Characterization and Expression Analysis of β-Glucosidase Gene Under Abiotic Stresses in Pepper (Capsicum annuum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. The Identification of the CaBGLU Gene Family
2.2.2. Physicochemical Characterization and Subcellular Localization Prediction of the CaBGLU Gene Family
2.2.3. Chromosomal Localization and Phylogenetic Analysis of the CaBGLU Gene Family
2.2.4. Prediction of Conserved Motifs, Gene Structures, and Cis-Acting Elements in the CaBGLU Gene Family
2.2.5. Collinearity Analysis of CaBGLU Gene Family
2.2.6. Protein–Protein Interaction Network Analysis of the CaBGLU Gene Family
2.2.7. Expression Profile Analysis of CaBGLU Gene Family Members
2.2.8. qRT-PCR Analysis
2.2.9. Subcellular Localization of CaBGLU21
3. Results
3.1. Identification and Physicochemical Characterization of the CaBGLU Gene Family
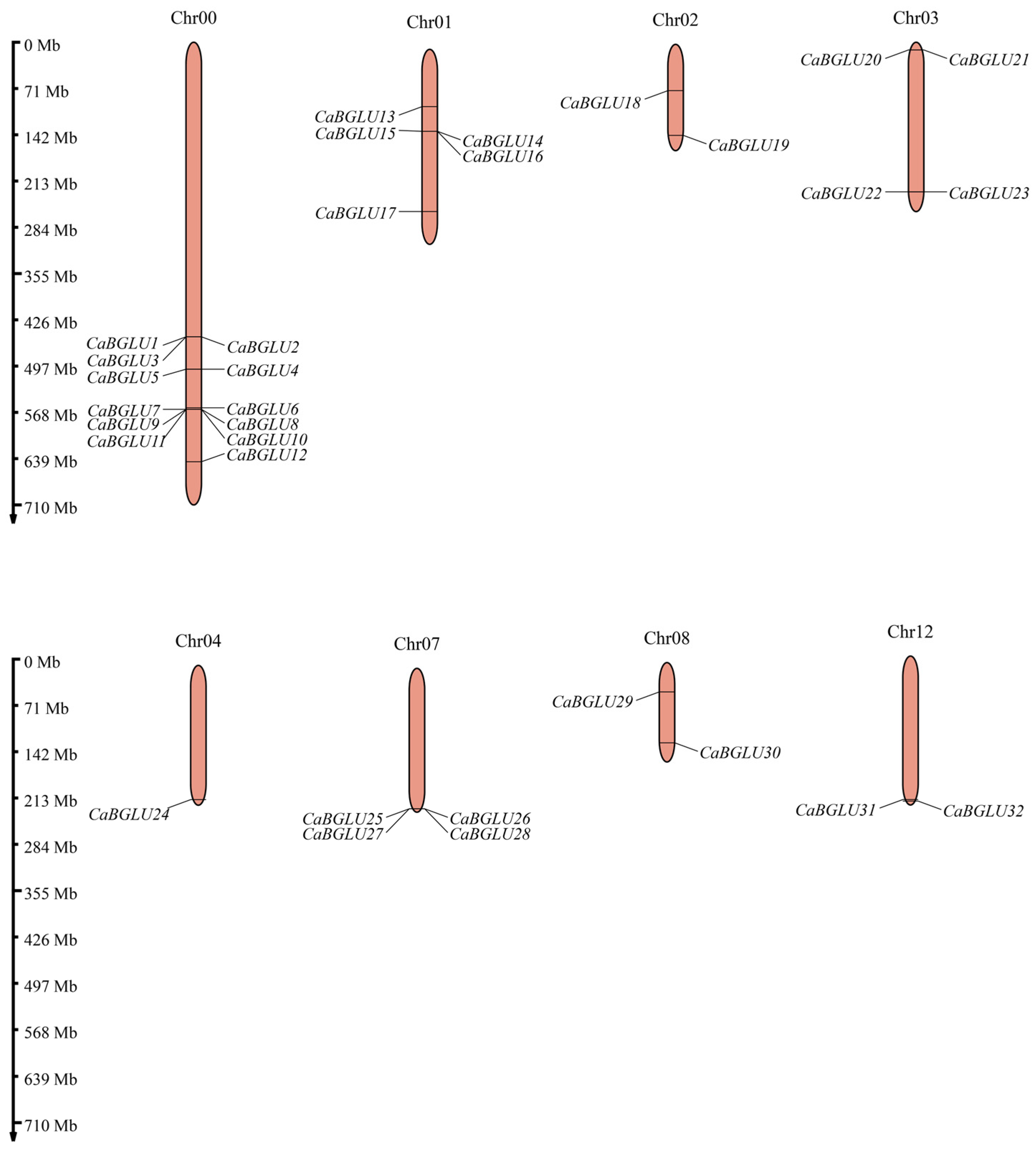
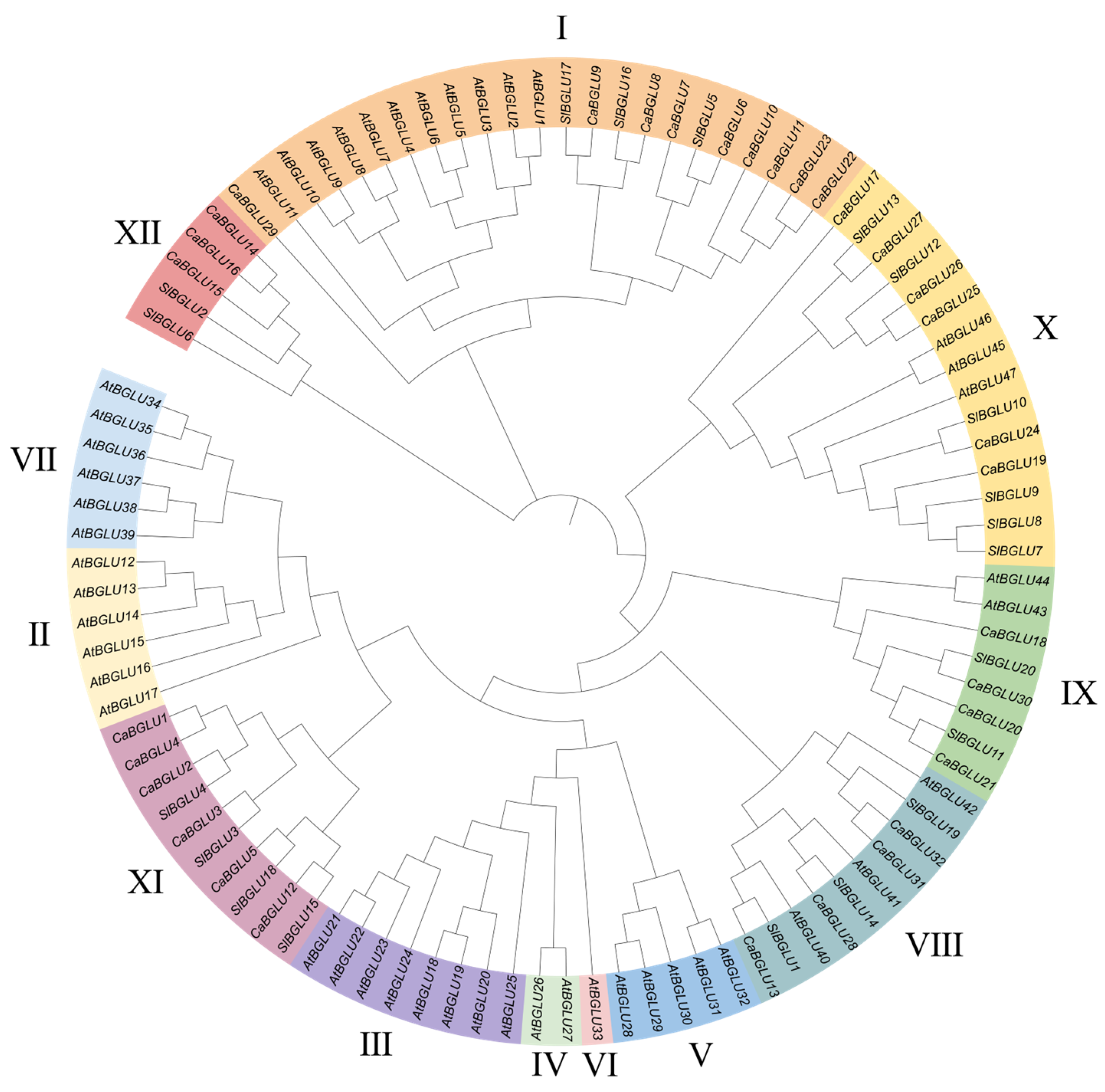
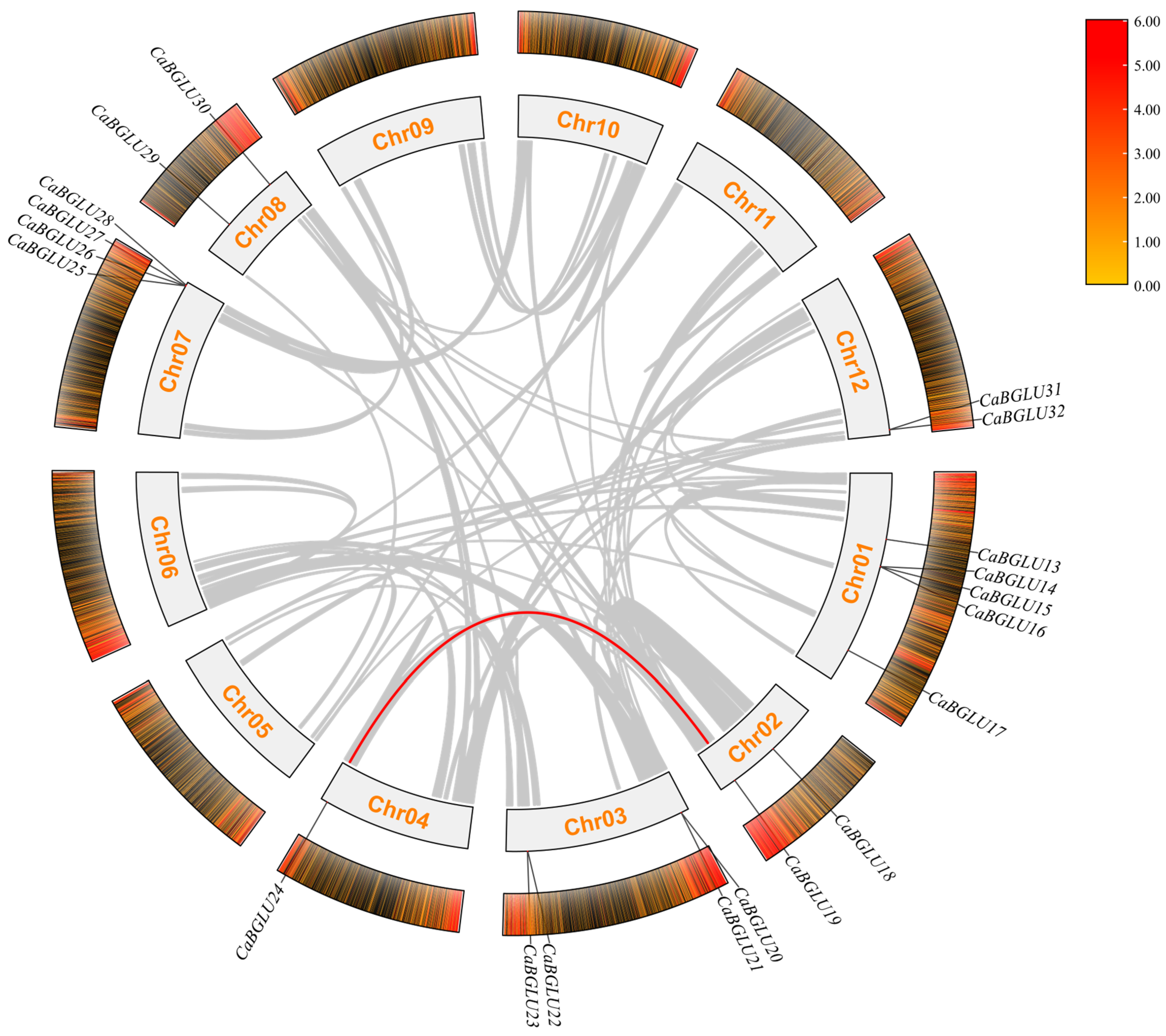
3.2. Chromosomal Localization and Phylogenetic Analysis
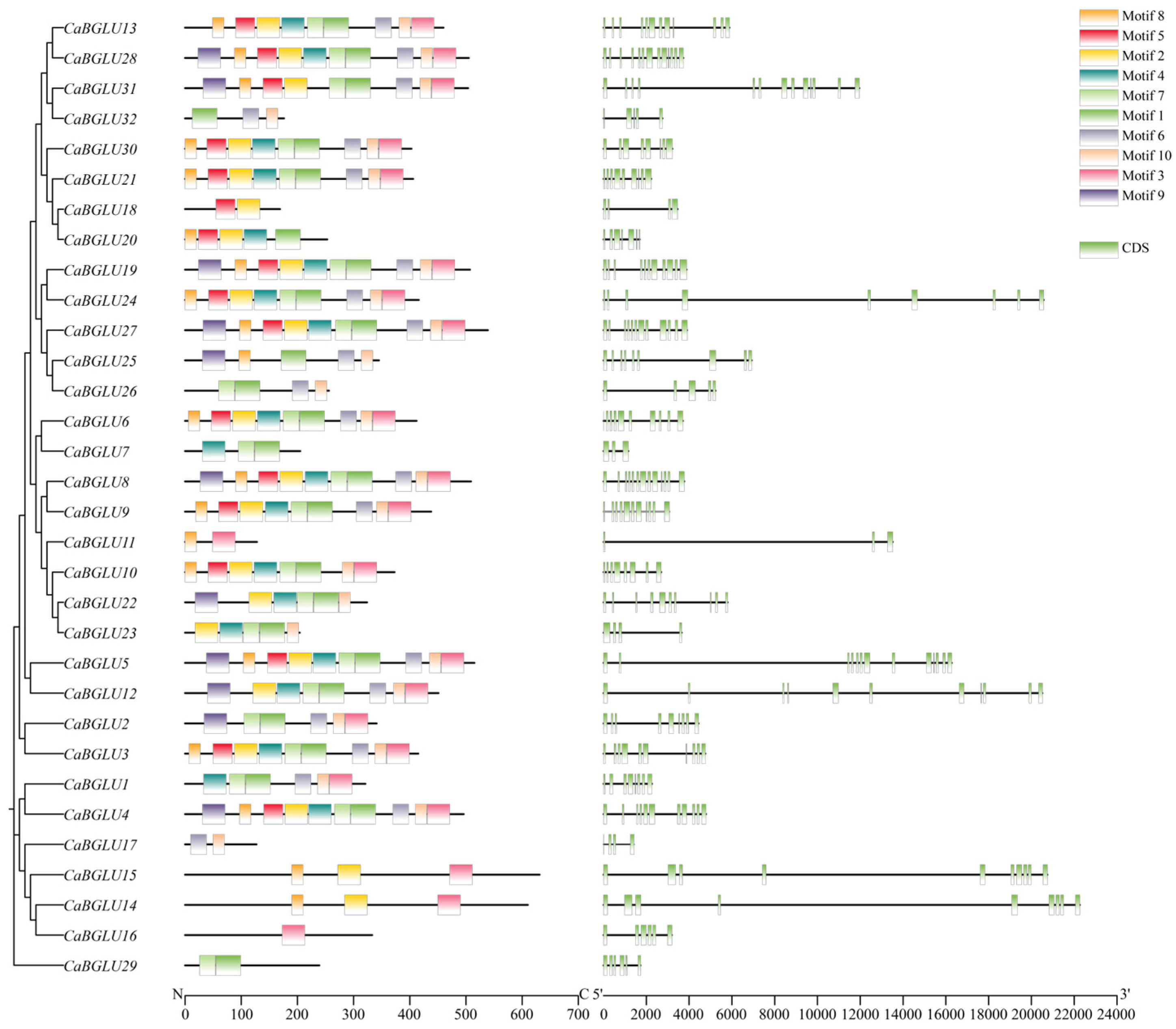
3.3. Analysis of Conserved Motifs and Exon-Intron Structures
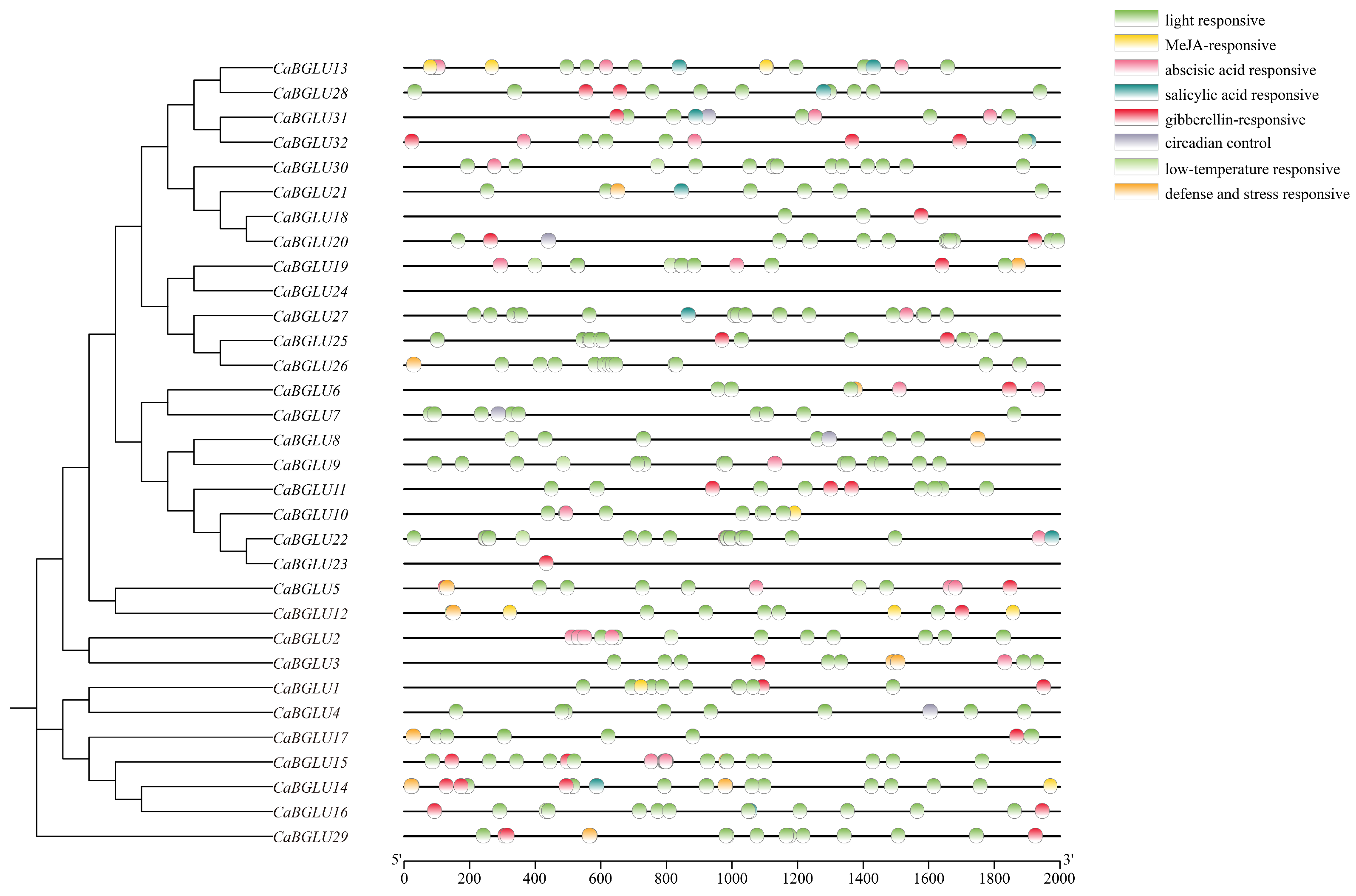
3.4. Analysis of Cis-Acting Elements in the Promoter Region
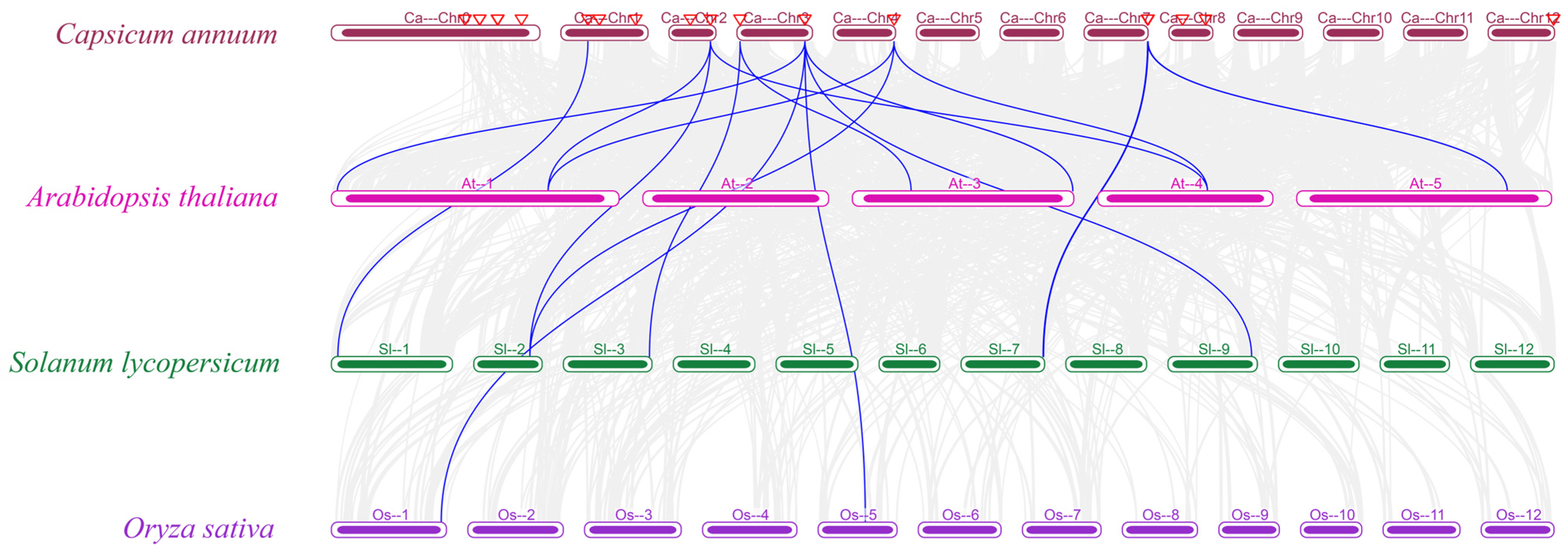
3.5. Collinearity Analysis of the CaBGLU Gene Family
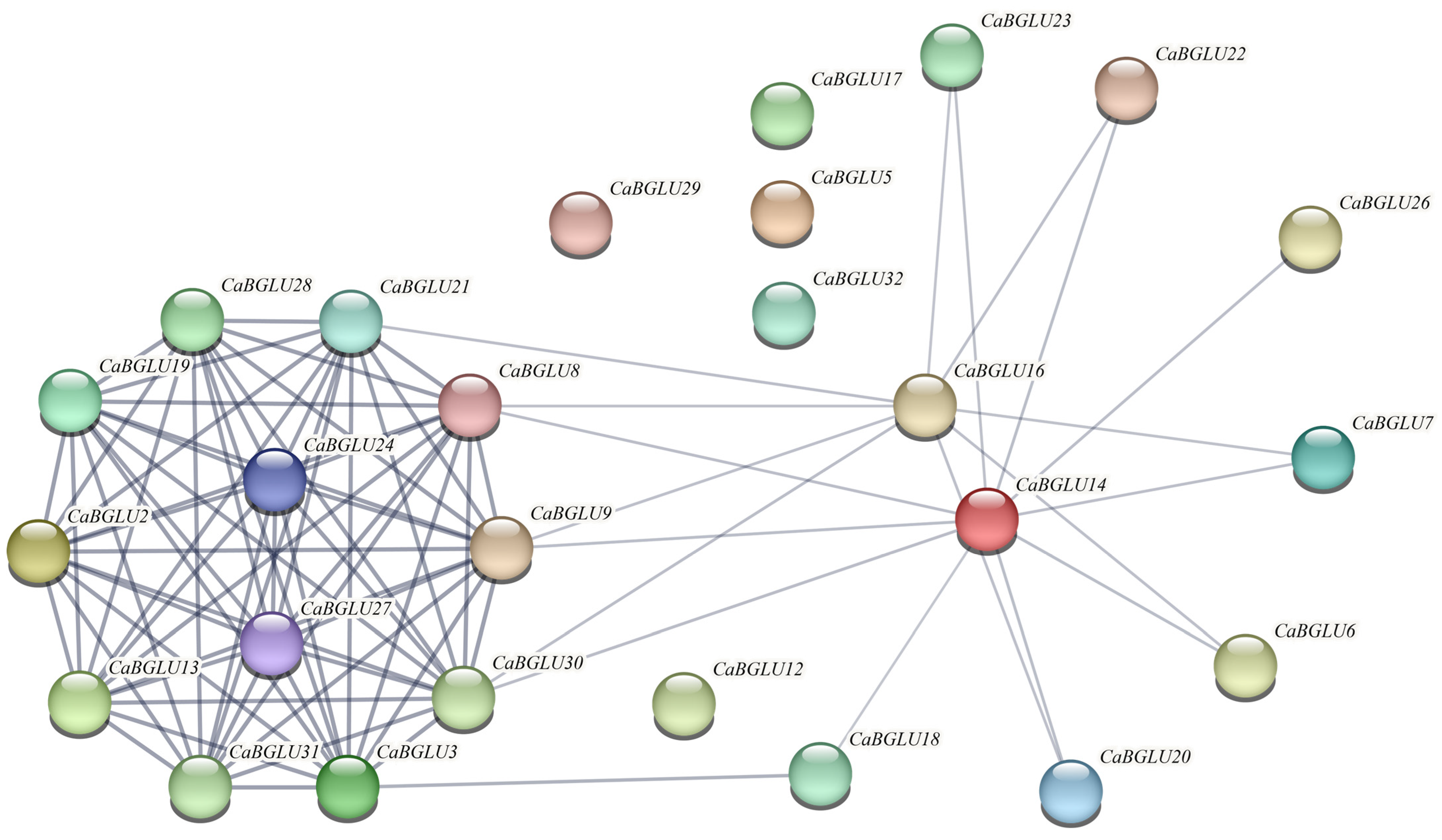
3.6. Protein–Protein Interaction Network Analysis of the CaBGLU Family
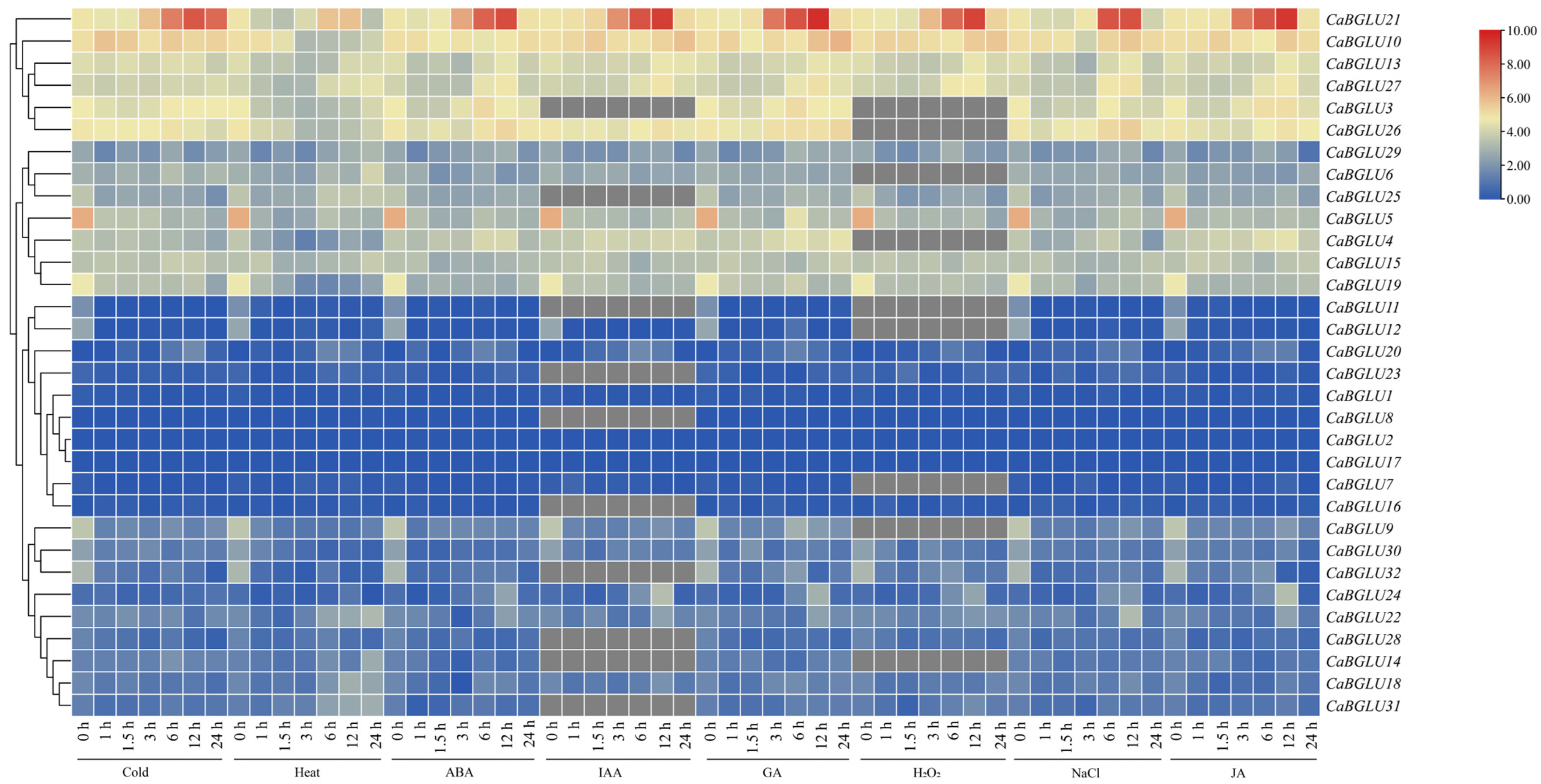
3.7. Expression Profiling of CaBGLU Genes Across Pepper Tissues and Stress Treatments
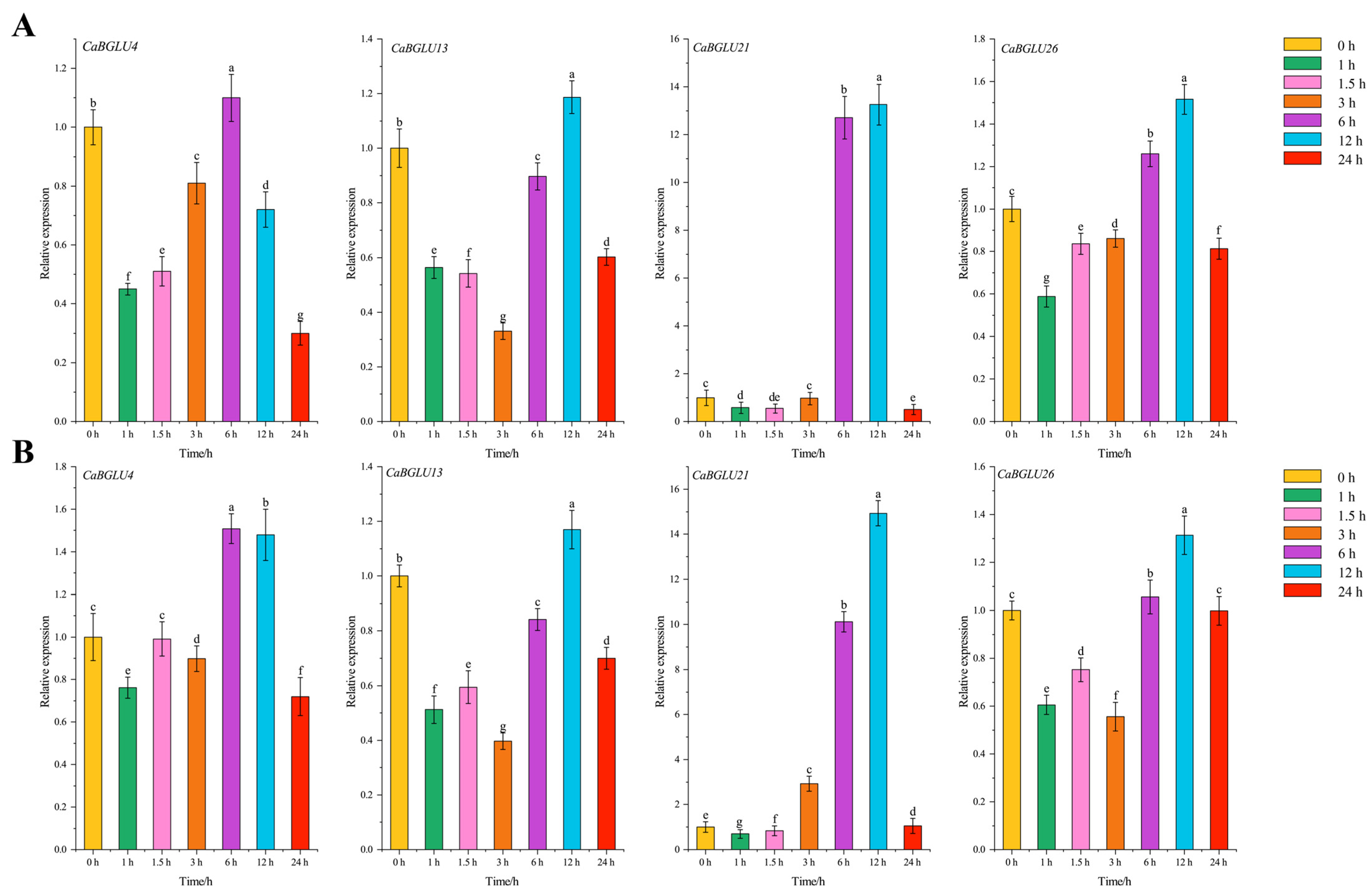
3.8. Relative Expression Levels of CaBGLU Genes in Pepper
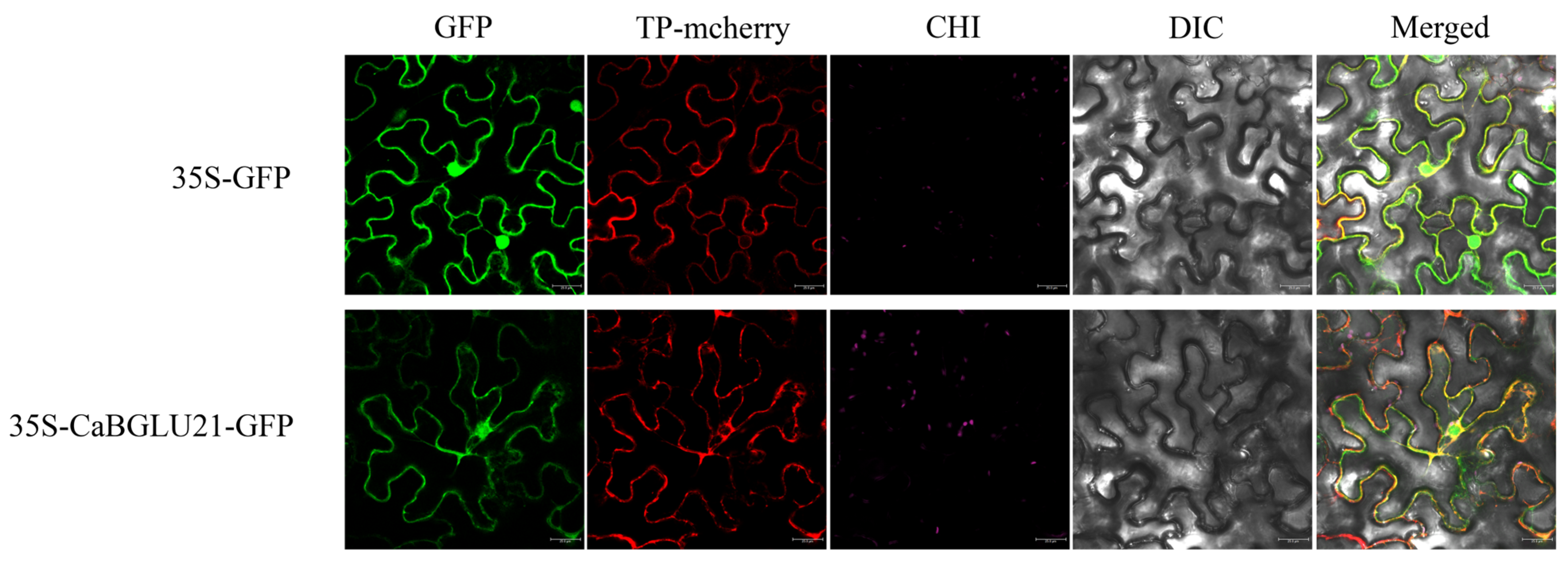
3.9. Subcellular Localization of CaBGLU21
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BGLU | β-Glucosidase |
| GH | glycoside hydrolase |
References
- Kim, S.; Park, M.; Yeom, S.-I.; Kim, Y.-M.; Lee, J.M.; Lee, H.-A.; Seo, E.; Choi, J.; Cheong, K.; Kim, K.-T.; et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nat. Genet. 2014, 46, 270–278. [Google Scholar] [CrossRef]
- Rodrigues, P.D.O.; Santos, B.V.D.; Costa, L.; Henrique, M.A.; Baffi, M.A. Xylanase and β-glucosidase production by Aspergillus fumigatus using commercial and lignocellulosic substrates submitted to chemical pre-treatments. Ind. Crops Prod. 2017, 95, 453–459. [Google Scholar] [CrossRef]
- González-Pombo, P.; Fariña, L.; Carrau, F.; Batista-Viera, F.; Brena, B.M. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process Biochem. 2011, 46, 385–389. [Google Scholar] [CrossRef]
- Duroux, L.; Delmotte, F.M.; Lancelin, J.-M.; KÉRavis, G.; Jay-Allemand, C. Insight into naphthoquinone metabolism: β-glucosidase-catalysed hydrolysis of hydrojuglone β-d-glucopyranoside. Biochem. J. 1998, 333, 275–283. [Google Scholar] [CrossRef]
- Dharmawardhana, D.P.; Ellis, B.E.; Carlson, J.E. A β-Glucosidase from lodgepole pine xylem specific for the lignin precursor coniferin. Plant Physiol. 1995, 107, 331–339. [Google Scholar] [CrossRef]
- Morant, A.V.; Bjarnholt, N.; Kragh, M.E.; Kjærgaard, C.H.; Jørgensen, K.; Paquette, S.M.; Piotrowski, M.; Imberty, A.; Olsen, C.E.; Møller, B.L.; et al. The β-Glucosidases responsible for bioactivation of hydroxynitrile glucosides in Lotus japonicus. Plant Physiol. 2008, 147, 1072–1091. [Google Scholar] [CrossRef]
- Ketudat Cairns, J.R.; Mahong, B.; Baiya, S.; Jeon, J.-S. β-Glucosidases: Multitasking, moonlighting or simply misunderstood? Plant Sci. 2015, 241, 246–259. [Google Scholar] [CrossRef]
- Sampedro, J.; Valdivia, E.R.; Fraga, P.; Iglesias, N.; Revilla, G.; Zarra, I. Soluble and membrane-bound β-glucosidases are involved in trimming the xyloglucan backbone. Plant Physiol. 2017, 173, 1017–1030. [Google Scholar] [CrossRef]
- Sun, H.; Xue, Y.; Lin, Y. Enhanced catalytic efficiency in quercetin-4′-glucoside hydrolysis of thermotoga maritima β-Glucosidase A by site-directed mutagenesis. J. Agric. Food Chem. 2014, 62, 6763–6770. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids. Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Henrissat, B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991, 280, 309–316. [Google Scholar] [CrossRef]
- Xu, Z.; Escamilla-Treviño, L.; Zeng, L.; Lalgondar, M.; Bevan, D.; Winkel, B.; Mohamed, A.; Cheng, C.-L.; Shih, M.-C.; Poulton, J.; et al. Functional genomic analysis of Arabidopsis thaliana glycoside hydrolase family 1. Plant Mol. Biol. 2004, 55, 343–367. [Google Scholar] [CrossRef]
- Opassiri, R.; Pomthong, B.; Onkoksoong, T.; Akiyama, T.; Esen, A.; Ketudat Cairns, J.R. Analysis of rice glycosyl hydrolase family 1 and expression of Os4bglu12 β-glucosidase. BMC Plant Biol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Yang, J.; Ma, L.; Jiang, W.; Yao, Y.; Tang, Y.; Pang, Y. Comprehensive identification and characterization of abiotic stress and hormone responsive glycosyl hydrolase family 1 genes in Medicago truncatula. Plant Physiol. Bioch. 2021, 158, 21–33. [Google Scholar] [CrossRef]
- Gómez-Anduro, G.; Ceniceros-Ojeda, E.A.; Casados-Vázquez, L.E.; Bencivenni, C.; Sierra-Beltrán, A.; Murillo-Amador, B.; Tiessen, A. Genome-wide analysis of the beta-glucosidase gene family in maize (Zea mays L. var B73). Plant Mol. Biol. 2011, 77, 159–183. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, Y.; Hur, Y. Genome-wide analysis of glycoside hydrolase family 1 β-glucosidase genes in Brassica rapa and their potential role in pollen development. Int. J. Mol. Sci. 2019, 20, 1663. [Google Scholar] [CrossRef]
- Escamilla-Treviño, L.L.; Chen, W.; Card, M.L.; Shih, M.-C.; Cheng, C.-L.; Poulton, J.E. Arabidopsis thaliana β-Glucosidases BGLU45 and BGLU46 hydrolyse monolignol glucosides. Phytochemistry 2006, 67, 1651–1660. [Google Scholar] [CrossRef]
- Wang, C.; Chen, S.; Dong, Y.; Ren, R.; Chen, D.; Chen, X. Chloroplastic Os3BGlu6 contributes significantly to cellular ABA pools and impacts drought tolerance and photosynthesis in rice. New Phytol. 2020, 226, 1042–1054. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, T.; Xu, X.; Sun, Y.; Zhang, Y.; Hou, M.; Huang, S.; Yuan, H.; Tong, H. Identification of GH1 gene family fgt members in Stevia rebaudiana and their expression when grown in darkness. Mol. Biol. Rep. 2020, 47, 8739–8746. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, M.; Zhang, X.; Deng, X.; Li, J.; Wang, M. Genome-wide identification and characterization of active ingredients related β-Glucosidases in Dendrobium catenatum. BMC Genom. 2022, 23, 612. [Google Scholar] [CrossRef]
- Kristoffersen, P.; Brzobohaty, B.; Höhfeld, I.; Bako, L.; Melkonian, M.; Palme, K. Developmental regulation of the maize Zm-p60.1 gene encoding a β-glucosidase located to plastids. Planta 2000, 210, 407–415. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Hua, H.; Wang, L.; Song, C.-P. A vacuole localized β-glucosidase contributes to drought tolerance in Arabidopsis. Chin. Sci. Bull. 2011, 56, 3538–3546. [Google Scholar] [CrossRef]
- Ren, R.; Li, D.; Zhen, C.; Chen, D.; Chen, X. Specific roles of Os4BGlu10, Os6BGlu24, and Os9BGlu33 in seed germination, root elongation, and drought tolerance in rice. Planta 2019, 249, 1851–1861. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, D.; Liu, Y.; Xi, Y.; Wang, X.; Meng, S. Analysis of Populus glycosyl hydrolase family I members and their potential role in the ABA treatment and drought stress response. Plant Physiol. Bioch. 2021, 163, 178–188. [Google Scholar] [CrossRef]
- Lee, K.H.; Piao, H.L.; Kim, H.-Y.; Choi, S.M.; Jiang, F.; Hartung, W.; Hwang, I.; Kwak, J.M.; Lee, I.-J.; Hwang, I. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 2006, 126, 1109–1120. [Google Scholar] [CrossRef]
- Xu, Z.-Y.; Lee, K.H.; Dong, T.; Jeong, J.C.; Jin, J.B.; Kanno, Y.; Kim, D.H.; Kim, S.Y.; Seo, M.; Bressan, R.A.; et al. A vacuolar β-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell 2012, 24, 2184–2199. [Google Scholar] [CrossRef]
- Kong, H.; Song, J.; Ma, S.; Yang, J.; Shao, Z.; Li, Q.; Li, Z.; Xie, Z.; Yang, P.; Cao, Y. Genome-wide identification and expression analysis of the glycosyl hydrolase family 1 genes in Medicago sativa revealed their potential roles in response to multiple abiotic stresses. BMC Genom. 2024, 25, 20. [Google Scholar] [CrossRef]
- Yang, H.; Yao, X.; Wu, W.; He, A.; Ma, C.; Yang, S.; Ruan, J. Genome-wide identification and gene expression pattern analysis of the glycoside hydrolase family 1 in Fagopyrum tataricum. BMC Plant Biol. 2024, 24, 1183. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Huang, J.; Jia, X.; Hao, Z.; Yang, Y.; Tian, R.; Liang, Y. Characterization and Expression Analysis of β-Glucosidase Gene Under Abiotic Stresses in Pepper (Capsicum annuum L.). Genes 2025, 16, 889. https://doi.org/10.3390/genes16080889
Wang J, Huang J, Jia X, Hao Z, Yang Y, Tian R, Liang Y. Characterization and Expression Analysis of β-Glucosidase Gene Under Abiotic Stresses in Pepper (Capsicum annuum L.). Genes. 2025; 16(8):889. https://doi.org/10.3390/genes16080889
Chicago/Turabian StyleWang, Jing, Jiaxin Huang, Xu Jia, Zhenxin Hao, Yuancai Yang, Ruxia Tian, and Yanping Liang. 2025. "Characterization and Expression Analysis of β-Glucosidase Gene Under Abiotic Stresses in Pepper (Capsicum annuum L.)" Genes 16, no. 8: 889. https://doi.org/10.3390/genes16080889
APA StyleWang, J., Huang, J., Jia, X., Hao, Z., Yang, Y., Tian, R., & Liang, Y. (2025). Characterization and Expression Analysis of β-Glucosidase Gene Under Abiotic Stresses in Pepper (Capsicum annuum L.). Genes, 16(8), 889. https://doi.org/10.3390/genes16080889




