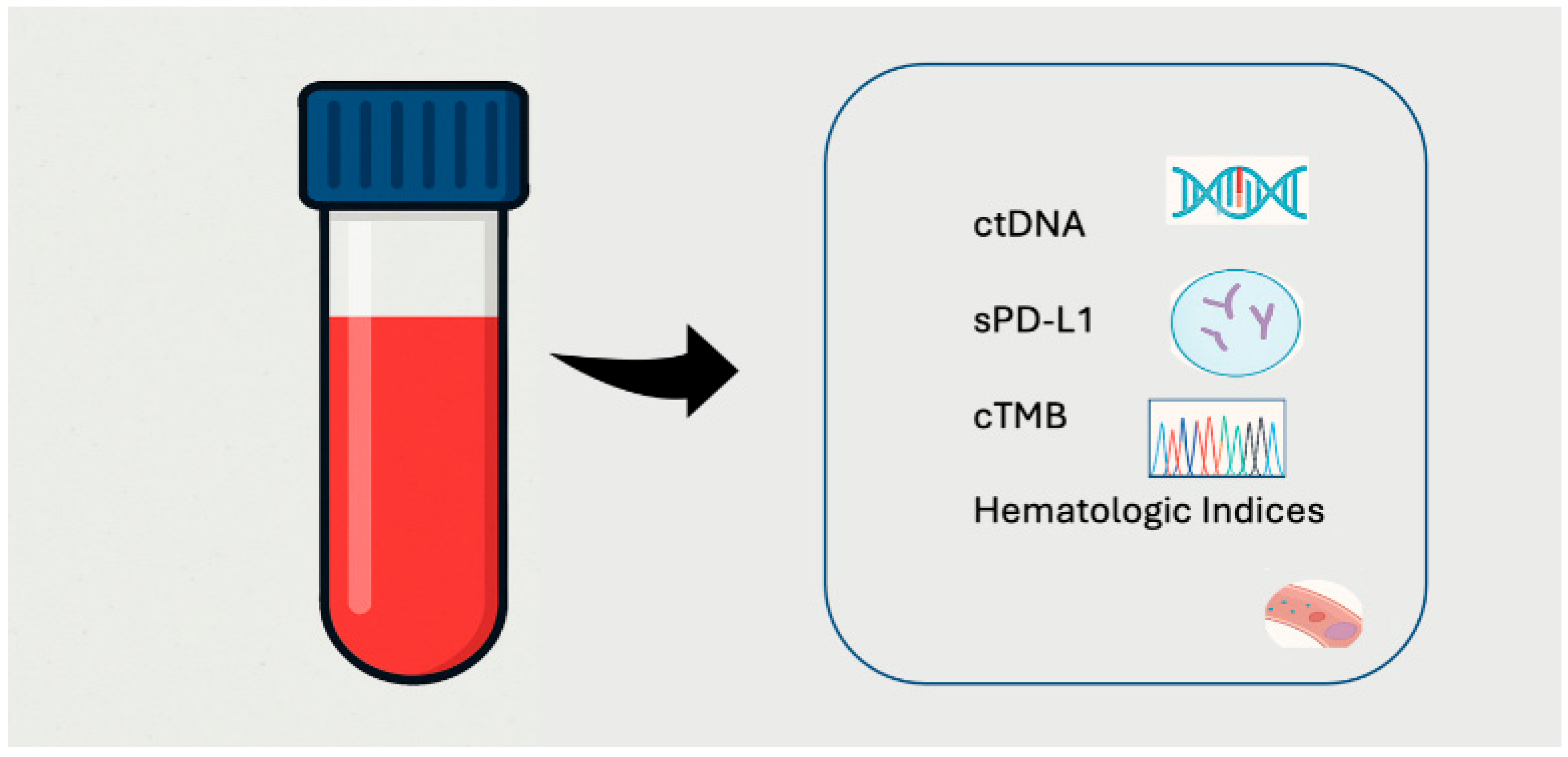
2. Biological Rationale and Technical Advances in Liquid Biopsy
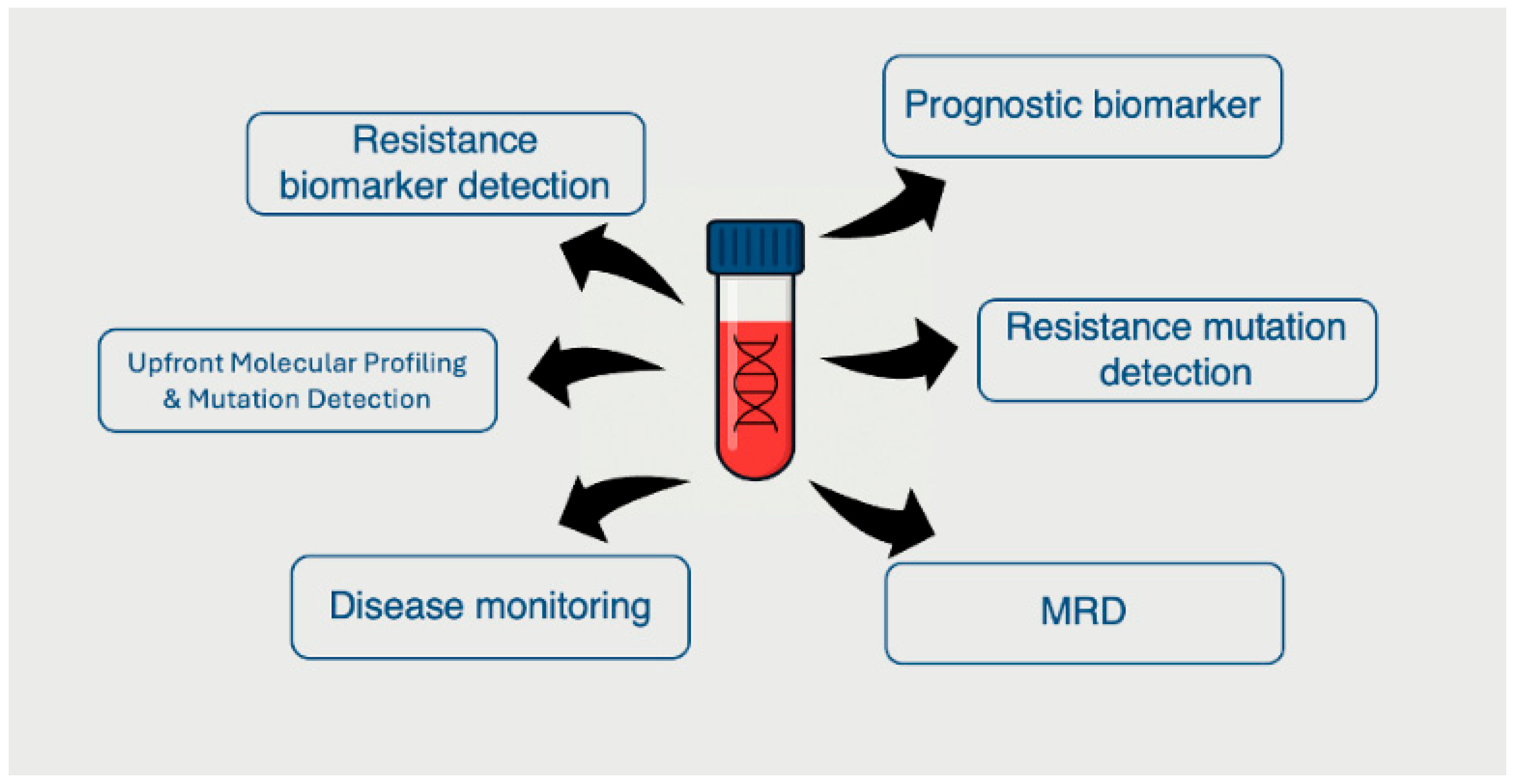
3. ctDNA Kinetics as a Predictor of Immunotherapy and Targeted Therapy Response
4. Mutation Profiling via ctDNA
4.1. Predictive and Resistance Biomarkers for Targeted Therapy
4.2. Predictive and Resistance Biomarkers for ICI
5. Soluble Immune Checkpoint Molecules in Peripheral Blood
6. Inflammatory Biomarkers and Hematologic Indices
7. Tumor Mutational Burden (TMB) from ctDNA (cTMB)
8. Clinical Utility and Implementation Challenges
9. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ibodeng, G.O.; Uche, I.N.; Mokua, R.; Galo, M.; Odigwe, B.; Galeas, J.N.; Dasgupta, S. A snapshot of lung cancer: Where are we now?—A narrative review. Ann. Transl. Med. 2023, 11, 261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, W.; Liu, J.B.; Hou, L.K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.M.; Sun, F.; Lu, H.M.; Deng, J.; et al. Liquid biopsy in lung cancer: Significance in diagnostics, prediction, and treatment monitoring. Mol. Cancer 2022, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Abascal, J.; Rennels, A.K.; Salehi-Rad, R.; Dubinett, S.M.; Liu, B. Tumor Heterogeneity and the Immune Response in Non-Small Cell Lung Cancer: Emerging Insights and Implications for Immunotherapy. Cancers 2025, 17, 1027. [Google Scholar] [CrossRef] [PubMed]
- Indini, A.; Rijavec, E.; Grossi, F. Circulating biomarkers of response and toxicity of immunotherapy in advanced non-small cell lung cancer (NSCLC): A comprehensive review. Cancers 2021, 13, 1794. [Google Scholar] [CrossRef]
- Rosenlund, L.; Guldbrandsen, K.; Ahlborn, L.B.; Bloch, M.; Skougaard, K.; Albrecht-Beste, E.; Nellemann, H.M.; Krakauer, M.; Gørtz, P.M.; Fledelius, J.; et al. ctDNA can detect minimal residual disease in curative treated non-small cell lung cancer patients using a tumor agnostic approach. Lung Cancer 2025, 203, 108528. [Google Scholar] [CrossRef]
- Marković, F.; Stjepanović, M.; Samardžić, N.; Kontić, M. The Association of Immune-Related Adverse Events with the Efficacy of Atezolizumab in Previously Treated Advanced Non-Small-Cell Lung Cancer Patients: A Single-Center Experience. Cancers 2024, 16, 2995. [Google Scholar] [CrossRef]
- Assaf, Z.J.F.; Zou, W.; Fine, A.D.; Socinski, M.A.; Young, A.; Lipson, D.; Freidin, J.F.; Kennedy, M.; Polisecki, E.; Nishio, M.; et al. A longitudinal circulating tumor DNA-based model associated with survival in metastatic non-small-cell lung cancer. Nat. Med. 2023, 29, 859–868. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldberg, S.B.; Narayan, A.; Kole, A.J.; Decker, R.H.; Teysir, J.; Carriero, N.J.; Lee, A.; Nemati, R.; Nath, S.K.; Mane, S.M.; et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin. Cancer Res. 2018, 24, 1872–1880. [Google Scholar] [CrossRef]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef]
- Chaudhuri, A.A.; Chabon, J.J.; Lovejoy, A.F.; Newman, A.M.; Stehr, H.; Azad, T.D.; Khodadoust, M.S.; Esfahani, M.S.; Liu, C.L.; Zhou, L.; et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017, 7, 1394–1403. [Google Scholar] [CrossRef]
- Nadal, E.; Massuti, B.; Dómine, M.; García-Campelo, R.; Cobo, M.; Felip, E. Immunotherapy with checkpoint inhibitors in non-small cell lung cancer: Insights from long-term survivors. Cancer Immunol. Immunother. 2019, 68, 341–352. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Nathanson, T.; Rizvi, H.; Creelan, B.C.; Sanchez-Vega, F.; Ahuja, A.; Ni, A.; Novik, J.B.; Mangarin, L.M.B.; Abu-Akeel, M.; et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell 2018, 33, 843–852.e4. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement from the International Association for the Study of Lung Cancer. J. Thorac. Oncol. 2021, 16, 1647–1662. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef]
- Gray, J.E.; Ahn, M.J.; Oxnard, G.R.; Shepherd, F.A.; Imamura, F.; Cheng, Y.; Okamoto, I.; Cho, B.C.; Lin, M.C.; Wu, Y.L.; et al. Early Clearance of Plasma Epidermal Growth Factor Receptor Mutations as a Predictor of Outcome on Osimertinib in Advanced Non-Small Cell Lung Cancer; Exploratory Analysis from AURA3 and FLAURA. Clin. Cancer Res. 2023, 29, 3340–3351. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Redman, M.W.; Lilenbaum, R.; Politi, K.; Stinchcombe, T.E.; Horn, L.; Chen, E.H.; Mashru, S.H.; Gettinger, S.N.; Melnick, M.A.; et al. Randomized trial of afatinib plus cetuximab versus afatinib alone for first-line treatment of EGFR-mutant non-small-cell lung cancer: Final results from SWOG S1403. J. Clin. Oncol. 2020, 38, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Mack, P.C.; Miao, J.; Redman, M.W.; Moon, J.; Goldberg, S.B.; Herbst, R.S.; Melnick, M.A.; Walther, Z.; Hirsch, F.R.; Politi, K.; et al. Circulating Tumor DNA Kinetics Predict Progression-Free and Overall Survival in EGFR TKI-Treated Patients with EGFR-Mutant NSCLC (SWOG S1403). Clin. Cancer Res. 2022, 28, 3752–3760. [Google Scholar] [CrossRef]
- Gray, J.E.; Okamoto, I.; Sriuranpong, V.; Vansteenkiste, J.; Imamura, F.; Lee, J.S.; Pang, Y.K.; Cobo, M.; Kasahara, K.; Cheng, Y.; et al. Tissue and plasma EGFR mutation analysis in the FLAURA trial: Osimertinib versus Comparator EGFR tyrosine kinase inhibitor as first-line treatment in patients with EGFR-mutated advanced non-small cell lung cancer. Clin. Cancer Res. 2019, 25, 6644–6652. [Google Scholar] [CrossRef]
- Stockhammer, P.; Grant, M.; Wurtz, A.; Foggetti, G.; Expósito, F.; Gu, J.; Zhao, H.; Choi, J.; Chung, S.; Li, F.; et al. Co-Occurring Alterations in Multiple Tumor Suppressor Genes Are Associated with Worse Outcomes in Patients With EGFR-Mutant Lung Cancer. J. Thorac. Oncol. 2024, 19, 240–251. [Google Scholar] [CrossRef]
- Gouda, M.A.; Janku, F.; Wahida, A.; Buschhorn, L.; Schneeweiss, A.; Abdel Karim, N.; De Miguel Perez, D.; Del Re, M.; Russo, A.; Curigliano, G.; et al. Liquid Biopsy Response Evaluation Criteria in Solid Tumors (LB-RECIST). Ann. Oncol. 2024, 35, 267–275. [Google Scholar] [CrossRef]
- Merker, J.D.; Oxnard, G.R.; Compton, C.; Diehn, M.; Hurley, P.; Lazar, A.J.; Lindeman, N.; Lockwood, C.M.; Rai, A.J.; Schilsky, R.L.; et al. Circulating tumor DNA analysis in patients with cancer: American society of clinical oncology and college of American pathologists joint review. J. Clin. Oncol. 2018, 36, 1631–1641. [Google Scholar] [CrossRef]
- Oya, Y.; Yoshida, T.; Asada, K.; Oguri, T.; Inui, N.; Morikawa, S.; Ito, K.; Kimura, T.; Kunii, E.; Matsui, T.; et al. Clinical utility of liquid biopsy for EGFR driver, T790M mutation and EGFR amplification in plasma in patients with acquired resistance to afatinib. BMC Cancer 2021, 21, 57. [Google Scholar] [CrossRef]
- Kwapisz, D. Signalling pathways in cancer—A report from the European Society for Medical Oncology symposium. Oncol. Clin. Pract. 2016, 12, 63–66. Available online: https://journals.viamedica.pl/oncology_in_clinical_practice/article/view/45086 (accessed on 13 July 2025).
- Oxnard, G.R.; Paweletz, C.P.; Kuang, Y.; Mach, S.L.; O’Connell, A.; Messineo, M.M.; Luke, J.J.; Butaney, M.; Kirschmeier, P.; Jackman, D.M.; et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin. Cancer Res. 2014, 20, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Samaha, R.; El Sayed, R.; Alameddine, R.; Florescu, M.; Tehfe, M.; Routy, B.; Elkrief, A.; Belkaid, W.; Desilets, A.; Weng, X.; et al. Clinical Utility of Liquid Biopsy for the Early Diagnosis of EGFR-Mutant Advanced Lung Cancer Patients in a Real-Life Setting (CLEAR Study). Curr. Oncol. 2025, 32, 57. [Google Scholar] [CrossRef] [PubMed]
- Nacchio, M.; Sgariglia, R.; Gristina, V.; Pisapia, P.; Pepe, F.; De Luca, C.; Migliatico, I.; Clery, E.; Greco, L.; Vigliar, E.; et al. KRAS mutations testing in non-small cell lung cancer: The role of Liquid biopsy in the basal setting. J. Thorac. Dis. 2020, 12, 3836. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, A.; Pisapia, P.; Pepe, F.; Sgariglia, R.; Nacchio, M.; Russo, G.; Gragnano, G.; De Luca, C.; Troncone, G.; Malapelle, U. Liquid biopsy for BRAF mutations testing in non-small cell lung cancer: A retrospective study. J. Clin. Pathol. 2022, 75, 58–60. [Google Scholar] [CrossRef]
- Munera-Maravilla, E.; Torres-Martínez, S.; Mosqueda, M.; Bellón, M.L.; Navarro, L.; Espinosa-Olarte, P.; Blasco-Cordellat, A.; Caballero, C.; Jantus-Lewintre, E.; Calabuig-Fariñas, S. Liquid biopsy for monitoring response in KRAS-mutated NSCLC patients treated with first-line immunotherapy. J. Liq. Biopsy 2024, 5, 100186. [Google Scholar] [CrossRef]
- Leonetti, A.; Minari, R.; Pluchino, M.; Passiglia, F.; Sartore Bianchi, A.; Cortinovis, D.L.; Toschi, L.; Gelsomino, F.; Frega, S.; Metro, G.; et al. 52P Liquid biopsy monitoring in BRAF V600E mutated NSCLC patients treated with dabrafenib plus trametinib: A prospective, explorative, multicentric study, LiBRA study (GOIRC-03-2020). ESMO Open 2024, 9, 102631. [Google Scholar] [CrossRef]
- Le, X.; Kowalski, D.; Cho, B.C.; Conte, P.; Felip, E.; Garassino, M.C.; Viteri, S.; Chang, G.-C.; Richart, J.; Paz-Ares, L.; et al. Abstract 3385: Liquid biopsy to detect MET exon 14 skipping (METex14) and MET amplification in patients with advanced NSCLC: Biomarker analysis from VISION study. Cancer Res. 2020, 80 (Suppl. S16), 3385. [Google Scholar] [CrossRef]
- Le, X.; Kowalski, D.; Cho, B.C.; Conte, P.; Felip, E.; Garassino, M.; Viteri, S.; Chang, G.-C.; Richart, J.; Paz-Ares, L.; et al. OFP01.01 Liquid Biopsy to Detect MET Alterations in Patients with Advanced NSCLC: Biomarker Analysis from the VISION Study. J. Thorac. Oncol. 2021, 16, S7–S8. [Google Scholar] [CrossRef]
- Rich, T.A.; Reckamp, K.L.; Chae, Y.K.; Doebele, R.C.; Iams, W.T.; Oh, M.; Raymond, V.M.; Lanman, R.B.; Riess, J.W.; Stinchcombe, T.E.; et al. Analysis of cell-free DNA from 32,989 advanced cancers reveals novel co-occurring activating RET alterations and oncogenic signaling pathway aberrations. Clin. Cancer Res. 2019, 25, 5832–5842. [Google Scholar] [CrossRef]
- Peled, M.; Bar, J.; Avni, L.; Chatterji, S.; Somech, D.; Dvir, A.; Soussan-Gutman, L.; Onn, A. Early Blood-based Liquid Biopsy in Patients with Treatment-naïve Metastatic Adenocarcinoma of the Lung: A Case Series. Isr. Med. Assoc. J. 2020, 22, 768–772. [Google Scholar] [PubMed]
- Dagogo-Jack, I.; Rooney, M.; Nagy, R.J.; Lin, J.J.; Chin, E.; Ferris, L.A.; Ackil, J.; Lennerz, J.K.; Lanman, R.B.; Gainor, J.F.; et al. Molecular Analysis of Plasma from Patients with ROS1-Positive NSCLC. J. Thorac. Oncol. 2019, 14, 816–824. [Google Scholar] [CrossRef]
- Mezquita, L.; Swalduz, A.; Jovelet, C.; Ortiz-Cuaran, S.; Howarth, K.; Planchard, D.; Avrillon, V.; Recondo, G.; Marteau, S.; Benitez, J.C.; et al. Clinical Relevance of an Amplicon-Based Liquid Biopsy for Detecting ALK and ROS1 Fusion and Resistance Mutations in Patients with Non-Small-Cell Lung Cancer. JCO Precis. Oncol. 2020, 4, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.M.; Lee, J.K.; Pasquina, L.W.; Decker, B.; Vanden Borre, P.; Pavlick, D.C.; Allen, J.M.; Parachoniak, C.; Quintanilha, J.C.F.; Graf, R.P.; et al. Circulating Tumor DNA Enables Sensitive Detection of Actionable Gene Fusions and Rearrangements Across Cancer Types. Clin. Cancer Res. 2024, 30, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Isozaki, H.; Takigawa, N.; Kiura, K. Mechanisms of Acquired Resistance to ALK Inhibitors and the Rationale for Treating ALK-positive Lung Cancer. Cancers 2015, 7, 763–783. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.; Pilling, A.; Aisner, D.; Kutateladze, T.; Le, A.; Weickhardt, A.; Kondo, K. Mechanisms of Resistance to Crizotinib in Patients with ALK Gene Rearranged Non-Small Cell Lung Cancer. Clin. Cancer Res. 2012, 18, 1472–1482. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef]
- Romero, R.; Sayin, V.I.; Davidson, S.M.; Bauer, M.R.; Singh, S.X.; LeBoeuf, S.E.; Karakousi, T.R.; Ellis, D.C.; Bhutkar, A.; Sánchez-Rivera, F.J.; et al. Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat. Med. 2017, 23, 1362–1368. [Google Scholar] [CrossRef]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016, 6, 202–216. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Zhong, W.Z.; Zhang, X.C.; Su, J.; Xie, Z.; Liu, S.Y.; Tu, H.Y.; Chen, H.J.; Sun, Y.L.; Zhou, Q.; et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin. Cancer Res. 2017, 23, 3012–3024. [Google Scholar] [CrossRef]
- Arbour, K.C.; Jordan, E.; Kim, H.R.; Dienstag, J.; Yu, H.A.; Sanchez-Vega, F.; Lito, P.; Berger, M.; Solit, D.B.; Hellmann, M.; et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin. Cancer Res. 2018, 24, 334–340. [Google Scholar] [CrossRef]
- Marković, F.; Milin-Lazović, J.; Nikolić, N.; Golubović, A.; Stjepanović, M.; Kontić, M. KRAS Mutations as Predictive Biomarkers for First-Line Immune Checkpoint Inhibitor Monotherapy in Advanced NSCLC: A Systematic Review and Meta-Analysis. Curr. Oncol. 2025, 32, 365. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ju, Z.; Zhao, W.; Wang, L.; Peng, Y.; Ge, Z.; Nagel, Z.D.; Zou, J.; Wang, C.; Kapoor, P.; et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat. Med. 2018, 24, 556–562. [Google Scholar] [CrossRef]
- Pan, D.; Kobayashi, A.; Jiang, P.; de Andrade, L.F.; Tay, R.E.; Luoma, A.M.; Tsoucas, D.; Qiu, X.; Lim, K.; Rao, P.; et al. A major chromatin regulator determines resistance of tumor cells to T cell–mediated killing. Science 2018, 359, 770–775. [Google Scholar] [CrossRef]
- Nikolic, N.; Golubovic, A.; Ratkovic, A.; Pandurevic, S.; Kontic, M. Brief Report: Predictive value of PD-L1 Expression in non–Small-Cell Lung Cancer-Should we Set the Bar Higher for Monotherapy? Clin. Lung Cancer 2023, 24, e214–e218. [Google Scholar] [CrossRef]
- Brun, S.S.; Hansen, T.F.; Wen, S.W.C.; Nyhus, C.H.; Bertelsen, L.; Jakobsen, A.; Nederby, L. Soluble programmed death ligand 1 as prognostic biomarker in non-small cell lung cancer patients receiving nivolumab, pembrolizumab or atezolizumab therapy. Sci. Rep. 2024, 14, 8993. [Google Scholar] [CrossRef]
- Wang, Y.; He, H. Prognostic value of soluble programmed cell death ligand-1 in patients with non-small-cell lung cancer: A meta-analysis. Immunotherapy 2022, 14, 945–956. [Google Scholar] [CrossRef]
- Raza, A.; Mohsen, R.; Kanbour, A.; Zar Gul, A.R.; Philip, A.; Vijayakumar, S.; Hydrose, S.; Prabhu, K.S.; Al-Suwaidi, A.K.; Inchakalody, V.P.; et al. Serum immune mediators as novel predictors of response to anti-PD-1/PD-L1 therapy in non-small cell lung cancer patients with high tissue-PD-L1 expression. Front. Immunol. 2023, 14, 1157100. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Azuma, K.; Hattori, S.; Ohtake, J.; Kawahara, A.; Ishii, H.; Tokito, T.; Yamada, K.; Shibata, Y.; Shimokawaji, T.; et al. Association between soluble immune mediators and tumor responses in patients with nonsmall cell lung cancer treated with anti‐PD‐1 inhibitor. Int. J. Cancer 2019, 144, 1170–1179. [Google Scholar] [CrossRef]
- Akbar, S.; Raza, A.; Mohsin, R.; Kanbour, A.; Qadri, S.; Parray, A.; Zar Gul, A.R.; Philip, A.; Vijayakumar, S.; Merhi, M.; et al. Circulating exosomal immuno-oncological checkpoints and cytokines are potential biomarkers to monitor tumor response to anti-PD-1/PD-L1 therapy in non-small cell lung cancer patients. Front. Immunol. 2023, 13, 1097117. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Yang, Y.-P.; Chien, C.-S.; Yarmishyn, A.A.; Ishola, A.A.; Chien, Y.; Chen, Y.-M.; Tsai, P.-H.; Lin, T.-W.; Wang, M.-L.; et al. Circular RNA hsa_circ_0000190 Facilitates the Tumorigenesis and Immune Evasion by Upregulating the Expression of Soluble PD-L1 in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 64. [Google Scholar] [CrossRef]
- Kokkotou, E.; Grapsa, D.; Papadopoulou, A.; Gaitanakis, S.; Bakakos, P.; Poulakou, G.; Moutsatsou, P.; Syrigos, K. Soluble PD-L1 and Serum Vascular Endothelial Growth Factor-B May Independently Predict Prognosis in Patients with Advanced Non-Small Cell Lung Cancer Treated with Pembrolizumab. Cancers 2025, 17, 421. [Google Scholar] [CrossRef]
- Bagley, S.J.; Kothari, S.; Aggarwal, C.; Bauml, J.M.; Alley, E.W.; Evans, T.L.; Kosteva, J.A.; Ciunci, C.A.; Gabriel, P.E.; Thompson, J.C.; et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer 2017, 106, 1–7. [Google Scholar] [CrossRef]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.; Yuan, X.; Fu, M.; Qian, H.; Xu, W. Neutrophils in cancer development and progression: Roles, mechanisms, and implications (Review). Int. J. Oncol. 2016, 49, 857–867. [Google Scholar] [CrossRef]
- Zhang, N.; Jiang, J.; Tang, S.; Sun, G. Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Int. Immunopharmacol. 2020, 85, 106677. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cui, Y.; Li, L.L.; Guan, Y.P.; Feng, D.F.; Yin, B.B.; Liang, X.F.; Yin, J.; Jiang, R.; Liang, J.; et al. Dynamics of early serum tumour markers and neutrophil-to-lymphocyte ratio predict response to PD-1/PD-L1 inhibitors in advanced non-small-cell lung cancer. Cancer Manag. Res. 2021, 13, 8241–8255. [Google Scholar] [CrossRef]
- Zhou, K.; Cao, J.; Lin, H.; Liang, L.; Shen, Z.; Wang, L.; Peng, Z.; Mei, J. Prognostic role of the platelet to lymphocyte ratio (PLR) in the clinical outcomes of patients with advanced lung cancer receiving immunotherapy: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 962173. [Google Scholar] [CrossRef]
- Zheng, F.; Meng, Q.; Zhang, L.; Chen, J.; Zhao, L.; Zhou, Z.; Liu, Y. Prognostic roles of hematological indicators for the efficacy and prognosis of immune checkpoint inhibitors in patients with advanced tumors: A retrospective cohort study. World J. Surg. Oncol. 2023, 21, 198. [Google Scholar] [CrossRef]
- Raphael, A.; Feldman, A.K.; Lazarev, I.; Kian, W.; Peled, N.; Hod, K.; Shalata, W.; Dudnik, E. Lung Immune Prognostic Index-Based Predictive Score in Advanced Non-Small Cell Lung Cancer with a Programmed Death Ligand-1 Tumor Proportion Score ≥ 50%. J. Clin. Med. 2025, 14, 3543. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Shao, M.M.; Xu, Y.P.; Zhang, J.J.; Mao, M.; Wang, M.C. Tumor mutational burden as a predictive biomarker for non-small cell lung cancer treated with immune checkpoint inhibitors of PD-1/PD-L1. Clin. Transl. Oncol. 2024, 26, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Callahan, M.K.; Awad, M.M.; Calvo, E.; Ascierto, P.A.; Atmaca, A.; Rizvi, N.A.; Hirsch, F.R.; Selvaggi, G.; Szustakowski, J.D.; et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2018, 33, 853–861.e4. [Google Scholar] [CrossRef]
- Kim, E.S.; Velcheti, V.; Mekhail, T.; Yun, C.; Shagan, S.M.; Hu, S.; Chae, Y.K.; Leal, T.A.; Dowell, J.E.; Tsai, M.L.; et al. Blood-based tumor mutational burden as a biomarker for atezolizumab in non-small cell lung cancer: The phase 2 B-F1RST trial. Nat. Med. 2022, 28, 939–945. [Google Scholar] [CrossRef]
- Zhu, H.; Xie, D.; Yu, Y.; Yao, L.; Xu, B.; Huang, L.; Wu, S.; Li, F.; Zheng, Y.; Liu, X.; et al. KEAP1/NFE2L2 Mutations of Liquid Biopsy as Prognostic Biomarkers in Patients with Advanced Non-Small Cell Lung Cancer: Results from Two Multicenter, Randomized Clinical Trials. Front. Oncol. 2021, 11, 659200. [Google Scholar] [CrossRef]
- Samol, J.; Ng, D.; Poh, J.; Tan, M.-H.; Dawar, R.; Carney, J.; Orsini, J.; Scilla, K.; Tan, Y.O.; Chin, T.M.; et al. Prospective Multicenter Study Evaluating a Combined Circulating Tumor DNA and Circulating Tumor RNA Liquid Biopsy in Metastatic Non–Small Cell Lung Cancer (LIQUIK). JCO Precis. Oncol. 2025, 9, e2500181. [Google Scholar] [CrossRef]
- Si, H.; Kuziora, M.; Quinn, K.J.; Helman, E.; Ye, J.; Liu, F.; Scheuring, U.; Peters, S.; Rizvi, N.A.; Brohawn, P.Z.; et al. A Blood-based Assay for Assessment of Tumor Mutational Burden in First-line Metastatic NSCLC Treatment: Results from the MYSTIC Study. Clin. Cancer Res. 2021, 27, 1631–1640. [Google Scholar] [CrossRef]
- Ye, L.; Chu, X.; Ni, J.; Chu, L.; Yang, X.; Zhu, Z. NGS-based Tissue-Blood TMB Comparison and Blood-TMB Monitoring in Stage-III Non-Small Cell Lung Cancer Treated with Concurrent Chemoradiotherapy. Cancer Investig. 2024, 42, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, M.; Chen, Y.; Cao, Y.; Liu, Y. Advances in predictive biomarkers associated with immunotherapy in extensive-stage small cell lung cancer. Cell Biosci. 2024, 14, 117. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Z.; Jiang, C.; Li, L.; Sun, X.; Bai, M.; Liu, M.; Xiong, K.; Shang, J.; Yu, J.; et al. Integration of Circulating Tumor DNA and Metabolic Parameters on 18F‐Fludeoxyglucose Positron Emission Tomography for Outcome Prediction in Unresectable Locally Advanced Non‐Small Cell Lung Cancer. Adv. Sci. 2025, 12, 2413125. [Google Scholar] [CrossRef]
- Costantini, A.; Kamga, P.T.; Dumenil, C.; Chinet, T.; Emile, J.F.; Leprieur, E.G. Plasma biomarkers and immune checkpoint inhibitors in non-small cell lung cancer: New tools for better patient selection? Cancers 2019, 11, 1269. [Google Scholar] [CrossRef]
- Heeke, S.; Benzaquen, J.; Long-Mira, E.; Audelan, B.; Lespinet, V.; Bordone, O.; Lalvée, S.; Zahaf, K.; Poudenx, M.; Humbert, O.; et al. In-house Implementation of Tumor Mutational Burden Testing to Predict Durable Clinical Benefit in Non-small Cell Lung Cancer and Melanoma Patients. Cancers 2019, 11, 1271. [Google Scholar] [CrossRef]
- Menzel, M.; Martis-Thiele, M.; Goldschmid, H.; Ott, A.; Romanovsky, E.; Siemanowski-Hrach, J.; Seillier, L.; Brüchle, N.O.; Maurer, A.; Lehmann, K.-V.; et al. Benchmarking whole exome sequencing in the German network for personalized medicine. Eur. J. Cancer 2024, 211, 114306. [Google Scholar] [CrossRef]
- Lockwood, C.M.; Merker, J.D.; Bain, E.; Compton, C.; Grossman, R.L.; Johann, D.; Jones, F.; Jones, G.; Kreifels, M.; LeBlang, S.; et al. Towards Preanalytical Best Practices for Liquid Biopsy Studies: A BLOOD-PAC Landscape Analysis. Clin. Pharmacol. Ther. 2025, 117, 28–33. [Google Scholar] [CrossRef]
- Simmons, K.; Thomas, J.V.; Ludford, K.; Willis, J.A.; Higbie, V.S.; Raghav, K.P.; Johnson, B.; Dasari, A.; Kee, B.K.; Parseghian, C.M.; et al. Sustained Disease Control in Immune Checkpoint Blockade Responders with Microsatellite Instability-high Colorectal Cancer after Treatment Termination. Cancer Res. Commun. 2023, 3, 2510–2517. [Google Scholar] [CrossRef]
- Cortiula, F.; Reymen, B.; Peters, S.; Van Mol, P.; Wauters, E.; Vansteenkiste, J.; De Ruysscher, D.; Hendriks, L. Immunotherapy in unresectable stage III non-small-cell lung cancer: State of the art and novel therapeutic approaches. Ann. Oncol. 2022, 33, 893–908. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontic, M.; Stjepanovic, M.; Markovic, F. Beyond the Tissue: Unlocking NSCLC Treatment Potential Through Liquid Biopsy. Genes 2025, 16, 954. https://doi.org/10.3390/genes16080954
Kontic M, Stjepanovic M, Markovic F. Beyond the Tissue: Unlocking NSCLC Treatment Potential Through Liquid Biopsy. Genes. 2025; 16(8):954. https://doi.org/10.3390/genes16080954
Chicago/Turabian StyleKontic, Milica, Mihailo Stjepanovic, and Filip Markovic. 2025. "Beyond the Tissue: Unlocking NSCLC Treatment Potential Through Liquid Biopsy" Genes 16, no. 8: 954. https://doi.org/10.3390/genes16080954
APA StyleKontic, M., Stjepanovic, M., & Markovic, F. (2025). Beyond the Tissue: Unlocking NSCLC Treatment Potential Through Liquid Biopsy. Genes, 16(8), 954. https://doi.org/10.3390/genes16080954






