Genetic Regulation of Immune Response in Dogs
Abstract
1. Introduction
2. Overview of the Canine Immune System Compared to Human
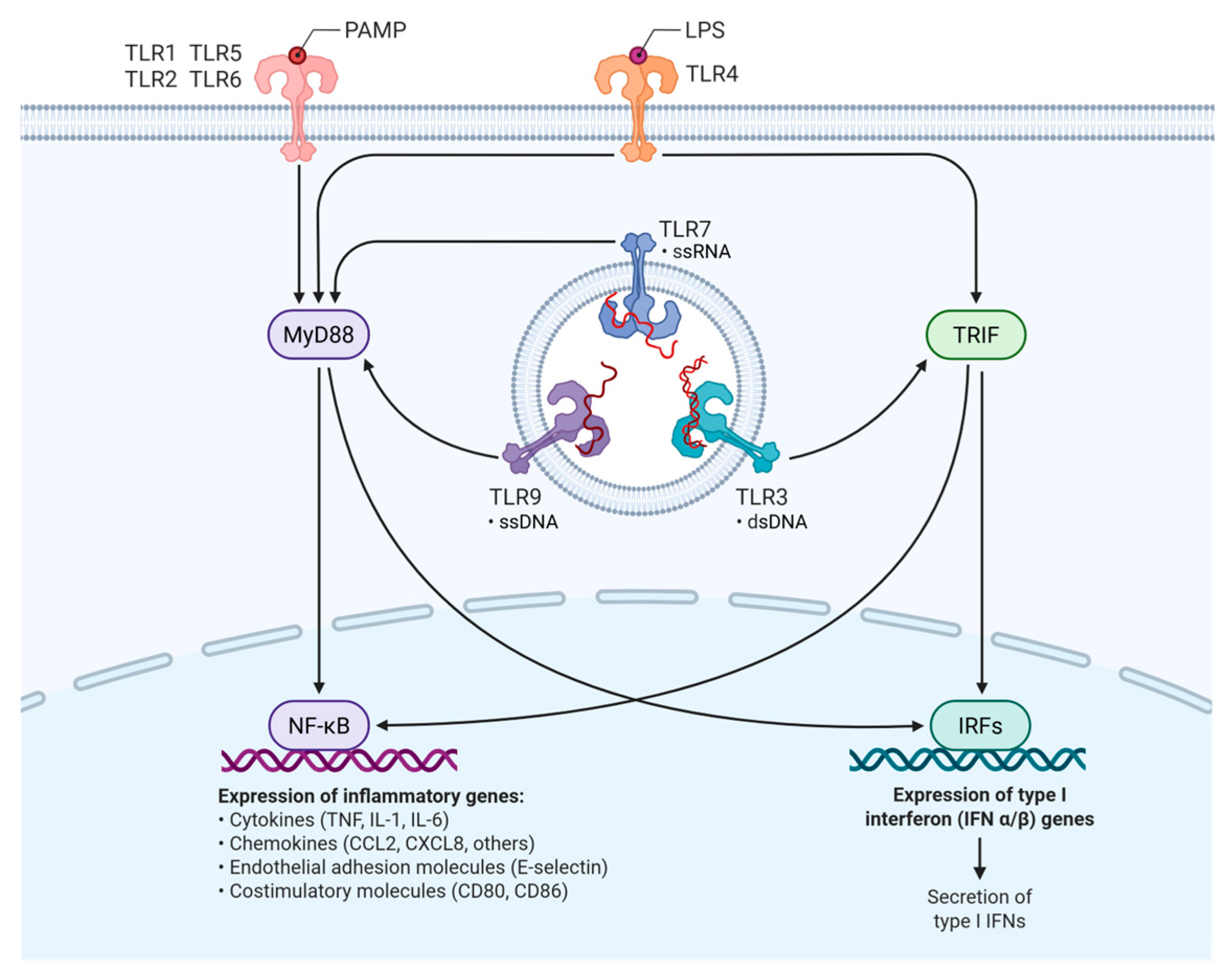
3. Toll-like Receptors (TLRs) and Innate Immune Gene Variability
| Diseases | Involved TLRs | Key Findings | References |
|---|---|---|---|
| Leptospira spp. infection | TLR-2, TLR-4 | Increase IL-1β production | [37,38] |
| Salmonella enteriditis | TLR-5 | Recognizes flagellin, increase IL-1β and IL-8 production | [39] |
| Canine leishmaniosis | TLR-2, -4, -5, and -9 | Stage-specific expression. TLR-4 increases in early stages and decreases in late stages | [40,41,42] |
| Demodicosis | TLR-2, -4, and -6 | Parasite modulates expression to evade immune response | [45] |
| Inflammatory bowel diseases (IBD) | TLR-4 and -5 | SNPs in TLR-5: G22A increases the risk and C100T and T1844C with protective effect | [23,46] |
| Inflammatory colorectal polyps (ICRP) | TLR-2, -4, and -9 | Increases the cytokine production after ligand exposure | [48] |
| Atopic dermatitis | TLR-1, -2, -4, -6, -9, and -10 | SNP in TLR-2 R753Q, decreases IFN-γ and increases IL-4 levels | [49,50,51,52,53,54,55,60,61,62,63] |
| Canine lupus (DLE) | TLR-4 | Overexpressed in skin; potential therapeutic target | [65] |
| Canine distemper (Lycaon pictus) | Multiple TLRs | Polymorphisms in TLR genes related to stronger immunity | [64] |
4. Cytokine Genes and Immune Modulation
5. Breed-Specific Immune Profiles and Heritable Disorders
6. Epigenetics and Immune Gene Regulation
7. Applications for Veterinary Medicine and Canine Health
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCL | Chemokine (C-C motif) Ligand |
| CDV | Canine Distemper Virus |
| CD4fh | Follicular helper CD4 T |
| CHV-1 | Canid Alphaherpesvirus 1 |
| DLA | Dog Leukocyte Antigen |
| DAMP | Endogenous Damage Signal |
| DNMT | DNA Methyltransferase |
| IBD | Inflammatory Bowel Disease |
| ICI | Immune Checkpoint Inhibitor |
| ICRP | Inflammatory Colorectal Polyp |
| IFN | Interferon |
| Ig | Immunoglobulin |
| IL | Interleukin |
| IMRD | Immune-Mediated Rheumatic Disease |
| iNOS | Inducible Nitric Oxide Synthase |
| GWAS | Genome-Wide Association Studies |
| mregCD | Unique Dendritic Dell |
| miRNA | MicroRNA |
| MHC | Major Histocompatibility Complex |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NK | Natural Killer |
| PAMP | Pathogen-Associated Molecular Pattern |
| PBMCs | Peripheral Mononuclear Cells |
| SNP | Single Nucleotide Polymorphism |
| SOCS3 | Suppressor of Cytokine Signaling 3 |
| TAM | Tumor-Associated Macrophage |
| TRIF | TIR-Domain-Containing Adapter-Inducing Interferon-β |
| TLR | Toll-Like Receptor |
| TNF | Tumor Necrosis Factor |
| Tregs | Regulatory T cells |
References
- Wagner, J.L. Molecular Organization of the Canine Major Histocompatibility Complex. J. Hered. 2003, 94, 23–26. [Google Scholar] [CrossRef][Green Version]
- Gershony, L.C.; Belanger, J.M.; Short, A.D.; Le, M.; Hytönen, M.K.; Lohi, H.; Famula, T.R.; Kennedy, L.J.; Oberbauer, A.M. DLA Class II Risk Haplotypes for Autoimmune Diseases in the Bearded Collie Offer Insight to Autoimmunity Signatures across Dog Breeds. Canine Genet. Epidemiol. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Liu, H.; Millon, L.; Greer, K. Dog Leukocyte Antigen Class II-Associated Genetic Risk Testing for Immune Disorders of Dogs: Simplified Approaches Using Pug Dog Necrotizing Meningoencephalitis as a Model. J. Vet. Diagn. Investig. 2011, 23, 68–76. [Google Scholar] [CrossRef] [PubMed]
- House, A.K.; Binns, M.M.; Gregory, S.P.; Catchpole, B. Analysis of NOD1, NOD2, TLR1, TLR2, TLR4, TLR5, TLR6 and TLR9 Genes in Anal Furunculosis of German Shepherd Dogs. Tissue Antigens 2009, 73, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Wilbe, M.; Jokinen, P.; Truvé, K.; Seppala, E.H.; Karlsson, E.K.; Biagi, T.; Hughes, A.; Bannasch, D.; Andersson, G.; Hansson-Hamlin, H.; et al. Genome-Wide Association Mapping Identifies Multiple Loci for a Canine SLE-Related Disease Complex. Nat. Genet. 2010, 42, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.-R.; Heo, M.; Lee, K.-H.; Kim, J.-Y.; Won, S.-H.; Cho, J.-Y. The Landscape of PBMC Methylome in Canine Mammary Tumors Reveals the Epigenetic Regulation of Immune Marker Genes and Its Potential Application in Predicting Tumor Malignancy. BMC Genom. 2023, 24, 403. [Google Scholar] [CrossRef]
- Paris, S.; Chapat, L.; Pasin, M.; Lambiel, M.; Sharrock, T.E.; Shukla, R.; Sigoillot-Claude, C.; Bonnet, J.-M.; Poulet, H.; Freyburger, L.; et al. β-Glucan-Induced Trained Immunity in Dogs. Front. Immunol. 2020, 11, 566893. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, X. Identifying Epigenetic Markers for Disease Resistance in Dogs. Anim. Mol. Breed. 2024, 14, 244–253. [Google Scholar]
- Nikbakht, G.; Houshmand, P.; Esmailnejad, A.; Abasabadi, F. Major Histocompatibility Complex as Marker Assisted Selection for Breeding Immunocompetent Animal. Iran. J. Vet. Med. 2022, 16, 211–227. [Google Scholar] [CrossRef]
- Tagawa, M.; Kurashima, C.; Takagi, S.; Maekawa, N.; Konnai, S.; Shimbo, G.; Matsumoto, K.; Inokuma, H.; Kawamoto, K.; Miyahara, K. Evaluation of Costimulatory Molecules in Dogs with B Cell High Grade Lymphoma. PLoS ONE 2018, 13, e0201222. [Google Scholar] [CrossRef]
- Chow, L.; Wheat, W.; Ramirez, D.; Impastato, R.; Dow, S. Direct Comparison of Canine and Human Immune Responses Using Transcriptomic and Functional Analyses. Sci. Rep. 2024, 14, 2207. [Google Scholar] [CrossRef]
- Gingrich, A.A.; Reiter, T.E.; Judge, S.J.; York, D.; Yanagisawa, M.; Razmara, A.; Sturgill, I.; Basmaci, U.N.; Brady, R.V.; Stoffel, K.; et al. Comparative Immunogenomics of Canine Natural Killer Cells as Immunotherapy Target. Front. Immunol. 2021, 12, 670309. [Google Scholar] [CrossRef] [PubMed]
- Regan, D.; Guth, A.; Coy, J.; Dow, S. Cancer Immunotherapy in Veterinary Medicine: Current Options and New Developments. Vet. J. 2016, 207, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Peiravan, A.; Allenspach, K.; Boag, A.M.; Soutter, F.; Holder, A.; Catchpole, B.; Kennedy, L.J.; Werling, D.; Procoli, F. Single Nucleotide Polymorphisms in Major Histocompatibility Class II Haplotypes Are Associated with Potential Resistance to Inflammatory Bowel Disease in German Shepherd Dogs. Vet. Immunol. Immunopathol. 2016, 182, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Peiravan, A.; Bertolini, F.; Rothschild, M.F.; Simpson, K.W.; Jergens, A.E.; Allenspach, K.; Werling, D. Genome-Wide Association Studies of Inflammatory Bowel Disease in German Shepherd Dogs. PLoS ONE 2018, 13, e0200685. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Davison, L.J.; Barnes, A.; Short, A.D.; Fretwell, N.; Jones, C.A.; Lee, A.C.; Ollier, W.E.R.; Catchpole, B. Identification of Susceptibility and Protective Major Histocompatibility Complex Haplotypes in Canine Diabetes Mellitus. Tissue Antigens 2006, 68, 467–476. [Google Scholar] [CrossRef]
- Kathrani, A.; House, A.; Catchpole, B.; Murphy, A.; German, A.; Werling, D.; Allenspach, K. Polymorphisms in the TLR4 and TLR5 Gene Are Significantly Associated with Inflammatory Bowel Disease in German Shepherd Dogs. PLoS ONE 2010, 5, e15740. [Google Scholar] [CrossRef]
- Mercier, E.; Peters, I.R.; Farnir, F.; Lavoué, R.; Day, M.; Clercx, C.; Peeters, D. Assessment of Toll-like Receptor 2, 4 and 9 SNP Genotypes in Canine Sino-Nasal Aspergillosis. BMC Vet. Res. 2014, 10, 187. [Google Scholar] [CrossRef]
- Kathrani, A.; House, A.; Catchpole, B.; Murphy, A.; Werling, D.; Allenspach, K. Breed-Independent Toll-like Receptor 5 Polymorphisms Show Association with Canine Inflammatory Bowel Disease. Tissue Antigens 2011, 78, 94–101. [Google Scholar] [CrossRef]
- Álvarez, L.; Marín-García, P.-J.; Rentero-Garrido, P.; Llobat, L. Immune and Genomic Analysis of Boxer Dog Breed and Its Relationship with Leishmania Infantum Infection. Vet. Sci. 2022, 9, 608. [Google Scholar] [CrossRef]
- Álvarez, L.; Marín-García, P.-J.; Llobat, L. Genetic Haplotypes Associated with Immune Response to Leishmania Infantum Infection in Dogs. Vet. Res. Commun. 2023, 47, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Ostrander, E.A.; Wang, G.-D.; Larson, G.; vonHoldt, B.M.; Davis, B.W.; Jagannathan, V.; Hitte, C.; Wayne, R.K.; Zhang, Y.-P.; Dog10K Consortium. Dog10K: An International Sequencing Effort to Advance Studies of Canine Domestication, Phenotypes and Health. Natl. Sci. Rev. 2019, 6, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Complement System and Innate Immunity. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Germain, R.N. MHC-Dependent Antigen Processing and Peptide Presentation: Providing Ligands for T Lymphocyte Activation. Cell 1994, 76, 287–299. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Barnes, A.; Short, A.; Brown, J.J.; Lester, S.; Seddon, J.; Happ, G.M.; Ollier, W.E.R. Canine DLA Diversity: 2. Family Studies. Tissue Antigens 2007, 69 (Suppl. 1), 289–291. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Barnes, A.; Short, A.; Brown, J.J.; Lester, S.; Seddon, J.; Fleeman, L.; Francino, O.; Brkljacic, M.; Knyazev, S.; et al. Canine DLA Diversity: 1. New Alleles and Haplotypes. Tissue Antigens 2007, 69 (Suppl. 1), 272–288. [Google Scholar] [CrossRef]
- Tizard, I.R. Veterinary Immunology; Elsevier: Amsterdam, The Netherlands, 2024; ISBN 978-0-443-10975-1. [Google Scholar]
- Turin, L.; Riva, F. Toll-like Receptor Family in Domestic Animal Species. Crit. Rev. Immunol. 2008, 28, 513–538. [Google Scholar] [CrossRef] [PubMed]
- Werling, D.; Jungi, T.W. TOLL-like Receptors Linking Innate and Adaptive Immune Response. Vet. Immunol. Immunopathol. 2003, 91, 1–12. [Google Scholar] [CrossRef]
- Angles, J.M.; Kennedy, L.J.; Pedersen, N.C. Frequency and Distribution of Alleles of Canine MHC-II DLA-DQB1, DLA-DQA1 and DLA-DRB1 in 25 Representative American Kennel Club Breeds. Tissue Antigens 2005, 66, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.J.; Barnes, A.; Happ, G.M.; Quinnell, R.J.; Bennett, D.; Angles, J.M.; Day, M.J.; Carmichael, N.; Innes, J.F.; Isherwood, D.; et al. Extensive Interbreed, but Minimal Intrabreed, Variation of DLA Class II Alleles and Haplotypes in Dogs. Tissue Antigens 2002, 59, 194–204. [Google Scholar] [CrossRef]
- Debenham, S.L.; Hart, E.A.; Ashurst, J.L.; Howe, K.L.; Quail, M.A.; Ollier, W.E.R.; Binns, M.M. Genomic Sequence of the Class II Region of the Canine MHC: Comparison with the MHC of Other Mammalian Species. Genomics 2005, 85, 48–59. [Google Scholar] [CrossRef]
- Roach, J.C.; Glusman, G.; Rowen, L.; Kaur, A.; Purcell, M.K.; Smith, K.D.; Hood, L.E.; Aderem, A. The Evolution of Vertebrate Toll-like Receptors. Proc. Natl. Acad. Sci. USA 2005, 102, 9577–9582. [Google Scholar] [CrossRef] [PubMed]
- Zaninoni, A.; Fattizzo, B.; Pettine, L.; Vercellati, C.; Marcello, A.P.; Barcellini, W. Cytokine Polymorphisms in Patients with Autoimmune Hemolytic Anemia. Front. Immunol. 2023, 14, 1221582. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.W.; Woods, K.; Wu, Y.; Glanemann, B.; Garden, O.A. Characterisation of the Immunophenotype of Dogs with Primary Immune-Mediated Haemolytic Anaemia. PLoS ONE 2016, 11, e0168296. [Google Scholar] [CrossRef]
- Denyer, A.L.; Massey, J.P.; Davison, L.J.; Ollier, W.E.R.; Catchpole, B.; Kennedy, L.J. Dog Leucocyte Antigen (DLA) Class II Haplotypes and Risk of Canine Diabetes Mellitus in Specific Dog Breeds. Canine Med. Genet. 2020, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, L.; Marín-García, P.-J.; Llobat, L. Immunological and Genomic Characterization of Ibizan Hound Dogs in an Endemic Leishmania Infantum Region. Parasit. Vectors 2022, 15, 445. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Robert, E.; Altet, L.; Sanchez, A.; Francino, O. Polymorphism of Slc11a1 (Nramp1) Gene and Canine Leishmaniasis in a Case-Control Study. J. Hered. 2005, 96, 755–758. [Google Scholar] [CrossRef]
- Ollier, W.E.; Kennedy, L.J.; Thomson, W.; Barnes, A.N.; Bell, S.C.; Bennett, D.; Angles, J.M.; Innes, J.F.; Carter, S.D. Dog MHC Alleles Containing the Human RA Shared Epitope Confer Susceptibility to Canine Rheumatoid Arthritis. Immunogenetics 2001, 53, 669–673. [Google Scholar] [CrossRef]
- Stromberg, S.J.; Thomasy, S.M.; Marangakis, A.D.; Kim, S.; Cooper, A.E.; Brown, E.A.; Maggs, D.J.; Bannasch, D.L. Evaluation of the Major Histocompatibility Complex (MHC) Class II as a Candidate for Sudden Acquired Retinal Degeneration Syndrome (SARDS) in Dachshunds. Vet. Ophthalmol. 2019, 22, 751–759. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Golenbock, D.; Bowie, A.G. The History of Toll-like Receptors—Redefining Innate Immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The Roles of TLRs, RLRs and NLRs in Pathogen Recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef]
- Federico, S.; Pozzetti, L.; Papa, A.; Carullo, G.; Gemma, S.; Butini, S.; Campiani, G.; Relitti, N. Modulation of the Innate Immune Response by Targeting Toll-like Receptors: A Perspective on Their Agonists and Antagonists. J. Med. Chem. 2020, 63, 13466–13513. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.H.; Pillai, S. Cellular and Molecular Immunology International Edition, 9th ed.; Elsevier Health Inspection Copies; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Akira, S.; Takeda, K. Toll-like Receptor Signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Balachandran, Y.; Knaus, S.; Caldwell, S.; Singh, B. Toll-like Receptor 10 Expression in Chicken, Cattle, Pig, Dog, and Rat Lungs. Vet. Immunol. Immunopathol. 2015, 168, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Lee, J.-O. Recognition of Lipopolysaccharide Pattern by TLR4 Complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef]
- Novak, A.; Pupo, E.; Van’t Veld, E.; Rutten, V.P.M.G.; Broere, F.; Sloots, A. Activation of Canine, Mouse and Human TLR2 and TLR4 by Inactivated Leptospira Vaccine Strains. Front. Immunol. 2022, 13, 823058. [Google Scholar] [CrossRef] [PubMed]
- Bolat, İ.; Bolat, M.; Kiliçlioğlu, M.; Yıldırım, S.; Sağlam, Y.S.; Çomaklı, S.; Gözegir, B.; Özmen, M.; Warda, M. Differential TLR2 and TLR4 Mediated Inflammatory and Apoptotic Responses in Asymptomatic and Symptomatic Leptospira Interrogans Infections in Canine Uterine Tissue. Microb. Pathog. 2025, 198, 107186. [Google Scholar] [CrossRef]
- Zhu, A.; Wei, L.; Hu, S.; Yang, C.; Chen, C.; Zhou, Z.; Pan, Z. Characterisation and Functional Analysis of Canine TLR5. Innate Immun. 2020, 26, 451–458. [Google Scholar] [CrossRef]
- Hosein, S.; Rodríguez-Cortés, A.; Blake, D.P.; Allenspach, K.; Alberola, J.; Solano-Gallego, L. Transcription of Toll-Like Receptors 2, 3, 4 and 9, FoxP3 and Th17 Cytokines in a Susceptible Experimental Model of Canine Leishmania Infantum Infection. PLoS ONE 2015, 10, e0140325. [Google Scholar] [CrossRef]
- Ordeix, L.; Montserrat-Sangrà, S.; Martínez-Orellana, P.; Baxarias, M.; Solano-Gallego, L. Toll-like Receptors 2, 4 and 7, Interferon-Gamma and Interleukin 10, and Programmed Death Ligand 1 Transcripts in Skin from Dogs of Different Clinical Stages of Leishmaniosis. Parasit. Vectors 2019, 12, 575. [Google Scholar] [CrossRef]
- Grano, F.G.; Dos, S.; Silva, J.E.; Melo, G.D.; de Souza, M.S.; Lima, V.M.F.; Machado, G.F. Toll-like Receptors and Cytokines in the Brain and in Spleen of Dogs with Visceral Leishmaniosis. Vet. Parasitol. 2018, 253, 30–38. [Google Scholar] [CrossRef]
- Carrillo, E.; Moreno, J. Cytokine Profiles in Canine Visceral Leishmaniasis. Vet. Immunol. Immunopathol. 2009, 128, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Orellana, P.; Marí-Martorell, D.; Montserrat-Sangrà, S.; Ordeix, L.; Baneth, G.; Solano-Gallego, L. Leishmania Infantum-Specific IFN-γ Production in Stimulated Blood from Dogs with Clinical Leishmaniosis at Diagnosis and during Treatment. Vet. Parasitol. 2017, 248, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Nigam, R.; Choudhury, S.; Singh, S.K.; Yadav, B.; Kumar, D.; Garg, S.K. Demodex Canis Targets TLRs to Evade Host Immunity and Induce Canine Demodicosis. Parasite Immunol. 2018, 40, e12509. [Google Scholar] [CrossRef] [PubMed]
- Cuscó, A.; Sánchez, A.; Altet, L.; Ferrer, L.; Francino, O. Non-Synonymous Genetic Variation in Exonic Regions of Canine Toll-like Receptors. Canine Genet. Epidemiol. 2014, 1, 11. [Google Scholar] [CrossRef][Green Version]
- Barreiro, L.B.; Ben-Ali, M.; Quach, H.; Laval, G.; Patin, E.; Pickrell, J.K.; Bouchier, C.; Tichit, M.; Neyrolles, O.; Gicquel, B.; et al. Evolutionary Dynamics of Human Toll-like Receptors and Their Different Contributions to Host Defense. PLoS Genet. 2009, 5, e1000562. [Google Scholar] [CrossRef]
- Yokoyama, N.; Ohta, H.; Kagawa, Y.; Nagata, N.; Nisa, K.; Morita, T.; Osuga, T.; Sasaki, N.; Morishita, K.; Nakamura, K.; et al. Stimulation of Colorectal Biopsies from Miniature Dachshunds with Inflammatory Colorectal Polyps with Toll-like Receptor Ligands: A Pilot Study. Vet. Immunol. Immunopathol. 2017, 188, 78–83. [Google Scholar] [CrossRef]
- Vafaeian, A.; Rajabi, F.; Rezaei, N. Toll-like Receptors in Atopic Dermatitis: Pathogenesis and Therapeutic Implications. Heliyon 2025, 11, e42226. [Google Scholar] [CrossRef]
- Kaesler, S.; Volz, T.; Skabytska, Y.; Köberle, M.; Hein, U.; Chen, K.-M.; Guenova, E.; Wölbing, F.; Röcken, M.; Biedermann, T. Toll-like Receptor 2 Ligands Promote Chronic Atopic Dermatitis through IL-4-Mediated Suppression of IL-10. J. Allergy Clin. Immunol. 2014, 134, 92–99. [Google Scholar] [CrossRef]
- Volz, T.; Kaesler, S.; Draing, C.; Hartung, T.; Röcken, M.; Skabytska, Y.; Biedermann, T. Induction of IL-10-Balanced Immune Profiles Following Exposure to LTA from Staphylococcus Epidermidis. Exp. Dermatol. 2018, 27, 318–326. [Google Scholar] [CrossRef]
- Tyurin, Y.A.; Shamsutdinov, A.F.; Kalinin, N.N.; Sharifullina, A.A.; Reshetnikova, I.D. Association of Toll-Like Cell Receptors TLR2 (p.Arg753GLN) and TLR4 (p.Asp299GLY) Polymorphisms with Indicators of General and Local Immunity in Patients with Atopic Dermatitis. J. Immunol. Res. 2017, 2017, 8493545. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Nejad, P.; Mrabet-Dahbi, S.; Breuer, K.; Klotz, M.; Werfel, T.; Herz, U.; Heeg, K.; Neumaier, M.; Renz, H. The Toll-like Receptor 2 R753Q Polymorphism Defines a Subgroup of Patients with Atopic Dermatitis Having Severe Phenotype. J. Allergy Clin. Immunol. 2004, 113, 565–567. [Google Scholar] [CrossRef]
- Mrabet-Dahbi, S.; Dalpke, A.H.; Niebuhr, M.; Frey, M.; Draing, C.; Brand, S.; Heeg, K.; Werfel, T.; Renz, H. The Toll-like Receptor 2 R753Q Mutation Modifies Cytokine Production and Toll-like Receptor Expression in Atopic Dermatitis. J. Allergy Clin. Immunol. 2008, 121, 1013–1019. [Google Scholar] [CrossRef]
- Niebuhr, M.; Langnickel, J.; Draing, C.; Renz, H.; Kapp, A.; Werfel, T. Dysregulation of Toll-like Receptor-2 (TLR-2)-Induced Effects in Monocytes from Patients with Atopic Dermatitis: Impact of the TLR-2 R753Q Polymorphism. Allergy 2008, 63, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Roduit, C.; Wohlgensinger, J.; Frei, R.; Bitter, S.; Bieli, C.; Loeliger, S.; Büchele, G.; Riedler, J.; Dalphin, J.-C.; Remes, S.; et al. Prenatal Animal Contact and Gene Expression of Innate Immunity Receptors at Birth Are Associated with Atopic Dermatitis. J. Allergy Clin. Immunol. 2011, 127, 179–185. [Google Scholar] [CrossRef]
- Inoue, J.; Aramaki, Y. Suppression of Skin Lesions by Transdermal Application of CpG-Oligodeoxynucleotides in NC/Nga Mice, a Model of Human Atopic Dermatitis. J. Immunol. 2007, 178, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Jassies-van der Lee, A.; Rutten, V.; Spiering, R.; van Kooten, P.; Willemse, T.; Broere, F. The Immunostimulatory Effect of CpG Oligodeoxynucleotides on Peripheral Blood Mononuclear Cells of Healthy Dogs and Dogs with Atopic Dermatitis. Vet. J. 2014, 200, 103–108. [Google Scholar] [CrossRef]
- Wagner, I.; Geh, K.J.; Hubert, M.; Winter, G.; Weber, K.; Classen, J.; Klinger, C.; Mueller, R.S. Preliminary Evaluation of Cytosine-Phosphate-Guanine Oligodeoxynucleotides Bound to Gelatine Nanoparticles as Immunotherapy for Canine Atopic Dermatitis. Vet. Rec. 2017, 181, 118. [Google Scholar] [CrossRef]
- Novak, N.; Yu, C.-F.; Bussmann, C.; Maintz, L.; Peng, W.-M.; Hart, J.; Hagemann, T.; Diaz-Lacava, A.; Baurecht, H.-J.; Klopp, N.; et al. Putative Association of a TLR9 Promoter Polymorphism with Atopic Eczema. Allergy 2007, 62, 766–772. [Google Scholar] [CrossRef]
- Zhou, B.; Liang, S.; Shang, S.; Li, L. Association of TLR2 and TLR9 Gene Polymorphisms with Atopic Dermatitis: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Immunol. Med. 2023, 46, 32–44. [Google Scholar] [CrossRef]
- Nagata, N.; Oshida, T.; Yoshida, N.L.; Yuyama, N.; Sugita, Y.; Tsujimoto, G.; Katsunuma, T.; Akasawa, A.; Saito, H. Analysis of Highly Expressed Genes in Monocytes from Atopic Dermatitis Patients. Int. Arch. Allergy Immunol. 2003, 132, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Santoro, D.; Rodrigues Hoffmann, A. Canine and Human Atopic Dermatitis: Two Faces of the Same Host-Microbe Interaction. J. Investig. Dermatol. 2016, 136, 1087–1089. [Google Scholar] [CrossRef][Green Version]
- Borish, L.C.; Steinke, J.W. 2. Cytokines and Chemokines. J. Allergy Clin. Immunol. 2003, 111, S460–S475. [Google Scholar] [CrossRef]
- Pereira, A.M.; de Pinheiro, C.G.M.; Dos Santos, L.R.; Teixeira, N.C.; Chang, Y.-F.; Pontes-de-Carvalho, L.C.; de Sá Oliveira, G.G. Requirement of Dual Stimulation by Homologous Recombinant IL-2 and Recombinant IL-12 for the in Vitro Production of Interferon Gamma by Canine Peripheral Blood Mononuclear Cells. BMC Res. Notes 2014, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Resende, L.A.; Roatt, B.M.; Aguiar-Soares, R.D.d.O.; Viana, K.F.; Mendonça, L.Z.; Lanna, M.F.; Silveira-Lemos, D.; Corrêa-Oliveira, R.; Martins-Filho, O.A.; Fujiwara, R.T.; et al. Cytokine and Nitric Oxide Patterns in Dogs Immunized with LBSap Vaccine, before and after Experimental Challenge with Leishmania Chagasi plus Saliva of Lutzomyia Longipalpis. Vet. Parasitol. 2013, 198, 371–381. [Google Scholar] [CrossRef][Green Version]
- Álvarez, L.; Marín-García, P.-J.; Rentero-Garrido, P.; Martinez-Jimenez, C.P.; Llobat, L. Interleukin 6 and Interferon Gamma Haplotypes Are Related to Cytokine Serum Levels in Dogs in an Endemic Leishmania Infantum Region. Infect. Dis. Poverty 2023, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-de-Albuquerque, S.d.C.; da Silva, L.G.; de Sousa-Paula, L.C.; Sales, K.G.d.S.; Boegel, A.; Dantas-Torres, F. Exploring IL-17 Gene Promoter Polymorphisms in Canine Leishmaniasis. Acta Trop. 2022, 232, 106452. [Google Scholar] [CrossRef]
- Faria, J.L.M.; Munhoz, T.D.; João, C.F.; Vargas-Hernández, G.; André, M.R.; Pereira, W.A.B.; Machado, R.Z.; Tinucci-Costa, M. Ehrlichia Canis (Jaboticabal Strain) Induces the Expression of TNF-α in Leukocytes and Splenocytes of Experimentally Infected Dogs. Rev. Bras. Parasitol. Vet. 2011, 20, 71–74. [Google Scholar] [CrossRef]
- Hu, W.-C. A Framework of All Discovered Immunological Pathways and Their Roles for Four Specific Types of Pathogens and Hypersensitivities. Front. Immunol. 2020, 11, 1992. [Google Scholar] [CrossRef]
- Jaramillo-Hernández, D.A.; Salazar Garcés, L.F.; Pacheco, L.G.C.; Pinheiro, C.S.; Alcantara-Neves, N.M. Protective Response Mediated by Immunization with Recombinant Proteins in a Murine Model of Toxocariasis and Canine Infection by Toxocara Canis. Vaccine 2022, 40, 912–923. [Google Scholar] [CrossRef]
- Louzada-Flores, V.N.; Latrofa, M.S.; Mendoza-Roldan, J.A.; Lucente, M.S.; Epis, S.; Varotto-Boccazzi, I.; Bandi, C.; Otranto, D. Expression of Key Cytokines in Dog Macrophages Infected by Leishmania Tarentolae Opening New Avenues for the Protection against Leishmania Infantum. Sci. Rep. 2024, 14, 27565. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Rao, D.S.; Baltimore, D. microRNA Regulation of Inflammatory Responses. Annu. Rev. Immunol. 2012, 30, 295–312. [Google Scholar] [CrossRef]
- Rebech, G.T.; Bragato, J.P.; Costa, S.F.; de Freitas, J.H.; Dos Santos, M.O.; Soares, M.F.; Eugênio, F.d.R.; Dos Santos, P.S.P.; de Lima, V.M.F. miR-148a Regulation Interferes in Inflammatory Cytokine and Parasitic Load in Canine Leishmaniasis. PLoS Negl. Trop. Dis. 2023, 17, e0011039. [Google Scholar] [CrossRef]
- Cavalcanti, A.S.; Ribeiro-Alves, M.; Pereira, L.d.O.R.; Mestre, G.L.; Ferreira, A.B.R.; Morgado, F.N.; Boité, M.C.; Cupolillo, E.; Moraes, M.O.; Porrozzi, R. Parasite Load Induces Progressive Spleen Architecture Breakage and Impairs Cytokine mRNA Expression in Leishmania Infantum-Naturally Infected Dogs. PLoS ONE 2015, 10, e0123009. [Google Scholar] [CrossRef]
- Outerbridge, C.A.; Jordan, T.J.M. Current Knowledge on Canine Atopic Dermatitis: Pathogenesis and Treatment. Adv. Small Anim. Care 2021, 2, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Schlotter, Y.M.; Rutten, V.P.M.G.; Riemers, F.M.; Knol, E.F.; Willemse, T. Lesional Skin in Atopic Dogs Shows a Mixed Type-1 and Type-2 Immune Responsiveness. Vet. Immunol. Immunopathol. 2011, 143, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nishikomori, R.; Usui, T.; Wu, C.-Y.; Morinobu, A.; O’Shea, J.J.; Strober, W. Activated STAT4 Has an Essential Role in Th1 Differentiation and Proliferation That Is Independent of Its Role in the Maintenance of IL-12R Beta 2 Chain Expression and Signaling. J. Immunol. 2002, 169, 4388–4398. [Google Scholar] [CrossRef]
- Zhou, M.; Ouyang, W. The Function Role of GATA-3 in Th1 and Th2 Differentiation. Immunol. Res. 2003, 28, 25–37. [Google Scholar] [CrossRef]
- Wu, K.; Bi, Y.; Sun, K.; Wang, C. IL-10-Producing Type 1 Regulatory T Cells and Allergy. Cell Mol. Immunol. 2007, 4, 269–275. [Google Scholar]
- Toichi, E.; Torres, G.; McCormick, T.S.; Chang, T.; Mascelli, M.A.; Kauffman, C.L.; Aria, N.; Gottlieb, A.B.; Everitt, D.E.; Frederick, B.; et al. An Anti-IL-12p40 Antibody down-Regulates Type 1 Cytokines, Chemokines, and IL-12/IL-23 in Psoriasis. J. Immunol. 2006, 177, 4917–4926. [Google Scholar] [CrossRef]
- Yoon, J.-S.; Park, J. Non-Invasive Evaluation of Cytokine Expression Using the Cerumen of Dogs with Otitis Externa. Front. Vet. Sci. 2024, 11, 1355569. [Google Scholar] [CrossRef]
- McGovern, D.P.B.; Kugathasan, S.; Cho, J.H. Genetics of Inflammatory Bowel Diseases. Gastroenterology 2015, 149, 1163–1176.e2. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Heilmann, R.M. Canine Chronic Enteropathy-Current State-of-the-Art and Emerging Concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.; Bernardazzi, C.; de Souza, H.S. Interleukin-33 and Inflammatory Bowel Diseases: Lessons from Human Studies. Mediat. Inflamm. 2014, 2014, 423957. [Google Scholar] [CrossRef]
- Osada, H.; Ogawa, M.; Hasegawa, A.; Nagai, M.; Shirai, J.; Sasaki, K.; Shimoda, M.; Itoh, H.; Kondo, H.; Ohmori, K. Expression of Epithelial Cell-Derived Cytokine Genes in the Duodenal and Colonic Mucosae of Dogs with Chronic Enteropathy. J. Vet. Med. Sci. 2017, 79, 393–397. [Google Scholar] [CrossRef][Green Version]
- Alvarez, F.; Istomine, R.; Shourian, M.; Pavey, N.; Al-Aubodah, T.A.-F.; Qureshi, S.; Fritz, J.H.; Piccirillo, C.A. The Alarmins IL-1 and IL-33 Differentially Regulate the Functional Specialisation of Foxp3+ Regulatory T Cells during Mucosal Inflammation. Mucosal Immunol. 2019, 12, 746–760. [Google Scholar] [CrossRef]
- Pastille, E.; Wasmer, M.-H.; Adamczyk, A.; Vu, V.P.; Mager, L.F.; Phuong, N.N.T.; Palmieri, V.; Simillion, C.; Hansen, W.; Kasper, S.; et al. The IL-33/ST2 Pathway Shapes the Regulatory T Cell Phenotype to Promote Intestinal Cancer. Mucosal Immunol. 2019, 12, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A. Cytokines Induce the Development of Functionally Heterogeneous T Helper Cell Subsets. Immunity 1998, 8, 275–283. [Google Scholar] [CrossRef]
- Albuquerque, C.; Morinha, F.; Requicha, J.; Dias, I.; Guedes-Pinto, H.; Viegas, C.; Bastos, E. A Case-Control Study between Interleukin-10 Gene Variants and Periodontal Disease in Dogs. Gene 2014, 539, 75–81. [Google Scholar] [CrossRef]
- Park, E.-S.; Uchida, K.; Nakayama, H. Th1-, Th2-, and Th17-Related Cytokine and Chemokine Receptor mRNA and Protein Expression in the Brain Tissues, T Cells, and Macrophages of Dogs with Necrotizing and Granulomatous Meningoencephalitis. Vet. Pathol. 2013, 50, 1127–1134. [Google Scholar] [CrossRef]
- Bujak, J.K.; Szopa, I.M.; Pingwara, R.; Kruczyk, O.; Krzemińska, N.; Mucha, J.; Majchrzak-Kuligowska, K. The Expression of Selected Factors Related to T Lymphocyte Activity in Canine Mammary Tumors. Int. J. Mol. Sci. 2020, 21, 2292. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Tseng, S.; Horner, R.M.; Tam, C.; Loda, M.; Rollins, B.J. Control of TH2 Polarization by the Chemokine Monocyte Chemoattractant Protein-1. Nature 2000, 404, 407–411. [Google Scholar] [CrossRef]
- Kaim, U.; Moritz, A.; Failing, K.; Baumgärtner, W. The Regression of a Canine Langerhans Cell Tumour Is Associated with Increased Expression of IL-2, TNF-Alpha, IFN-Gamma and iNOS mRNA. Immunology 2006, 118, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. A Review of Immunologic Diseases of the Dog. Vet. Immunol. Immunopathol. 1999, 69, 251–342. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.J.; Wotton, P.; Eastwood, J.; Swift, S.T.; Jones, B.; Day, M.J. Immunoglobulin Deficiency in Cavalier King Charles Spaniels with Pneumocystis Pneumonia. J. Vet. Intern. Medicne 2006, 20, 523–527. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Huson, H.J.; Leonard, J.; Angles, J.M.; Fox, L.E.; Wojciechowski, J.W.; Yuncker, C.; Happ, G.M. Association of Hypothyroid Disease in Doberman Pinscher Dogs with a Rare Major Histocompatibility Complex DLA Class II Haplotype. Tissue Antigens 2006, 67, 53–56. [Google Scholar] [CrossRef]
- Wilbe, M.; Sundberg, K.; Hansen, I.R.; Strandberg, E.; Nachreiner, R.F.; Hedhammar, A.; Kennedy, L.J.; Andersson, G.; Björnerfeldt, S. Increased Genetic Risk or Protection for Canine Autoimmune Lymphocytic Thyroiditis in Giant Schnauzers Depends on DLA Class II Genotype. Tissue Antigens 2010, 75, 712–719. [Google Scholar] [CrossRef]
- Short, A.D.; Catchpole, B.; Kennedy, L.J.; Barnes, A.; Lee, A.C.; Jones, C.A.; Fretwell, N.; Ollier, W.E.R. T Cell Cytokine Gene Polymorphisms in Canine Diabetes Mellitus. Vet. Immunol. Immunopathol. 2009, 128, 137–146. [Google Scholar] [CrossRef]
- Wilbe, M.; Andersson, G. MHC Class II Is an Important Genetic Risk Factor for Canine Systemic Lupus Erythematosus (SLE)-Related Disease: Implications for Reproductive Success. Reprod. Domest. Anim. 2012, 47 (Suppl. 1), 27–30. [Google Scholar] [CrossRef]
- Siering, O.; Langbein, M.; Herrmann, M.; Wittwer, K.; von Messling, V.; Sawatsky, B.; Pfaller, C.K. Genetic Diversity Accelerates Canine Distemper Virus Adaptation to Ferrets. J. Virol. 2024, 98, e0065724. [Google Scholar] [CrossRef]
- Olsson, M.; Frankowiack, M.; Tengvall, K.; Roosje, P.; Fall, T.; Ivansson, E.; Bergvall, K.; Hansson-Hamlin, H.; Sundberg, K.; Hedhammar, Å.; et al. The Dog as a Genetic Model for Immunoglobulin A (IgA) Deficiency: Identification of Several Breeds with Low Serum IgA Concentrations. Vet. Immunol. Immunopathol. 2014, 160, 255–259. [Google Scholar] [CrossRef]
- Angles, J.M.; Famula, T.R.; Pedersen, N.C. Uveodermatologic (VKH-like) Syndrome in American Akita Dogs Is Associated with an Increased Frequency of DQA1*00201. Tissue Antigens 2005, 66, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Perryman, L.E. Molecular Pathology of Severe Combined Immunodeficiency in Mice, Horses, and Dogs. Vet. Pathol. 2004, 41, 95–100. [Google Scholar] [CrossRef]
- Röthig, A.; Rüfenacht, S.; Welle, M.M.; Thom, N. Dermatomyositis in a Family of Working Kelpies. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 2015, 43, 331–336. [Google Scholar] [CrossRef]
- Evans, J.M.; Noorai, R.E.; Tsai, K.L.; Starr-Moss, A.N.; Hill, C.M.; Anderson, K.J.; Famula, T.R.; Clark, L.A. Beyond the MHC: A Canine Model of Dermatomyositis Shows a Complex Pattern of Genetic Risk Involving Novel Loci. PLoS Genet. 2017, 13, e1006604. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Jones, P.A.; Takai, D. The Role of DNA Methylation in Mammalian Epigenetics. Science 2001, 293, 1068–1070. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef]
- Montaner-Angoiti, E.; Marín-García, P.J.; Llobat, L. Epigenetic Alterations in Canine Malignant Lymphoma: Future and Clinical Outcomes. Animals 2023, 13, 468. [Google Scholar] [CrossRef]
- Basso, K.; Sumazin, P.; Morozov, P.; Schneider, C.; Maute, R.L.; Kitagawa, Y.; Mandelbaum, J.; Haddad, J.; Chen, C.-Z.; Califano, A.; et al. Identification of the Human Mature B Cell miRNome. Immunity 2009, 30, 744–752. [Google Scholar] [CrossRef]
- Liu, G.; Abraham, E. MicroRNAs in Immune Response and Macrophage Polarization. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Cañavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine Leishmaniasis. In Advances in Parasitology; Academic Press: Cambridge, MA, USA, 2004; Volume 57, pp. 1–88. [Google Scholar]
- Barbiéri, C.L. Immunology of Canine Leishmaniasis. Parasite Immunol. 2006, 28, 329–337. [Google Scholar] [CrossRef]
- Soares, M.F.; Melo, L.M.; Bragato, J.P.; Furlan, A.d.O.; Scaramele, N.F.; Lopes, F.L.; Lima, V.M.F. de Differential Expression of miRNAs in Canine Peripheral Blood Mononuclear Cells (PBMC) Exposed to Leishmania Infantum in Vitro. Res. Vet. Sci. 2021, 134, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Bragato, J.P.; Rebech, G.T.; de Freitas, J.H.; Santos, M.O.D.; Costa, S.F.; Eugênio, F.d.R.; Santos, P.S.P.D.; de Lima, V.M.F. miRNA-21 Regulates CD69 and IL-10 Expression in Canine Leishmaniasis. PLoS ONE 2022, 17, e0265192. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.M.; Bragato, J.P.; Venturin, G.L.; Rebech, G.T.; Costa, S.F.; Garcia, L.E.; Lopes, F.L.; Eugênio, F.d.R.; Patto dos Santos, P.S.; de Lima, V.M.F. Induction of miR 21 Impairs the Anti-Leishmania Response through Inhibition of IL-12 in Canine Splenic Leukocytes. PLoS ONE 2019, 14, e0226192. [Google Scholar] [CrossRef]
- Zou, Y.; Zheng, W.-B.; He, J.-J.; Elsheikha, H.M.; Zhu, X.-Q.; Lu, Y.-X. Toxocara Canis Differentially Affects Hepatic MicroRNA Expression in Beagle Dogs at Different Stages of Infection. Front. Vet. Sci. 2020, 7, 587273. [Google Scholar] [CrossRef]
- Zheng, Y.; Fu, X.; Wang, L.; Zhang, W.; Zhou, P.; Zhang, X.; Zeng, W.; Chen, J.; Cao, Z.; Jia, K.; et al. Comparative Analysis of MicroRNA Expression in Dog Lungs Infected with the H3N2 and H5N1 Canine Influenza Viruses. Microb. Pathog. 2018, 121, 252–261. [Google Scholar] [CrossRef]
- Xu, C.; He, X.; Zheng, Z.; Zhang, Z.; Wei, C.; Guan, K.; Hou, L.; Zhang, B.; Zhu, L.; Cao, Y.; et al. Downregulation of microRNA miR-526a by Enterovirus Inhibits RIG-I-Dependent Innate Immune Response. J. Virol. 2014, 88, 11356–11368. [Google Scholar] [CrossRef]
- Ben Hamouda, M.; Pearson, A. Small RNA Sequencing Analysis Reveals Regulation of microRNA Expression in Madin-Darby Canine Kidney Epithelial Cells Infected with Canid Alphaherpesvirus 1. Virus Genes. 2024, 60, 537–548. [Google Scholar] [CrossRef]
- Holder, A.; Jones, G.; Soutter, F.; Palmer, D.B.; Aspinall, R.; Catchpole, B. Polymorphisms in the Canine IL7R 3′UTR Are Associated with Thymic Output in Labrador Retriever Dogs and Influence Post-Transcriptional Regulation by microRNA 185. Dev. Comp. Immunol. 2018, 81, 244–251. [Google Scholar] [CrossRef]
- Vasconcelos, C.R.S.; de Almeida, M.B.; de Oliveira, C.P.; Silva, J.L.; Dias, F.G.G.; Rodrigues, M.A. Nuclear Morphology, Chromatin Compaction, and Epigenetic Changes in Lymphocytes of Dogs Infected with Ehrlichia Canis. Vet. Parasitol. 2025, 334, 110385. [Google Scholar] [CrossRef] [PubMed]
- Menachery, V.D.; Schäfer, A.; Burnum-Johnson, K.E.; Mitchell, H.D.; Eisfeld, A.J.; Walters, K.B.; Nicora, C.D.; Purvine, S.O.; Casey, C.P.; Monroe, M.E.; et al. MERS-CoV and H5N1 Influenza Virus Antagonize Antigen Presentation by Altering the Epigenetic Landscape. Proc. Natl. Acad. Sci. USA 2018, 115, E1012–E1021. [Google Scholar] [CrossRef]
- Liu, S.; Liu, L.; Xu, G.; Cao, Z.; Wang, Q.; Li, S.; Peng, N.; Yin, J.; Yu, H.; Li, M.; et al. Epigenetic Modification Is Regulated by the Interaction of Influenza A Virus Nonstructural Protein 1 with the De Novo DNA Methyltransferase DNMT3B and Subsequent Transport to the Cytoplasm for K48-Linked Polyubiquitination. J. Virol. 2019, 93, e01587-18. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, T.; Feng, N.; Meng, X.; Sun, W.; Wang, T.; Zhao, Y.; Yang, S.; Song, X.; Li, W.; et al. A Highly Efficient Recombinant Canarypox Virus-Based Vaccine against Canine Distemper Virus Constructed Using the CRISPR/Cas9 Gene Editing Method. Vet. Microbiol. 2020, 251, 108920. [Google Scholar] [CrossRef]
- Rendon-Marin, S.; Rincón-Tabares, D.-S.; Tabares-Guevara, J.H.; Arbeláez, N.; Forero-Duarte, J.E.; Díaz, F.J.; Robledo, S.M.; Hernandez, J.C.; Ruiz-Saenz, J. Evaluation of the Safety and Immunogenicity of a Multiple Epitope Polypeptide from Canine Distemper Virus (CDV) in Mice. Vaccines 2024, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Kocatürk, M.; Öz, A.D.; Muñoz, A.; Martinez, J.D.; Ceron, J.J.; Yilmaz, Z. Changes in Immuno-Inflammatory and Antioxidant Biomarkers in Serum and Cerebrospinal Fluid of Dogs with Distemper. Microb. Pathog. 2025, 198, 107160. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Watanabe-Takahashi, M.; Nishimura, H.; Omi, J.; Tamada, M.; Saitoh, T.; Maenaka, K.; Okuda, Y.; Ikegami, A.; Kitagawa, A.; et al. CaMKII-Dependent Non-Canonical RIG-I Pathway Promotes Influenza Virus Propagation in the Acute-Phase of Infection. mBio 2025, 16, e00087-24. [Google Scholar] [CrossRef] [PubMed]
- Ariyarathna, H.; Thomson, N.A.; Aberdein, D.; Perrott, M.R.; Munday, J.S. Increased Programmed Death Ligand (PD-L1) and Cytotoxic T-Lymphocyte Antigen-4 (CTLA-4) Expression Is Associated with Metastasis and Poor Prognosis in Malignant Canine Mammary Gland Tumours. Vet. Immunol. Immunopathol. 2020, 230, 110142. [Google Scholar] [CrossRef]
- Giuliano, A.; Pimentel, P.A.B.; Horta, R.S. Checkpoint Inhibitors in Dogs: Are We There Yet? Cancers 2024, 16, 2003. [Google Scholar] [CrossRef]
- Lenz, J.A.; Peng, B.; Assenmacher, C.; King, A.; Zhang, P.J.; Maki, R.G.; Blanco, M.A.; Radaelli, E.; Atherton, M.J. Identification of Immune Suppressor Candidates Utilizing Comparative Transcriptional Profiling in Histiocytic Sarcoma. Cancer Immunol. Immunother. 2025, 74, 61. [Google Scholar] [CrossRef]
- Ammons, D.T.; Hopkins, L.S.; Cronise, K.E.; Kurihara, J.; Regan, D.P.; Dow, S. Single-Cell RNA Sequencing Reveals the Cellular and Molecular Heterogeneity of Treatment-Naïve Primary Osteosarcoma in Dogs. Commun. Biol. 2024, 7, 496. [Google Scholar] [CrossRef] [PubMed]
- Mucignat, G.; Montanucci, L.; Elgendy, R.; Giantin, M.; Laganga, P.; Pauletto, M.; Mutinelli, F.; Vascellari, M.; Leone, V.; Dacasto, M.; et al. A Whole-Transcriptomic Analysis of Canine Oral Melanoma: A Chance to Disclose the Radiotherapy Effect and Outcome-Associated Gene Signature. Genes. 2024, 15, 1065. [Google Scholar] [CrossRef] [PubMed]

| Diseases | Breed | Resistance/Susceptibility | References |
|---|---|---|---|
| Pneumocystis pneumonia infection | Cavalier King Charles Spaniels | Susceptibility due to immunoglobulin deficiencies | [103] |
| Chronic infections | Beagle | Susceptibility due to IgA deficiency | [102] |
| Hypothyroid disorders | Doberman Pinschers | Predisposition | [104] |
| Autoimmune lymphocytic thyroiditis | Giant Schnauzers | Predisposition | [105] |
| Diabetes mellitus | Collie, Cairn Terrier, Schnauzer, Cavalier King Charles Spaniel | Susceptibility associated with SNPs in cytokine genes | [106] |
| Lupus-like autoimmune disease | Nova Scotia Duck Tolling Retriever | Susceptibility related to haplotypes in DLA genes | [107] |
| Systemic lupus erythematosus | Nova Scotia Duck Tolling Retriever | Predisposition related to IgA deficiency | [110] |
| Uveodermatologic syndrome | American Akita | Risk due to allele of DLA | [111] |
| Severe combined immunodeficiency | Jack Russell Terriers | Predisposition due to SNP in kinase catalytic subunit | [112] |
| Severe combined immunodeficiency | Cardigan Welsh Corgis and Basset Hounds | Predisposition due to SNPs in gene-encoding interleukin receptors | [112] |
| Dermatomyositis | Collie, Shetland Sheepdog, and Beauceron | Susceptibility due to SNPs in different genes | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barragán-Sánchez, P.; Balastegui, M.T.; Marín-García, P.J.; Llobat, L. Genetic Regulation of Immune Response in Dogs. Genes 2025, 16, 764. https://doi.org/10.3390/genes16070764
Barragán-Sánchez P, Balastegui MT, Marín-García PJ, Llobat L. Genetic Regulation of Immune Response in Dogs. Genes. 2025; 16(7):764. https://doi.org/10.3390/genes16070764
Chicago/Turabian StyleBarragán-Sánchez, Pablo, María Teresa Balastegui, Pablo Jesús Marín-García, and Lola Llobat. 2025. "Genetic Regulation of Immune Response in Dogs" Genes 16, no. 7: 764. https://doi.org/10.3390/genes16070764
APA StyleBarragán-Sánchez, P., Balastegui, M. T., Marín-García, P. J., & Llobat, L. (2025). Genetic Regulation of Immune Response in Dogs. Genes, 16(7), 764. https://doi.org/10.3390/genes16070764







