Abstract
Background: MYB is a key transcription factor that plays an essential role in regulating hematopoiesis, particularly influencing cell proliferation, differentiation, and apoptosis. It has been extensively implicated in the pathogenesis and progression of leukemia, as well as in determining patient prognosis and responsiveness to chemotherapy. Despite these well-documented roles, the precise molecular mechanisms by which MYB contributes to chemotherapy resistance in leukemia remain largely undefined. Methods: In this study, we investigated the potential role of MYB in regulating ferroptosis, a form of regulated cell death driven by iron-dependent lipid peroxidation, which has recently emerged as a novel therapeutic target in cancer. We overexpressed and knockdown MYB in human leukemia K562 cells and evaluated changes in ferroptosis-related markers, as well as cell proliferation and migration capacities, in the context of treatment with the chemotherapeutic agent sorafenib. Results: Our findings demonstrated that MYB overexpression significantly enhanced the resistance of human leukemia cells to sorafenib, while MYB knockdown increased their drug sensitivity. Mechanistically, MYB was found to upregulate ferritin heavy chain 1 (FTH1), thereby suppressing sorafenib-induced ferroptosis and cell death. Further, FTH1 knockdown significantly reduced the proliferation and migration ability of K562 cells and enhanced sorafenib-induced ferroptosis. Rescue experiments confirmed that FTH1 is required for MYB induced sorafenib resistance and ferroptosis inhibition in human leukemia cells. Conclusions: Collectively, this study identifies the MYB-FTH1 axis as a novel regulatory pathway modulating ferroptosis and chemoresistance in leukemia cells, providing potential therapeutic targets for improving treatment precision and preventing disease relapse.
1. Introduction
The transcription factor MYB is a key regulator for the hematopoietic process [1], and precise regulation of MYB is necessary for every stage of the development of thymocytes [2], red blood cells [3], B lymphocytes [4], and the self-renewal of hematopoietic stem cells (HSCs) [5]. Aberrant MYB expression plays a critical role in the onset and progression of various hematologic malignancies, particularly leukemia [6]. In addition, MYB dysregulation contributes to drug resistance in leukemia as well as gastrointestinal and breast cancers [7]. Thus, understanding the underlying mechanisms of MYB-mediated drug resistance is of great significance for the treatment of leukemia.
Sorafenib is an orally administered multikinase inhibitor that targets both Raf serine/threonine kinases and various receptor tyrosine kinases [8]. Sorafenib has been approved for liver cancer and kidney cancer [9], and has also shown therapeutic efficacy in acute myeloid leukemia (AML) patients with FLT3-ITD mutations, leading to improved patient survival [10]. In addition, a persistently low percentage of blasts, along with CD3+ cell infiltration in the skin, increased CD8+ lymphocytes in the bone marrow, and elevated expression of COL4A3, TLR9, FGF1, and IL-12 genes have been observed in sorafenib-treated AML patients [11]. A combination of sorafenib and decitabine has been used in preclinical and clinical trials to treat FLT3/ITD-mutated AML [12]. Unfortunately, most patients failed to experience a long-term benefit, largely because of the early occurrence of sorafenib resistance [13]. Since sorafenib can exhibit its anticancer effect through inducing apoptosis and ferroptosis, desensitivity of cancer cells to ferroptosis will lead to resistance.
Ferroptosis is an iron-driven cell death modality characterized by iron accumulation and excessive lipid peroxidation [14]. Ferroptosis dysfunction is involved in diseases such as degenerative diseases [15], solid tumors [16], and leukemia [17]. Ferroptosis is also associated with drug resistance in cancer therapy, and inducing ferroptosis has been demonstrated to reverse drug resistance [18]. Ferritin heavy chain 1 (FTH1) is an iron storage protein with ferroxidase activity, which converts the ferrous form (Fe2+) to the ferric form (Fe3+) [19]. High expression of FTH1 can promote tumor cell proliferation and migration and inhibit ferroptosis in leukemia [20]. FTH1 has also been identified as a key gene associated with poor prognosis in AML [21]. In addition, chemotherapy resistance progression of various diseases is closely related to FTH1 [22].
In this study, we found that MYB can inhibit sorafenib induced ferroptosis in human leukemia K562 cells through upregulating FTH1, conferring sorafenib resistance to leukemia cells. Our results help understanding the mechanism of MYB-mediated resistance to anticancer drugs, and suggest that targeting ferroptosis may be a promising therapeutic strategy for MYB-mediated drug resistance in leukemia.
2. Materials and Methods
2.1. Cell Culture
The K562, HL-60, and HEK 293T cell lines were obtained from ATCC (Manassas, VA, USA). K562 (CCL-243) and HL-60 (CCL-240) cells were grown in RPMI-1640 Medium (C11875500BT, Gibco, Waltham, MA, USA), while HEK 293T cells (CRL-3216) were maintained in DMEM (C11965500BT, Gibco, USA). All cell lines were supplemented with 10% fetal bovine serum (FBS, 10099141, Gibco, USA) and incubated in a humidified atmosphere at 37 °C with 5% CO2.
2.2. Plasmid Construction
The pLVX-IRES-neo plasmid (632181, Clontech, Mountain View, CA, USA), containing the sequence of MYB, was constructed and used to upregulate MYB expression. Two specific short hairpin RNAs (shRNAs) targeting the covalent closed junction of MYB and FTH1 were cloned into the pLKO.1-puro plasmid (8453, Addgene, Watertown, MA, USA). Sequences of the above oligos are listed in Table 1.

Table 1.
The sequences of shRNAs.
2.3. RNA Extraction and Quantitative Real-Time PCR (RT-qPCR)
RNA was extracted using TRIzol (15596018, Invitrogen, Carlsbad, CA, USA). The RNA was converted into cDNA using the PrimeScript™ RT Reagent Kit (RR037A, Takara, Shiga, Japan) according to the manufacturer’s instructions. RT-qPCR was performed in a LightCycler 480 II instrument using SYBR Green Master Mix (AQ601, TransGen Biotech, Beijing, China). GAPDH was used as the internal control, and the relative expression levels of specific genes were calculated using the 2−ΔΔCt method. The primers used for RT-qPCR are listed in Table 2.

Table 2.
Primer sequences for quantitative real-time PCR.
2.4. Lentivirus Preparation and Infection
Lentiviruses for MYB overexpression and MYB or FTH1 knockdown were produced by co-transfecting constructed plasmids and the packaging plasmids pCMV-dR8.91 and pCMV-VSV-G into HEK 293T cells using Lipofectamine 3000 Reagent (L3000015, Thermo Scientific, Waltham, MA, USA) for 72 h. Culture supernatants containing lentivirus were collected, filtered, and concentrated. K562 cells were infected with lentivirus in the presence of 8 µg/mL polybrene (Sigma-Aldrich, St. Louis, MO, USA).
2.5. Western Blot
Cells were lysed using RIPA buffer (P0013B, Beyotime, Shanghai, China), and lysates were centrifuged at 12,000× g for 10 min at 4 °C to collect the supernatant. Equal amounts of protein were separated by SDS-PAGE, and transferred to 0.2 μm PVDF membranes (LC2002, Thermo Scientific, USA). Membranes were blocked with 5% (w/v) skim milk at room temperature for 2 h and then incubated overnight at 4 °C with primary antibodies. After washing, membranes were incubated with HRP-conjugated secondary antibodies at room temperature for 2 h. Protein bands were visualized using Clarity™ Western ECL Substrate (170–5061, Bio-Rad, Hercules, CA, USA). Primary antibodies used included anti-c-MYB (sc-74512, Santa Cruz Biotechnology, Dallas, TX, USA), anti-FTH1 (MA5-43829, Thermo Fisher Scientific, USA), and anti-beta actin (A13685, HUABIO, Hangzhou, China); the secondary antibody was HRP-conjugated goat anti-mouse IgG (31420, Thermo Scientific, USA).
2.6. Determination of Reactive Oxygen Species (ROS)
K562 cells were collected by centrifuge and ROS levels were determined using a Reactive Oxygen Species Assay Kit (S0033S, Beyotime, China). The DCFH-DA was diluted with serum-free medium to reach a final concentration of 10 μM. Then, 1 mL of DCFH-DA was added into cells for incubation at 37 °C for 30 min, blending every 3 min. Cells were washed (×3 times) and the absorbance was determined at an excitation wavelength of 485 nm and an emission wavelength of 528 nm using a microplate reader (SYNERGY2, BIO-Tek, Shoreline, WA, USA).
2.7. Determination of Fe2+
K562 cells were washed three times with cell staining buffer. Fe2+ levels were determined using a Cell Ferrous Iron (Fe2+) Fluorometric Assay Kit (MA0647, MeilunBio, Dalian, China). The ferrous ion probe was diluted with cell staining buffer to reach a final concentration of 2 μM. Then, 1 mL of staining working solution was added into cells for incubation at 37 °C for 45 min. Absorbance was determined at an excitation wavelength of 543 nm and an emission wavelength of 580 nm using a microplate reader (SYNERGY2, BIO-Tek, USA).
2.8. CCK8 Assay
Cell proliferation was assessed using the Enhanced Cell Counting Kit-8 (C0042, Beyotime, China). Briefly, cells were plated in 96-well plates and incubated for 24, 48, 72, and 96 h. Then, 10 µL of CCK8 solution was added, and the cells were incubated at 37 °C for 2 h. Absorbance was measured at 450 nm using a microplate reader (ReadMax1900, Shanpu, Wuxi, China).
2.9. Transwell Assay
K562 cells (5 × 105/mL) were seeded into the upper chamber of 24-well Transwell plates with 8 μm pore size (CLS3422, Corning, Corning, NY, USA), while the lower chamber was filled with RPMI-1640 medium containing 10% FBS as a chemoattractant. After incubating for 16 h, non-migrating cells on the upper side of the chamber were removed by scrubbing, and migrating cells on the lower side were fixed with 4% paraformaldehyde and stained with Giemsa (E607315-0001, Sangon Biotech, Shanghai, China). The number of migrating cells was assessed in 3 random microscope fields.
2.10. Statistical Analysis
Each experiment was performed thrice for technical consistency. Statistical analyses were performed using GraphPad Prism version 8.0. Student’s t-test was used to determine the significance of differences between two groups.
3. Results
3.1. MYB Induces Drug Resistance in Human Leukemia Cells
To determine the sensitivity of human leukemia cells to sorafenib treatment, K562 and HL-60 cells were treated with different concentrations of sorafenib for 36 h, then cell viability was detected using a CCK8 assay (Figure 1a,b). In line with expectations, sorafenib treatment led to a dose-dependent decrease in the viability of K562 and HL-60 cells. Then, we selected 5 μM sorafenib for further study. To explore the effect of MYB on sorafenib treatment in K562 and HL-60 cells, MYB was either overexpressed or silenced using two different shRNAs (shMYB-1 and shMYB-2) (Figure 1c–e). The sequences of these shRNAs are listed in Table 1. shMYB-2 (hereafter referred to as shMYB) was used for further experiments. The CCK8 assay showed MYB overexpression partially reversed the decrease in cell viability caused by sorafenib, while MYB knockdown enhanced the inhibitory effect of sorafenib in K562 cells (Figure 1f). Similarly, MYB overexpression attenuated the sorafenib-induced reduction in cell viability in HL-60 cells. (Figure 1g). The Transwell assay showed that sorafenib inhibited the migration of K562 cells, MYB overexpression partially reversed the inhibition of cell migration caused by sorafenib, and MYB knockdown enhanced the inhibitory effect of sorafenib on the migration (Figure 1h,i). The above results show that MYB confers sorafenib resistance in human leukemia cells.
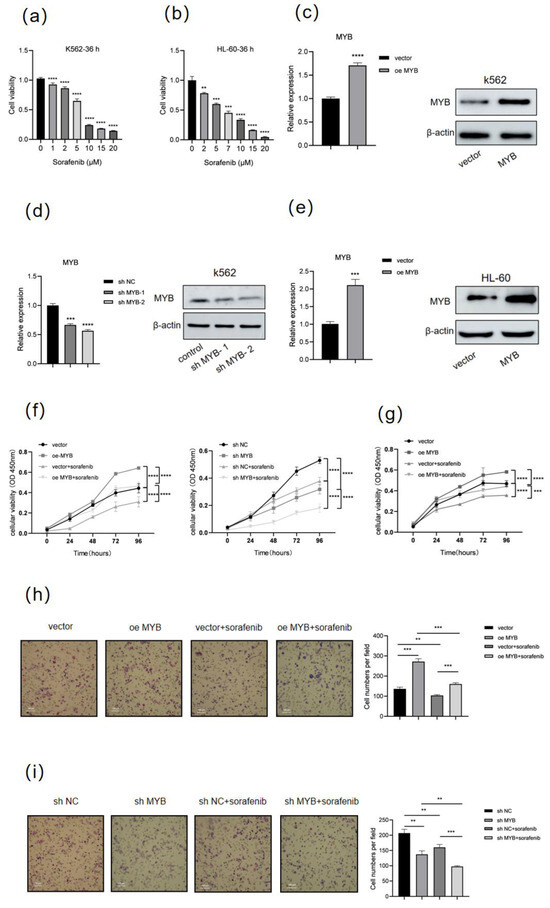
Figure 1.
MYB induces sorafenib resistance in human leukemia cells. (a) K562 cells were treated with sorafenib at the indicated doses (0, 1, 2, 5, 10, 15, 20 μM) for 36 h, and cell viability was measured by a CCK8 assay. (b) HL-60 cells were treated with sorafenib at indicated doses for 36 h, and cell viability was measured by a CCK8 assay. (c,d) K562 cells were infected with control lentivirus (vector), lentivirus expressing MYB (oe MYB), negative control shRNA (sh NC), or MYB-targeting shRNA (sh MYB-1 and sh MYB-2) for 48 h, then the expression level of MYB was determined by RT-qPCR and Western blot. (e) HL-60 cells were infected with lentivirus expressing MYB or control lentivirus (vector) for 48 h, then MYB expression was evaluated by RT-qPCR and Western blot. (f) K562 cells were treated with 5 μM sorafenib for 36 h after infection with the indicated lentivirus, and cell viability was measured by a CCK8 assay. (g) HL-60 cells were treated with 5 μM sorafenib for 36 h after MYB overexpression, and cell viability was measured by a CCK8 assay. (h,i) A Transwell migration assay was performed on K562 cells after MYB overexpression or knockdown and sorafenib treatment. Scale bar: 100 μm. Data for graphing were obtained from the means ± SD of three independent experiments (n = 3). p values were calculated using Student’s t-test (** p < 0.01, *** p < 0.001, **** p < 0.0001).
3.2. MYB Inhibits Ferroptosis in K562 Cells
To investigate the underlying mechanism, we performed RNA-seq in K562 cells after MYB overexpression; the results showed that MYB is closely related to the regulation of ferroptosis, and sorafenib is also a ferroptosis inducer. To further confirm the role of MYB in ferroptosis, we detected ROS and Fe2+ in K562 cells after MYB overexpression and knockdown. As shown in Figure 2a,b, sorafenib treatment increased the levels of ROS and Fe2+, while MYB overexpression restrained the increase of ROS and Fe2+ induced by sorafenib in K562 cells. In contrast, sh MYB led to a further increase in the levels of ROS and Fe2+ in sorafenib-treated K562 cells. This indicates that MYB inhibits sorafenib induced ferroptosis in K562 cells.
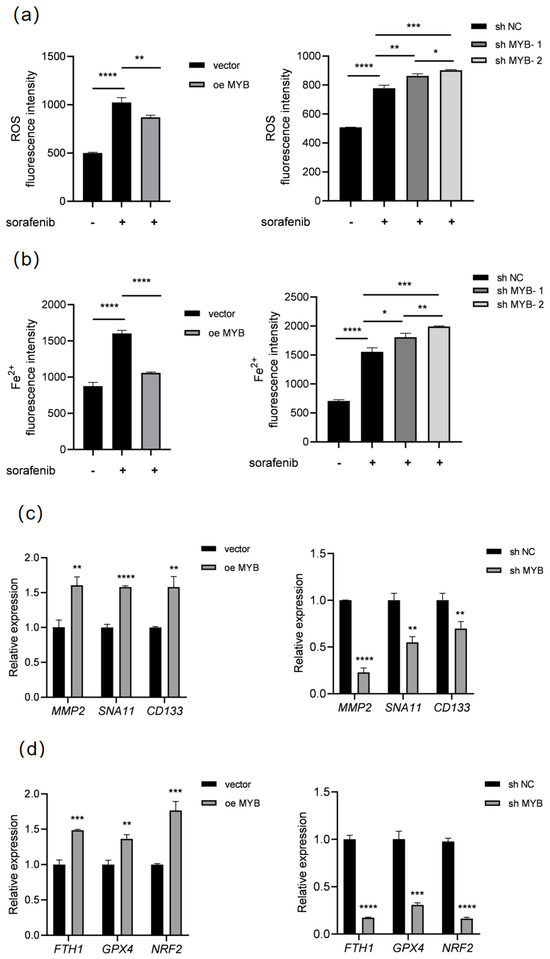
Figure 2.
MYB inhibits ferroptosis in K562 cells. K562 cells were treated with 5 μM sorafenib for 36 h after infection with a control lentivirus (vector), lentivirus expressing MYB, negative control shRNA (sh NC), or MYB targeting shRNA (sh MYB-1 and sh MYB-2), then ROS (a) and Fe2+ levels (b) were determined. K562 cells were infected with the indicated lentivirus for 48 h, then the expression of proliferation and migration related genes (c) and ferroptosis related genes (d) was examined using RT-qPCR. Data for graphing were obtained from the means ± SD of three individual experiments (n = 3), and p values were calculated using Student’s t-test (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001).
Meanwhile, the expression of proliferation and migration-related genes were detected. As shown in Table 2, RT-qPCR showed that MYB overexpression significantly upregulated MMP2, SNA11, and CD133, while sh MYB downregulated these genes (Figure 2c). Subsequently, we further analyzed the expression of ferroptosis-related genes. RT-qPCR showed that MYB overexpression significantly upregulated FTH1, GPX4, and NRF2, three negative regulators of ferroptosis, when sh MYB significantly downregulated these genes (Figure 2d). Since FTH1 is an important negative regulator of ferroptosis, the above data show that MYB can inhibit ferroptosis through regulating ferroptosis-related genes and confer resistance to sorafenib in K562 cells.
3.3. FTH1 Knockdown Induces Ferroptosis and Enhances Sorafenib Sensitivity in K562 Cells
Next, we investigated the role of FTH1 in the proliferation and migration of K562 cells. FTH1 was knocked down by a shRNA (sh FTH1-1 and sh FTH1-2)-expressing lentivirus in K562 cells. RT-qPCR and Western blot showed that FTH1 was successfully knocked down (Figure 3a). shFTH1-2 (hereafter referred to as sh FTH1) was selected for subsequent experiments. CCK8 assays and Transwell assays showed that knocking down FTH1 significantly inhibited the proliferation and migration of K562 cells (Figure 3b,c), suggesting that FTH1 plays a vital role in the growth and migration of K562 cells. Next, we explored the role of FTH1 in ferroptosis and sorafenib sensitivity of K562 cells. The results showed that ROS increased significantly after FTH1 knockdown or sorafenib treatment, and FTH1 knockdown further enhanced the elevation of ROS and Fe2+ caused by sorafenib treatment (Figure 3d,e). These data indicate that FTH1 can support the proliferation and migration of K562 cells, and inhibition of FTH1 causes decreased proliferation and migration and enhanced sorafenib sensitivity in K562 cells.
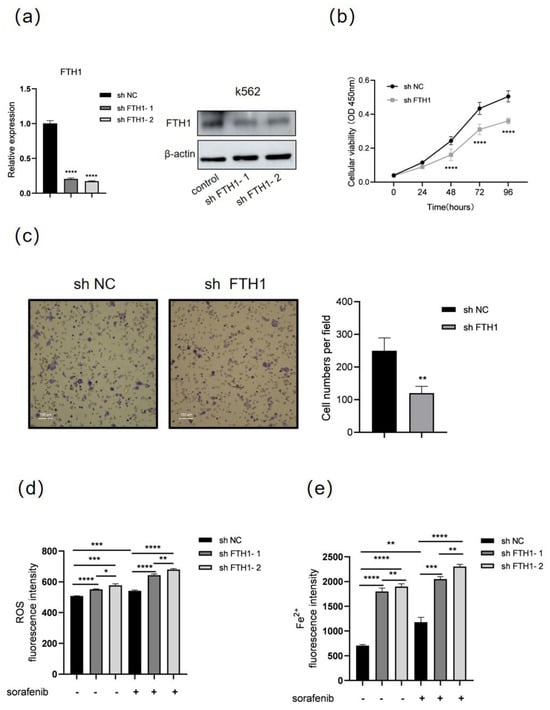
Figure 3.
FTH1 knockdown induces ferroptosis and enhances sorafenib sensitivity in K562 cells. K562 cells were infected with lentivirus expressing negative control shRNA (sh NC) or FTH1-targeting shRNA (sh FTH1-1 and sh FTH1-2) for 48 h, then RT-qPCR, Western blot (a), CCK8 assay (b), and Transwell assay (c) were performed. Scale bar: 100 μm. K562 cells were treated with 5 μM sorafenib for 36 h after infected with the indicated sh RNA lentivirus (sh FTH1-1 and sh FTH1-2), then ROS levels (d) and Fe2+ levels (e) were measured. Data for graphing were obtained from the means ± SD of three individual experiments (n = 3), and p values were calculated using Student’s t-test (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001).
3.4. MYB Inhibits Ferroptosis and Induces Sorafenib Resistance Through FTH1 in Leukemia Cells
To investigate the role of FTH1 in MYB-induced sorafenib resistance in leukemia cells, FTH1 was knocked down in K562 cells with MYB overexpression prior to sorafenib treatment. CCK8 and Transwell assays demonstrated that MYB overexpression significantly promoted the proliferation and migration of K562 cells, whereas FTH1 knockdown markedly suppressed these effects. When FTH1 knockdown and MYB overexpression occurred simultaneously, FTH1 knockdown could partially abolished the increase in cell proliferation and migration caused by MYB overexpression (Figure 4a,b). This finding could indicate that MYB promotes the proliferation and migration of K562 cells via FTH1. Meanwhile, we detected ROS and Fe2+ in K562 cells after FTH1 knockdown and MYB overexpression. The results show that MYB overexpression reduced the ROS level and Fe2+ content of K562 cells, while FTH1 knockdown increased the ROS level and Fe2+ content of K562 cells. Meanwhile, FTH1 knockdown abolished the reduction of cellular ROS levels and Fe2+ content caused by MYB overexpression (Figure 4c,d). The above data indicate the presence of MYB-induced sorafenib resistance through the upregulation of FTH1 and inhibition of ferroptosis.
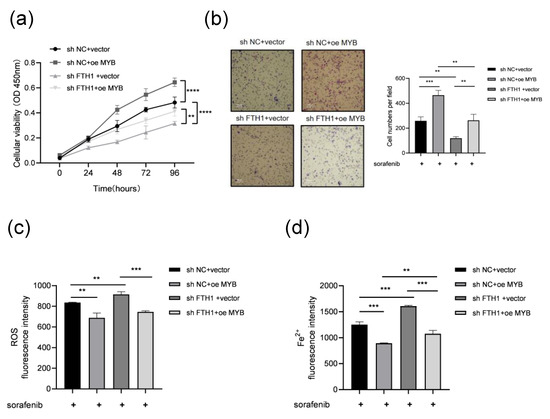
Figure 4.
MYB inhibits ferroptosis and induces sorafenib resistance through FTH1 in K562 cells. K562 cells coinfected with the indicated lentivirus were treated with 5 μM sorafenib for 36 h, then a CCK8 assay (a) and Transwell assay (b) were performed, and ROS levels (c) and Fe2+ levels (d) were examined. Scale bar: 100 μm. Data for graphing were obtained from the means ± SD of three individual experiments (n = 3), and p values were calculated using Student’s t-test (** p < 0.01, *** p < 0.001, **** p < 0.0001).
4. Discussion
MYB functions as a pivotal regulator of hematopoiesis by modulating various cellular processes such as proliferation [23], differentiation, and programmed cell death [5]. Abnormal expression of MYB is closely related to leukemia progression, poor prognosis, and anticancer drug resistance [7]. It has been reported that MYB silencing reduces the resistance of AML cells to doxorubicin [24]. Our work here shows that MYB can confer resistance to sorafenib via upregulating FTH1 and inhibiting sorafenib-induced ferroptosis in human leukemia. The above results support that targeting the ferroptosis pathway can overcome MYB-mediated drug resistance in leukemia.
It is well known that MYB is highly expressed in various leukemia cells, which often leads to drug resistance. Knockdown of MYB sensitized acute lymphoblastic leukemia (ALL) cells to doxorubicin and 6-mercaptopurine by downregulating anti-apoptotic BCL2 [25]. In ovarian cancer (OC) cells, MYB overexpression induces cisplatin resistance by activating the NF-κB and STAT3 signaling pathways, whereas MYB silencing or inhibition restores cisplatin sensitivity [26]. Here, we showed that MYB inhibits sorafenib-induced ferroptosis in human leukemia, desensitizing these cells to sorafenib. The above results demonstrate that MYB can induce drug resistance to multiple drugs via multiple pathways, and targeting these pathways will help overcoming MYB-related drug resistance in leukemia.
Ferroptosis is frequently dysregulated in cancers and closely related to drug resistance. Ferroptosis induction can alleviate sorafenib resistance in various cancers [27]. FTH1 prevents iron-dependent lipid peroxidation by storing free iron and attenuating ferroptosis [28]. FTH1 promotes leukemia cell proliferation via the ferroptosis pathway, serving as a potential risk factor influencing the prognosis of pediatric non-M3 AML [21]. High FTH1 expression is usually accompanied by the activation of NF-κB-related genes, thereby reducing the sensitivity of AML cells to chemotherapy [29]. This is consistent with our findings that FTH1 knockout inhibits cell proliferation and migration while enhancing the effect of sorafenib, thereby inducing ferroptosis in leukemia cells.
In tumor progression, multiple key genes are involved in cell proliferation and migration. Matrix metalloproteinase-2 (MMP-2) enhances invasion and metastasis in cancer cells by degrading the extracellular matrix (ECM) and promoting angiogenesis [30]. Zinc finger transcription factor (SNA11) induces epithelial–mesenchymal transition (EMT) in various cancers and promotes cell migration and invasion [31]. Cell membrane glycoprotein (CD133), is closely related to cancer cell proliferation, autophagy, and apoptosis [32]. Glutathione peroxidase 4 (GPX4) can effectively remove lipid peroxides and inhibit ferroptosis [33]. Nuclear factor erythroid 2-related factor 2 (NRF2), as a core regulator of cellular antioxidant stress, can activate multiple anti-ferroptosis pathways [34]. This study found that MYB can significantly upregulate proliferation and migration-related genes (MMP2, SNA11, CD133) and negative regulatory genes of ferroptosis (FTH1, GPX4, NRF2). These data indicate that MYB supports cell survival through multiple downstream target genes.
The regulation of FTH1 is important for ferroptosis. METTL1 can upregulate mature miR-26a-5p, which then inhibits FTH1 mRNA translation efficiency; the decrease of FTH1 in turn increases ferroptosis and promotes chemotherapy sensitivity in osteosarcoma cells [35]. LncRNA CACNA1G-AS1 can upregulate FTH1 to inhibit ferroptosis and promote malignant phenotypes in ovarian cancer cells [36]. The circPIAS1/NUPR1 axis suppresses ferroptosis activity in hepatocellular carcinoma (HCC) cells by upregulating FTH1, thereby accelerating HCC progression [37]. Here, we showed that MYB can upregulate FTH1, inhibit ferroptosis in human leukemia cells. Consistent with our findings, previous CUT&RUN data indicate that MYB binds directly to the promoter region of the FTH1 gene in K562 cells, suggesting that MYB directly regulates FTH1 transcription [38]. Collectively, these findings show that FTH1 is under the control of multiple upstream regulators.
Since FTH1 and MYB inhibition can sensitize human leukemia cells to sorafenib in vitro, combination therapy of sorafenib with FTH1 or MYB inhibitor may overcome drug resistance in sorafenib-resistant AML patients; further research using primary AML patient samples and in vivo models is required to support combining FTH1 or MYB inhibitors with sorafenib as a therapeutic strategy in the treatment of AML.
In summary, MYB plays a critical role in regulating chemoresistance through multiple mechanisms in various cancers, particularly in leukemia. Our results showed that MYB inhibits sorafenib-induced ferroptosis by positively regulating FTH1 expression, thereby enhancing the resistance of K562 cells to sorafenib. These findings emphasize the key role of MYB-FTH1 in inhibiting chemotherapy-induced ferroptosis and drug resistance.
Author Contributions
X.T.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft. Y.W.: Formal analysis, Methodology, Investigation. S.S.: Methodology, Investigation. H.F.: Methodology. H.S.: Methodology. J.Z.: Conceptualization, Methodology, Writing—review & editing. B.H.: Formal analysis, Funding acquisition, Methodology, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81770165]. This work was also supported by the First Class Discipline Program for Fishery from the Shanghai municipal government.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the use of commercially available human cell lines (K562, HL-60, and HEK 293T), approved code SHOU-DW-2025-021, approved date 17 February 2025.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated or analyzed during this study are included in the published article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheiness, D.; Gardinier, M. Expression of a proto-oncogene (proto-myb) in hemopoietic tissues of mice. Mol. Cell. Biol. 1984, 4, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Westin, E.H.; Gallo, R.C.; Arya, S.K.; Eva, A.; Souza, L.M.; Baluda, M.A.; Aaronson, S.A.; Wong-Staal, F. Differential expression of the amv gene in human hematopoietic cells. Proc. Natl. Acad. Sci. USA 1982, 79, 2194–2198. [Google Scholar] [CrossRef]
- Oh, I.H.; Reddy, E.P. The myb gene family in cell growth, differentiation and apoptosis. Oncogene 1999, 18, 3017–3033. [Google Scholar] [CrossRef]
- Pattabiraman, D.R.; Gonda, T.J. Role and potential for therapeutic targeting of MYB in leukemia. Leukemia 2013, 27, 269–277. [Google Scholar] [CrossRef]
- Zhou, Y.; Ness, S.A. Myb proteins: Angels and demons in normal and transformed cells. Front. Biosci. 2011, 16, 1109–1131. [Google Scholar] [CrossRef]
- Stenman, G.; Andersson, M.K.; Andrén, Y. New tricks from an old oncogene: Gene fusion and copy number alterations of MYB in human cancer. Cell Cycle 2010, 9, 2986–2995. [Google Scholar] [CrossRef]
- Biersack, B.; Höpfner, M. Emerging role of MYB transcription factors in cancer drug resistance. Cancer Drug Resist. 2024, 7, 15. [Google Scholar] [CrossRef]
- Iyer, R.; Fetterly, G.; Lugade, A.; Thanavala, Y. Sorafenib: A clinical and pharmacologic review. Expert Opin. Pharmacother. 2010, 11, 1943–1955. [Google Scholar] [CrossRef]
- Abdelgalil, A.A.; Alkahtani, H.M.; Al-Jenoobi, F.I. Sorafenib. Profiles Drug Subst. Excip. Relat. Methodol. 2019, 44, 239–266. [Google Scholar] [CrossRef]
- Metzelder, S.K.; Schroeder, T.; Lübbert, M.; Ditschkowski, M.; Götze, K.; Scholl, S.; Meyer, R.G.; Dreger, P.; Basara, N.; Fey, M.F.; et al. Long-term survival of sorafenib-treated FLT3-ITD-positive acute myeloid leukaemia patients relapsing after allogeneic stem cell transplantation. Eur. J. Cancer 2017, 86, 233–239. [Google Scholar] [CrossRef]
- Lange, A.; Jaskula, E.; Lange, J.; Dworacki, G.; Nowak, D.; Simiczyjew, A.; Mordak-Domagala, M.; Sedzimirska, M. The sorafenib anti-relapse effect after alloHSCT is associated with heightened alloreactivity and accumulation of CD8+PD-1+ (CD279+) lymphocytes in marrow. PLoS ONE 2018, 13, e0190525. [Google Scholar] [CrossRef]
- Muppidi, M.R.; Portwood, S.; Griffiths, E.A.; Thompson, J.E.; Ford, L.A.; Freyer, C.W.; Wetzler, M.; Wang, E.S. Decitabine and Sorafenib Therapy in FLT-3 ITD-Mutant Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2015, 15, S73–S79. [Google Scholar] [CrossRef]
- Xia, S.; Pan, Y.; Liang, Y.; Xu, J.; Cai, X. The microenvironmental and metabolic aspects of sorafenib resistance in hepatocellular carcinoma. EBioMedicine 2020, 51, 102610. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.C.; Ding, Y.X.; Bai, J.; Cao, F.; Li, F. The crosstalk between ferroptosis and mitochondrial dynamic regulatory networks. Int. J. Biol. Sci. 2023, 19, 2756–2771. [Google Scholar] [CrossRef]
- Raven, E.P.; Lu, P.H.; Tishler, T.A.; Heydari, P.; Bartzokis, G. Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J. Alzheimers Dis. 2013, 37, 127–136. [Google Scholar] [CrossRef]
- Eling, N.; Reuter, L.; Hazin, J.; Hamacher-Brady, A.; Brady, N.R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2015, 2, 517–532. [Google Scholar] [CrossRef]
- Birsen, R.; Larrue, C.; Decroocq, J.; Johnson, N.; Guiraud, N.; Gotanegre, M.; Cantero-Aguilar, L.; Grignano, E.; Huynh, T.; Fontenay, M.; et al. APR-246 induces early cell death by ferroptosis in acute myeloid leukemia. Haematologica 2022, 107, 403–416. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, M.; Liu, M.; Yu, Y.; Zhang, Y.; Yang, J.; Xing, L.; Shao, Z.; Wang, H. Loss of FTH1 Induces Ferritinophagy-Mediated Ferroptosis in Anaemia of Myelodysplastic Syndromes. J. Cell Mol. Med. 2025, 29, e70350. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Wei, J.; Wu, X.; Luo, J.; Wei, H.; Ning, L.; He, Y. High expression level of the FTH1 gene is associated with poor prognosis in children with non-M3 acute myeloid leukemia. Front. Oncol. 2022, 12, 1068094. [Google Scholar] [CrossRef]
- Ali, A.; Shafarin, J.; Abu Jabal, R.; Aljabi, N.; Hamad, M.; Sualeh Muhammad, J.; Unnikannan, H.; Hamad, M. Ferritin heavy chain (FTH1) exerts significant antigrowth effects in breast cancer cells by inhibiting the expression of c-MYC. FEBS Open Bio 2021, 11, 3101–3114. [Google Scholar] [CrossRef]
- Mucenski, M.L.; McLain, K.; Kier, A.B.; Swerdlow, S.H.; Schreiner, C.M.; Miller, T.A.; Pietryga, D.W.; Scott, W.J., Jr.; Potter, S.S. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell 1991, 65, 677–689. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J. Long noncoding RNA ZFAS1 enhances adriamycin resistance in pediatric acute myeloid leukemia through the miR-195/Myb axis. RSC Adv. 2019, 9, 28126–28134. [Google Scholar] [CrossRef]
- Sarvaiya, P.J.; Schwartz, J.R.; Hernandez, C.P.; Rodriguez, P.C.; Vedeckis, W.V. Role of c-Myb in the survival of pre B-cell acute lymphoblastic leukemia and leukemogenesis. Am. J. Hematol. 2012, 87, 969–976. [Google Scholar] [CrossRef]
- Tian, M.; Tian, D.; Qiao, X.; Li, J.; Zhang, L. Modulation of Myb-induced NF-kB -STAT3 signaling and resulting cisplatin resistance in ovarian cancer by dietary factors. J. Cell. Physiol. 2019, 234, 21126–21134. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Sun, Q.; Li, Y.; Zhou, C.; Sun, C. Susceptibility of acute myeloid leukemia cells to ferroptosis and evasion strategies. Front. Mol. Biosci. 2023, 10, 1275774. [Google Scholar] [CrossRef] [PubMed]
- Wolosowicz, M.; Prokopiuk, S.; Kaminski, T.W. The Complex Role of Matrix Metalloproteinase-2 (MMP-2) in Health and Disease. Int. J. Mol. Sci. 2024, 25, 13691. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, T.; Bian, H.; Li, F.; Ju, Y.; Gao, S.; Su, J.; Ren, W.; Qin, C. SNAI1 promotes the development of HCC through the enhancement of proliferation and inhibition of apoptosis. FEBS Open Bio 2016, 6, 326–337. [Google Scholar] [CrossRef]
- Gisina, A.; Kim, Y.; Yarygin, K.; Lupatov, A. Can CD133 Be Regarded as a Prognostic Biomarker in Oncology: Pros and Cons. Int. J. Mol. Sci. 2023, 24, 17398. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, Y.; Jiang, Y.; Zhang, L.; Cheng, W. GPX4: The hub of lipid oxidation, ferroptosis, disease and treatment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188890. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- He, M.; Wang, Y.; Xie, J.; Pu, J.; Shen, Z.; Wang, A.; Li, T.; Wang, T.; Li, G.; Liu, Y.; et al. M(7)G modification of FTH1 and pri-miR-26a regulates ferroptosis and chemotherapy resistance in osteosarcoma. Oncogene 2024, 43, 341–353. [Google Scholar] [CrossRef]
- Jin, Y.; Qiu, J.; Lu, X.; Ma, Y.; Li, G. LncRNA CACNA1G-AS1 up-regulates FTH1 to inhibit ferroptosis and promote malignant phenotypes in ovarian cancer cells. Oncol. Res. 2023, 31, 169–179. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, S.S.; Gu, Y.R.; Xiao, L.X.; Ma, X.Y.; Chen, X.R.; Wang, J.L.; Liao, C.H.; Lin, B.L.; Huang, Y.H.; et al. CircPIAS1 promotes hepatocellular carcinoma progression by inhibiting ferroptosis via the miR-455-3p/NUPR1/FTH1 axis. Mol. Cancer 2024, 23, 113. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.A.G.; Bouyssou, J.M.; Togami, K.; Armand, O.; Rivas, H.G.; Yan, K.; Rice, S.; Cheng, S.; Lachtara, E.M.; Bourquin, J.P.; et al. BPDCN MYB fusions regulate cell cycle genes, impair differentiation, and induce myeloid-dendritic cell leukemia. JCI Insight 2024, 9, e183889. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).