Abstract
Background/Objectives: Endplate lesions of the lumbar spine are often asymptomatic and frequently observed incidentally by radiological assessment. Variants in the vitamin D receptor gene (VDR) and an increase in some biochemical markers related to the osteo-cartilaginous metabolism were found in patients with endplate lesions. The aim of this study was to identify biochemical and genetic markers putatively associated with the presence of endplate lesions of the lumbar spine. Methods: Quantification of circulating bone remodeling proteins was obtained from 10 patients with endplate lesions and compared with age- and sex-matched controls. Whole exome sequencing (WES) was performed on patient genomic DNA using the Novaseq 6000 platform (Illumina, San Diego, CA, USA), obtaining a median read depth of 117×–200×, with ≥98% of regions covering at least 20×. The sequencing product was aligned to the reference genome (GRCh38.p13-hg38) and analyzed with Geneyx software. Results: We observed modifications in the levels of circulating proteins involved in bone remodeling and angiogenesis. We identified variants of interest in aggrecan (ACAN), bone morphogenetic protein 4 (BMP4), cytochrome P450 family 3 subfamily A member 4 (CYP3A4), GLI family zinc finger 2 (GLI2), heparan sulfate proteoglycan 2 (HSPG2), and mesoderm posterior bHLH transcription factor 2 (MESP2). VDR polymorphism (rs2228570) was present in nine patients, with the homozygotic ones having more severe endplate lesions and higher levels of the analyzed circulating markers in comparison with heterozygotic patients. Conclusions: These data represent interesting evidence of genetic variants, particularly in VDR, and altered levels of circulating markers of bone remodeling associated with endplate lesions, which should be confirmed in a larger population. The hypothesis suggested by our results is that the endplate lesions could be the consequence of an altered ossification mechanism at the vertebral level.
1. Introduction
Familial Scheuermann’s disease or spinal osteochondrosis is described in the rare disease portal Orphanet with the ORPHA code 3135. The clinical/radiological features of this pathology include irregularities of the vertebral endplates, subchondral sclerosis, presence of Schmorl’s nodes (upper- or lower-disc herniation into the spongious bone of the vertebral body), disc-space narrowing and vertebral wedging [1,2]. This disorder is not considered generalized per se, as the typical lesions of osteochondrosis are focal in nature. However, osteochondrosis may occur in multifocal locations in the same individual, with lesions often being bilaterally symmetrical [3].
Vertebral osteochondrosis involves defects in the cartilage endplate of vertebrae, which is devoted to the vertical growth of the vertebral body. Morphological studies of spinal osteochondrosis showed the presence of sparse disorganized fibrils in the cartilage matrix, which are likely associated with disturbed collagen synthesis and an abnormal collagen/proteoglycan ratio [2,4].
The etiology of osteochondrosis is likely multifactorial [5]. Evidence highlights the involvement of an impaired blood supply causing oxygen and nutrition insufficiency at the vertebral ossification nuclei and consequent cell necrosis, but mechanical damage/repeated microtrauma can also contribute [6].
An autosomal dominant mode of inheritance [7], with high prevalence in male monozygotic twins [8,9], supports the hypothesis of genetic etiology.
In some cases, especially when the pathology is present at the lumbar level, spine osteochondrosis is asymptomatic, and the related low back pain (LBP) appears only in adulthood [6,10].
We supposed that the degree of the lesion, from low to high, is a progressive or continuous process [11]. Recently, a study on 750 healthy Chinese subjects aged 20–60 has supported that the occurrence and development of endplate lesions is a cumulative process, showing that people with higher grades of lesions are older in both sexes and that the majority of endplate lesions are asymptomatic and frequently observed incidentally by radiological assessment [12].
An Italian cohort study performed on adult patients with LBP found that 26.8% of male patients and 8.5% of female patients had spine osteochondrosis [13,14]. Studies of the same group of researchers were dedicated to the development of a score based on the imaging features of vertebral osteochondrosis [11] and to the identification of peculiar biochemical and genetic markers putatively associated with the severity of the pathology [13,14,15,16,17]. In these studies, vertebral osteochondrosis showed peculiar genotypic and biochemical features related to vitamin D and osteo-cartilaginous metabolism. It was observed that being a male, having a high BMI and bearing specific genotypes of the vitamin D receptor gene (VDR) may represent risk factors for the development of higher magnetic resonance imaging (MRI) scores of endplate lesions, and that higher total MRI score directly correlates with higher levels of markers of type I and II collagen degradation [15,17]. On the contrary, a direct association between the severity of the score with more serious injuries and LBP was not found. These aspects suggest that osteochondrosis is an insidious pathology, asymptomatic at the beginning and for a long time, but provoking progressive structural damage of vertebral endplates and lasting in painful degeneration of the vertebral units.
The endplates involved in this pathology represent peculiar structures, essential to provide nutrients from the bloodstream to the avascular disc and for the molecular connection between discs and vertebrae [12].
Since osteochondrosis is acknowledged as a condition presenting degenerative-necrotic alteration of the epiphyseal and apophyseal growing ossification nuclei, the understanding of the biological mechanisms of the endochondral ossification is essential to identify the possible causes of endplate damages. In fact, the lesions related to osteochondrosis involve both the bony and cartilaginous tissues and are likely the consequence of an altered ossification mechanism at the vertebral level during skeletal growth, consisting of the replacing of the growth cartilage by bone through a sequential process of cell proliferation, extracellular matrix synthesis, cellular hypertrophy, matrix mineralization, and vascular invasion [3].
Another possible biological mechanism underlying the development of endplate lesions is strictly connected with disc degeneration. The pro-inflammatory cytokines secreted in degenerated disc tissues are known to promote osteo-progenitor differentiation and angiogenesis, which eventually activate ectopic ossification [18] with loss of joint space, subchondral sclerosis and the formation of osteophytes.
On the basis of these considerations, the aim of the present study is to identify peculiar imaging and biochemical and genetic markers putatively associated with the presence of endplate lesions of the lumbar spine.
2. Materials and Methods
2.1. Enrolment of Patients and Sample Collection
The study was approved by the local ethics committee (San Raffaele Hospital, Milan, Italy—approval number 150/int/2019). Patients were enrolled at IRCCS Istituto Ortopedico Galeazzi (Milan, Italy) after signing an informed consent form. The inclusion criteria were as follows: age over 18 years old, no previous spine surgeries, no previous diagnosis of scoliosis or other spine diseases, absence of pregnancy and absence of obesity (BMI < 30 kg/m2).
From the enrolled patients, 10 were selected (5 females and 5 males; age 48.9 ± 13.5; BMI 23.2 ± 2.5 kg/m2) for the presence of severe endplate lesions. From these patients, whole blood was obtained and used for peripheral blood mononuclear cell (PBMC) isolation and serum collection.
Data concerning circulating bone remodeling markers in 10 matched controls (5 females and 5 males, 49.6 ± 8.6; BMI 23.2 ± 1.4 kg/m2) were already published by our group [19] and used as a reference control in this study.
2.2. Patient MRI Evaluation
T2-weighted sagittal MRI scans of the thoracolumbar spine were acquired using a 1.5T MR system. All images were evaluated by a single experienced spine surgeon. Endplate lesions at intervertebral levels from T12L1 to L5S1 were assessed according to a previously validated shape-based classification scheme, which demonstrated acceptable inter- and intra-observer reliability [11]. The scoring system ranges from 0 to 3, reflecting the increasing severity of endplate lesions, and is defined as follows (see an illustrative example from a single subject at the mid-slice image in Figure 1):
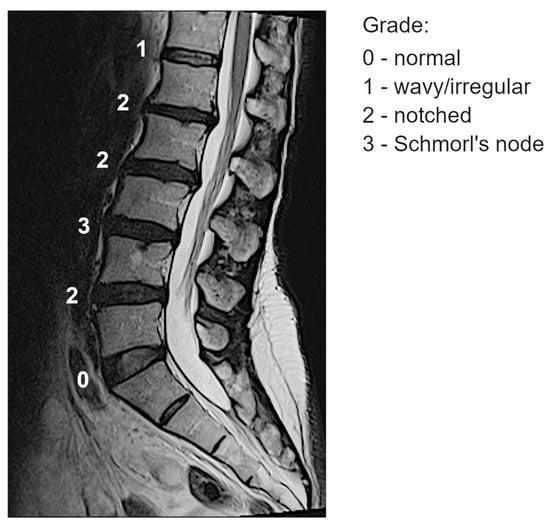
Figure 1.
Example of classification of the endplate lesions at spinal levels from one subject at the mid-slice image.
- -
- Grade 0 (“normal”): No lesions are visually identified in the sagittal MRI slices encompassing the intervertebral space.
- -
- Grade 1 (“wavy/irregular”): No specific lesions are detectable in the intervertebral space, but at least one endplate exhibits an altered shape compared to the typical curvature of a healthy intervertebral space. The endplate may appear wavy or irregular.
- -
- Grade 2 (“notched”): A small lesion is visible in at least one sagittal MRI slice. The lesion has a V-shaped or circular appearance and is present on one or both endplates, suggesting small defects or indentations.
- -
- Grade 3 (“Schmorl’s node”): A deep focal defect is observed in the vertebral endplate, characterized by a smooth margin and rounded appearance. Schmorl’s nodes involve disc tissue protruding through the endplate into the vertebral marrow.
The representative score at each spinal level was determined by identifying the highest severity grade observed across the image slices (e.g., if grades 0, 2, and 3 were present, the assigned score for that level was 3).
2.3. Protein Array of Bone Remodeling Markers
Custom Human Quantibody® Array was used to determine the concentration in serum of nine circulating bone remodeling factors (RayBiotech, Norcross, GA, USA): bone morphogenic proteins (BMPs) -2, -5, -7, -9, Dickkopf-1 (Dkk-1), matrix metalloprotease (MMP)-3, platelet-derived growth factor (PDGF)-BB, transforming growth factor (TGF)β3 and tumor necrosis factor-related activation-induced cytokine (TRANCE).
2.4. Whole Exome Sequencing (WES)
Genomic DNA was extracted from PBMCs according to the procedure of the DNeasy Midi kit (Qiagen, Duesseldorf, Germany). WES was performed on genomic DNA using the Novaseq 6000 platform (Illumina), obtaining a median read depth between 117× and 200× with regions covered at least 20× > 98%. The sequencing product was aligned to the reference genome (GRCh38.p13-hg38), and analysis and interpretation were carried out using Geneyx software v 5.15 [20]. We identified variants of interest both by analyzing total WES data and by filtering the WES data with a dedicated gene panel and the following human phenotype ontology clinical signatures: abnormality of the vertebral endplates, abnormal form of the vertebral bodies, abnormal vertebral morphology, abnormal intervertebral disk morphology, and morbus Scheuermann.
2.5. Statistical Analysis
GraphPad Prism v.8.0.2 (GraphPad Software) was used for statistical analysis. Protein levels are expressed as the mean ± SD. The normal distribution of values was assayed by the Kolmogorov–Smirnov normality test. Unpaired comparisons between patients and controls were performed by using a two-tailed t-test. In the case of non-normally distributed values, the Mann–Whitney test was used. We set significance levels at p ≤ 0.05.
3. Results
3.1. Characteristics of the Enrolled Patients
All the enrolled patients were Caucasian, 3 out of 10 were overweight (BMI ≥ 25 kg/m²), and the other 7 patients were normal weight. One patient had a hyperthyroid, one had Hashimoto’s thyroiditis and one was hypertensive. All but one of the enrolled patients reported a family history of back pain. Regarding lifestyle, four patients were ex-smokers, two were non-smokers and four were smokers (one heavy). Half of the patients were doing sedentary work, while the other half were doing medium to heavy work. Furthermore, 3 of 10 patients reported vibration exposure for more than 2 h per day. Seven patients reported minimal disability, one moderate and two severe, inflicted by back pain.
3.2. Endplate Lesions in Patients Through MRI Score
Lesions were present at all spinal levels in almost all patients (Table 1).

Table 1.
Classification of endplate lesion types along spinal levels in evaluated subjects: grade 0 (normal), 1 (wavy/irregular), 2 (notched), and 3 (Schmorl’s node).
Among the 60 evaluated intervertebral levels, 2 (3%) were classified as grade 0 (normal), 25 (42%) as grade 1 (wavy/irregular), 25 (42%) as grade 2 (notched), and 8 (13%) as grade 3 (Schmorl’s node), with the latter present in half of the subjects. Overall, wavy/irregular and notched lesions were observed at both upper and lower spinal levels, showing no clear association with either cranial or caudal intervertebral discs in the lumbar region. However, Schmorl’s nodes, the most severe lesion type, were absent in the most cranial upper levels from T12L1 to L1L2.
3.3. Circulating Marker Levels
We observed a significant increase in patients versus controls of the bone degradation marker Dkk-1 (p = 0.005, with 4/10 values in patients below the limit of detection vs. 9/10 values in controls), of the angiogenic marker PDGF-BB (p = 0.01) and of BMP-5 (p = 0.01). Data concerning all the evaluated markers are shown in Table 2.

Table 2.
Circulating levels of bone remodeling markers.
3.4. Genetic of the Endplate Lesions
WES analysis allowed us to identify variants in the 10 selected patients. In 9 out of 10 patients, a VDR polymorphism (rs2228570) was observed as present in homozygosis (three patients) and heterozygosis (six patients). Furthermore, we observed different variants: three in aggrecan (ACAN), four in heparan sulfate proteoglycan 2 (HSPG2) (two in the same patient), two in bone morphogenetic protein 4 (BMP)4 and three in GLI family zinc finger 2 (GLI2) (two in the same patient). Interestingly, one variant of mesoderm posterior bHLH transcription factor 2 (MESP2) was found in three patients, and one variant of cytochrome P450 family 3 subfamily A member 4 (CYP3A4) was found in two patients (Table 3).

Table 3.
Relevant variants identified through WES analysis.
Finally, other variants were found represented in only one patient (Supplementary Table S1) and can be grouped on the basis of the biological function of the genes. They comprise genes involved in signaling pathways such as nuclear factor kappa B subunit 1 (NFKB1) regulator of the NFkB complex; natriuretic peptide receptor 2 (NPR2), ETS variant transcription factor 2 (ETV2) and RUNX family transcription factor 1 (RUNX1), regulators of transcription; LDL receptor-related protein 4 (LRP4) involved in Wnt signaling and SMAD family member 3 (SMAD3) involved in TGFβ signaling. Other variants were found in genes related to the cellular cytoskeleton, such as filamin A (FLNA), myosin heavy chain 11 (MYH11), myosin light chain kinase (MYLK), nebulin (NEB) and thrombospondin type 1 domain containing 4 (THSD4). Finally, a group of variants were found in genes involved in the synthesis and remodeling of extracellular matrix components such as collagens and proteoglycans: collagen type XI α 1 chain (COL11A1), collagen type I α 1 chain (COL1A1), fibrillin 1 (FBN1), fibronectin 1 (FN1), galactosamine (N-acetyl)-6-sulfatase (GALNS), matrilin 3 (MATN3), matrix metalloprotease (MMP2), prolyl 3-hydroxylase 1 (P3H1), solute carrier family 26 member 2 (SLC26A2) and thrombospondin type 1 domain containing 4 (THBS2).
3.5. MRI Score and Circulating Marker Levels in Patients with VDR Variant rs2228570
The MRI score and the circulating marker levels of the cohort of nine patients presenting VDR variant rs2228570 were re-analyzed considering the presence of this variant in homozygosis (n = 3) or heterozygosis (n = 6). The data reported in Table 4 show that the homozygotic patients have a higher total MRI score for endplate lesions (p = 0.02) and higher levels of all the analyzed circulating markers, with significantly higher levels of BMP-5 (p = 0.05) in comparison with heterozygotic patients.

Table 4.
MRI score and circulating marker levels in patients with VDR variant rs2228570 in homozygotis (Hom; n = 3) and heterozygotis (Het; n = 6) patients.
Figure 2 summarizes the main findings of the study.
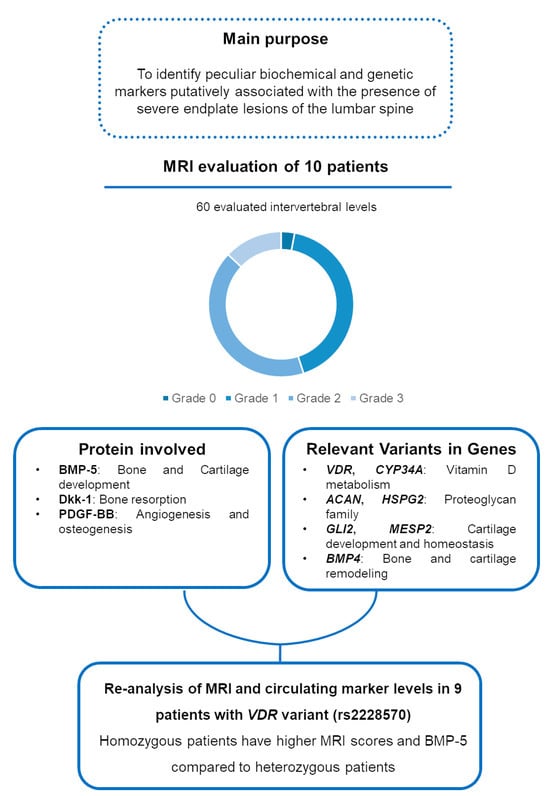
Figure 2.
Visual diagram summarizing the main purpose and findings of the study.
4. Discussion
The main findings of the present study are that the genetic variants identified in patients with endplate lesions support a multifactorial hypothesis for the pathogenesis of this condition and for intervertebral disc degeneration, with implications in the development, homeostasis, and metabolism of bone and cartilage.
The three proteins identified as associated with severe endplate lesions are involved in bone and cartilage development (BMP-5), in bone resorption (Dkk-1) and in angiogenesis (PDGF-BB). This is particularly relevant since the endplate defects observed in patients with osteochondrosis likely result from an altered ossification mechanism.
In particular, BMP-5 is a secreted ligand of the TGFβ superfamily of proteins that, through the activation of SMAD family transcription factors, plays a role in bone and cartilage development [21,22,23]. Another protein involved in bone remodeling is Dkk-1. It functions as an endogenous suppressor of the canonical Wnt signaling pathway [24]. Both preclinical and clinical data were suggestive that high DKK1 expression can impair osteoblast activity and cause bone loss [25].
Finally, PDGF-BB/platelet-derived growth factor receptor β signaling has a well-established role in blood vessel formation [26,27]. It correlates with the stabilization of newly formed vessels, the orchestration of cellular components for osteogenesis, and an increase in vascularity [27,28,29]. Excessive secretion of PDGF-BB from pre-osteoclasts promoted aberrant angiogenesis-dependent bone formation in subchondral bone [30,31].
In the present work, the WES analysis showed some variants from a panel of genes involved in vitamin D metabolism, osteo-cartilaginous extracellular matrix formation, cartilage development and homeostasis and endochondral ossification signaling. In a previously published paper, we observed an association between the VDR polymorphism rs2228570, previously named FokI, and discopathies in general and/or osteochondrosis concomitant with disc herniation [23]. In particular, the F allele and FF genotype were observed as risk factors for these conditions. In the present research, we found that this VDR polymorphism was present in the majority of patients (9 out of 10), and this SNP was present in homozygosis in three patients and in heterozygosis in six patients, confirming the association of this polymorphism with the presence of severe endplate defects. Interestingly, the homozygous patients showed higher MRI scores for endplate lesions and higher circulating BMP-5 levels than heterozygotes, strengthening the hypothesis that this variant is associated with the presence of more severe pathology with osteo-cartilaginous involvement. In the present work, we found two patients with a variant in CYP3A4, a gene potentially involved in vitamin D metabolism [32,33].
Three different variants were found in ACAN, encoding for a member of the aggrecan/versican proteoglycan family and four variants in HSPG2, and both are members of the proteoglycan family. In 3 out of 10 patients, we observed variants in these genes previously associated with spinal degeneration [34,35,36,37]. In the other two and three patients, we observed variants in GLI2 and MESP2, respectively, encoding proteins involved in SHH and Notch signaling, and these genes participate in joint cartilage development and homeostasis [38,39,40,41].
In two patients, we observed a variant in BMP4, and the protein encoded by this gene acts through the activation of the same signaling pathway as BMP-5 and is involved in the remodeling of bone and cartilage [42].
Among the variants identified in each patient are those in COL11A1 and THBS2, and these genes were already identified as potentially associated with disc-related disorders [34]. The other genes in which we observed variants in at least one patient such as COL1A1, MATN3, GALNS, MMP2, P3H1 and SLC2GA2 are involved in extracellular matrix remodeling and in the development and homeostasis of cartilage and bone and in collagen and proteoglycan synthesis and assembly as cartilaginous matrix components.
MRI evaluation of the enrolled patients identified the presence of endplate lesions in all subjects, with the presence of Schmorl’s nodes, the most severe types of lesions, in half of the subjects. The questionnaires allowed the collection of clinical and lifestyle-related characteristics of the enrolled patients, highlighting the presence of back pain-related disability in all subjects.
The main limitation of this study lies in the small sample size, which constrains the ability to correlate resonance scores and patient characteristics with biochemical and genetic data. Functional studies involving a larger patient cohort are necessary to better elucidate the genetic underpinnings of the disease. Additionally, the cross-sectional design of the data should be taken into account when planning future research, especially considering the potential progression of endplate lesions over time.
The strength of the study resides in the integrative approach, combining genetic analysis with biochemical and imaging evaluation to uncover a strong genotype–phenotype link. By identifying key genetic variants—particularly in the VDR gene—and associating them with MRI-detected endplate lesions and biochemical markers like BMP-5, the research supports a pathogenesis involving bone and cartilage metabolism.
5. Conclusions
Although preliminary, these findings provide compelling evidence of genetic variants—particularly in VDR—and altered circulating levels of cartilage and bone remodeling markers associated with endplate lesions. These results warrant confirmation in a larger patient cohort, as the endplate alterations are likely the result of disrupted ossification processes at the vertebral level.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes16070738/s1, Table S1: Variants found represented in only one patient.
Author Contributions
Conceptualization, A.C.; methodology, A.C., V.R., A.E.C., T.B. and L.P.; formal analysis, A.C., A.E.C., T.B. and L.P.; resources, A.C.; data curation, A.C., A.E.C., T.B. and L.P.; writing—original draft preparation, A.C., A.E.C. and T.B.; writing—review and editing, D.C., S.C. and M.B.-B.; project administration, A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported and funded by the Italian Ministry of Health—“Ricerca Corrente”. The APC was funded by the Italian Ministry of Health—“Ricerca Corrente”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local Ethics Committee (San Raffaele Hospital, Milan, Italy—approval number 150/int/2019; date of approval 12/09/2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this study are stored in a data repository at the following link: https://osf.io/2kehj/?view_only=593aca436fd44e2db0590410b5c2379b, accessed on 20 May 2025.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| VDR | vitamin D receptor |
| WES | whole exome sequencing |
| DNA | deoxyribonucleic acid |
| ACAN | aggrecan |
| BMP4 | bone morphogenetic protein 4 |
| CYP3A4 | cytochrome P450 family 3 subfamily A member 4 |
| GLI2 | GLI family zinc finger 2 |
| HSPG2 | heparan sulfate proteoglycan 2 |
| MESP2 | mesoderm posterior bHLH transcription factor 2 |
| LBP | low back pain |
| MRI | magnetic resonance imaging |
| BMI | body mass index |
| BMP | bone morphogenic protein |
| Dkk-1 | Dickkopf-1 |
| MMP | matrix metalloprotease |
| PDGF | platelet-derived growth factor |
| TGF | transforming growth factor |
| TRANCE | tumor necrosis factor-related activation-induced cytokine |
| PBMC | peripheral blood mononuclear cell |
| SD | standard deviation |
| NFKB1 | nuclear factor kappa B subunit 1 |
| NPR2 | natriuretic peptide receptor 2 |
| ETV2 | ETS variant transcription factor 2 |
| RUNX1 | RUNX family transcription factor 1 |
| LRP4 | LDL receptor-related protein 4 |
| SMAD3 | SMAD family member 3 |
| FLNA | filamin A |
| MYH11 | myosin heavy chain 11 |
| MYLK | myosin light chain kinase |
| NEB | nebulin |
| THSD4 | thrombospondin type 1 domain containing 4 |
| COL11A1 | collagen type XI α 1 chain |
| COL1A1 | collagen type I α 1 chain |
| FBN1 | fibrillin 1 |
| FN1 | fibronectin 1 |
| GALNS | galactosamine (N-acetyl)-6-sulfatase |
| MATN3 | matrilin 3 |
| P3H1 | prolyl 3-hydroxylase 1 |
| SLC26A2 | solute carrier family 26 member 2 |
| THBS2 | thrombospondin 2 |
References
- Lowe, T.G. Scheuermann Disease. J. Bone Jt. Surg. Am. 1990, 72, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Guo, X.; Chen, Z.; Qi, Q.; Li, W.; Guo, Z.; Zeng, Y.; Sun, C.; Liu, Z. Radiological Signs of Scheuermann Disease and Low Back Pain: Retrospective Categorization of 188 Hospital Staff Members with 6-Year Follow-Up. Spine 2014, 39, 1666–1675. [Google Scholar] [CrossRef]
- Ytrehus, B.; Carlson, C.S.; Ekman, S. Etiology and Pathogenesis of Osteochondrosis. Vet. Pathol. 2007, 44, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Dommisse, G.F. The Vulnerable, Rapidly Growing Thoracic Spine of the Adolescent. S. Afr. Med. J. Suid-Afr. Tydskr. Vir Geneeskd. 1990, 78, 211–213. [Google Scholar]
- Ekman, S.; Carlson, C.S. The Pathophysiology of Osteochondrosis. Vet. Clin. N. Am. Small Anim. Pract. 1998, 28, 17–32. [Google Scholar] [CrossRef]
- Trotta, A.; Corrado, A.; Soragnese, M.F.; Santoro, N.; Cantatore, F.P. [Adult Scheuermann’s disease as cause of mechanic dorsalgia]. Reumatismo 2008, 60, 14–21. [Google Scholar] [CrossRef]
- McKenzie, L.; Sillence, D. Familial Scheuermann Disease: A Genetic and Linkage Study. J. Med. Genet. 1992, 29, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Graat, H.C.A.; van Rhijn, L.W.; Schrander-Stumpel, C.T.R.M.; van Ooij, A. Classical Scheuermann Disease in Male Monozygotic Twins: Further Support for the Genetic Etiology Hypothesis. Spine 2002, 27, E485–E487. [Google Scholar] [CrossRef]
- Damborg, F.; Engell, V.; Andersen, M.; Kyvik, K.O.; Thomsen, K. Prevalence, Concordance, and Heritability of Scheuermann Kyphosis Based on a Study of Twins. J. Bone Jt. Surg. Am. 2006, 88, 2133–2136. [Google Scholar] [CrossRef]
- Tribus, C.B. Scheuermann’s Kyphosis in Adolescents and Adults: Diagnosis and Management. J. Am. Acad. Orthop. Surg. 1998, 6, 36–43. [Google Scholar] [CrossRef]
- Brayda-Bruno, M.; Albano, D.; Cannella, G.; Galbusera, F.; Zerbi, A. Endplate Lesions in the Lumbar Spine: A Novel MRI-Based Classification Scheme and Epidemiology in Low Back Pain Patients. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2018, 27, 2854–2861. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Yu, A.; Geng, J.; Liu, Y.; Wang, L.; Shi, J.; Zhou, F.; Zhang, T.; Huang, P.; Cheng, X. The lumbar spinal endplate lesions grades and association with lumbar disc disorders, and lumbar bone mineral density in a middle-young general Chinese population. BMC Musculoskelet. Disord. 2023, 24, 258. [Google Scholar] [CrossRef]
- Colombini, A.; Brayda-Bruno, M.; Lombardi, G.; Croiset, S.J.; Vrech, V.; Maione, V.; Banfi, G.; Cauci, S. FokI Polymorphism in the Vitamin D Receptor Gene (VDR) and Its Association with Lumbar Spine Pathologies in the Italian Population: A Case-Control Study. PLoS ONE 2014, 9, e97027. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Brayda-Bruno, M.; Ferino, L.; Lombardi, G.; Maione, V.; Banfi, G.; Cauci, S. Gender Differences in the VDR-FokI Polymorphism and Conventional Non-Genetic Risk Factors in Association with Lumbar Spine Pathologies in an Italian Case-Control Study. Int. J. Mol. Sci. 2015, 16, 3722–3739. [Google Scholar] [CrossRef] [PubMed]
- Colombini, A.; Galbusera, F.; Cortese, M.C.; Gallazzi, E.; Viganò, M.; Albano, D.; Cauci, S.; Sconfienza, L.M.; Brayda-Bruno, M. Classification of Endplate Lesions in the Lumbar Spine and Association with Risk Factors, Biochemistry, and Genetics. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2021, 30, 2231–2237. [Google Scholar] [CrossRef]
- Cauci, S.; Viganò, M.; de Girolamo, L.; De Luca, P.; Perucca Orfei, C.; Banfi, G.; Lombardi, G.; Brayda-Bruno, M.; Colombini, A. High Levels of Circulating Type II Collagen Degradation Marker (CTx-II) Are Associated with Specific VDR Polymorphisms in Patients with Adult Vertebral Osteochondrosis. Int. J. Mol. Sci. 2017, 18, 2073. [Google Scholar] [CrossRef]
- Colombini, A.; Galbusera, F.; Gallazzi, E.; Cortese, M.C.; Albano, D.; Sconfienza, L.M.; Cauci, S.; Brayda-Bruno, M. Letter to the Editor Concerning “Classification of Endplate Lesions in the Lumbar Spine and Association with Risk Factors, Biochemistry, and Genetics” by Alessandra Colombini et al. (Eur Spine J;. https://doi.org/10.1007/S00586-021-06719-1). Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2022, 31, 2822–2823. [Google Scholar] [CrossRef]
- Zehra, U.; Tryfonidou, M.; Iatridis, J.C.; Illien-Jünger, S.; Mwale, F.; Samartzis, D. Mechanisms and Clinical Implications of Intervertebral Disc Calcification. Nat. Rev. Rheumatol. 2022, 18, 352–362. [Google Scholar] [CrossRef]
- Marasco, E.; Fabbriciani, G.; Rotunno, L.; Longhi, M.; De Luca, P.; De Girolamo, L.; Colombini, A. Identification of Biomarkers in Patients with Rheumatoid Arthritis Responsive to DMARDs but with Progressive Bone Erosion. Front. Immunol. 2023, 14, 1254139. [Google Scholar] [CrossRef]
- Dahary, D.; Golan, Y.; Mazor, Y.; Zelig, O.; Barshir, R.; Twik, M.; Stein, T.I.; Rosner, G.; Kariv, R.; Chen, F.; et al. Genome analysis and knowledge-driven variant interpretation with TGex. BMC Med. Genomics 2019, 12, 200. [Google Scholar] [CrossRef]
- King, J.A.; Marker, P.C.; Seung, K.J.; Kingsley, D.M. BMP5 and the Molecular, Skeletal, and Soft-Tissue Alterations in Short Ear Mice. Dev. Biol. 1994, 166, 112–122. [Google Scholar] [CrossRef]
- Mailhot, G.; Yang, M.; Mason-Savas, A.; Mackay, C.A.; Leav, I.; Odgren, P.R. BMP-5 Expression Increases during Chondrocyte Differentiation in Vivo and in Vitro and Promotes Proliferation and Cartilage Matrix Synthesis in Primary Chondrocyte Cultures. J. Cell. Physiol. 2008, 214, 56–64. [Google Scholar] [CrossRef]
- Snelling, S.J.B.; Hulley, P.A.; Loughlin, J. BMP5 Activates Multiple Signaling Pathways and Promotes Chondrogenic Differentiation in the ATDC5 Growth Plate Model. Growth Factors 2010, 28, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, Z.; Yu, Y.; Chu, H.Y.; Yu, S.; Yao, S.; Zhang, G.; Zhang, B.-T. Drug Discovery of DKK1 Inhibitors. Front. Pharmacol. 2022, 13, 847387. [Google Scholar] [CrossRef]
- Zhang, W.; Drake, M.T. Potential Role for Therapies Targeting DKK1, LRP5, and Serotonin in the Treatment of Osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 93–100. [Google Scholar] [CrossRef]
- Andrae, J.; Gallini, R.; Betsholtz, C. Role of Platelet-Derived Growth Factors in Physiology and Medicine. Genes Dev. 2008, 22, 1276–1312. [Google Scholar] [CrossRef]
- Rolny, C.; Nilsson, I.; Magnusson, P.; Armulik, A.; Jakobsson, L.; Wentzel, P.; Lindblom, P.; Norlin, J.; Betsholtz, C.; Heuchel, R.; et al. Platelet-Derived Growth Factor Receptor-Beta Promotes Early Endothelial Cell Differentiation. Blood 2006, 108, 1877–1886. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Correa, D. PDGF in Bone Formation and Regeneration: New Insights into a Novel Mechanism Involving MSCs. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2011, 29, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Battegay, E.J.; Rupp, J.; Iruela-Arispe, L.; Sage, E.H.; Pech, M. PDGF-BB Modulates Endothelial Proliferation and Angiogenesis in Vitro via PDGF Beta-Receptors. J. Cell Biol. 1994, 125, 917–928. [Google Scholar] [CrossRef]
- Su, W.; Liu, G.; Liu, X.; Zhou, Y.; Sun, Q.; Zhen, G.; Wang, X.; Hu, Y.; Gao, P.; Demehri, S.; et al. Angiogenesis Stimulated by Elevated PDGF-BB in Subchondral Bone Contributes to Osteoarthritis Development. JCI Insight 2020, 5, 135446. [Google Scholar] [CrossRef]
- Clarke, J. PDGF-BB Is the Key to Unlocking Pathological Angiogenesis in OA. Nat. Rev. Rheumatol. 2020, 16, 298. [Google Scholar] [CrossRef]
- Qin, X.; Wang, X. Role of Vitamin D Receptor in the Regulation of CYP3A Gene Expression. Acta Pharm. Sin. B 2019, 9, 1087–1098. [Google Scholar] [CrossRef]
- Wang, Z.; Schuetz, E.G.; Xu, Y.; Thummel, K.E. Interplay between Vitamin D and the Drug Metabolizing Enzyme CYP3A4. J. Steroid Biochem. Mol. Biol. 2013, 136, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Näkki, A.; Battié, M.C.; Kaprio, J. Genetics of Disc-Related Disorders: Current Findings and Lessons from Other Complex Diseases. Eur. Spine J. Off. Publ. Eur. Spine Soc. Eur. Spinal Deform. Soc. Eur. Sect. Cerv. Spine Res. Soc. 2014, 23 (Suppl. 3), S354–S363. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.S.; Dissanayake, P.H.; Senarath, U.; Wijayaratne, L.S.; Karunanayake, A.L.; Dissanayake, V.H.W. Variants of ACAN Are Associated with Severity of Lumbar Disc Herniation in Patients with Chronic Low Back Pain. PLoS ONE 2017, 12, e0181580. [Google Scholar] [CrossRef]
- Yaltirik, C.K.; Timirci-Kahraman, Ö.; Gulec-Yilmaz, S.; Ozdogan, S.; Atalay, B.; Isbir, T. The Evaluation of Proteoglycan Levels and the Possible Role of ACAN Gene (c.6423T>C) Variant in Patients with Lumbar Disc Degeneration Disease. In Vivo 2019, 33, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Kraatari, M.; Skarp, S.; Niinimäki, J.; Karppinen, J.; Männikkö, M. A Whole Exome Study Identifies Novel Candidate Genes for Vertebral Bone Marrow Signal Changes (Modic Changes). Spine 2017, 42, 1201–1206. [Google Scholar] [CrossRef]
- Kohn, A.; Dong, Y.; Mirando, A.J.; Jesse, A.M.; Honjo, T.; Zuscik, M.J.; O’Keefe, R.J.; Hilton, M.J. Cartilage-Specific RBPjκ-Dependent and -Independent Notch Signals Regulate Cartilage and Bone Development. Development 2012, 139, 1198–1212. [Google Scholar] [CrossRef]
- Mead, T.J.; Yutzey, K.E. Notch Pathway Regulation of Chondrocyte Differentiation and Proliferation during Appendicular and Axial Skeleton Development. Proc. Natl. Acad. Sci. USA 2009, 106, 14420–14425. [Google Scholar] [CrossRef]
- Karlsson, C.; Lindahl, A. Notch Signaling in Chondrogenesis. Int. Rev. Cell Mol. Biol. 2009, 275, 65–88. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, C.; Lu, W.; He, T.; Fan, J.; Wang, C.; Jie, Q.; Chan, D.; Cheah, K.S.E.; Yang, L. Hedgehog Signaling Orchestrates Cartilage-to-Bone Transition Independently of Smoothened. Matrix Biol. J. Int. Soc. Matrix Biol. 2022, 110, 76–90. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, Z.; Ye, Y.; Zhu, D.; Li, N.; Yang, G.; Wang, Y. Role and Mechanism of BMP4 in Regenerative Medicine and Tissue Engineering. Ann. Biomed. Eng. 2023, 51, 1374–1389. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).