Uridine Kinase-like Protein (GhUKL4) Positively Regulates Resistance to Verticillium Wilt in Cotton
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
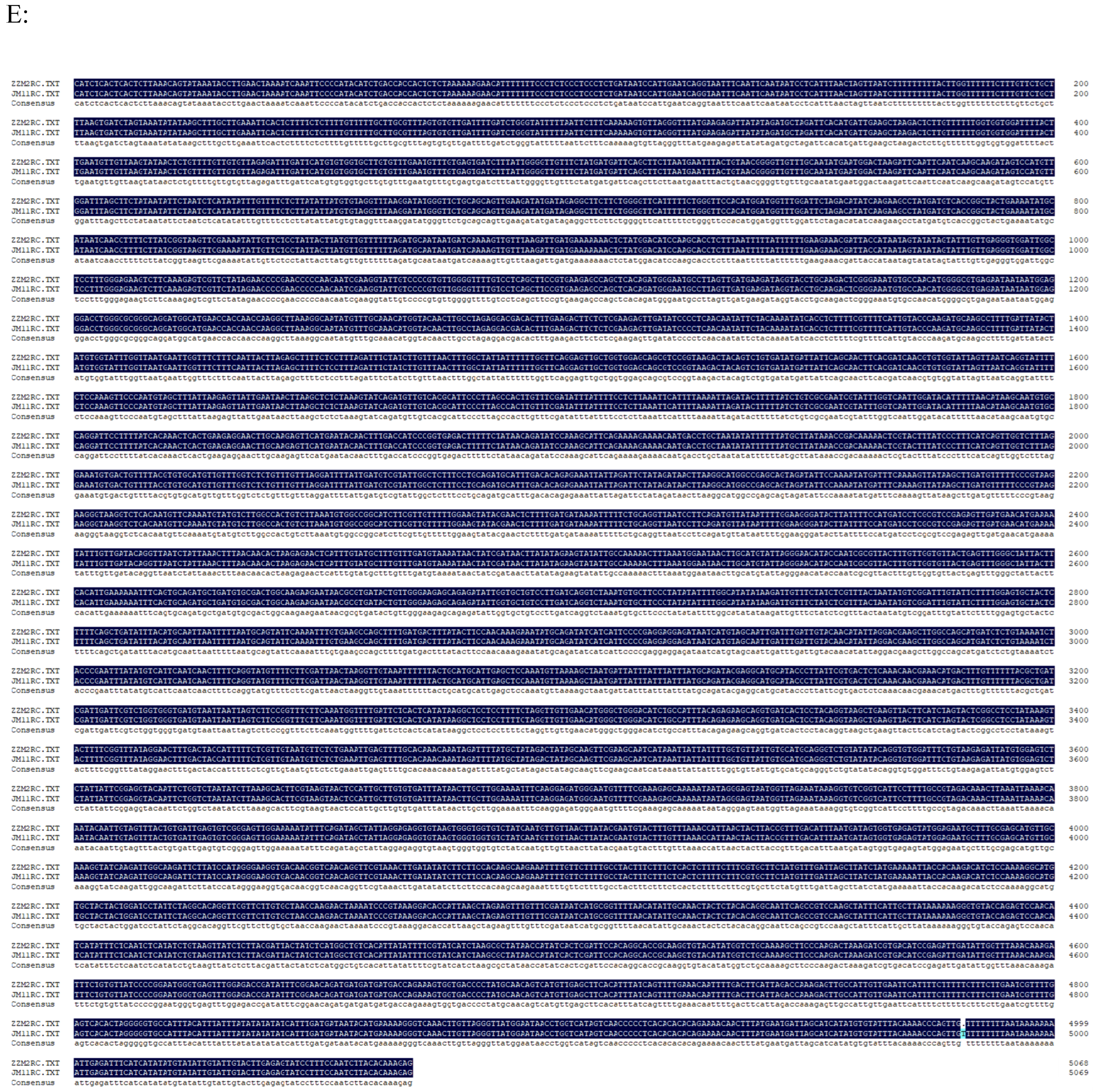
2.2. Cloning the gDNA and cDNA of Gene GhUKL4
2.3. V. dahliae Materials and Inoculation Methods
2.4. Vector Construction for Virus-Induced Gene Silencing (VIGS) in Cotton and VIGS Experiments
2.5. Morbidity Situation Analysis
2.6. qRT-PCR
2.7. V. dahliae Recovery
2.8. Chemical Staining
3. Results
3.1. Candidate Gene Differential Expression Analysis
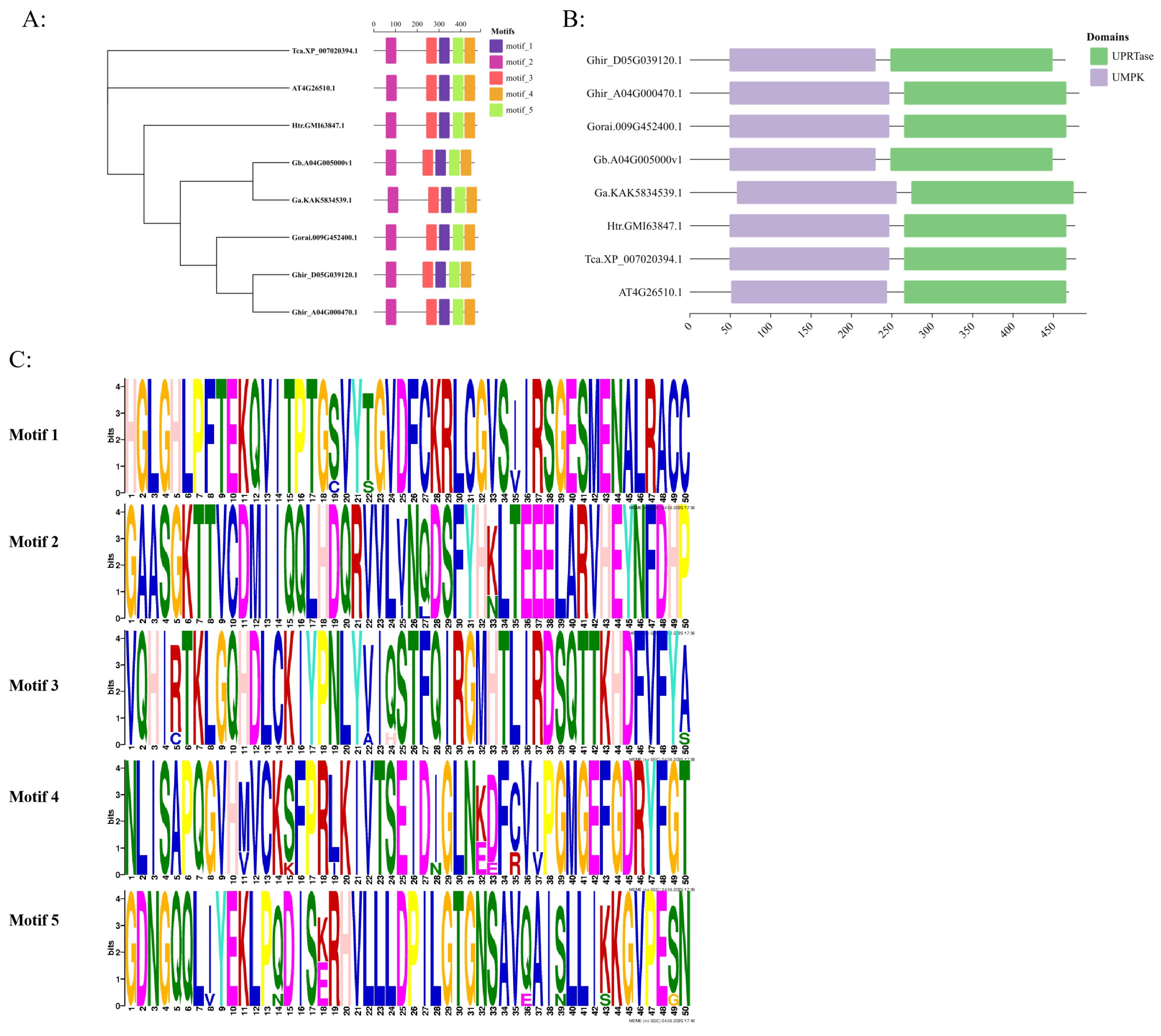
3.2. Characterization of GhUKL4
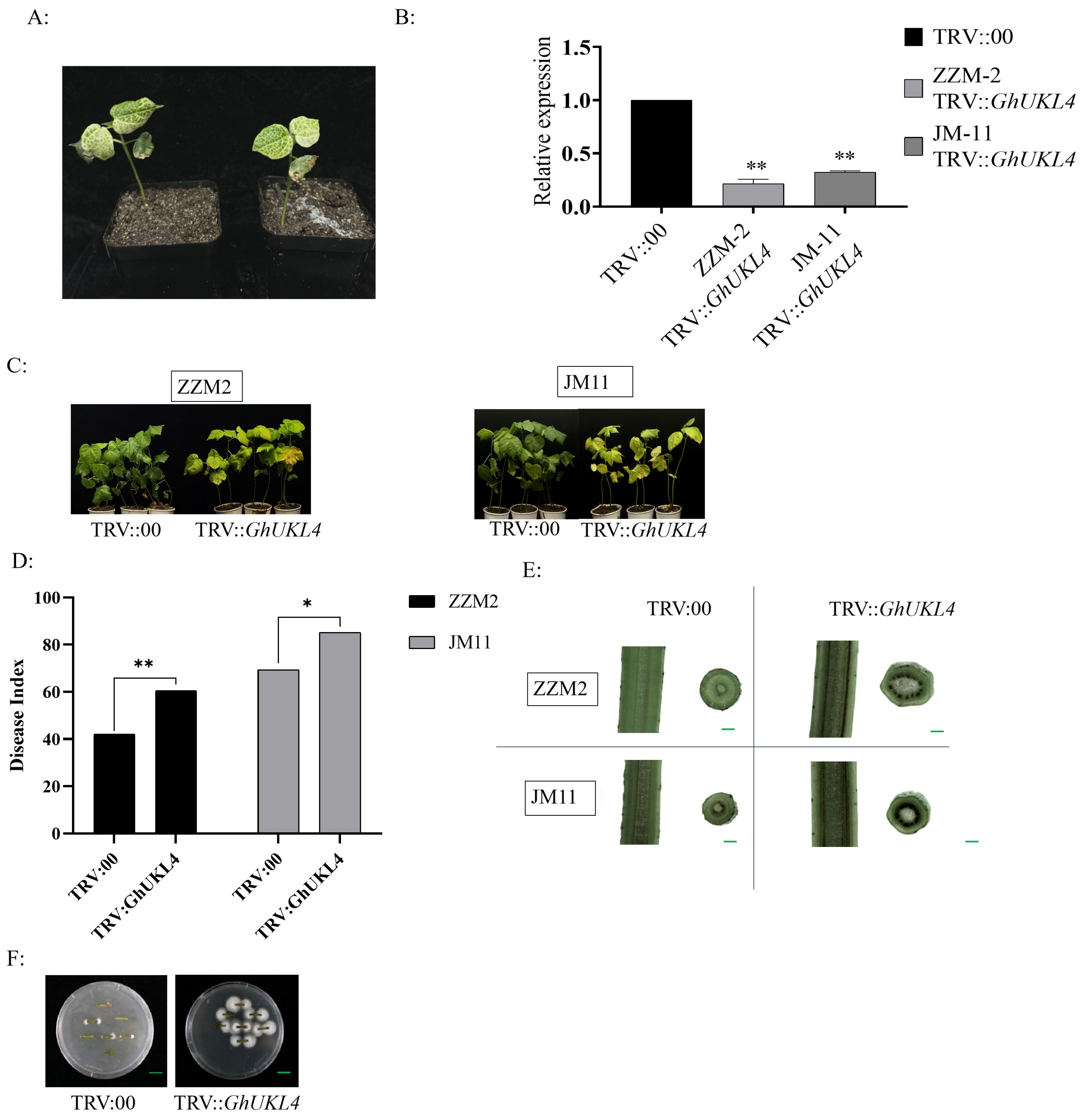
3.3. The Silencing of GhUKL4 Reduced Cotton’s Resistance to VW
3.4. Silencing the GhUKL4 Gene Reduces Resistance Through Multiple Pathways
4. Discussion
GhUKL4 Is Associated with Verticillium Wilt Resistance in Cotton
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VW | Verticillium wilt |
| UMPK | Uidine 5′-monophosphate kinase |
| UPRT | Uacil phosphoribosyltransferase |
| UKL | Uridine kinase like |
| VIGS | Virus-Induced Gene Silencing |
| ROS | Reactive Oxygen Species |
| JA | Reactive Oxygen Species |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
References
- Witte, C.-P.; Herde, M. Nucleotides and nucleotide derivatives as signal molecules in plants. J. Exp. Bot. 2024, 75, 6918–6938. [Google Scholar] [CrossRef]
- Zrenner, R.; Stitt, M.; Sonnewald, U.; Boldt, R. Pyrimidine and purine biosynthesis and degradation in plants. Annu. Rev. Plant Biol. 2006, 57, 805–836. [Google Scholar] [CrossRef] [PubMed]
- Kafer, C.; Zhou, L.; Santoso, D.; Guirgis, A.; Weers, B.; Park, S.; Thornburg, R. Regulation of pyrimidine metabolism in plants. Front. Biosci. 2004, 9, 1611–1625. [Google Scholar] [CrossRef]
- Shambaugh, G.E., 3rd. Pyrimidine biosynthesis. Am. J. Clin. Nutr. 1979, 32, 1290–1297. [Google Scholar] [CrossRef]
- Marco-Marín, C.; Gil-Ortiz, F.; Pérez-Arellano, I.; Cervera, J.; Fita, I.; Rubio, V. A novel two-domain architecture within the amino acid kinase enzyme family revealed by the crystal structure of Escherichia coli glutamate 5-kinase. J. Mol. Biol. 2007, 367, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Wang, M.M.; Huang, J.; Tang, H.J.; Lan, H.X.; Zhang, H.S. The OsDHODH1 gene is involved in salt and drought tolerance in rice. J. Integr. Plant Biol. 2009, 51, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Hu, S.; Wu, L.; Ge, C.; Cui, Y.; Chen, P.; Wang, X.; Xu, J.; Ren, D.; Dong, G.; et al. White stripe leaf 12 (WSL12), encoding a nucleoside diphosphate kinase 2 (OsNDPK2), regulates chloroplast development and abiotic stress response in rice (Oryza sativa L.). Mol. Breed. 2016, 36, 57. [Google Scholar] [CrossRef]
- Zhou, K.; Xia, J.; Wang, Y.; Ma, T.; Li, Z. A Young Seedling Stripe2 phenotype in rice is caused by mutation of a chloroplast-localized nucleoside diphosphate kinase 2 required for chloroplast biogenesis. Genet. Mol. Biol. 2017, 40, 630–642. [Google Scholar] [CrossRef]
- Geigenberger, P.; Regierer, B.; Nunes-Nesi, A.; Leisse, A.; Urbanczyk-Wochniak, E.; Springer, F.; van Dongen, J.T.; Kossmann, J.; Fernie, A.R. Inhibition of de novo pyrimidine synthesis in growing potato tubers leads to a compensatory stimulation of the pyrimidine salvage pathway and a subsequent increase in biosynthetic performance. Plant Cell 2005, 17, 2077–2088. [Google Scholar] [CrossRef]
- Chen, C.T.; Slocum, R.D. Expression and functional analysis of aspartate transcarbamoylase and role of de novo pyrimidine synthesis in regulation of growth and development in Arabidopsis. Plant Physiol. Biochem. 2008, 46, 150–159. [Google Scholar] [CrossRef]
- Feng, X.; Yang, R.; Zheng, X.; Zhang, F. Identification of a novel nuclear-localized adenylate kinase 6 from Arabidopsis thaliana as an essential stem growth factor. Plant Physiol. Biochem. 2012, 61, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.R.; Geserick, C.; Tischendorf, G.; Zrenner, R. Functions of chloroplastic adenylate kinases in Arabidopsis. Plant Physiol. 2008, 146, 492–504. [Google Scholar] [CrossRef]
- Daumann, M.; Hickl, D.; Zimmer, D.; DeTar, R.A.; Kunz, H.H.; Möhlmann, T. Characterization of filament-forming CTP synthases from Arabidopsis thaliana. Plant J. 2018, 96, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.Y.; Li, M.W.; Yung, Y.L.; Wen, C.Q.; Lam, H.M. The unconventional P-loop NTPase OsYchF1 and its regulator OsGAP1 play opposite roles in salinity stress tolerance. Plant Cell Environ. 2013, 36, 2008–2020. [Google Scholar] [CrossRef]
- Cheung, M.Y.; Li, X.; Miao, R.; Fong, Y.H.; Li, K.P.; Yung, Y.L.; Yu, M.H.; Wong, K.B.; Chen, Z.; Lam, H.M. ATP binding by the P-loop NTPase OsYchF1 (an unconventional G protein) contributes to biotic but not abiotic stress responses. Proc. Natl. Acad. Sci. USA 2016, 113, 2648–2653. [Google Scholar] [CrossRef]
- Wu, J.; Steinebrunner, I.; Sun, Y.; Butterfield, T.; Torres, J.; Arnold, D.; Gonzalez, A.; Jacob, F.; Reichler, S.; Roux, S.J. Apyrases (nucleoside triphosphate-diphosphohydrolases) play a key role in growth control in Arabidopsis. Plant Physiol. 2007, 144, 961–975. [Google Scholar] [CrossRef]
- Deng, S.; Sun, J.; Zhao, R.; Ding, M.; Zhang, Y.; Sun, Y.; Wang, W.; Tan, Y.; Liu, D.; Ma, X.; et al. Populus euphratica APYRASE2 Enhances Cold Tolerance by Modulating Vesicular Trafficking and Extracellular ATP in Arabidopsis Plants. Plant Physiol. 2015, 169, 530–548. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Thelen, J.J. Plastid uridine salvage activity is required for photoassimilate allocation and partitioning in Arabidopsis. Plant Cell 2011, 23, 2991–3006. [Google Scholar] [CrossRef]
- Riegler, H.; Geserick, C.; Zrenner, R. Arabidopsis thaliana nucleosidase mutants provide new insights into nucleoside degradation. New Phytol. 2011, 191, 349–359. [Google Scholar] [CrossRef]
- Cornelius, S.; Witz, S.; Rolletschek, H.; Möhlmann, T. Pyrimidine degradation influences germination seedling growth and production of Arabidopsis seeds. J. Exp. Bot. 2011, 62, 5623–5632. [Google Scholar] [CrossRef]
- Islam, M.R.; Kim, H.; Kang, S.W.; Kim, J.S.; Jeong, Y.M.; Hwang, H.J.; Lee, S.Y.; Woo, J.C.; Kim, S.G. Functional characterization of a gene encoding a dual domain for uridine kinase and uracil phosphoribosyltransferase in Arabidopsis thaliana. Plant Mol. Biol. 2007, 63, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Mainguet, S.E.; Gakière, B.; Majira, A.; Pelletier, S.; Bringel, F.; Guérard, F.; Caboche, M.; Berthomé, R.; Renou, J.P. Uracil salvage is necessary for early Arabidopsis development. Plant J. 2009, 60, 280–291. [Google Scholar] [CrossRef]
- Henderson, J.F.; Paterson, A.R.; Caldwell, I.C.; Paul, B.; Chan, M.C.; Lau, K.F. Inhibitors of nucleoside and nucleotide metabolism. Cancer Chemother Rep 2 1972, 3, 71–85. [Google Scholar] [PubMed]
- Matchett, E.C.; Ambrose, E.C.; Kornbluth, J. Characterization of uridine-cytidine kinase like-1 nucleoside kinase activity and its role in tumor growth. Biochem. J. 2022, 479, 1149–1164. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S. Rice versus Xanthomonas oryzae pv. oryzae: A unique pathosystem. Curr. Opin. Plant Biol. 2013, 16, 188–195. [Google Scholar] [CrossRef]
- Kim, S.H.; Witte, C.P.; Rhee, S. Structural basis for the substrate specificity and catalytic features of pseudouridine kinase from Arabidopsis thaliana. Nucleic Acids Res. 2021, 49, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, J.Q.; Chen, X.Y.; Zhu, Y.X. Recent Advances and Future Perspectives in Cotton Research. Annu. Rev. Plant Biol. 2021, 72, 437–462. [Google Scholar] [CrossRef]
- Man, M.; Zhu, Y.; Liu, L.; Luo, L.; Han, X.; Qiu, L.; Li, F.; Ren, M.; Xing, Y. Defense Mechanisms of Cotton Fusarium and Verticillium Wilt and Comparison of Pathogenic Response in Cotton and Humans. Int. J. Mol. Sci. 2022, 23, 12217. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, C.; Zhang, Y.; Yan, Q.; Hu, W.; Yang, L.; Wang, Z.; Li, F. Recent progression and future perspectives in cotton genomic breeding. J. Integr. Plant Biol. 2023, 65, 548–569. [Google Scholar] [CrossRef]
- Inderbitzin, P.; Subbarao, K.V. Verticillium systematics and evolution: How confusion impedes Verticillium wilt management and how to resolve it. Phytopathology 2014, 104, 564–574. [Google Scholar] [CrossRef]
- An, Q.; Ehlers, K.; Kogel, K.H.; van Bel, A.J.; Hückelhoven, R. Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol. 2006, 172, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Eichmann, R.; Dechert, C.; Kogel, K.H.; Hückelhoven, R. Transient over-expression of barley BAX Inhibitor-1 weakens oxidative defence and MLA12-mediated resistance to Blumeria graminis f.sp. hordei. Mol. Plant Pathol. 2006, 7, 543–552. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Jin, L.; Long, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef]
- Liu, F.; Cai, S.; Ma, Z.; Yue, H.; Xing, L.; Wang, Y.; Feng, S.; Wang, L.; Dai, L.; Wan, H.; et al. RVE2, a new regulatory factor in jasmonic acid pathway, orchestrates resistance to Verticillium wilt. Plant Biotechnol. J. 2023, 21, 2507–2524. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, W.; Cui, Y.; Sang, X.; Lu, J.; Jing, H.; Wang, W.; Zhao, P.; Wang, H. Detection of candidate genes and development of KASP markers for Verticillium wilt resistance by combining genome-wide association study, QTL-seq and transcriptome sequencing in cotton. Theor. Appl. Genet. 2021, 134, 1063–1081. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Chen, W.; Wang, H.; Zhu, X.; Yang, J.; Lu, N.; Zhao, P.; Sang, X.; Cui, Y.; et al. Identification of major QTLfor Verticillium wilt resistance in cotton by using, F2 and RIL. Cotton Sci. 2023, 35, 101–116. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Chen, W.; Li, Y. Genetic structure, linkage disequilibrium and association mapping of Verticillium wilt resistance in elite cotton (Gossypium hirsutum L.) germplasm population. PLoS ONE 2014, 9, e86308. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Huang, L.; Yu, L.J.; Zhang, X.; Fan, B.; Wang, F.Z.; Dai, Y.S.; Qi, H.; Zhou, Y.; Xie, L.J.; Xiao, S. Autophagy regulates glucose-mediated root meristem activity by modulating ROS production in Arabidopsis. Autophagy 2019, 15, 407–422. [Google Scholar] [CrossRef]
- Li, F.; Zang, M.; Hou, J.; Luo, Q.; Yu, S.; Sun, H.; Xu, J.; Liu, J. Cascade catalytic nanoplatform constructed by laterally-functionalized pillar (5)arenes for antibacterial chemodynamic therapy. J. Mater. Chem. B 2021, 9, 5069–5075. [Google Scholar] [CrossRef]
- Alariqi, M.; Ramadan, M.; Wang, Q.; Yang, Z.; Hui, X.; Nie, X.; Ahmed, A.; Chen, Q.; Wang, Y.; Zhu, L.; et al. Cotton 4-coumarate-CoA ligase 3 enhanced plant resistance to Verticillium dahliae by promoting jasmonic acid signaling-mediated vascular lignification and metabolic flux. Plant J. 2023, 115, 190–204. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, D.; Liu, D.; Cheng, Y.; Zhang, X.; Song, L.; Hu, M.; Dong, J.; Shen, F. Comprehensive analysis of the Gossypium hirsutum L. respiratory burst oxidase homolog (Ghrboh) gene family. BMC Genom. 2020, 21, 91. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wu, J.; Li, Y.; Nan, Z.; Guo, X.; Wang, Y.; Zhang, A.; Wang, Z.; Xia, G.; Tian, Y. Synergistic effects of GhSOD1 and GhCAT1 overexpression in cotton chloroplasts on enhancing tolerance to methyl viologen and salt stresses. PLoS ONE 2013, 8, e54002. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, D.; Wang, H.; Kong, J.; Chen, Z.; Ruan, C.; Deng, C.; Zheng, Q.; Guo, Z.; Liu, H.; et al. Natural SNP variation in GbOSM1 promoter enhances Verticillium wilt resistance in cotton. Adv. Sci. 2024, 11, 2406522. [Google Scholar] [CrossRef]
- Mu, T.; Luo, S.; Li, L.; Zhang, R.; Wang, P.; Zhang, G. A review of the interaction mechanisms between jasmonic acid (JA) and various plant hormones, as well as the core regulatory role of MYC2. Plant Sci. 2025, 353, 112407. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, Z.; He, P.; Wang, J.; Li, D.; Meng, J.; Zhang, D.; You, J.; Luo, Y.; Wang, X.; et al. The synergistic roles of MsRCI2B and MsRCI2E in the regulation of ion balance and ROS homeostasis in alfalfa under salt stress. Int. J. Biol. Macromol. 2025, 300, 140093. [Google Scholar] [CrossRef]





| Gene ID | Gene Name | Description | Start | End | Strand |
|---|---|---|---|---|---|
| Ghir_D05G039040 | SFT2 | Protein transport protein SFT2 | 62,511,198 | 62,514,773 | + |
| Ghir_D05G039050 | recX | Regulatory protein RecX | 62,526,388 | 62,534,437 | - |
| Ghir_D05G039070 | UGT76A2 | UDP-glucose iridoid glucosyltransferase | 62,535,623 | 62,537,817 | - |
| Ghir_D05G039080 | UGT76A2 | UDP-glucose iridoid glucosyltransferase | 62,540,122 | 62,541,578 | - |
| Ghir_D05G039090 | UGT76A2 | UDP-glucose iridoid glucosyltransferase | 62,549,851 | 62,551,307 | - |
| Ghir_D05G039100 | NA | NA | 62,552,046 | 62,553,225 | - |
| Ghir_D05G039110 | KIN14F | Kinesin-like protein KIN-14F | 62,553,683 | 62,560,508 | - |
| Ghir_D05G039120 | UKL4 | Uridine kinase-like protein 4 | 62,576,759 | 62,581,764 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Sun, Y.; Sang, X.; Lu, J.; Zhao, P.; Chen, W.; Zhao, Y.; Wang, H. Uridine Kinase-like Protein (GhUKL4) Positively Regulates Resistance to Verticillium Wilt in Cotton. Genes 2025, 16, 819. https://doi.org/10.3390/genes16070819
Cheng B, Sun Y, Sang X, Lu J, Zhao P, Chen W, Zhao Y, Wang H. Uridine Kinase-like Protein (GhUKL4) Positively Regulates Resistance to Verticillium Wilt in Cotton. Genes. 2025; 16(7):819. https://doi.org/10.3390/genes16070819
Chicago/Turabian StyleCheng, Baimei, Yanmeng Sun, Xiaohui Sang, Jianhua Lu, Pei Zhao, Wei Chen, Yunlei Zhao, and Hongmei Wang. 2025. "Uridine Kinase-like Protein (GhUKL4) Positively Regulates Resistance to Verticillium Wilt in Cotton" Genes 16, no. 7: 819. https://doi.org/10.3390/genes16070819
APA StyleCheng, B., Sun, Y., Sang, X., Lu, J., Zhao, P., Chen, W., Zhao, Y., & Wang, H. (2025). Uridine Kinase-like Protein (GhUKL4) Positively Regulates Resistance to Verticillium Wilt in Cotton. Genes, 16(7), 819. https://doi.org/10.3390/genes16070819






