Molecular Network Analysis and Effector Gene Prioritization of Endurance-Training-Influenced Modulation of Cardiac Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition and Preprocessing
2.2. Gene Set Network and Enrichment Analysis
2.3. Gene Prioritization and Linear Integration
3. Results
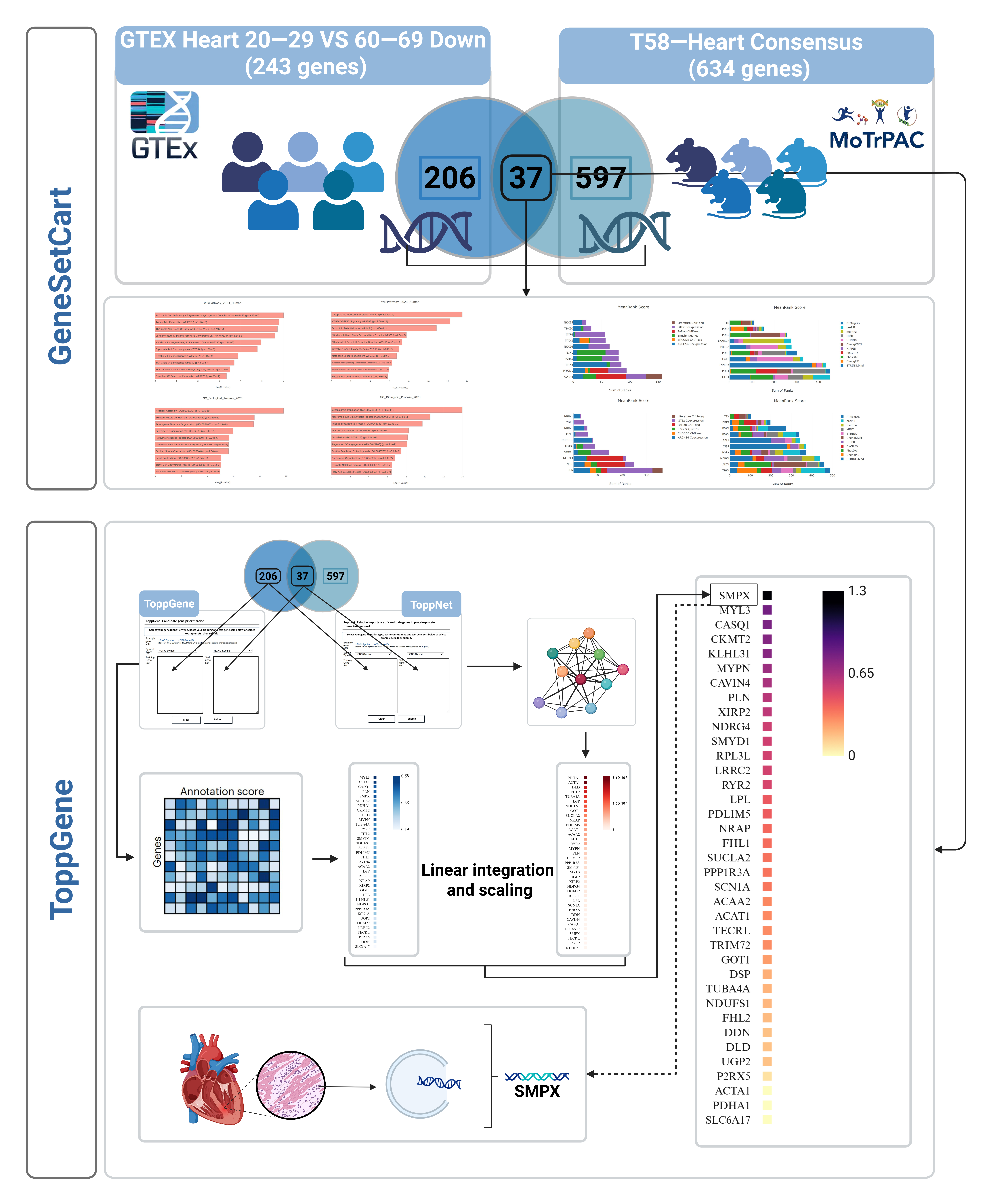
3.1. Gene Set Overlap and Enrichment Analysis
3.1.1. Gene Set Overlap
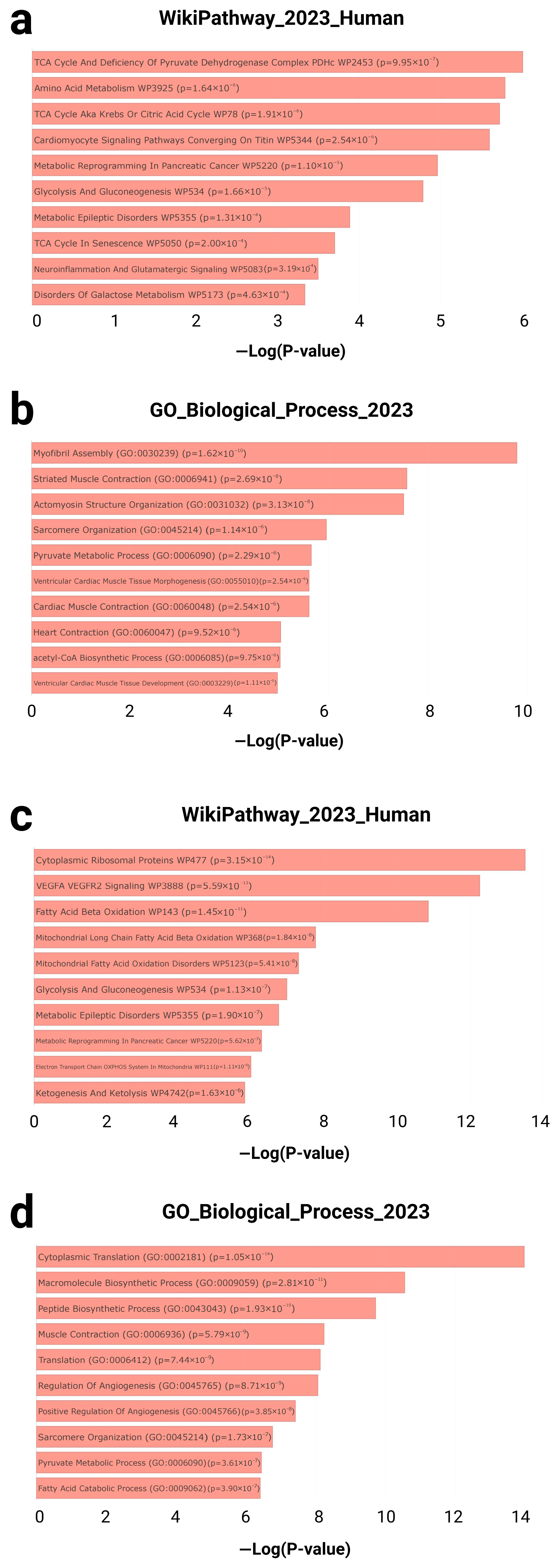
3.1.2. Functional Enrichment Analysis
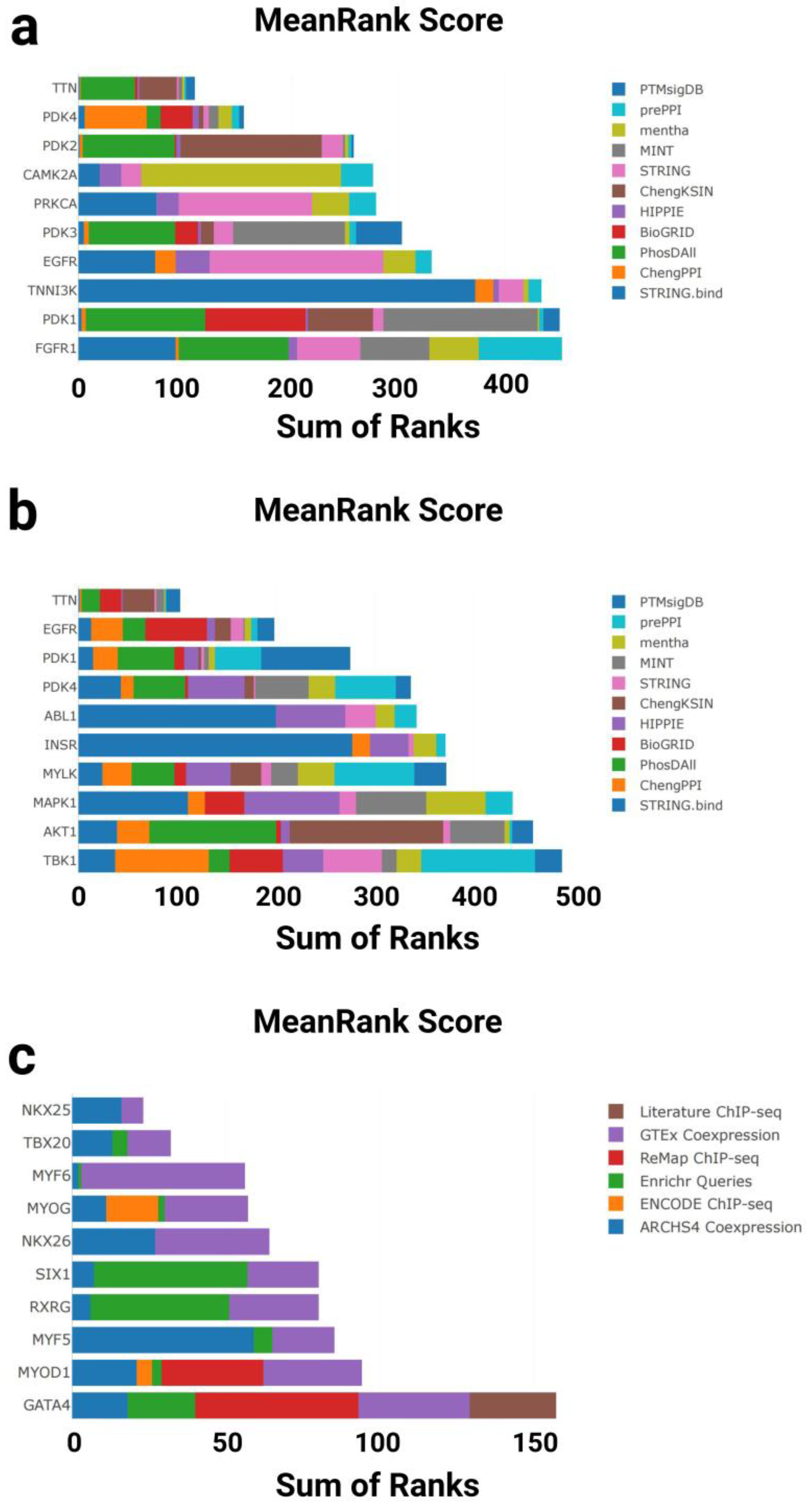
3.1.3. Kinase Enrichment Analysis
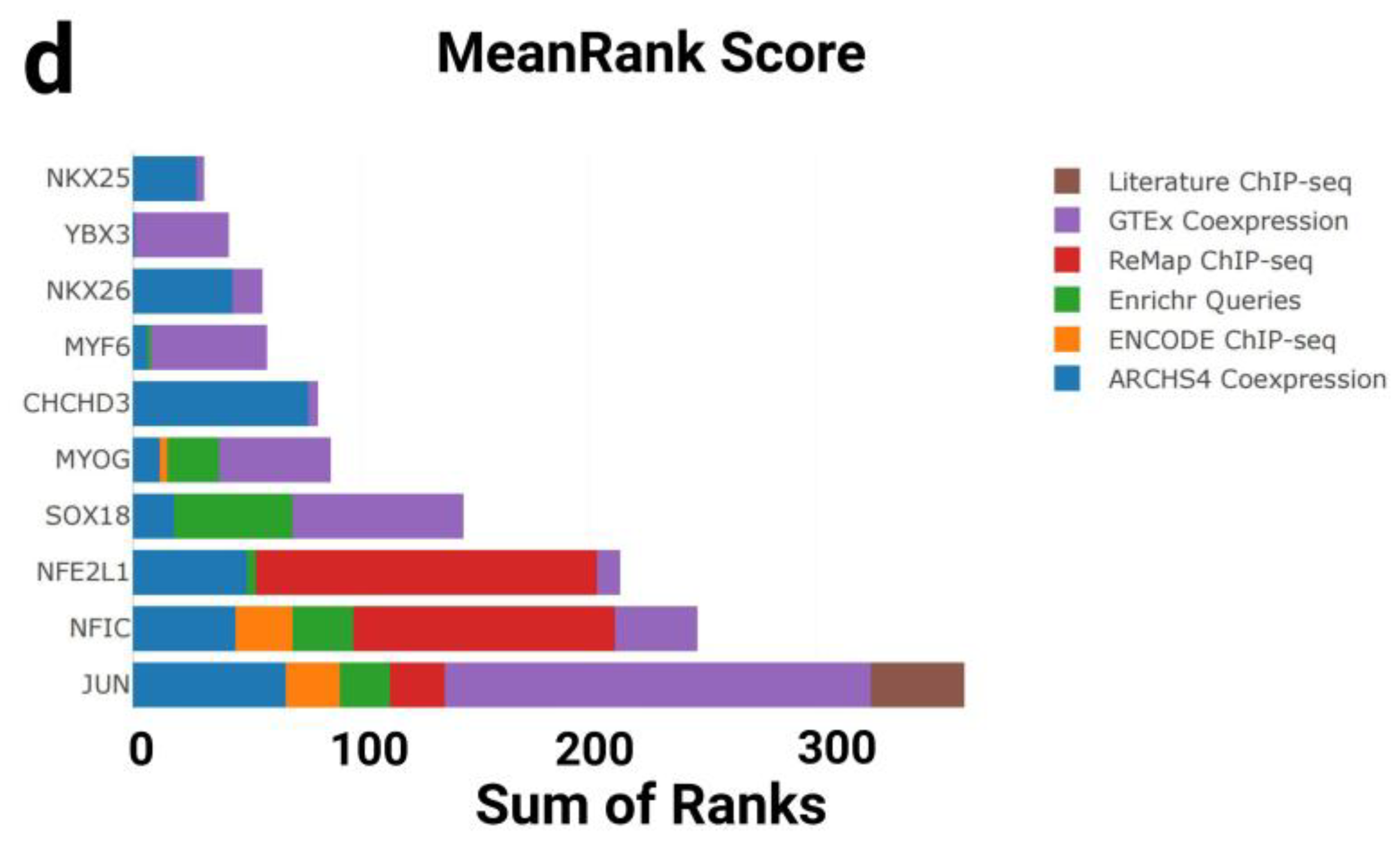
3.1.4. Transcription Factor Enrichment Analysis
3.1.5. Effector Gene Prioritization
4. Discussion
4.1. Functional Enrichment Analysis
4.2. Kinase Enrichment Analysis
4.3. Transcription Factor Enrichment Analysis
4.4. Effector Gene Prioritization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABL1 | ABL Proto-Oncogene 1, Non-Receptor Tyrosine Kinase |
| ACAA2 | Acetyl-CoA Acyltransferase 2 |
| ACAT1 | Acetyl-CoA Acetyltransferase 1 |
| ACTA1 | Actin Alpha 1, Skeletal Muscle |
| ACTN2 | Alpha Actinin 2 |
| AKT1 | AKT Serine/Threonine Kinase 1 |
| AMPK | AMP-Activated Protein Kinase |
| ARCHS4 Coexpression | All RNA-seq and ChIP-seq Sample and Signature Search Coexpression |
| ATP | Adenosine Triphosphate |
| BioGRID | Biological General Repository for Interaction Datasets |
| CAGE | Cap Analysis of Gene Expression |
| CAMK2A | Calcium/Calmodulin-Dependent Protein Kinase II Alpha |
| CASQ1 | Calsequestrin 1 |
| CAVIN4 | Caveolae Associated Protein 4 |
| CHCHD3 | Coiled-Coil-Helix-Coiled-Coil-Helix Domain Containing 3 |
| ChEA3 | ChIP-X Enrichment Analysis 3 |
| ChengKSIN | Cheng Kinase–Substrate Interaction Network |
| ChengPPI | Cheng Protein–Protein Interaction Dataset |
| CKMT2 | Creatine Kinase, Mitochondrial 2 |
| CVDs | Cardiovascular Diseases |
| dbGaP | Database of Genotypes and Phenotypes |
| DDN | Dendrin |
| DLD | Dihydrolipoamide Dehydrogenase |
| DNA | Deoxyribonucleic Acid |
| DSP | Desmoplakin |
| ENCODE ChIP-seq | ENCODE ChIP-Sequencing |
| Enrichr Queries | Enrichment Queries from Enrichr |
| FDR | False Discovery Rate |
| FGFR1 | Fibroblast Growth Factor Receptor 1 |
| FHL1 | Four and a Half LIM Domains 1 |
| FHL2 | Four and a Half LIM Domains 2 |
| FLAMES | Functional and Network Linear Model for Multi-dimensional Effector Scoring |
| GATA4 | GATA Binding Protein 4 |
| GO | Gene Ontology |
| GOT1 | Glutamic-Oxaloacetic Transaminase 1 |
| GSEA | Gene Set Enrichment Analysis |
| GTEx | Genotype-Tissue Expression |
| GTEx Coexpression | Gene Coexpression from GTEx Dataset |
| HIPPIE | Human Integrated Protein–Protein Interaction rEference |
| ID | Identifier |
| INSR | Insulin Receptor |
| JUN | Jun Proto-Oncogene, AP-1 Transcription Factor Subunit |
| KEA3 | Kinase Enrichment Analysis 3 |
| KLHL31 | Kelch Like Family Member 31 |
| Literature ChIP-seq | Literature-Curated Chromatin Immunoprecipitation Sequencing |
| LPL | Lipoprotein Lipase |
| LRRC2 | Leucine Rich Repeat Containing 2 |
| MAPK1 | Mitogen-Activated Protein Kinase 1 |
| mentha | MEtabolic Network Through Heterogeneous Analysis |
| MINT | Molecular INTeraction Database |
| MoTrPAC | Molecular Transducers of Physical Activity Consortium |
| MYF5 | Myogenic Factor 5 |
| MYF6 | Myogenic Factor 6 |
| MYL3 | Myosin Light Chain 3 |
| MYLK | Myosin Light Chain Kinase |
| MYOD1 | Myogenic Differentiation 1 |
| MYOG | Myogenin |
| MYPN | Myopalladin |
| NDRG4 | N-Myc Downstream Regulated 4 |
| NDUFS1 | NADH:Ubiquinone Oxidoreductase Core Subunit S1 |
| NFE2L1 | Nuclear Factor, Erythroid 2 Like 1 |
| NFIC | Nuclear Factor I C-Type |
| NKKX2-6 | NK2 Homeobox 6 |
| NKX2-5 | NK2 Homeobox 5 |
| NRAP | Nebulin Related Anchoring Protein |
| P2RX5 | Purinergic Receptor P2X 5 |
| PAXgene | PreAnalytix PaxGene Tissue/Tube System |
| PDHA1 | Pyruvate Dehydrogenase E1 Alpha 1 Subunit |
| PDK1 | Pyruvate Dehydrogenase Kinase Isozyme 1 |
| PDK2 | Pyruvate Dehydrogenase Kinase Isozyme 2 |
| PDK3 | Pyruvate Dehydrogenase Kinase Isozyme 3 |
| PDK4 | Pyruvate Dehydrogenase Kinase Isozyme 4 |
| PDLIM5 | PDZ and LIM Domain 5 |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| PhosDAll | Phosphorylation Data Aggregated from All Sources |
| PLN | Phospholamban |
| PPI | Protein–Protein Interaction |
| PPP1R3A | Protein Phosphatase 1 Regulatory Subunit 3A |
| prePPI | Predicted Protein–Protein Interactions Database |
| PRKCA | Protein Kinase C Alpha |
| PTMsigDB | Post-Translational Modification Signature Database |
| ReMap ChIP-seq | Regulatory Map ChIP-seq |
| RIN | RNA Integrity Number |
| RNA | Ribonucleic Acid |
| RPL3L | Ribosomal Protein L3-Like |
| RXRG | Retinoid X Receptor Gamma |
| RYR2 | Ryanodine Receptor 2 |
| SCN1A | Sodium Voltage-Gated Channel Alpha Subunit 1 |
| SIRT1 | Sirtuin 1 |
| SIX1 | SIX Homeobox 1 |
| SLC6A17 | Solute Carrier Family 6 Member 17 |
| SMPX | Small Muscle Protein, X-Linked |
| SMYD1 | SET and MYND Domain Containing 1 |
| SOX18 | SRY-Box Transcription Factor 18 |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| STRING.bind | STRING Binding Evidence Subset |
| SUCLA2 | Succinate-CoA Ligase ADP-Forming Beta Subunit |
| TBK1 | TANK-Binding Kinase 1 |
| TBX20 | T-box Transcription Factor 20 |
| TCA | Tricarboxylic Acid Cycle |
| TECRL | Trans-2,3-Enoyl-CoA Reductase Like |
| TF | Transcription Factor |
| TNNI3K | Troponin I-Interacting Kinase |
| ToppGene | ToppGene Suite Functional Enrichment Analysis Tool |
| ToppNet | ToppGene Suite Network Prioritization Tool |
| TRIM72 | Tripartite Motif Containing 72 |
| TTN | Titin |
| TUBA4A | Tubulin Alpha 4a |
| UGP2 | UDP-Glucose Pyrophosphorylase 2 |
| XIRP2 | Xin Actin Binding Repeat Containing 2 |
| YBX3 | Y-box Binding Protein 3 |
Appendix A
| Gene Symbol | Full Name | ToppGene Score | ToppNet Score | FinalScore | Primary Functional Annotation |
|---|---|---|---|---|---|
| ACAA2 | Acetyl-CoA Acyltransferase 2 | 0.370033773 | 9.37 × 10−5 | 0.326818946 | Fatty acid breakdown and mitochondrial lipid metabolism [135] |
| ACAT1 | Acetyl-CoA Acetyltransferase 1 | 0.376042415 | 1.02 × 10−4 | 0.324654235 | Cholesterol esterification and lipid metabolism [136] |
| ACTA1 | Actin Alpha 1, Skeletal Muscle | 0.565635952 | 3.07 × 10−4 | 0.000948507 | Contractile cytoskeleton and myopathy/cardiomyopathy [74] |
| CASQ1 | Calsequestrin 1 | 0.516803064 | 2.85 × 10−6 | 0.838524706 | Calcium handling in fast-twitch muscle via sarcoplasmic reticulum [112] |
| CAVIN4 | Caveolae Associated Protein 4 | 0.458419609 | 2.96 × 10−6 | 0.689971039 | T-tubule development and function [118] |
| CKMT2 | Creatine Kinase, Mitochondrial 2 | 0.556345232 | 4.38 × 10−5 | 0.812833829 | Fast-twitch muscle calcium regulation [115] |
| DDN | Dendrin | 0.254432411 | 4.23 × 10−6 | 0.171287172 | Neural signaling and synaptic plasticity [130] |
| DLD | Dihydrolipoamide Dehydrogenase | 0.468299535 | 2.36 × 10−4 | 0.167994144 | Energy and pyruvate metabolism, redox balance [142] |
| DSP | Desmoplakin | 0.401244892 | 1.82 × 10−4 | 0.22412071 | Cell adhesion and myocardial stability [141] |
| FHL1 | Four And A Half LIM Domains 1 | 0.413964729 | 9.28 × 10−5 | 0.406741081 | Mechanical–transcriptional integration and muscle structure [123] |
| FHL2 | Four And A Half LIM Domains 2 | 0.459343846 | 2.26 × 10−4 | 0.184490101 | Muscle development, signaling and disease mechanisms [124] |
| GOT1 | Glutamic-Oxaloacetic Transaminase 1 | 0.415075731 | 1.66 × 10−4 | 0.268865825 | Amino acid metabolism and TCA anaplerosis [129] |
| KLHL31 | Kelch Like Family Member 31 | 0.394484874 | 0 | 0.799148858 | Sarcomere integrity and SR coupling [116] |
| LPL | Lipoprotein Lipase | 0.377975045 | 1.69 × 10−5 | 0.463416222 | Triglyceride hydrolysis and lipid metabolism [137] |
| LRRC2 | Leucine Rich Repeat Containing 2 | 0.333212286 | 1.03 × 10−6 | 0.562245597 | Mitochondrial function and cardiac remodeling [127] |
| MYL3 | Myosin Light Chain 3 | 0.576717614 | 3.90 × 10−5 | 0.873185812 | Sarcomere contraction and cardiac mechanics [112] |
| MYPN | Myopalladin | 0.541188005 | 5.81 × 10−5 | 0.737031654 | Sarcomere maintenance and signaling regulation [117] |
| NDRG4 | NDRG Family Member 4 | 0.433742708 | 2.40 × 10−5 | 0.583950809 | Stress response and cardiac maintenance [131] |
| NDUFS1 | NADH:Ubiquinone Oxidoreductase Core Subunit S1 | 0.369426305 | 1.77 × 10−4 | 0.198506148 | Mitochondrial metabolism, remodeling, and oxidative stress [84] |
| NRAP | Nebulin Related Anchoring Protein | 0.466523641 | 1.29 × 10−4 | 0.415352267 | Cardiac development and force transmission [139] |
| P2RX5 | Purinergic Receptor P2X 5 | 0.218041283 | 1.22 × 10−5 | 0.077196996 | ATP-dependent ion transport and multisystem regulation [143] |
| PDHA1 | Pyruvate Dehydrogenase E1 Subunit Alpha 1 | 0.47683771 | 3.07 × 10−4 | 0 | Glycolysis–TCA linkage and cardiac metabolic stability [72] |
| PDLIM5 | PDZ And LIM Domain 5 | 0.481768861 | 1.23 × 10−4 | 0.452752432 | Z-disc stability and signaling [122] |
| PLN | Phospholamban | 0.498915554 | 5.74 × 10−5 | 0.650992399 | Cardiac Ca2+ homeostasis and contraction [119] |
| PPP1R3A | Protein Phosphatase 1 Regulatory Subunit 3A | 0.356625144 | 4.07 × 10−5 | 0.377933825 | PP1 regulation, Ca2+ homeostasis, and rhythm control [133] |
| RPL3L | Ribosomal Protein L3 Like | 0.422976684 | 1.94 × 10−5 | 0.567611129 | Cardiac-specific translation elongation [126] |
| RYR2 | Ryanodine Receptor 2 | 0.491528815 | 9.08 × 10−5 | 0.550565627 | Ca2+ release and excitation–contraction coupling [120] |
| SCN1A | Sodium Voltage-Gated Channel Alpha Subunit 1 | 0.340497171 | 1.48 × 10−5 | 0.375326735 | Action potential and neuro-cardiac rhythm [134] |
| SLC6A17 | Solute Carrier Family 6 Member 17 | 0.186693123 | 2.72 × 10−6 | 0 | Glutamine transport, synaptic function, and neurodevelopment [145] |
| SMPX | Small Muscle Protein, X-linked | 0.527740575 | 1.55 × 10−6 | 1.307228059 | Sarcolemmal stability and stress response [147,150,151] |
| SMYD1 | SET And MYND Domain Containing 1 | 0.444368961 | 3.94 × 10−5 | 0.57604553 | Cardiac development and epigenetic regulation [125] |
| SUCLA2 | Succinate-CoA Ligase ADP-Forming Beta Subunit | 0.452569875 | 1.32 × 10−4 | 0.387985714 | TCA cycle catalysis and mitochondrial metabolism [140] |
| TECRL | Trans-2,3-Enoyl-CoA Reductase Like | 0.268486979 | 1.16 × 10−6 | 0.313783778 | Ca2+ homeostasis and rhythm stability [132] |
| TRIM72 | Tripartite Motif Containing 72 | 0.317477311 | 2.07 × 10−5 | 0.312757183 | Membrane repair and muscle cell integrity [128] |
| TUBA4A | Tubulin Alpha 4a | 0.443812358 | 2.11 × 10−4 | 0.205730526 | Microtubule structure and cardiac stress response [144] |
| UGP2 | UDP-Glucose Pyrophosphorylase 2 | 0.261004361 | 3.85 × 10−5 | 0.166646811 | Glucose metabolism and protein glycosylation [138] |
| XIRP2 | Xin Actin Binding Repeat Containing 2 | 0.457391502 | 2.76 × 10−5 | 0.631808969 | Actin structure and cardiac remodeling [121] |
References
- Hastings, M.H.; Zhou, Q.; Wu, C.; Shabani, P.; Huang, S.; Yu, X.; Singh, A.P.; Guseh, J.S.; Li, H.; Lerchenmuller, C.; et al. Cardiac aging: From hallmarks to therapeutic opportunities. Cardiovasc. Res. 2024, cvae124, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, M.; Schmid, S.T.; Fuerlinger, A.; Kroemer, G. Anti-ageing interventions for the treatment of cardiovascular disease. Cardiovasc. Res. 2024, cvae177. [Google Scholar] [CrossRef]
- Leuchtmann, A.B.; Furrer, R.; Steurer, S.A.; Schneider-Heieck, K.; Karrer-Cardel, B.; Sagot, Y.; Handschin, C. Interleukin-6 potentiates endurance training adaptation and improves functional capacity in old mice. J. Cachexia Sarcopenia Muscle 2022, 13, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Feng, P.; Feng, L.; Shi, L.; Song, Y.; Yang, J.; Duan, W.; Gao, E.; Liu, J.; Yi, D.; et al. Low-dose exercise protects the heart against established myocardial infarction via IGF-1-upregulated CTRP9 in male mice. MedComm 2023, 4, e411. [Google Scholar] [CrossRef]
- Jin, L.; Geng, L.; Ying, L.; Shu, L.; Ye, K.; Yang, R.; Liu, Y.; Wang, Y.; Cai, Y.; Jiang, X.; et al. FGF21-sirtuin 3 axis confers the protective effects of exercise against diabetic cardiomyopathy by governing mitochondrial integrity. Circulation 2022, 146, 1537–1557. [Google Scholar] [PubMed]
- Forbes, L.M.; Bull, T.M.; Lahm, T.; Make, B.J.; Cornwell, W.K., III. Exercise Testing in the Risk Assessment of Pulmonary Hypertension. Chest 2023, 164, 736–746. [Google Scholar] [CrossRef]
- Schenk, S.; Sagendorf, T.J.; Many, G.M.; Lira, A.K.; de Sousa, L.G.O.; Bae, D.; Cicha, M.; Kramer, K.S.; Muehlbauer, M.; Hevener, A.L.; et al. Physiological adaptations to progressive endurance exercise training in adult and aged rats: Insights from the Molecular Transducers of Physical Activity Consortium (MoTrPAC). Function 2024, 5, zqae014. [Google Scholar] [CrossRef]
- Vetr, N.G.; Gay, N.R.; MoTr, P.A.C.S.G.; Montgomery, S.B. The impact of exercise on gene regulation in association with complex trait genetics. Nat. Commun. 2024, 15, 3346. [Google Scholar]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Sanford, J.A.; Nogiec, C.D.; Lindholm, M.E.; Adkins, J.N.; Amar, D.; Dasari, S.; Drugan, J.K.; Fernandez, F.M.; Radom-Aizik, S.; Schenk, S.; et al. Molecular Transducers of Physical Activity Consortium (MoTrPAC): Mapping the Dynamic Responses to Exercise. Cell 2020, 181, 1464–1474. [Google Scholar] [CrossRef]
- Kim, D.S.; Wheeler, M.T.; Ashley, E.A. The genetics of human performance. Nat. Rev. Genet. 2022, 23, 40–54. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 2020, 16, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Roberts, F.L.; Markby, G.R. New Insights into molecular mechanisms mediating adaptation to exercise; a review focusing on mitochondrial biogenesis, mitochondrial function, mitophagy and autophagy. Cells 2021, 10, 2639. [Google Scholar] [CrossRef]
- MoTrPAC Study Group; Lead Analysts; MoTrPAC Study Group. Temporal dynamics of the multi-omic response to endurance exercise training. Nature 2024, 629, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Carrasquilla, G.D.; Ingerslev, L.R.; Hochreuter, M.Y.; Hansson, S.; Pillon, N.J.; Donkin, I.; Versteyhe, S.; Zierath, J.R.; Kilpelainen, T.O.; et al. Epigenetic rewiring of skeletal muscle enhancers after exercise training supports a role in whole-body function and human health. Mol. Metab. 2021, 53, 101290. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.D.; Pincas, H.; Smith, G.R.; Zaslavsky, E.; Ge, Y.; Amper, M.A.S.; Vasoya, M.; Chikina, M.; Sun, Y.; Raja, A.N.; et al. Molecular adaptations in response to exercise training are associated with tissue-specific transcriptomic and epigenomic signatures. Cell Genom. 2024, 4, 100421. [Google Scholar] [CrossRef]
- Bosslau, T.K.; Wasserfurth, P.; Reichel, T.; Weyh, C.; Palmowski, J.; Nebl, J.; Joisten, N.; Belen, S.; Schenk, A.; Hahn, A.; et al. 12-week combined strength and endurance exercise attenuates CD8(+) T-cell differentiation and affects the kynurenine pathway in the elderly: A randomized controlled trial. Immun. Ageing 2023, 20, 19. [Google Scholar] [CrossRef]
- Warner, A.W.; Moore, H.; Reinhard, D.; Ball, L.A.; Knoppers, B.M. Harmonizing global biospecimen consent practices to advance translational research: A call to action. Clin. Pharmacol. Ther. 2017, 101, 317–319. [Google Scholar] [CrossRef]
- Deviatiiarov, R.M.; Gams, A.; Kulakovskiy, I.V.; Buyan, A.; Meshcheryakov, G.; Syunyaev, R.; Singh, R.; Shah, P.; Tatarinova, T.V.; Gusev, O.; et al. An atlas of transcribed human cardiac promoters and enhancers reveals an important role of regulatory elements in heart failure. Nat. Cardiovasc. Res. 2023, 2, 58–75. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Jang, W.B.; Lee, Y.; Kim, Y.H.; Lim, H.J.; Lee, E.J.; Nguyen, T.M.T.; Choi, E.J.; Kwon, S.M.; Oh, J.W. Non-intrusive quality appraisal of differentiation-induced cardiovascular stem cells using E-Nose sensor technology. Biosens. Bioelectron. 2024, 246, 115838. [Google Scholar] [CrossRef]
- Gladka, M.M.; Molenaar, B.; de Ruiter, H.; van der Elst, S.; Tsui, H.; Versteeg, D.; Lacraz, G.P.A.; Huibers, M.M.H.; van Oudenaarden, A.; van Rooij, E. Single-cell sequencing of the healthy and diseased heart reveals cytoskeleton-associated protein 4 as a new modulator of fibroblasts activation. Circulation 2018, 138, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Ang, G.C.; Low, S.L.; How, C.H. Approach to falls among the elderly in the community. Singapore Med. J. 2020, 61, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Bloch, F.; Thibaud, M.; Dugué, B.; Brèque, C.; Rigaud, A.S.; Kemoun, G. Episodes of falling among elderly people: A systematic review and meta-analysis of social and demographic pre-disposing characteristics. Clinics 2010, 65, 895–903. [Google Scholar] [CrossRef]
- Schwendner, K.I.; Mikesky, A.E.; Holt, W.S., Jr.; Peacock, M.; Burr, D.B. Differences in muscle endurance and recovery between fallers and nonfallers, and between young and older women. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M155–M160. [Google Scholar] [CrossRef]
- Hottenrott, L.; Ketelhut, S.; Schneider, C.; Wiewelhove, T.; Ferrauti, A. Age- and sex-related differences in recovery from high-intensity and endurance exercise: A brief review. Int. J. Sports Physiol. Perform. 2021, 16, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Putri, N.R.I.A.T.; Rekawati, E.; Wati, D.N.K. Relationship of age, gender, hypertension history, and vulnerability perception with physical exercise compliance in elderly. Enferm. Clin. 2019, 29, 541–545. [Google Scholar] [CrossRef]
- Zhu, Y.; Cheng, K.; Wang, H.; Xu, Z.; Zhang, R.; Cheng, W.; Wang, Y.; Lyu, W. Exercise adherence and compliance and its related factors among elderly patients with type 2 diabetes in china: A cross-sectional study. Patient Prefer. Adherence 2022, 16, 3329–3339. [Google Scholar] [CrossRef]
- Taylor, D.; Binns, E.; Signal, N. Upping the ante: Working harder to address physical inactivity in older adults. Curr. Opin. Psychiatry 2017, 30, 352–357. [Google Scholar] [CrossRef]
- Haynes, A.; Naylor, L.H.; Carter, H.H.; Spence, A.L.; Robey, E.; Cox, K.L.; Maslen, B.A.; Lautenschlager, N.T.; Ridgers, N.D.; Green, D.J. Land-walking vs. water-walking interventions in older adults: Effects on aerobic fitness. J. Sport Health Sci. 2020, 9, 274–282. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Z.; He, L.; Xiao, Y.; Zhao, H.; Zhang, L.; Liu, T.; Chen, R.; Zhang, K.; Luo, B. Optimal lifestyle patterns for delaying ageing and reducing all-cause mortality: Insights from the UK Biobank. Eur. Rev. Aging Phys. Act. 2024, 21, 27. [Google Scholar] [CrossRef]
- Linscheid, N.; Santos, A.; Poulsen, P.C.; Mills, R.W.; Calloe, K.; Leurs, U.; Ye, J.Z.; Stolte, C.; Thomsen, M.B.; Bentzen, B.H.; et al. Quantitative proteome comparison of human hearts with those of model organisms. PLoS Biol. 2021, 19, e3001144. [Google Scholar] [CrossRef] [PubMed]
- Arduini, A.; Fleming, S.J.; Xiao, L.; Hall, A.W.; Akkad, A.D.; Chaffin, M.D.; Bendinelli, K.J.; Tucker, N.R.; Papangeli, I.; Mantineo, H.; et al. Transcriptional profile of the rat cardiovascular system at single-cell resolution. Cell Rep. 2025, 44, 115091. [Google Scholar] [CrossRef]
- Bugger, H.; Byrne, N.J.; Abel, E.D. Animal models of dysregulated cardiac metabolism. Circ. Res. 2022, 130, 1965–1993. [Google Scholar] [CrossRef] [PubMed]
- Liška, F.; Landa, V.; Zídek, V.; Mlejnek, P.; Šilhavý, J.; Šimáková, M.; Strnad, H.; Trnovská, J.; Škop, V.; Kazdová, L.; et al. Downregulation of Plzf gene ameliorates metabolic and cardiac traits in the spontaneously hypertensive rat. Hypertension 2017, 69, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, H.; Dou, J.; Wang, Y.; Liao, Y.; Huang, X.; Tang, Z.; Xu, J.; Yin, D.; Zhu, S.; et al. IAnimal: A cross-species omics knowledgebase for animals. Nucleic Acids Res. 2023, 51, D1312–D1324. [Google Scholar] [CrossRef]
- Russell-Hallinan, A.; Cappa, O.; Kerrigan, L.; Tonry, C.; Edgar, K.; Glezeva, N.; Ledwidge, M.; McDonald, K.; Collier, P.; Simpson, D.A.; et al. Single-cell RNA sequencing reveals cardiac fibroblast-specific transcriptomic changes in dilated cardiomyopathy. Cells 2024, 13, 752. [Google Scholar] [CrossRef]
- Koutsandreas, T.; Tsafou, K.; Horn, H.; Barrett, I.; Petsalaki, E. Network-based approaches for drug target identification. Annu. Rev. Biomed. Data Sci. 2025, 8, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Ewald, J.D.; Zhou, G.; Lu, Y.; Kolic, J.; Ellis, C.; Johnson, J.D.; Macdonald, P.E.; Xia, J. Web-based multi-omics integration using the Analyst software suite. Nat. Protoc. 2024, 19, 1467–1497. [Google Scholar] [CrossRef]
- Dhillon, B.K.; Smith, M.; Baghela, A.; Lee, A.H.Y.; Hancock, R.E.W. Systems Biology Approaches to Understanding the Human Immune System. Front. Immunol. 2020, 11, 1683. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Marino, G.B.; Olaiya, S.; Evangelista, J.E.; Clarke, D.J.B.; Ma’ayan, A. GeneSetCart: Assembling, augmenting, combining, visualizing, and analyzing gene sets. Gigascience 2025, 14, giaf025. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bardes, E.E.; Aronow, B.J.; Jegga, A.G. ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res. 2009, 37, W305–W311. [Google Scholar] [CrossRef] [PubMed]
- Consortium, G.T. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar]
- Schipper, M.; de Leeuw, C.A.; Maciel, B.; Wightman, D.P.; Hubers, N.; Boomsma, D.I.; O’Donovan, M.C.; Posthuma, D. Prioritizing effector genes at trait-associated loci using multimodal evidence. Nat. Genet. 2025, 57, 323–333. [Google Scholar] [CrossRef]
- Merino, G.A.; Conesa, A.; Fernandez, E.A. A benchmarking of workflows for detecting differential splicing and differential expression at isoform level in human RNA-seq studies. Brief Bioinform. 2019, 20, 471–481. [Google Scholar] [CrossRef]
- Sofer, T.; Kurniansyah, N.; Aguet, F.; Ardlie, K.; Durda, P.; Nickerson, D.A.; Smith, J.D.; Liu, Y.; Gharib, S.A.; Redline, S.; et al. Benchmarking association analyses of continuous exposures with RNA-seq in observational studies. Brief Bioinform. 2021, 22, bbab194. [Google Scholar] [CrossRef]
- Rangwala, S.H.; Kuznetsov, A.; Ananiev, V.; Asztalos, A.; Borodin, E.; Evgeniev, V.; Joukov, V.; Lotov, V.; Pannu, R.; Rudnev, D.; et al. Accessing NCBI data using the NCBI sequence viewer and genome data viewer (GDV). Genome Res. 2021, 31, 159–169. [Google Scholar] [CrossRef]
- Agrawal, A.; Balci, H.; Hanspers, K.; Coort, S.L.; Martens, M.; Slenter, D.N.; Ehrhart, F.; Digles, D.; Waagmeester, A.; Wassink, I.; et al. WikiPathways 2024: Next generation pathway database. Nucleic Acids Res. 2024, 52, D679–D689. [Google Scholar] [CrossRef]
- Harris, M.A.; Clark, J.; Ireland, A.; Lomax, J.; Ashburner, M.; Foulger, R.; Eilbeck, K.; Lewis, S.; Marshall, B.; Mungall, C.; et al. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 2004, 32, D258–D261. [Google Scholar]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Falcon, S.; Gentleman, R. Hypergeometric testing used for gene set enrichment analysis. In Bioconductor Case Studies; Hahne, F., Huber, W., Gentleman, R., Falcon, S., Eds.; Springer: New York, NY, USA, 2008; pp. 207–220. [Google Scholar]
- Kuleshov, M.V.; Xie, Z.; London, A.B.K.; Yang, J.; Evangelista, J.E.; Lachmann, A.; Shu, I.; Torre, D.; Ma’ayan, A. KEA3: Improved kinase enrichment analysis via data integration. Nucleic Acids Res 2021, 49, W304–W316. [Google Scholar] [CrossRef]
- Keenan, A.B.; Torre, D.; Lachmann, A.; Leong, A.K.; Wojciechowicz, M.L.; Utti, V.; Jagodnik, K.M.; Kropiwnicki, E.; Wang, Z.; Ma’ayan, A. ChEA3: Transcription factor enrichment analysis by orthogonal omics integration. Nucleic Acids Res. 2019, 47, W212–W224. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, H.; Aronow, B.J.; Jegga, A.G. Improved human disease candidate gene prioritization using mouse phenotype. BMC Bioinform. 2007, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Scott, W.; Padhraic, S. Algorithms for estimating relative importance in networks. In Proceedings of the Ninth ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, Washington, DC, USA, 24–27 August 2003; pp. 266–275. [Google Scholar]
- Chen, J.; Aronow, B.J.; Jegga, A.G. Disease candidate gene identification and prioritization using protein interaction networks. BMC Bioinform. 2009, 10, 73. [Google Scholar]
- Xu, J.; Li, Y. Discovering disease-genes by topological features in human protein-protein interaction network. Bioinformatics 2006, 22, 2800–2805. [Google Scholar] [CrossRef]
- Eder, R.A.; van den Boomen, M.; Yurista, S.R.; Rodriguez-Aviles, Y.G.; Islam, M.R.; Chen, Y.I.; Trager, L.; Coll-Font, J.; Cheng, L.; Li, H.; et al. Exercise-induced CITED4 expression is necessary for regional remodeling of cardiac microstructural tissue helicity. Commun. Biol. 2022, 5, 656. [Google Scholar]
- Lerchenmuller, C.; Hastings, M.H.; Rabolli, C.P.; Betge, F.; Roshan, M.; Liu, L.X.; Liu, X.; Hess, C.; Roh, J.D.; Platt, C.; et al. CITED4 gene therapy protects against maladaptive cardiac remodeling after ischemia/reperfusion injury in mice. Mol. Ther. 2024, 32, 3683–3694. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, S.; Huang, G.; Zhang, L.; Zhong, L.; Feng, Y.; Wen, P.; Liu, J. Transcriptome analysis reveals EBF1 ablation-induced injuries in cardiac system. Theranostics 2024, 14, 4894–4915. [Google Scholar] [CrossRef]
- Ploeg, M.C.; Munts, C.; Prinzen, F.W.; Turner, N.A.; van Bilsen, M.; van Nieuwenhoven, F.A. Piezo1 mechanosensitive ion channel mediates stretch-induced Nppb expression in adult rat cardiac fibroblasts. Cells 2021, 10, 1745. [Google Scholar] [CrossRef]
- Ruan, C.C.; Kong, L.R.; Chen, X.H.; Ma, Y.; Pan, X.X.; Zhang, Z.B.; Gao, P.J. A(2A) Receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab. 2018, 28, 476–489.e5. [Google Scholar] [CrossRef]
- Feng, N.; Yu, H.; Wang, Y.; Zhang, Y.; Xiao, H.; Gao, W. Exercise training attenuates angiotensin II-induced cardiac fibrosis by reducing POU2F1 expression. J. Sport Health Sci. 2023, 12, 464–476. [Google Scholar] [CrossRef]
- Wang, H.; Xie, Y.; Guan, L.; Elkin, K.; Xiao, J. Targets identified from exercised heart: Killing multiple birds with one stone. NPJ Regen. Med. 2021, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.C.; Fernandes, T.; Soci, U.P.R.; Gomes, J.L.P.; Barretti, D.L.; Mota, G.G.F.; Negrao, C.E.; Oliveira, E.M. Exercise training restores cardiac MicroRNA-1 and MicroRNA-29c to nonpathological levels in obese rats. Oxid. Med. Cell Longev. 2017, 2017, 1549014. [Google Scholar] [CrossRef]
- Spaulding, H.R.; Yan, Z. AMPK and the Adaptation to Exercise. Annu. Rev. Physiol. 2022, 84, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Quan, N.; Sun, W.; Chen, X.; Cates, C.; Rousselle, T.; Zhou, X.; Zhao, X.; Li, J. Cardiomyocyte-specific deletion of Sirt1 gene sensitizes myocardium to ischaemia and reperfusion injury. Cardiovasc. Res. 2018, 114, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.; Yang, D.; Chen, Z.L.; Xiao, J.L.; Xiao, Q.; Li, C.L.; Zhou, Z.Q.; Peng, X.Y.; Tang, C.F.; Zheng, L. Exercise intervention mitigates zebrafish age-related sarcopenia via alleviating mitochondrial dysfunction. FEBS J. 2023, 290, 1519–1530. [Google Scholar] [CrossRef]
- Packer, M. Longevity genes, cardiac ageing, and the pathogenesis of cardiomyopathy: Implications for understanding the effects of current and future treatments for heart failure. Eur. Heart J. 2020, 41, 3856–3861. [Google Scholar] [CrossRef]
- Neto, I.V.S.; Pinto, A.P.; Munoz, V.R.; de Cassia Marqueti, R.; Pauli, J.R.; Ropelle, E.R.; Silva, A. Pleiotropic and multi-systemic actions of physical exercise on PGC-1alpha signaling during the aging process. Ageing Res. Rev. 2023, 87, 101935. [Google Scholar] [CrossRef]
- Stacpoole, P.W. Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. J. Natl. Cancer Inst. 2017, 109, 116008. [Google Scholar] [CrossRef]
- Sun, J.; Hua, C.; Zhang, J.; Ding, N.; Liu, Y.; Liu, M.; Tao, H.; Dong, J.; Zhao, X.; Li, X. Decreased energy production and Ca(2+) homeostasis imbalance induce myocardial hypertrophy in PDHA1-deficient human pluripotent stem cell derived cardiomyocytes. Life Sci. 2025, 364, 123439. [Google Scholar] [CrossRef]
- Alvi, S.B.; Sridharan, D.; Shalaan, M.T.; Sanghvi, S.K.; Mergaye, M.; Ahmed, U.; Mikula, S.K.; Singh, H.; Khan, M. Modulation of mitochondrial bioenergetics by polydopamine nanoparticles in human iPSC-derived cardiomyocytes. ACS Appl. Mater. Interfaces 2022, 14, 53451–53461. [Google Scholar] [CrossRef]
- Garg, A.; Jansen, S.; Greenberg, L.; Zhang, R.; Lavine, K.J.; Greenberg, M.J. Dilated cardiomyopathy-associated skeletal muscle actin (ACTA1) mutation R256H disrupts actin structure and function and causes cardiomyocyte hypocontractility. Proc. Natl. Acad. Sci. USA 2024, 121, e2405020121. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Chen, Q.; Hoppel, C.L. Mitochondrial metabolism in aging heart. Circ. Res. 2016, 118, 1593–1611. [Google Scholar] [CrossRef] [PubMed]
- Serio, S.; Pagiatakis, C.; Musolino, E.; Felicetta, A.; Carullo, P.; Laura Frances, J.; Papa, L.; Rozzi, G.; Salvarani, N.; Miragoli, M.; et al. Cardiac aging is promoted by pseudohypoxia increasing p300-induced glycolysis. Circ. Res. 2023, 133, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Faakye, A.; Harold, K.M.; Matsuzaki, S.; Pranay, A.; Mendez Garcia, M.F.; Loveland, B.L.; Rigsby, S.N.; Peelor, F.F., III; Eyster, C.; Miller, B.F.; et al. The effect of enhanced glycolysis on cardiac aging. Geroscience 2025, 47, 1–18. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Fritzen, A.M.; Kiens, B. Molecular regulation of fatty acid oxidation in skeletal muscle during aerobic exercise. Trends Endocrinol. Metab. 2018, 29, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Gemmink, A.; Schrauwen, P.; Hesselink, M.K.C. Exercising your fat (metabolism) into shape: A muscle-centred view. Diabetologia 2020, 63, 1453–1463. [Google Scholar] [CrossRef]
- Granata, C.; Caruana, N.J.; Botella, J.; Jamnick, N.A.; Huynh, K.; Kuang, J.; Janssen, H.A.; Reljic, B.; Mellett, N.A.; Laskowski, A.; et al. High-intensity training induces non-stoichiometric changes in the mitochondrial proteome of human skeletal muscle without reorganisation of respiratory chain content. Nat. Commun. 2021, 12, 7056. [Google Scholar] [CrossRef]
- Granata, C.; Jamnick, N.A.; Bishop, D.J. Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sports Med. 2018, 48, 1809–1828. [Google Scholar] [CrossRef]
- van Weeghel, M.; Abdurrachim, D.; Nederlof, R.; Argmann, C.A.; Houtkooper, R.H.; Hagen, J.; Nabben, M.; Denis, S.; Ciapaite, J.; Kolwicz, S.C., Jr.; et al. Increased cardiac fatty acid oxidation in a mouse model with decreased malonyl-CoA sensitivity of CPT1B. Cardiovasc. Res. 2018, 114, 1324–1334. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Warfel, J.D.; Wicks, S.E.; Ghosh, S.; Salbaum, J.M.; Burk, D.; Dubuisson, O.S.; Mendoza, T.M.; Zhang, J.; Noland, R.C.; et al. Impaired mitochondrial fat oxidation induces FGF21 in muscle. Cell Rep. 2016, 15, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Tao, J.; Qiu, J.; Shi, W.; Zou, M.; Chen, W.; Li, W.; Zhou, N.; Wang, S.; Ma, L.; et al. Ndufs1 deficiency aggravates the mitochondrial membrane potential dysfunction in pressure overload-induced myocardial hypertrophy. Oxid. Med. Cell. Longev. 2021, 2021, 5545261. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Song, L.; Hu, L.; Guo, D.; Ren, G.; Peng, T.; Liu, M.; Fang, Y.; Li, C.; Zhang, M.; et al. Cardiac-specific overexpression of Ndufs1 ameliorates cardiac dysfunction after myocardial infarction by alleviating mitochondrial dysfunction and apoptosis. Exp. Mol. Med. 2022, 54, 946–960. [Google Scholar] [CrossRef]
- Brunchault, M.R.; Hesse, A.M.; Schaeffer, J.; Frohlich, A.; Saintpierre, A.; Decourt, C.; Combes, F.; Nawabi, H.; Coute, Y.; Belin, S. Proteomics-based characterization of ribosome heterogeneity in adult mouse organs. Cell. Mol. Life Sci. 2025, 82, 175. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; da Costa, H.F.R.; Bierhoff, H. The circuitry between ribosome biogenesis and translation in stem cell function and ageing. Mech. Ageing Dev. 2020, 189, 111282. [Google Scholar] [CrossRef]
- Saba, J.A.; Liakath-Ali, K.; Green, R.; Watt, F.M. Translational control of stem cell function. Nat. Rev. Mol. Cell Biol. 2021, 22, 671–690. [Google Scholar] [CrossRef]
- Swist, S.; Unger, A.; Li, Y.; Voge, A.; von Frieling-Salewsky, M.; Skarlen, A.; Cacciani, N.; Braun, T.; Larsson, L.; Linke, W.A. Maintenance of sarcomeric integrity in adult muscle cells crucially depends on Z-disc anchored titin. Nat. Commun. 2020, 11, 4479. [Google Scholar] [CrossRef] [PubMed]
- Bogomolovas, J.; Fleming, J.R.; Franke, B.; Manso, B.; Simon, B.; Gasch, A.; Markovic, M.; Brunner, T.; Knoll, R.; Chen, J.; et al. Titin kinase ubiquitination aligns autophagy receptors with mechanical signals in the sarcomere. EMBO Rep. 2021, 22, e48018. [Google Scholar] [CrossRef]
- Sheeran, F.L.; Angerosa, J.; Liaw, N.Y.; Cheung, M.M.; Pepe, S. Adaptations in protein expression and regulated activity of pyruvate dehydrogenase multienzyme complex in human systolic heart failure. Oxid. Med. Cell. Longev. 2019, 2019, 4532592. [Google Scholar] [CrossRef]
- Fatmi, M.K.; Ren, D.; Fedorova, J.; Zoungrana, L.I.; Wang, H.; Davitt, K.; Li, Z.; Iglesias, M.; Lesnefsky, E.J.; Krause-Hauch, M.; et al. Cardiomyocyte Pdk4 response is associated with metabolic maladaptation in aging. Aging Cell 2023, 22, e13800. [Google Scholar] [CrossRef]
- Kim, M.J.; Sinam, I.S.; Siddique, Z.; Jeon, J.H.; Lee, I.K. The Link between Mitochondrial Dysfunction and Sarcopenia: An Update Focusing on the Role of Pyruvate Dehydrogenase Kinase 4. Diabetes Metab. J. 2023, 47, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Valverde, C.A.; Mazzocchi, G.; Di Carlo, M.N.; Ciocci Pardo, A.; Salas, N.; Ragone, M.I.; Felice, J.I.; Cely-Ortiz, A.; Consolini, A.E.; Portiansky, E.; et al. Ablation of phospholamban rescues reperfusion arrhythmias but exacerbates myocardium infarction in hearts with Ca2+/calmodulin kinase II constitutive phosphorylation of ryanodine receptors. Cardiovasc. Res. 2019, 115, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Guo, X.; Zhang, Z.; Xu, H.; Ji, Y.W.; Wang, R.J.; Zhu, J.H.; Chen, Q.H. Activation of CaMKIIdeltaA promotes Ca2+ leak from the sarcoplasmic reticulum in cardiomyocytes of chronic heart failure rats. Acta Pharmacol. Sin. 2018, 39, 1604–1612. [Google Scholar] [CrossRef]
- Gekle, M.; Dubourg, V.; Schwerdt, G.; Benndorf, R.A.; Schreier, B. The role of EGFR in vascular AT1R signaling: From cellular mechanisms to systemic relevance. Biochem. Pharmacol. 2023, 217, 115837. [Google Scholar] [CrossRef]
- Manickam, N.; Sultan, I.; Panthel, J.; Kujundzic, H.; Fischer, A.; Schmitz, K.; Ruz Jurado, M.; Morales, D.R.; John, D.; Glaser, S.F.; et al. Beneficial effects of vascular endothelial growth factor B gene transfer in the aged heart. Cardiovasc. Res. 2025, cvaf046. [Google Scholar] [CrossRef]
- Borlak, J.; Ciribilli, Y.; Bisio, A.; Selvaraj, S.; Inga, A.; Oh, J.H.; Spanel, R. The Abl1 tyrosine kinase is a key player in doxorubicin-induced cardiomyopathy and its p53/p73 cell death mediated signaling differs in atrial and ventricular cardiomyocytes. J. Transl. Med. 2024, 22, 845. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Charng, W.L.; Chen, C.A.; Rosenfeld, J.A.; Al Shamsi, A.; Al-Gazali, L.; McGuire, M.; Mew, N.A.; Arnold, G.L.; Qu, C.; et al. Germline mutations in ABL1 cause an autosomal dominant syndrome characterized by congenital heart defects and skeletal malformations. Nat. Genet. 2017, 49, 613–617. [Google Scholar] [CrossRef]
- Naderi, N.; Hemmatinafar, M.; Gaeini, A.A.; Bahramian, A.; Ghardashi-Afousi, A.; Kordi, M.R.; Darbandi-Azar, A.; Karimzade, F.; Mohebbi, H.; Barati, M. High-intensity interval training increase GATA4, CITED4 and c-Kit and decreases C/EBPbeta in rats after myocardial infarction. Life Sci. 2019, 221, 319–326. [Google Scholar] [CrossRef]
- de Sena-Tomas, C.; Aleman, A.G.; Ford, C.; Varshney, A.; Yao, D.; Harrington, J.K.; Saude, L.; Ramialison, M.; Targoff, K.L. Activation of Nkx2.5 transcriptional program is required for adult myocardial repair. Nat. Commun. 2022, 13, 2970. [Google Scholar] [CrossRef]
- Tang, Y.; Aryal, S.; Geng, X.; Zhou, X.; Fast, V.G.; Zhang, J.; Lu, R.; Zhou, Y. TBX20 Improves Contractility and Mitochondrial Function During Direct Human Cardiac Reprogramming. Circulation 2022, 146, 1518–1536. [Google Scholar] [CrossRef]
- Boogerd, C.J.; Aneas, I.; Sakabe, N.; Dirschinger, R.J.; Cheng, Q.J.; Zhou, B.; Chen, J.; Nobrega, M.A.; Evans, S.M. Probing chromatin landscape reveals roles of endocardial TBX20 in septation. J. Clin. Investig. 2016, 126, 3023–3035. [Google Scholar] [CrossRef] [PubMed]
- Lazure, F.; Blackburn, D.M.; Corchado, A.H.; Sahinyan, K.; Karam, N.; Sharanek, A.; Nguyen, D.; Lepper, C.; Najafabadi, H.S.; Perkins, T.J.; et al. Myf6/MRF4 is a myogenic niche regulator required for the maintenance of the muscle stem cell pool. EMBO Rep. 2020, 21, e49499. [Google Scholar] [CrossRef] [PubMed]
- Fahrner, A.; Luca, E.; Krutzfeldt, J. microRNA-501 controls myogenin+/CD74+ myogenic progenitor cells during muscle regeneration. Mol. Metab. 2023, 71, 101704. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Wang, L.W.; Wen, J.; Cao, J.D.; Zhou, R.; Yang, J.L.; Xiao, Y.; Su, T.; Huang, Y.; Guo, Q.; et al. RNA-binding protein YBX3 promotes PPARgamma-SLC3A2 mediated BCAA metabolism fueling brown adipogenesis and thermogenesis. Mol. Metab. 2024, 90, 102053. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.S.; Lang Lehrskov, L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-induced changes in visceral adipose tissue mass are regulated by il-6 signaling: A randomized controlled trial. Cell Metab. 2019, 29, 844–855.e3. [Google Scholar] [CrossRef]
- An, J.; Thorson, A.S.; Wasserman, D.H.; Stafford, J.M.; Zhu, L. Sex- and endurance training-mediated cardiovascular protection through lipids during exercise. Trends Endocrinol. Metab. 2024, 12, 1–15. [Google Scholar] [CrossRef]
- Wang, W.; Chen, L.; Zhao, Y.; Zhang, S.; Zhou, X. Circ-0001283 Aggravates Cardiac Hypertrophy by Targeting Myosin Light Chain 3 Protein. Research 2025, 8, 0626. [Google Scholar] [CrossRef]
- Osborn, D.P.S.; Emrahi, L.; Clayton, J.; Tabrizi, M.T.; Wan, A.Y.B.; Maroofian, R.; Yazdchi, M.; Garcia, M.L.E.; Galehdari, H.; Hesse, C.; et al. Autosomal recessive cardiomyopathy and sudden cardiac death associated with variants in MYL3. Genet. Med. 2021, 23, 787–792. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, J.Z.; Itzhaki, I.; Zhang, S.L.; Chen, H.; Haddad, F.; Kitani, T.; Wilson, K.D.; Tian, L.; Shrestha, R.; et al. Determining the pathogenicity of a genomic variant of uncertain significance using CRISPR/Cas9 and human-induced pluripotent stem cells. Circulation 2018, 138, 2666–2681. [Google Scholar] [CrossRef]
- Woo, J.S.; Jeong, S.Y.; Park, J.H.; Choi, J.H.; Lee, E.H. Calsequestrin: A well-known but curious protein in skeletal muscle. Exp. Mol. Med. 2020, 52, 1908–1925. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, L.; Han, L.; Wang, Y.; Zhou, Y.; Li, Q.; Wu, Y.; Talabieke, A.; Hou, Y.; Wu, L.; et al. Functional calsequestrin-1 is expressed in the heart and its deficiency is causally related to malignant hyperthermia-like arrhythmia. Circulation 2021, 144, 788–804. [Google Scholar] [CrossRef] [PubMed]
- Guarnier, F.A.; Serano, M.; Michelucci, A.; Pietrangelo, L.; Boncompagni, S.; Protasi, F. Aerobic training prevents heatstrokes in calsequestrin-1 knockout mice by reducing oxidative stress. Oxid. Med. Cell. Longev. 2018, 2018, 4652480. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Roca, D.; Guimaraes, D.; Pendergrast, L.A.; Di Leo, N.; Chibalin, A.V.; Maqdasy, S.; Ryden, M.; Naslund, E.; Zierath, J.R.; Krook, A. Decreased mitochondrial creatine kinase 2 impairs skeletal muscle mitochondrial function independently of insulin in type 2 diabetes. Sci. Transl. Med. 2024, 16, eado3022. [Google Scholar] [CrossRef] [PubMed]
- Papizan, J.B.; Garry, G.A.; Brezprozvannaya, S.; McAnally, J.R.; Bassel-Duby, R.; Liu, N.; Olson, E.N. Deficiency in Kelch protein Klhl31 causes congenital myopathy in mice. J. Clin. Investig. 2017, 127, 3730–3740. [Google Scholar] [CrossRef]
- Filomena, M.C.; Yamamoto, D.L.; Caremani, M.; Kadarla, V.K.; Mastrototaro, G.; Serio, S.; Vydyanath, A.; Mutarelli, M.; Garofalo, A.; Pertici, I.; et al. Myopalladin promotes muscle growth through modulation of the serum response factor pathway. J. Cachexia Sarcopenia Muscle 2020, 11, 169–194. [Google Scholar] [CrossRef]
- Lo, H.P.; Lim, Y.W.; Xiong, Z.; Martel, N.; Ferguson, C.; Ariotti, N.; Giacomotto, J.; Rae, J.; Floetenmeyer, M.; Moradi, S.V.; et al. Cavin4 interacts with Bin1 to promote T-tubule formation and stability in developing skeletal muscle. J. Cell Biol. 2021, 220, e201905065. [Google Scholar] [CrossRef] [PubMed]
- Rathod, N.; Lemieux, M.J.; Chipot, C.; Roux, B.; Young, H.S. Probing the formation of a hetero-dimeric membrane transport complex with dual in vitro and in silico mutagenesis. Chem. Sci. 2024, 15, 14310–14322. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, Y.; Jardin, B.D.; Zhang, X.; Zhou, P.; Guatimosim, S.; Lin, J.; Chen, Z.; Zhang, Y.; Mazumdar, N.; et al. Ryanodine receptor 2 (RYR2) dysfunction activates the unfolded protein response and perturbs cardiomyocyte maturation. Cardiovasc. Res. 2023, 119, 221–235. [Google Scholar] [CrossRef]
- McCalmon, S.A.; Desjardins, D.M.; Ahmad, S.; Davidoff, K.S.; Snyder, C.M.; Sato, K.; Ohashi, K.; Kielbasa, O.M.; Mathew, M.; Ewen, E.P. Modulation of angiotensin II-mediated cardiac remodeling by the MEF2A target gene Xirp2. Circ. Res. 2010, 106, 952–960. [Google Scholar] [CrossRef]
- Gan, P.; Wang, Z.; Bezprozvannaya, S.; McAnally, J.R.; Tan, W.; Li, H.; Bassel-Duby, R.; Liu, N.; Olson, E.N. RBPMS regulates cardiomyocyte contraction and cardiac function through RNA alternative splicing. Cardiovasc. Res. 2024, 120, 56–68. [Google Scholar] [CrossRef]
- Friedrich, F.W.; Wilding, B.R.; Reischmann, S.; Crocini, C.; Lang, P.; Charron, P.; Müller, O.J.; McGrath, M.J.; Vollert, I.; Hansen, A.; et al. Evidence for FHL1 as a novel disease gene for isolated hypertrophic cardiomyopathy. Hum. Mol. Genet. 2012, 21, 3237–3254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Han, S.; Wang, Y.; Cui, C.; Zhu, Q.; Jiang, X.; Yang, C.; Du, H.; Yu, C.; Li, Q.; et al. The LIM-only protein FHL2 is involved in autophagy to regulate the development of skeletal muscle cell. Int. J. Biol. Sci. 2019, 15, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.S.; Tracy, C.M.; Miller, M.R.; Makaju, A.; Szulik, M.W.; Oka, S.I.; Yuzyuk, T.N.; Cox, J.E.; Kumar, A.; Lozier, B.K.; et al. Histone methyltransferase Smyd1 regulates mitochondrial energetics in the heart. Proc. Natl. Acad. Sci. USA 2018, 115, E7871–E7880. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, C.; Matsumoto, A.; Ichihara, K.; Yamamoto, T.; Yokoyama, T.; Mizoo, T.; Hatano, A.; Matsumoto, M.; Tanaka, Y.; Matsuura-Suzuki, E.; et al. RPL3L-containing ribosomes determine translation elongation dynamics required for cardiac function. Nat. Commun. 2023, 14, 2131. [Google Scholar] [CrossRef]
- McDermott-Roe, C.; Leleu, M.; Rowe, G.C.; Palygin, O.; Bukowy, J.D.; Kuo, J.; Rech, M.; Hermans-Beijnsberger, S.; Schaefer, S.; Adami, E.; et al. Transcriptome-wide co-expression analysis identifies LRRC2 as a novel mediator of mitochondrial and cardiac function. PLoS ONE 2017, 12, e0170458. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, L.; Li, Z.; Zhou, C. Structural basis for TRIM72 oligomerization during membrane damage repair. Nat. Commun. 2023, 14, 1555. [Google Scholar] [CrossRef]
- Zhao, X.H.; Han, M.M.; Yan, Q.Q.; Yue, Y.M.; Ye, K.; Zhang, Y.Y.; Teng, L.; Xu, L.; Shi, X.J.; La, T.; et al. DNA replication stress underpins the vulnerability to oxidative phosphorylation inhibition in colorectal cancer. Cell Death Dis. 2025, 16, 16. [Google Scholar] [CrossRef]
- Ji, Z.; Li, H.; Yang, Z.; Huang, X.; Ke, X.; Ma, S.; Lin, Z.; Lu, Y.; Zhang, M. Kibra modulates learning and memory via binding to dendrin. Cell Rep. 2019, 26, 2064–2077. [Google Scholar] [CrossRef]
- Zhou, R.H.; Kokame, K.; Tsukamoto, Y.; Yutani, C.; Kato, H.; Miyata, T. Characterization of the human NDRG gene family: A newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics 2001, 73, 86–97. [Google Scholar] [CrossRef]
- Devalla, H.D.; Gélinas, R.; Aburawi, E.H.; Beqqali, A.; Goyette, P.; Freund, C.; Chaix, M.A.; Tadros, R.; Jiang, H.; Le Béchec, A.; et al. TECRL, a new life-threatening inherited arrhythmia gene associated with overlapping clinical features of both LQTS and CPVT. EMBO Mol. Med. 2016, 8, 1390–1408. [Google Scholar] [CrossRef]
- Alsina, K.M.; Hulsurkar, M.; Brandenburg, S.; Kownatzki-Danger, D.; Lenz, C.; Urlaub, H.; Abu-Taha, I.; Kamler, M.; Chiang, D.Y.; Lahiri, S.K.; et al. Loss of protein phosphatase 1 regulatory subunit PPP1R3A promotes atrial fibrillation. Circulation 2019, 140, 681–693. [Google Scholar] [CrossRef]
- Frasier, C.R.; Zhang, H.; Offord, J.; Dang, L.T.; Auerbach, D.S.; Shi, H.; Chen, C.; Goldman, A.M.; Eckhardt, L.L.; Bezzerides, V.J.; et al. Channelopathy as a SUDEP biomarker in Dravet syndrome patient-derived cardiac myocytes. Stem Cell Rep. 2018, 11, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Chang, J.P.; Lin, Y.S.; Pan, K.L.; Ho, W.C.; Liu, W.H.; Chang, T.H.; Huang, Y.K.; Fang, C.Y.; Chen, C.J. Deciphering the gene expression profile of peroxisome proliferator-activated receptor signaling pathway in the left atria of patients with mitral regurgitation. J. Transl. Med. 2016, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Takahashi, M.; Yamamuro, D.; Karasawa, T.; Takei, A.; Takei, S.; Yamazaki, H.; Nagashima, S.; Ebihara, K.; Takahashi, M.; et al. Inflammasome activation aggravates cutaneous xanthomatosis and atherosclerosis in ACAT1 (acyl-CoA cholesterol acyltransferase 1) deficiency in bone marrow. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2576–2589. [Google Scholar] [CrossRef]
- Nguyen, L.P.; Song, W.; Yang, Y.; Tran, A.P.; Weston, T.A.; Jung, H.; Tu, Y.; Kim, P.H.; Kim, J.R.; Xie, K.; et al. Distinct strategies for intravascular triglyceride metabolism in hearts of mammals and lower vertebrate species. JCI Insight 2024, 9, e184940. [Google Scholar] [CrossRef]
- Wolfe, A.L.; Zhou, Q.; Toska, E.; Galeas, J.; Ku, A.A.; Koche, R.P.; Bandyopadhyay, S.; Scaltriti, M.; Lebrilla, C.B.; McCormick, F.; et al. UDP-glucose pyrophosphorylase 2, a regulator of glycogen synthesis and glycosylation, is critical for pancreatic cancer growth. Proc. Natl. Acad. Sci. USA 2021, 118, e2103592118. [Google Scholar] [CrossRef] [PubMed]
- Truszkowska, G.T.; Bilińska, Z.T.; Muchowicz, A.; Pollak, A.; Biernacka, A.; Kozar-Kamińska, K.; Stawiński, P.; Gasperowicz, P.; Kosińska, J.; Zieliński, T.; et al. Homozygous truncating mutation in NRAP gene identified by whole exome sequencing in a patient with dilated cardiomyopathy. Sci. Rep. 2017, 7, 3362. [Google Scholar] [CrossRef]
- Gut, P.; Matilainen, S.; Meyer, J.G.; Pällijeff, P.; Richard, J.; Carroll, C.J.; Euro, L.; Jackson, C.B.; Isohanni, P.; Minassian, B.A.; et al. SUCLA2 mutations cause global protein succinylation contributing to the pathomechanism of a hereditary mitochondrial disease. Nat. Commun. 2020, 11, 5927. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Cheng, L.T.; Wang, Z.F.; Wu, Y.Q. Desmoplakin and clinical manifestations of desmoplakin cardiomyopathy. Chin. Med. J. 2021, 134, 1771–1779. [Google Scholar] [CrossRef]
- Broxton, C.N.; Kaur, P.; Lavorato, M.; Ganesh, S.; Xiao, R.; Mathew, N.D.; Nakamaru-Ogiso, E.; Anderson, V.E.; Falk, M.J. Dichloroacetate and thiamine improve survival and mitochondrial stress in a C. elegans model of dihydrolipoamide dehydrogenase deficiency. JCI Insight 2022, 7, e156222. [Google Scholar] [CrossRef]
- Tam, S.W.; Huffer, K.; Li, M.; Swartz, K.J. Ion permeation pathway within the internal pore of P2X receptor channels. eLife 2023, 12, e84796. [Google Scholar] [CrossRef]
- Liu, S.; Deshmukh, V.; Meng, F.; Wang, Y.; Morikawa, Y.; Steimle, J.D.; Li, R.G.; Wang, J.; Martin, J.F. Microtubules sequester acetylated YAP in the cytoplasm and inhibit heart regeneration. Circulation 2025, 151, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhu, J.; Bian, X.; Liu, S.; Yu, S.; Liang, W.; Jiang, L.; Mao, R.; Zhang, W.; Rao, Y. Importance of glutamine in synaptic vesicles revealed by functional studies of SLC6A17 and its mutations pathogenic for intellectual disability. eLife 2023, 12, e86972. [Google Scholar] [CrossRef] [PubMed]
- Ranta-Aho, J.; Johari, M.; Udd, B. Current advance on distal myopathy genetics. Curr. Opin. Neurol. 2024, 37, 515–522. [Google Scholar] [CrossRef]
- Palmer, S.; Groves, N.; Schindeler, A.; Yeoh, T.; Biben, C.; Wang, C.C.; Sparrow, D.B.; Barnett, L.; Jenkins, N.A.; Copeland, N.G.; et al. The small muscle-specific protein Csl modifies cell shape and promotes myocyte fusion in an insulin-like growth factor 1-dependent manner. J. Cell Biol. 2001, 153, 985–998. [Google Scholar] [CrossRef]
- Hoelzl, S.; Hasenbein, T.P.; Engelhardt, S.; Andergassen, D. Aging promotes reactivation of the Barr body at distal chromosome regions. Nat. Aging 2025, 5, 984–996. [Google Scholar] [CrossRef] [PubMed]
- Savarese, M.; Palmio, J.; Poza, J.J.; Weinberg, J.; Olive, M.; Cobo, A.M.; Vihola, A.; Jonson, P.H.; Sarparanta, J.; García-Bragado, F.; et al. Actininopathy: A new muscular dystrophy caused by ACTN2 dominant mutations. Ann. Neurol. 2019, 85, 899–906. [Google Scholar] [CrossRef]
- Ervasti, J.M. Costameres: The Achilles’ heel of Herculean muscle. J. Biol. Chem. 2003, 278, 13591–13594. [Google Scholar] [CrossRef]
- Johari, M.; Sarparanta, J.; Vihola, A.; Jonson, P.H.; Savarese, M.; Jokela, M.; Torella, A.; Piluso, G.; Said, E.; Vella, N.; et al. Missense mutations in small muscle protein X-linked (SMPX) cause distal myopathy with protein inclusions. Acta Neuropathol. 2021, 142, 375–393. [Google Scholar] [CrossRef]
- Kim, J.C.; Son, M.J.; Wang, J.; Woo, S.H. Regulation of cardiac Ca2+ and ion channels by shear mechanotransduction. Arch. Pharm. Res. 2017, 40, 783–795. [Google Scholar] [CrossRef]
- Riaz, M.; Park, J.; Sewanan, L.R.; Ren, Y.; Schwan, J.; Das, S.K.; Pomianowski, P.T.; Huang, Y.; Ellis, M.W.; Luo, J.; et al. Muscle LIM protein force-sensing mediates sarcomeric biomechanical signaling in human familial hypertrophic cardiomyopathy. Circulation 2022, 145, 1238–1253. [Google Scholar] [CrossRef]
- Lindholm, M.E.; Jimenez-Morales, D.; Zhu, H.; Seo, K.; Amar, D.; Zhao, C.; Raja, A.; Madhvani, R.; Abramowitz, S.; Espenel, C.; et al. Mono- and biallelic protein-truncating variants in Alpha-Actinin 2 cause cardiomyopathy through distinct mechanisms. Circ. Genom. Precis. Med. 2021, 14, e003419. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hao, X.; Shang, P.; Nie, J.; Chamba, Y.; Zhang, B.; Zhang, H. MUSTN1 interaction with SMPX regulates muscle development and regeneration. Cell Prolif. 2025, 58, e13809. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B. Relationships between stress granules, oxidative stress, and neurodegenerative diseases. Oxid. Med. Cell. Longev. 2017, 2017, 1809592. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.; Patel, D.; Moran, J.L.; Lian, X.J.; Di Marco, S.; Gallouzi, I.E. Autophagy and heat-shock response impair stress granule assembly during cellular senescence. Mech. Ageing Dev. 2020, 192, 111382. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kuk, M.U.; So, M.K.; Song, E.S.; Lee, H.; Ahn, S.K.; Kwon, H.W.; Park, J.T.; Park, S.C. Targeting mitochondrial oxidative stress as a strategy to treat aging and age-related diseases. Antioxidants 2023, 12, 837. [Google Scholar] [CrossRef]
- Garcia, I.; Innis-Whitehouse, W.; Lopez, A.; Keniry, M.; Gilkerson, R. Oxidative insults disrupt OPA1-mediated mitochondrial dynamics in cultured mammalian cells. Redox Rep. 2018, 23, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Ash, D.; Nagarkoti, S.; Belin de Chantemele, E.J.; Fulton, D.J.R.; Fukai, T. Interplay between reactive oxygen/reactive nitrogen species and metabolism in vascular biology and disease. Antioxid. Redox Signal. 2021, 34, 1319–1354. [Google Scholar] [CrossRef]
- Zhao, R. Exercise mimetics: A novel strategy to combat neuroinflammation and Alzheimer’s disease. J. Neuroinflammation 2024, 21, 40. [Google Scholar] [CrossRef]
- Weihrauch, M.; Handschin, C. Pharmacological targeting of exercise adaptations in skeletal muscle: Benefits and pitfalls. Biochem. Pharmacol. 2018, 147, 211–220. [Google Scholar] [CrossRef]
- Cento, A.S.; Leigheb, M.; Caretti, G.; Penna, F. Exercise and exercise mimetics for the treatment of musculoskeletal disorders. Curr. Osteoporos. Rep. 2022, 20, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.; Gardiner, P.A.; Harvey, J.A.; Leask, C.F.; Jerez-Roig, J.; Rosenberg, D.; Ashe, M.C.; Helbostad, J.L.; Skelton, D.A. Interventions for reducing sedentary behaviour in community-dwelling older adults. Cochrane Database Syst. Rev. 2021, 6, CD012784. [Google Scholar] [CrossRef] [PubMed]
- Raffin, J.; Rolland, Y.; Aubertin-Leheudre, M.; Aragoni da Silva, J.; Guyonnet, S.; Pillard, F.; Vellas, B.; de Souto Barreto, P. Cross-sectional interactive associations of physical activity and sedentary behaviour with physical capacity across adulthood. J. Cachexia Sarcopenia Muscle 2024, 15, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Kehler, D.S.; Theou, O. The impact of physical activity and sedentary behaviors on frailty levels. Mech. Ageing Dev. 2019, 180, 29–41. [Google Scholar] [CrossRef]
- Ozemek, C.; Lavie, C.J.; Rognmo, O. Global physical activity levels—Need for intervention. Prog. Cardiovasc. Dis. 2019, 62, 102–107. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Azhati, S.; Chen, H.; Zhang, Y.; Shi, L. Molecular Network Analysis and Effector Gene Prioritization of Endurance-Training-Influenced Modulation of Cardiac Aging. Genes 2025, 16, 814. https://doi.org/10.3390/genes16070814
Wang M, Azhati S, Chen H, Zhang Y, Shi L. Molecular Network Analysis and Effector Gene Prioritization of Endurance-Training-Influenced Modulation of Cardiac Aging. Genes. 2025; 16(7):814. https://doi.org/10.3390/genes16070814
Chicago/Turabian StyleWang, Mingrui, Samuhaer Azhati, Hangyu Chen, Yanyan Zhang, and Lijun Shi. 2025. "Molecular Network Analysis and Effector Gene Prioritization of Endurance-Training-Influenced Modulation of Cardiac Aging" Genes 16, no. 7: 814. https://doi.org/10.3390/genes16070814
APA StyleWang, M., Azhati, S., Chen, H., Zhang, Y., & Shi, L. (2025). Molecular Network Analysis and Effector Gene Prioritization of Endurance-Training-Influenced Modulation of Cardiac Aging. Genes, 16(7), 814. https://doi.org/10.3390/genes16070814






