Development and Evaluation of a Molecular Test for Monkeypox Virus in the Federal District, Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. DNA Extraction
2.3. Real Time PCR
2.4. Sanger Sequencing
2.5. Limit of Detection (LoD)
2.6. Specificity
2.7. Analytical Precision and Reproducibility
2.8. Epidemiological Data and Analysis
3. Results
3.1. DNA Extraction
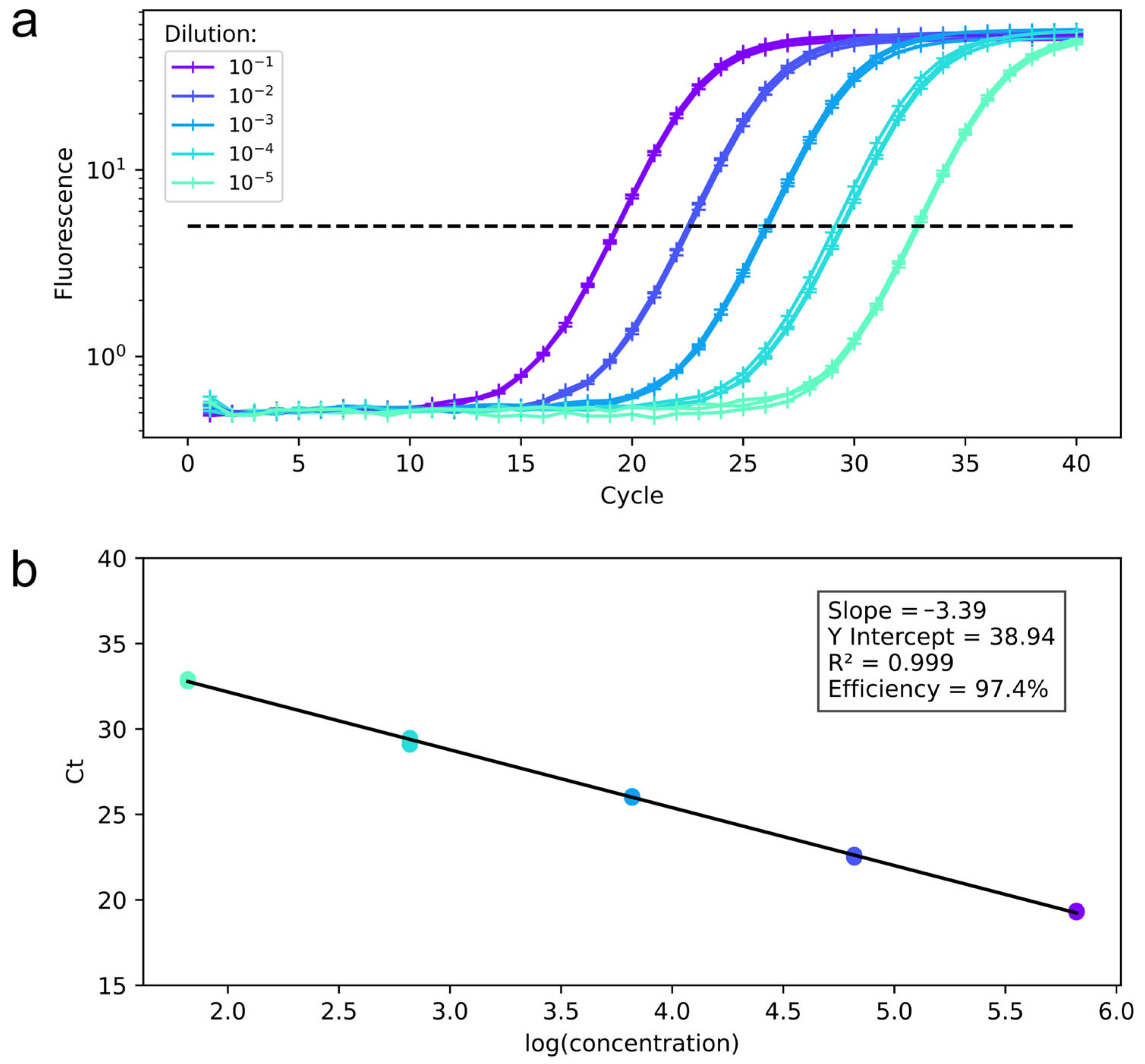
3.2. qPCR Assay Standardization
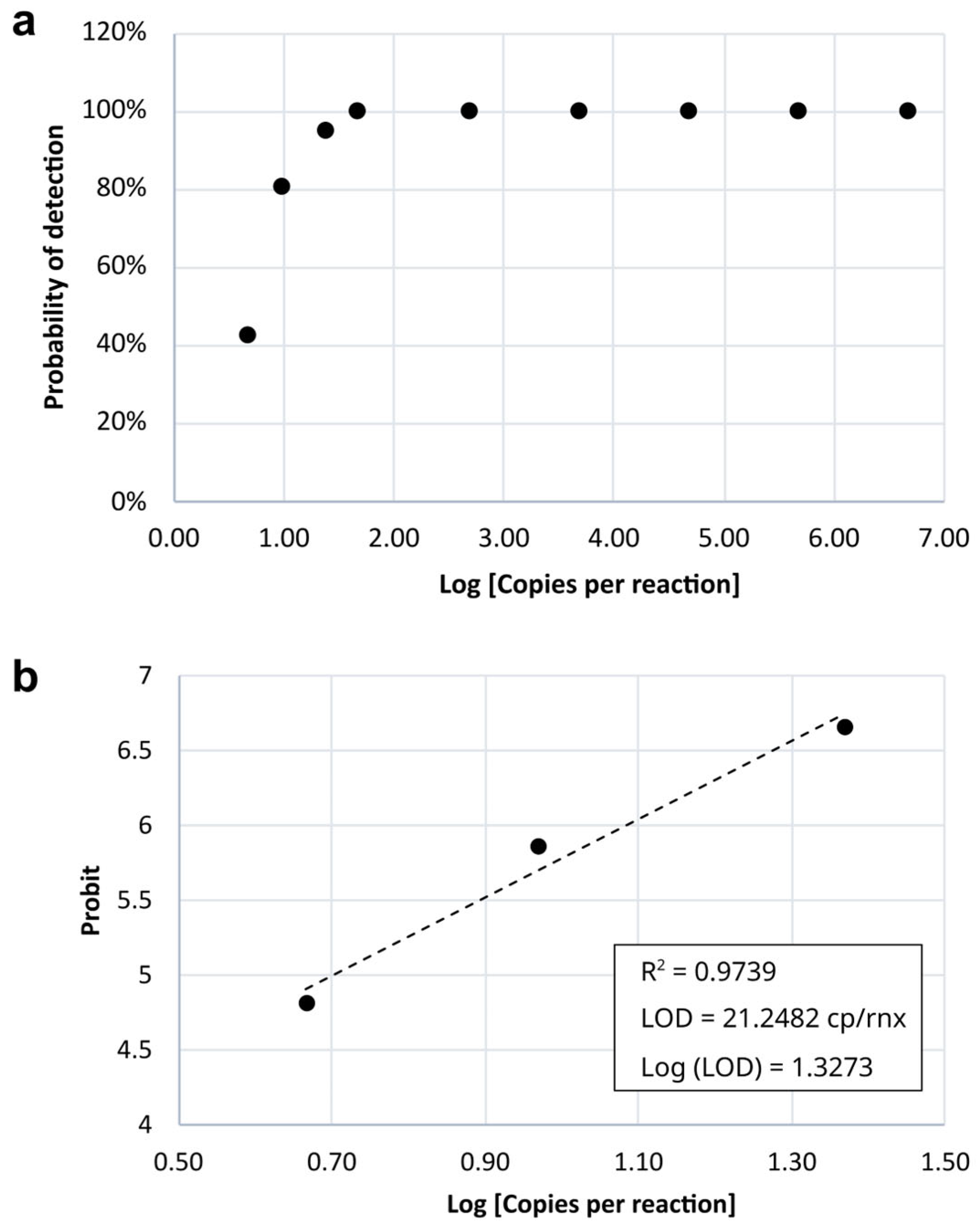
3.3. Sensitivity (Limit of Detection—LoD)
3.4. Specificity
3.5. Analytical Precision and Reproducibility
3.6. Sanger Sequencing
3.7. Accuracy
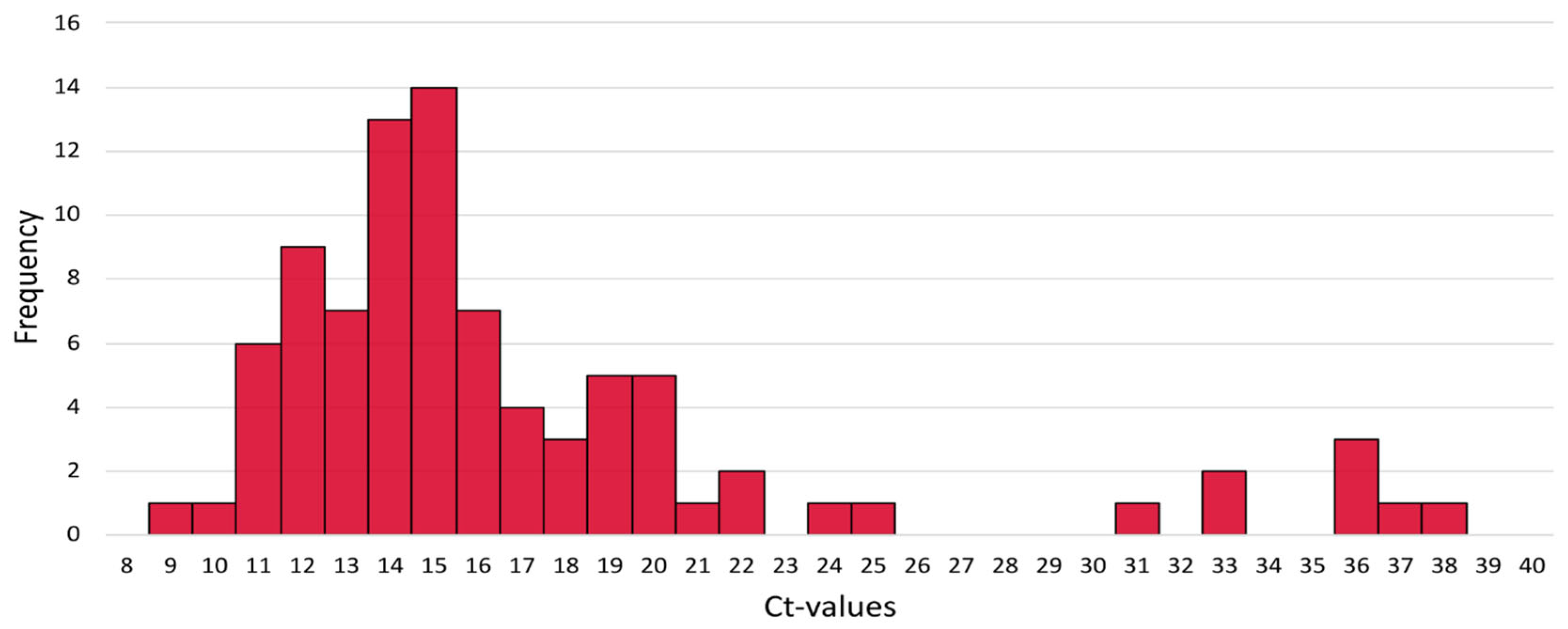
3.8. Monkeypox Ct Values
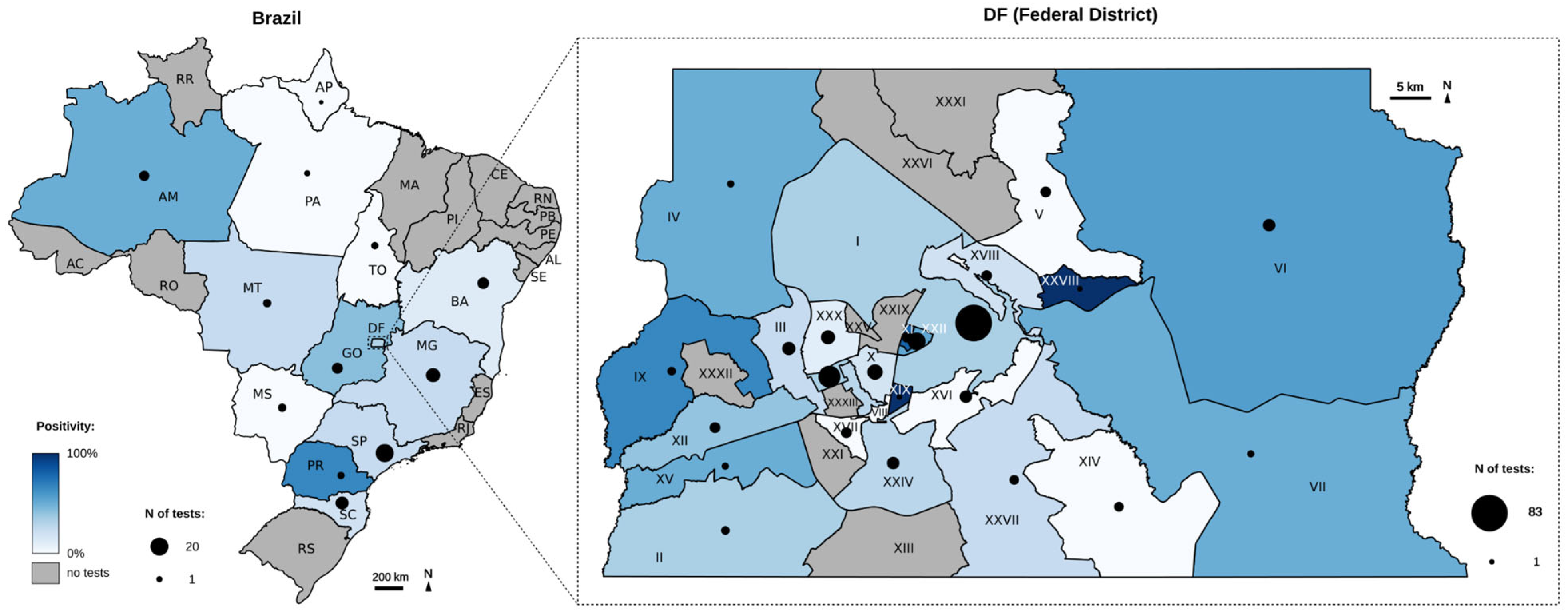
3.9. Spatial and Temporal Distribution of Monkeypox Detection
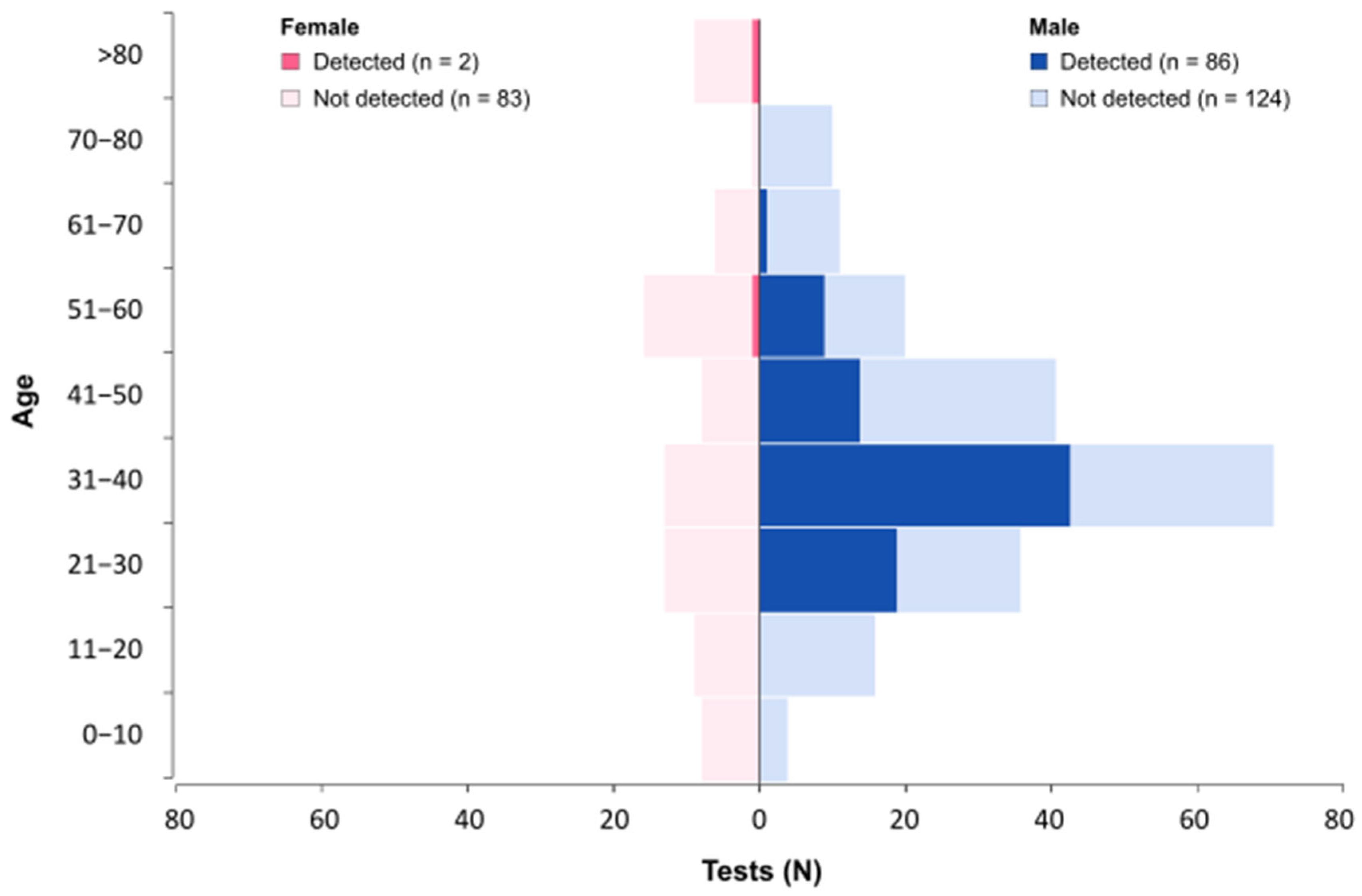
3.10. Demographic Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPOX | Re-emerging disease caused by the monkeypox virus |
| MPXV | Monkeypox virus |
| qPCR | Quantitative polymerase chain reaction |
| WHO | World Health Organization |
| DF | Federal District (Brazil) |
| NTC | Non-template control |
| NC | Negative control |
| Ct | Cycle threshold |
| LACEN-DF | Central Laboratory in the Federal District reference laboratory of the public Healthcare |
| MSM | Men who have sex with men |
References
- Kmiec, D.; Kirchhoff, F. Monkeypox: A New Threat? Int. J. Mol. Sci. 2022, 23, 7866. [Google Scholar] [CrossRef]
- Lopera, J.G.; Falendysz, E.A.; Rocke, T.E.; Osorio, J.E. Attenuation of Monkeypox Virus by Deletion of Genomic Regions. Virology 2015, 475, 129–138. [Google Scholar] [CrossRef]
- Damhorst, G.L.; McLendon, K.; Morales, E.; Solis, Z.M.; Fitts, E.; Bowers, H.B.; Sabino, C.; Sullivan, J.; Greenleaf, M.; Roback, J.D.; et al. Performance of the XpertTM Mpox PCR Assay with Oropharyngeal, Anorectal, and Cutaneous Lesion Swab Specimens. J. Clin. Virol. 2024, 171, 105659. [Google Scholar] [CrossRef] [PubMed]
- Hutson, C.L.; Carroll, D.S.; Gallardo-Romero, N.; Drew, C.; Zaki, S.R.; Nagy, T.; Hughes, C.; Olson, V.A.; Sanders, J.; Patel, N.; et al. Comparison of Monkeypox Virus Clade Kinetics and Pathology within the Prairie Dog Animal Model Using a Serial Sacrifice Study Design. Biomed. Res. Int. 2015, 2015, 965710. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Martínez, Y.; Rodríguez-Morales, A.J.; Franco-Paredes, C.; Chastain, D.B.; Gharamti, A.A.; Vargas Barahona, L.; Henao-Martínez, A.F. Monkeypox-A Description of the Clinical Progression of Skin Lesions: A Case Report from Colorado, USA. Ther. Adv. Infect. Dis. 2022, 9, 20499361221117726. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: Epidemiology, Pathogenesis, Treatment and Prevention. Signal Transduct. Target. Ther. 2022, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Wang, C.; Chuai, X.; Chiu, S. Monkeypox Virus: A Re-Emergent Threat to Humans. Virol. Sin. 2022, 37, 477–482. [Google Scholar] [CrossRef]
- Saxena, S.K.; Ansari, S.; Maurya, V.K.; Kumar, S.; Jain, A.; Paweska, J.T.; Tripathi, A.K.; Abdel-Moneim, A.S. Re-Emerging Human Monkeypox: A Major Public-Health Debacle. J. Med. Virol. 2023, 95, e27902. [Google Scholar] [CrossRef]
- Nuzzo, J.B.; Borio, L.L.; Gostin, L.O. The WHO Declaration of Monkeypox as a Global Public Health Emergency. JAMA 2022, 328, 615–617. [Google Scholar] [CrossRef]
- Claro, I.M.; Romano, C.M.; da Candido, D.S.; de Lima, E.L.; Lindoso, J.A.L.; Ramundo, M.S.; Moreira, F.R.R.; Barra, L.A.C.; Borges, L.M.S.; Medeiros, L.A.; et al. Shotgun Metagenomic Sequencing of the First Case of Monkeypox Virus in Brazil, 2022. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e48. [Google Scholar] [CrossRef]
- Ministério Da Saúde. Boletim Epidemiológico de Mpox No 25. Available online: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/variola-dos-macacos/boletim-epidemiologico-de-monkeypox-no-25/view (accessed on 6 May 2025).
- Maksyutov, R.A.; Gavrilova, E.V.; Shchelkunov, S.N. Species-Specific Differentiation of Variola, Monkeypox, and Varicella-Zoster Viruses by Multiplex Real-Time PCR Assay. J. Virol. Methods 2016, 236, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Emery, S.L.; Erdman, D.D.; Bowen, M.D.; Newton, B.R.; Winchell, J.M.; Meyer, R.F.; Tong, S.; Cook, B.T.; Holloway, B.P.; McCaustland, K.A.; et al. Real-Time Reverse Transcription-Polymerase Chain Reaction Assay for SARS-Associated Coronavirus. Emerg. Infect. Dis. 2004, 10, 311–316. [Google Scholar] [CrossRef]
- de Lima, E.L.; Barra, L.A.C.; Borges, L.M.S.; Medeiros, L.A.; Tomishige, M.Y.S.; de Santos, L.S.L.A.; da Silva, A.J.D.; Rodrigues, C.C.M.; de Azevedo, L.C.F.; Villas-Boas, L.S.; et al. First Case Report of Monkeypox in Brazil: Clinical Manifestations and Differential Diagnosis with Sexually Transmitted Infections. Rev. Inst. Med. Trop. Sao Paulo 2022, 64, e54. [Google Scholar] [CrossRef]
- Mills, M.G.; Juergens, K.B.; Gov, J.P.; McCormick, C.J.; Sampoleo, R.; Kachikis, A.; Amory, J.K.; Fang, F.C.; Pérez-Osorio, A.C.; Lieberman, N.A.P.; et al. Evaluation and Clinical Validation of Monkeypox (Mpox) Virus Real-Time PCR Assays. J. Clin. Virol. 2023, 159, 105373. [Google Scholar] [CrossRef] [PubMed]
- Paran, N.; Yahalom-Ronen, Y.; Shifman, O.; Lazar, S.; Ben-Ami, R.; Yakubovsky, M.; Levy, I.; Wieder-Feinsod, A.; Amit, S.; Katzir, M.; et al. Monkeypox DNA Levels Correlate with Virus Infectivity in Clinical Samples, Israel, 2022. Eurosurveillance 2022, 27, 2200636. [Google Scholar] [CrossRef] [PubMed]
- Chelsky, Z.L.; Dittmann, D.; Blanke, T.; Chang, M.; Vormittag-Nocito, E.; Jennings, L.J. Validation Study of a Direct Real-Time PCR Protocol for Detection of Monkeypox Virus. J. Mol. Diagn. 2022, 24, 1155–1159. [Google Scholar] [CrossRef]
- Paniz-Mondolfi, A.; Guerra, S.; Muñoz, M.; Luna, N.; Hernandez, M.M.; Patino, L.H.; Reidy, J.; Banu, R.; Shrestha, P.; Liggayu, B.; et al. Evaluation and Validation of an RT-PCR Assay for Specific Detection of Monkeypox Virus (MPXV). J. Med. Virol. 2023, 95, e28247. [Google Scholar] [CrossRef]
- Rosa, G.L.; Mancini, P.; Veneri, C.; Ferraro, G.B.; Lucentini, L.; Iaconelli, M.; Suffredini, E. Detection of Monkeypox Virus DNA in Airport Wastewater, Rome, Italy. Emerging Infectious Diseases 2023, 29, 193. [Google Scholar] [CrossRef]
- Fan, Z.; Xie, Y.; Huang, B.; Zhao, F.; Hu, Y.; Huang, Y.; Mei, S.; Wei, L.; Wang, L.; Wang, L.; et al. Development of a Multiplex Real-Time PCR Assay for the Simultaneous Detection of Mpox Virus and Orthopoxvirus Infections. J. Virol. Methods 2024, 328, 114957. [Google Scholar] [CrossRef]
- Lim, C.K.; McKenzie, C.; Deerain, J.; Chow, E.P.F.; Towns, J.; Chen, M.Y.; Fairley, C.K.; Tran, T.; Williamson, D.A. Correlation between Monkeypox Viral Load and Infectious Virus in Clinical Specimens. J. Clin. Virol. 2023, 161, 105421. [Google Scholar] [CrossRef]
- Iñigo Martínez, J.; Gil Montalbán, E.; Jiménez Bueno, S.; Martín Martínez, F.; Nieto Juliá, A.; Sánchez Díaz, J.; García Marín, N.; Córdoba Deorador, E.; Nunziata Forte, A.; Alonso García, M.; et al. Monkeypox Outbreak Predominantly Affecting Men Who Have Sex with Men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance 2022, 27, 2200471. [Google Scholar] [CrossRef] [PubMed]
- Pascom, A.R.P.; de Souza, I.N.; Krummenauer, A.; Duarte, M.M.S.; Sallas, J.; Rohlfs, D.B.; Pereira, G.M.; de Medeiros, A.C.; Miranda, A.E. Epidemiological and Clinical Characteristics of Monkeypox Cases in Brazil in 2022: A Cross-Sectional Study. Epidemiol. Serv. Saude 2022, 31, e2022851. [Google Scholar] [CrossRef] [PubMed]
- Selb, R.; Werber, D.; Falkenhorst, G.; Steffen, G.; Lachmann, R.; Ruscher, C.; McFarland, S.; Bartel, A.; Hemmers, L.; Koppe, U.; et al. A Shift from Travel-Associated Cases to Autochthonous Transmission with Berlin as Epicentre of the Monkeypox Outbreak in Germany, May to June 2022. Eurosurveillance 2022, 27, 2200499. [Google Scholar] [CrossRef] [PubMed]


| Name | Application | Sequence (5′-3′) | Reference |
|---|---|---|---|
| MPX-Fwd MPX-Rev Probe_MPX | qPCR | CATCTATTATAGCATCAGCATCAG | MAKSYUTOV, 2016 [12] |
| qPCR | GATACTCCTCCTCGTTGGTCTAC | ||
| qPCR | /56-FAM/TGTAGGCCG/ZEN/TGTATCAGCATCCATT/3IABkFQ/ | ||
| RNase-P-Fwd RNase-P-Rev Probe_RNase-P | qPCR | AGATTTGGACCTGCGAGCG | adapted from Emery, et.al, 2004 [13] |
| qPCR qPCR | GAGCGGCTGTCTCCACAA | ||
| /HEX/TTC TGA CCT/ZEN/GAA GGC TCT GCG CG-3IBkFQ | |||
| Mpox_2139-F (MPX_SEQ_1) Mpox_2620-R (MPX_SEQ_1) | Sanger | GGATTCGCTGAGACCGGTAG | designed in this study |
| Sanger | TATCGTGTCCTCCGGGAACT | designed in this study | |
| Mpox_19,722-F (MPX_SEQ_2) Mpox_20,266-R (MPX_SEQ_2) | Sanger | TGGCAAATCTAACTGCGGGT | designed in this study |
| Sanger | AATGACGCTATCCGACGGTC | designed in this study | |
| Mpox_61,272-F (MPX_SEQ_3) Mpox_61,801-R (MPX_SEQ_3R) | Sanger | AGACCTATTCCCCCTGCCAT | designed in this study |
| Sanger | TATGCCATTCTAGCCGCCAG | designed in this study | |
| Mpox_193,245-F (MPX_SEQ_4) Mpox_193,792-R (MPX_SEQ_4) | Sanger | CCTCGTGTGGTGTATGCTCT | designed in this study |
| Sanger | ACGTAGTGATCGTCGTAGGG | designed in this study |
| Kit | Sample Input | Elution | Average of Triplicates (Ct) | Minimum (Ct) | Maximum (Ct) |
|---|---|---|---|---|---|
| DSP virus/Pathogen MINI | 200 µL | 60 µL | 18.66 | 18.49 | 18.88 |
| 85 µL | 19.34 | 18.94 | 19.91 | ||
| 110 µL | 19.12 | 18.94 | 19.39 | ||
| DSP virus/Pathogen MIDI | 400 µL | 60 µL | 17.54 | 17.34 | 17.91 |
| 85 µL | 18.08 | 17.82 | 18.7 | ||
| 110 µL | 18.22 | 18.07 | 18.4 |
| Dilution Factor | Copies per Reaction | Log [Copies per Reaction] | Number of Replicates | Number of ‘Detected’ Results | Number of ‘Not Detected’ Results | Percentage of ‘Detected’ Results | Probit |
|---|---|---|---|---|---|---|---|
| 1/10 | 4,632,308 | 6.67 | 3 | 3 | 0 | 100.00% | - |
| 1/10 | 463,230.77 | 5.67 | 3 | 3 | 0 | 100.00% | - |
| 1/10 | 46,323.08 | 4.67 | 3 | 3 | 0 | 100.00% | - |
| 1/10 | 4632.31 | 3.67 | 3 | 3 | 0 | 100.00% | - |
| 1/10 | 463.23 | 2.67 | 3 | 3 | 0 | 100.00% | - |
| 1/10 | 46.32 | 1.67 | 21 | 21 | 0 | 100.00% | - |
| 1/2 | 23.16 | 1.36 | 21 | 20 | 1 | 95.24% | 6.67 |
| 1/2 | 9.26 | 0.97 | 21 | 17 | 4 | 80.95% | 5.88 |
| 1/2 | 4.63 | 0.67 | 21 | 9 | 12 | 42.86% | 4.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.P.d.; Hurtado, F.A.; Belmok, A.; Correa, R.; Sousa, C.F.; Gil, G.P.; Velasco, L.; Jácomo, R.H.; Nery, L.F.; Rodrigues, M.T.d.O.; et al. Development and Evaluation of a Molecular Test for Monkeypox Virus in the Federal District, Brazil. Genes 2025, 16, 779. https://doi.org/10.3390/genes16070779
Silva LPd, Hurtado FA, Belmok A, Correa R, Sousa CF, Gil GP, Velasco L, Jácomo RH, Nery LF, Rodrigues MTdO, et al. Development and Evaluation of a Molecular Test for Monkeypox Virus in the Federal District, Brazil. Genes. 2025; 16(7):779. https://doi.org/10.3390/genes16070779
Chicago/Turabian StyleSilva, Lucas Pereira da, Fabián Andrés Hurtado, Aline Belmok, Rafael Correa, Claudia F. Sousa, Gislene P. Gil, Lara Velasco, Rafael H. Jácomo, Lídia F. Nery, Maria Tereza de Oliveira Rodrigues, and et al. 2025. "Development and Evaluation of a Molecular Test for Monkeypox Virus in the Federal District, Brazil" Genes 16, no. 7: 779. https://doi.org/10.3390/genes16070779
APA StyleSilva, L. P. d., Hurtado, F. A., Belmok, A., Correa, R., Sousa, C. F., Gil, G. P., Velasco, L., Jácomo, R. H., Nery, L. F., Rodrigues, M. T. d. O., Andrade, M. S., & Andrade, R. V. d. (2025). Development and Evaluation of a Molecular Test for Monkeypox Virus in the Federal District, Brazil. Genes, 16(7), 779. https://doi.org/10.3390/genes16070779






