Dark Rearing Does Not Alter Developmental Retinoschisis Cavity Formation in Rs1 Gene Knockout Rat Model of X-Linked Retinoschisis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ocular Tissue Collection and Processing
2.3. Histology and Immunohistochemistry
2.4. Measurement of Cavity Size
2.5. Statistical Analysis of Cavity Size
2.6. Study Design
3. Results
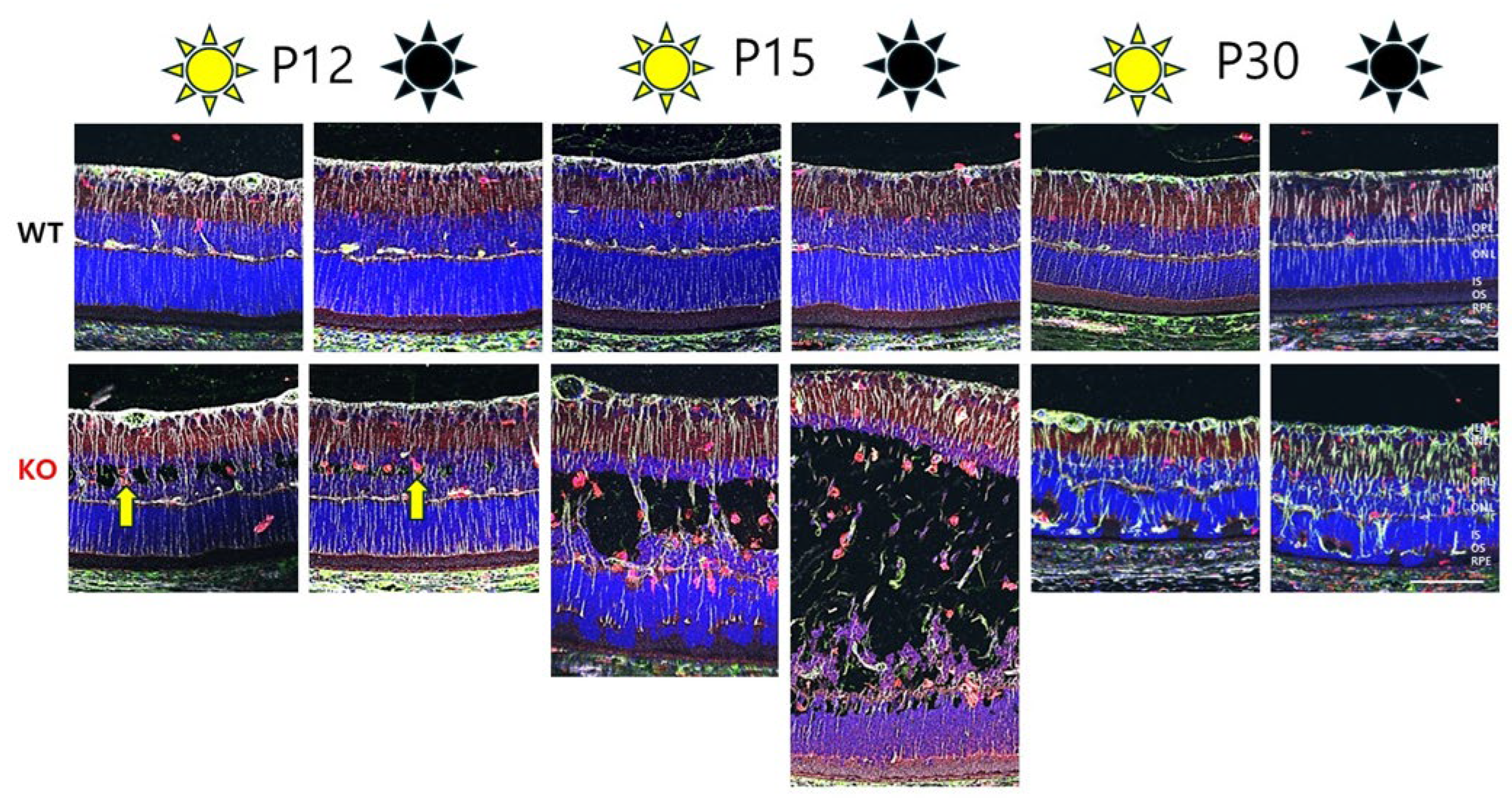
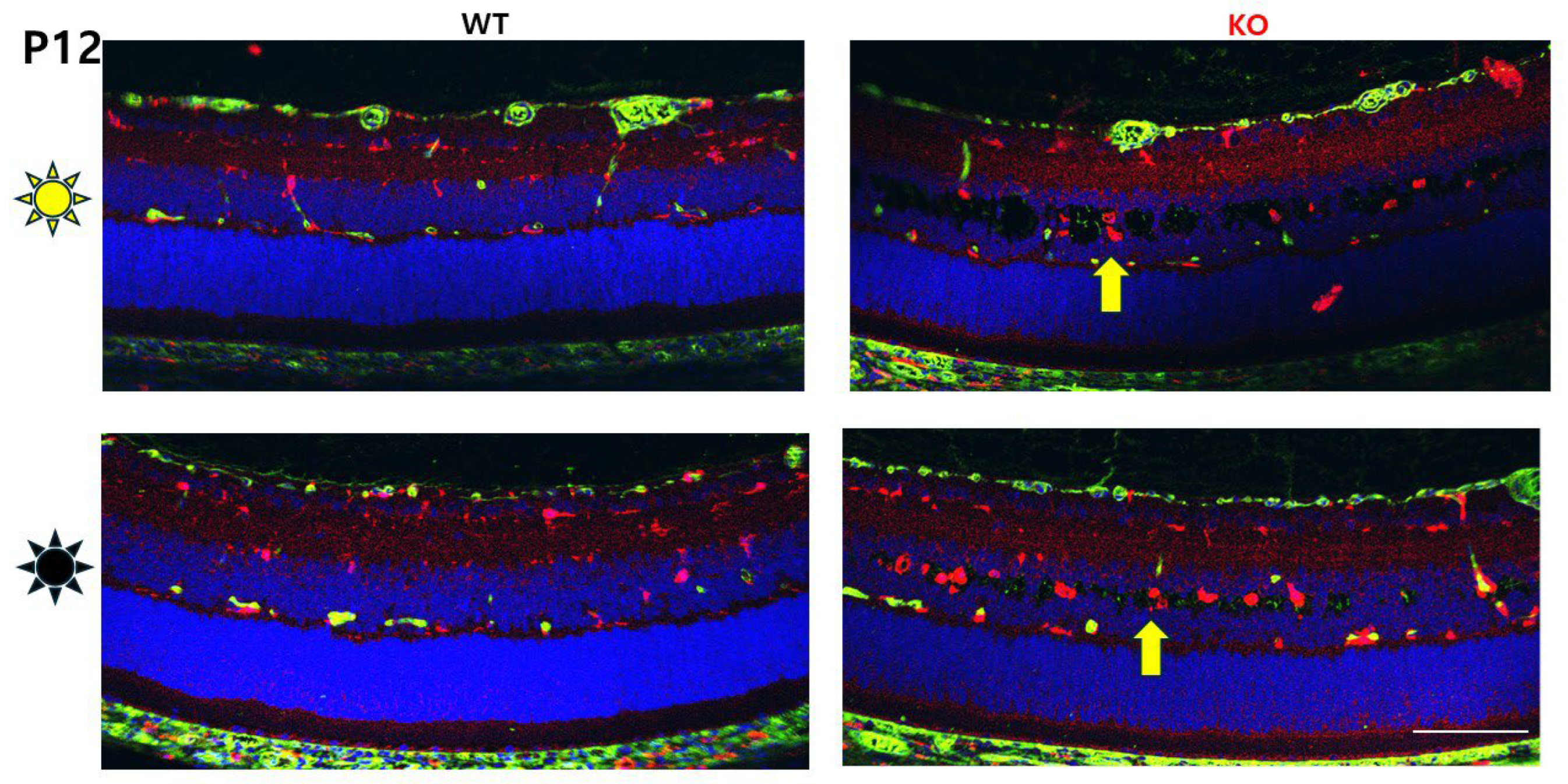
3.1. Tiny Schisis Cavities Begin to Form in the INL of Dark-Reared Animals by P12
3.2. Dark Rearing Does Not Reduce Cavity Formation Versus Standard Cyclic Lighting
3.3. Immune Cells (Iba and GFAP) Distribution and Activation in Dark vs. Light Rearing
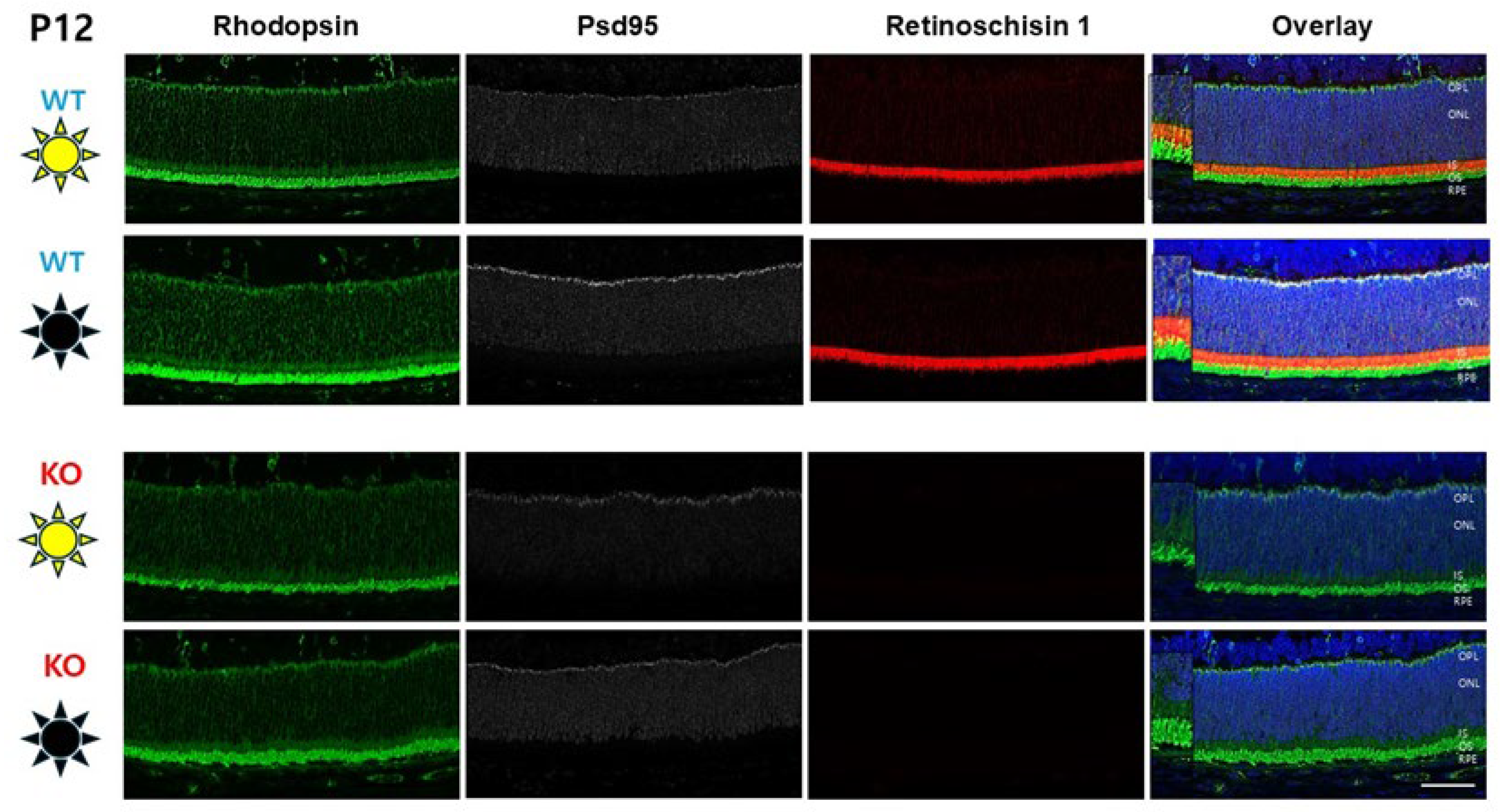
3.4. Photoreceptor Rhodopsin Expression at P12
4. Discussion
4.1. Eye Opening and Light Exposure
4.2. Diurnal Variation in XLRS Cavities
4.3. Microglial Activation in XLRS Retina
5. Conclusions
- Retinoschisis cavities formed at the same time in dark- and light-reared XLRS rats, indicating that the timing and extent of schisis formation are not driven by the metabolic activity of light exposure but apparently by developmental events.
- In our small sample, cavity size tended to be larger under dark-rearing conditions than under cyclic light/dark rearing, possibly due to reduced RPE fluid pumping activity that is normally stimulated by light.
- Dark-reared Rs1KO and WT rats have longer photoreceptor OSs (previously reported by Matt LaVail [19]) as RPE phagocytosis is decreased in the dark in adult rodents, allowing for ROS elongation in the absence of shedding of the distal tip.
- These results highlight the complexity of XLRS pathology, but we found no evidence that light-driven metabolic activity accounted for schisis cavity formation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marangoni, D.; Yong, Z.; Kjellstrom, S.; Vijayasarathy, C.; Sieving, P.A.; Bush, R.A. Rearing Light Intensity Affects Inner Retinal Pathology in a Mouse Model of X-Linked Retinoschisis but Does Not Alter Gene Therapy Outcome. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Takada, Y.; Kjellstrom, S.; Hiriyanna, K.; Tanikawa, A.; Wawrousek, E.; Smaoui, N.; Caruso, R.; Bush, R.A.; Sieving, P.A. RS-1 Gene Delivery to an Adult Rs1h Knockout Mouse Model Restores ERG b-Wave with Reversal of the Electronegative Waveform of X-Linked Retinoschisis. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3279–3285. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Cao, J.W.; Liu, L.Y.; Zhao, Z.H.; Fu, Y.; Yu, Y.C. Eye opening differentially modulates inhibitory synaptic transmission in the developing visual cortex. eLife 2017, 6, e32337. [Google Scholar] [CrossRef] [PubMed]
- Davis, Z.W.; Chapman, B.; Cheng, H.J. Increasing Spontaneous Retinal Activity before Eye Opening Accelerates the Development of Geniculate Receptive Fields. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 14612–14623. [Google Scholar] [CrossRef]
- Akerman, C.J.; Smyth, D.; Thompson, I.D. Visual experience before eye-opening and the development of the retinogeniculate pathway. Neuron 2002, 36, 869–879. [Google Scholar] [CrossRef]
- Tian, N.; Copenhagen, D.R. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron 2001, 32, 439–449. [Google Scholar] [CrossRef]
- Tian, N.; Copenhagen, D.R. Visual stimulation is required for refinement of ON and OFF pathways in postnatal retina. Neuron 2003, 39, 85–96. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef]
- Van Norren, D.; Vos, J.J. Light damage to the retina: An historical approach. Eye 2016, 30, 169–172. [Google Scholar] [CrossRef]
- Ye, E.A.; Zeng, Y.; Thomas, S.; Sun, N.; Smit-McBride, Z.; Sieving, P.A. XLRS Rat with Rs1-/Y Exon-1-Del Shows Failure of Early Postnatal Outer Retina Development. Genes 2022, 13, 1995. [Google Scholar] [CrossRef]
- Ziccardi, L.; Vijayasarathy, C.; Bush, R.A.; Sieving, P.A. Loss of retinoschisin (RS1) cell surface protein in maturing mouse rod photoreceptors elevates the luminance threshold for light-driven translocation of transducin but not arrestin. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 13010–13021. [Google Scholar] [CrossRef] [PubMed]
- Tiriac, A.; Smith, B.E.; Feller, M.B. Light Prior to Eye Opening Promotes Retinal Waves and Eye-Specific Segregation. Neuron 2018, 100, 1059–1065.e4. [Google Scholar] [CrossRef] [PubMed]
- Smit-McBride, Z.; Sun, N.; Thomas, S.; Cho, I.H.; Sieving, P.A. Dark-rearing of XLRS Rs1 rat pups during development and effects on early post-natal age pathology. In Proceedings of the ARVO 2025, Salt Lake City, UT, USA, 4–8 May 2025. [Google Scholar]
- Smit-McBride, Z.; Sun, N.; Thomas, S.; Cho, I.H.; Stricklin, R.G.; Sieving, P.A. Kir4.1 and Aqp4 Contribution to Schisis Cystic Water Accumulation and Clearance in the Rs1 Exon-1 Del XLRS Rat Model. Genes 2024, 15, 1583. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.N.; Yamashita, C.; Farber, D.B. Retinoschisin, a photoreceptor-secreted protein, and its interaction with bipolar and muller cells. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 6030–6040. [Google Scholar] [CrossRef]
- Sun, N.; Shibata, B.; Hess, J.F.; FitzGerald, P.G. An alternative means of retaining ocular structure and improving immunoreactivity for light microscopy studies. Mol. Vis. 2015, 21, 428–442. [Google Scholar]
- Bosco, A.; Steele, M.R.; Vetter, M.L. Early microglia activation in a mouse model of chronic glaucoma. J. Comp. Neurol. 2011, 519, 599–620. [Google Scholar] [CrossRef]
- Fernandez-Sanchez, L.; Lax, P.; Campello, L.; Pinilla, I.; Cuenca, N. Astrocytes and Muller Cell Alterations During Retinal Degeneration in a Transgenic Rat Model of Retinitis Pigmentosa. Front. Cell Neurosci. 2015, 9, 484. [Google Scholar] [CrossRef]
- Battelle, B.A.; LaVail, M.M. Rhodopsin content and rod outer segment length in albino rat eyes: Modification by dark adaptation. Exp. Eye Res. 1978, 26, 487–497. [Google Scholar] [CrossRef]
- Osswald, I.K.; Galan, A.; Bowie, D. Light triggers expression of philanthotoxin-insensitive Ca2+-permeable AMPA receptors in the developing rat retina. J. Physiol. 2007, 582, 95–111. [Google Scholar] [CrossRef]
- Zhang, R.W.; Wei, H.P.; Xia, Y.M.; Du, J.L. Development of light response and GABAergic excitation-to-inhibition switch in zebrafish retinal ganglion cells. J. Physiol. 2010, 588, 2557–2569. [Google Scholar] [CrossRef]
- Thompson, I. Visual development: From darkness into light. Curr. Biol. 1994, 4, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Linton, J.D.; Holzhausen, L.C.; Babai, N.; Song, H.; Miyagishima, K.J.; Stearns, G.W.; Lindsay, K.; Wei, J.; Chertov, A.O.; Peters, T.A.; et al. Flow of energy in the outer retina in darkness and in light. Proc. Natl. Acad. Sci. USA 2010, 107, 8599–8604. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.Y.; Acosta, M.L.; Ready, S.; Cheong, Y.L.; Kalloniatis, M. Light exposure causes functional changes in the retina: Increased photoreceptor cation channel permeability, photoreceptor apoptosis, and altered retinal metabolic function. J. Neurochem. 2007, 103, 714–724. [Google Scholar] [CrossRef]
- Friedrich, U.; Stohr, H.; Hilfinger, D.; Loenhardt, T.; Schachner, M.; Langmann, T.; Weber, B.H. The Na/K-ATPase is obligatory for membrane anchorage of retinoschisin, the protein involved in the pathogenesis of X-linked juvenile retinoschisis. Hum. Mol. Genet. 2011, 20, 1132–1142. [Google Scholar] [CrossRef]
- Plossl, K.; Royer, M.; Bernklau, S.; Tavraz, N.N.; Friedrich, T.; Wild, J.; Weber, B.H.F.; Friedrich, U. Retinoschisin is linked to retinal Na/K-ATPase signaling and localization. Mol. Biol. Cell 2017, 28, 2178–2189. [Google Scholar] [CrossRef]
- Molday, L.L.; Wu, W.W.; Molday, R.S. Retinoschisin (RS1), the protein encoded by the X-linked retinoschisis gene, is anchored to the surface of retinal photoreceptor and bipolar cells through its interactions with a Na/K ATPase-SARM1 complex. J. Biol. Chem. 2007, 282, 32792–32801. [Google Scholar] [CrossRef]
- Eleftheriou, C.G.; Corona, C.; Khattak, S.; Alam, N.M.; Ivanova, E.; Bianchimano, P.; Liu, Y.; Sun, D.; Singh, R.; Batoki, J.C.; et al. Retinoschisin Deficiency Induces Persistent Aberrant Waves of Activity Affecting Neuroglial Signaling in the Retina. J. Neurosci. Off. J. Soc. Neurosci. 2022, 42, 6983–7000. [Google Scholar] [CrossRef]
- Tarchick, M.J.; Beight, C.; Bonezzi, P.B.; Peachey, N.S.; Renna, J.M. Photoreceptor deficits appear at eye opening in Rs1 mutant mouse models of X-linked retinoschisis. Exp. Eye Res. 2024, 242, 109872. [Google Scholar] [CrossRef]
- Hassan, S.; Stanley, S.; Brandauer, E.; Laird, J.; Hsu, Y.; Drack, A.V. Retinal Changes in Rs1-KO Mice: Role of Light and Dark Adaptation. In Proceedings of the ARVO 2025, Salt Lake City, UT, USA, 4–8 May 2025. [Google Scholar]
- Marmor, M.F. Control of subretinal fluid: Experimental and clinical studies. Eye 1990, 4 Pt 2, 340–344. [Google Scholar] [CrossRef]
- Vijayasarathy, C.; Zeng, Y.; Brooks, M.J.; Fariss, R.N.; Sieving, P.A. Genetic Rescue of X-Linked Retinoschisis Mouse (Rs1-/y) Retina Induces Quiescence of the Retinal Microglial Inflammatory State Following AAV8-RS1 Gene Transfer and Identifies Gene Networks Underlying Retinal Recovery. Hum. Gene Ther. 2021, 32, 667–681. [Google Scholar] [CrossRef]


| Primary Antibody | Species | Vendor | Cat# | Dilution |
|---|---|---|---|---|
| Vimentin (Vim) | Chicken | Custom Ab, Fitzgerald Lab | n/a | 1:1000 |
| Iba1 | Rabbit | Wako Chemicals, Richmond, VA, USA | 019-19741 | 1:500 |
| GFAP | Mouse | Cell Signaling Technology, Inc. Danvers, MA | 36705 | 1:500 |
| Rhodopsin (Rho) | Mouse | Santa Cruz Biotech | sc-57432 | 1:500 |
| Psd-95 | Rabbit | Cell Signaling Technology, Inc. Danvers, MA | 34505 | 1:500 |
| Retinoschisin 1 (RS-1) | Guinea Pig | Custom Ab, Sieving Lab | n/a | 1:1000 |
| Secondary Antibody | Vendor | Cat# | Dilution |
|---|---|---|---|
| Goat anti-chicken, AF488-conjugated | Invitrogen, Carlsbad, CA, USA | A-32931 | 1:1000 |
| Goat anti-mouse, AF488-conjugated | Invitrogen, Carlsbad, CA, USA | A-10667 | 1:1000 |
| Goat anti-rabbit, AF568-conjugated | Invitrogen, Carlsbad, CA, USA | A-11011 | 1:1000 |
| Goat anti-mouse, AF647-conjugated | Invitrogen, Carlsbad, CA, USA | A-21235 | 1:1000 |
| Goat anti-guinea pig, AF647-conjugated | Invitrogen, Carlsbad, CA, USA | A-21450 | 1:1000 |
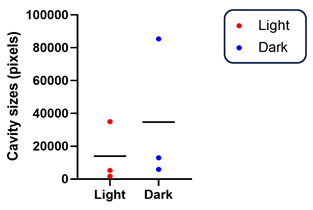 | |||
| Light-Rearing (L/D) | Dark-Rearing (D/D) | ||
| Cavity Size (Pixels) | Cavity Size (Pixels) | ||
| A | 1741 | G | 5860 |
| B | 5263 | H | 12,979 |
| C | 34,970 | I | 85,401 |
| Mean Values of Cavity Size in Rs1KO Rats Under L/D and D/D | |||
| Light-Rearing (L/D) | Dark-Rearing (D/D) | p-Value | |
| Mean ± SEM | 13,991 ± 10,538 | 34,747 ± 25,410 | 0.511 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smit-McBride, Z.; Cho, I.H.; Sun, N.; Thomas, S.; Sieving, P.A. Dark Rearing Does Not Alter Developmental Retinoschisis Cavity Formation in Rs1 Gene Knockout Rat Model of X-Linked Retinoschisis. Genes 2025, 16, 815. https://doi.org/10.3390/genes16070815
Smit-McBride Z, Cho IH, Sun N, Thomas S, Sieving PA. Dark Rearing Does Not Alter Developmental Retinoschisis Cavity Formation in Rs1 Gene Knockout Rat Model of X-Linked Retinoschisis. Genes. 2025; 16(7):815. https://doi.org/10.3390/genes16070815
Chicago/Turabian StyleSmit-McBride, Zeljka, In Hwan Cho, Ning Sun, Serafina Thomas, and Paul A. Sieving. 2025. "Dark Rearing Does Not Alter Developmental Retinoschisis Cavity Formation in Rs1 Gene Knockout Rat Model of X-Linked Retinoschisis" Genes 16, no. 7: 815. https://doi.org/10.3390/genes16070815
APA StyleSmit-McBride, Z., Cho, I. H., Sun, N., Thomas, S., & Sieving, P. A. (2025). Dark Rearing Does Not Alter Developmental Retinoschisis Cavity Formation in Rs1 Gene Knockout Rat Model of X-Linked Retinoschisis. Genes, 16(7), 815. https://doi.org/10.3390/genes16070815







