Abstract
Background/Objectives: Rhodococcus rhodochrous IEGM 107 cells exhibit pronounced catalytic activity toward mono- and diterpenoids. However, the genetics and enzymatic foundations underlying this activity remain poorly understood. Methods: Using new-generation sequencing, the R. rhodochrous IEGM 107 whole genome was sequenced. Bioinformatic analysis and PCR were employed to identify and characterize genes, with a focus on cytochromes P450 (CYP450s). Results: The catalytic potential of R rhodochrous IEGM 107 was revealed. Its CYP450 genes were detected and analyzed, providing information on the enzymatic base of the strain related to the biotransformation of terpenoids. Conclusions: These findings enhance the understanding of the molecular and genetic basis for terpenoid transformations in R. rhodochrous actinomycetes. The results provide a foundation for future studies on gene expression and enzyme characterization aimed at developing efficient and selective biocatalysts for mono- and diterpenoid transformations.
1. Introduction
The biotransformation of plant terpenoids is one of the promising approaches used to obtain biologically active compounds. Actinomycetes of the genus Rhodococcus are highly effective catalysts for the targeted biotransformation of terpenoids of different structural groups, ranging from acyclic monoterpenoids to polycyclic triterpenoids [1]. The primary enzymes responsible for the degradation of complex organic compounds in bacterial cells are cytochromes P450 (CYP450s). However, current knowledge about the specific enzymes and corresponding genes involved in terpenoid biotransformation remains limited. To date, only a few enzymes from Rhodococcus species have been characterized: P450cin in R. jostii TMP1, which catalyzes the conversion of 1,8-cineole [2]; limonene-1,2-epoxide hydrolase (limC), involved in the transformation of limonene-1,2-epoxide and (6S)-carveol in R. erythropolis DCL14 [3,4]; and CYP108N12 and CYP108N14 from R. globerulus, which participate in the oxidation of p-cymene, (R)- and (S)-limonene, (S)-α-terpineol, and (S)-4-terpineol [5,6].
The strain R. rhodochrous IEGM 107 was isolated from the water of the Dnieper River and is deposited in the Regional Specialised Collection of Alkanotrophic Microorganisms. This strain exhibits unique biotransformation activity toward both the monoterpene alcohol trans-carveol and the diterpenoid dehydroabietic acid (Figure 1) [7,8]. Terpenoid derivatives are recognized as highly effective bioactive compounds and serve as key precursors for pharmaceutical synthesis [9,10]. Сarvone and dihydrocarvone, for example, as components of essential oils, exhibit pronounced antibacterial and fungicidal activity [11,12]. 7-Oxo-dehydroabietic acid has long been known for its fungicidal activity [13,14]. Thus, the strain’s activity toward both trans-carveol and dehydroabietic acid may offer advantages for green chemistry and fine organic synthesis.
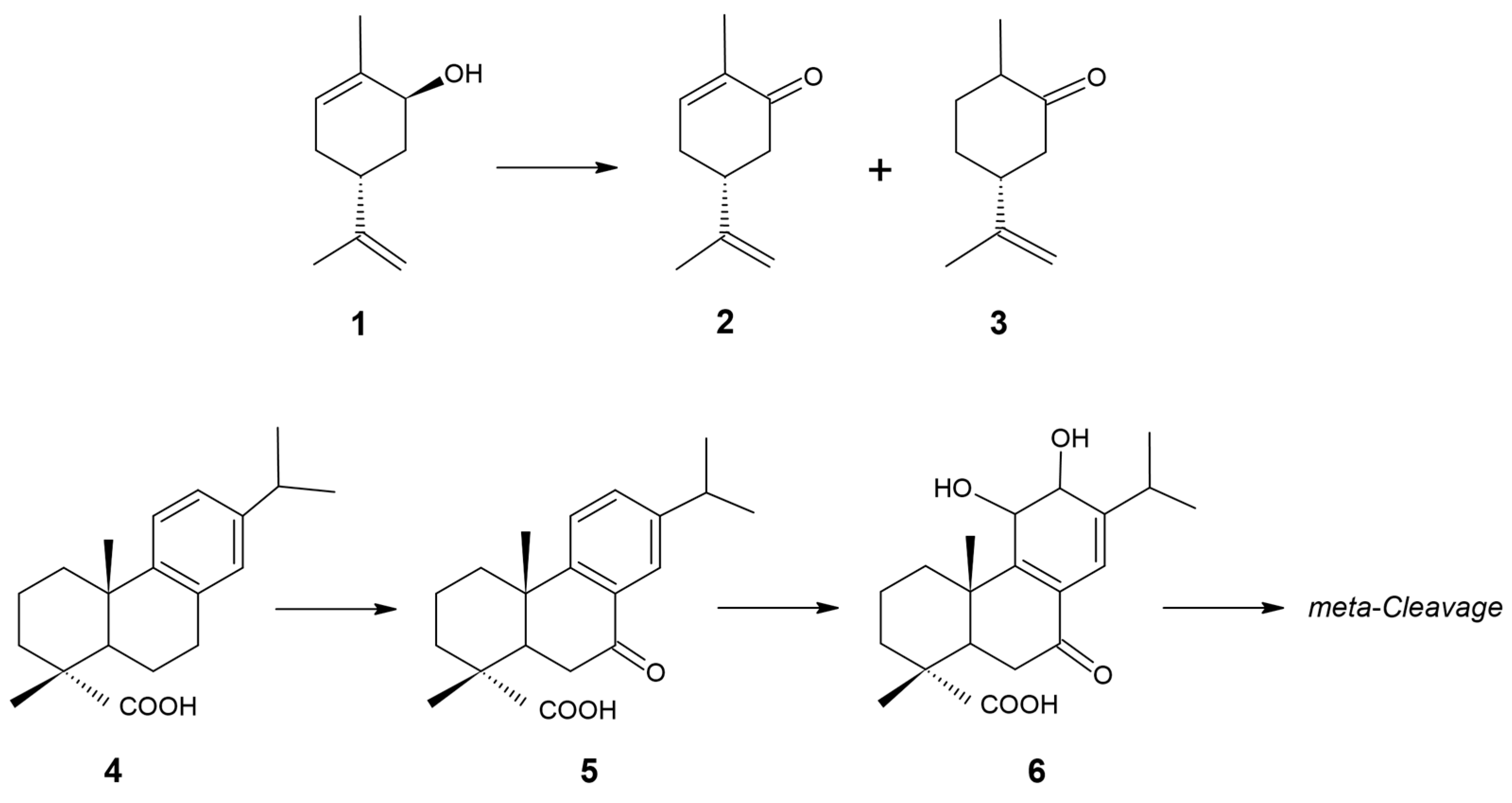
Figure 1.
Schemes of biotransformations catalyzed by R. rhodochrous IEGM 107 cells: (1) trans-carveol; (2) carvone; (3) dihydrocarvone; (4) dehydroabietic acid; (5) 7-oxo-dehydroabietic acid; (6) 11,12-dihydroxy-7-oxo-abieta-8,13-dien-18-oic acid. Modified from [7,8].
The aim of this work was to assess the catalytic potential of R. rhodochrous IEGM 107 and to identify the candidate genes involved in terpenoid transformation using bioinformatic tools.
2. Materials and Methods
2.1. Culture
R. rhodochrous IEGM 107 was isolated from the Dnieper River and is deposited in the Regional Specialized Collection of Alkanotrophic Microorganisms (acronym IEGM, WFCC number 285, WDCM number 768). In addition to transforming trans-carveol and dehydroabietic acid, the strain can utilize n-hexadecane as a sole carbon source and exhibits resistance to Pb2+ (10.0 mM), Cr6+, and Cu2+ (5.0 mM) (http://www.iegmcol.ru/strains/rhodoc/rhodoch/r_rhod107.html, accessed on 26 May 2025).
2.2. Whole Genome Sequencing
DNA extraction was performed using R. rhodochrous IEGM 107 cells revived from a nine-year-old lyophilized culture. The cells were cultivated in LB broth at 28 °C with shaking (160 rpm) for 24 h. Genomic DNA was isolated using the MagMAX™ Microbiome Ultra Nucleic Acid Isolation Kit (Thermo Fisher Scientific, Waltham, MA, USA) on a KingFisher™ Flex automated platform (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instructions.
A paired-end sequencing library (2 × 100 nt) was prepared using the Illumina DNA Prep Kit (Illumina, San Diego, CA, USA) and sequenced on the Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA). Library quality was assessed via fluorescence-based quantification and the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Sequencing reads were demultiplexed using Illumina bcl2fastq v2.20 (https://support.illumina.com/downloads/bcl2fastq-conversion-software-v2-20.html, accessed on 5 May 2025), and adapter trimming was performed with Skewer v. 0.2.2 [15]. FASTQ file quality was evaluated using FastQC v. 0.11.5-cegat [16], and genome assembly (contig level) was conducted using SPAdes v. 3.14.1 [17].
The whole genome sequence (WGS) of the strain is available in the NCBI database under GenBank accession number JAJNCP000000000.1.
2.3. Bioinformatic Analysis
For taxonomic identification, the whole genome was analyzed using the Type (Strain) Genome Server (TYGS) (https://tygs.dsmz.de, accessed on 5 May 2025) [18], a freely available bioinformatic platform that incorporates updated methodologies and features for genomic comparison [19]. Taxonomic nomenclature and synonymy were verified using the List of Prokaryotic Names with Standing in Nomenclature (LPSN, https://lpsn.dsmz.de, accessed on 5 May 2025) [19]. Pairwise genome comparisons were performed using the Genome BLAST Distance Phylogeny (GBDP) approach with the ‘trimming’ algorithm and distance formula d5 (100 distance replicates per analysis) [20]. Digital DNA–DNA hybridization (dDDH) and confidence intervals were calculated using GGDC 4.0 (Genome-to-Genome Distance Calculator 4.0, https://ggdc.dsmz.de/ggdc.php#, accessed on 5 May 2025) [19,20]. The resulting intergenomic distances were used to infer a balanced minimum evolution tree with branch support via FASTME 2.1.6.1 including SPR postprocessing [21]. Phylogenomic trees were constructed using FASTME 2.1.6.1 with SPR postprocessing and branch support estimated from 100 pseudo-bootstrap replicates. The resulting tree was midpoint-rooted [22] and visualized using PhyD3 (http://phyd3.bits.vib.be, accessed on 5 May 2025) [23]. Average Nucleotide Identity (ANI) was determined using the EZBioCloud ANI calculator (https://www.ezbiocloud.net/tools/ani, accessed on 5 May 2025) [24].
The search for the candidate genes involved in mono- and diterpenoid biotransformation, along with rRNA genes, was performed using the RAST (Rapid Annotation using Subsystem Technology, https://rast.nmpdr.org/, accessed on 5 May 2025) [25]. The analysis was performed through the automated annotation of the complete genome sequence of the biotransformer strain [26,27].
Target gene sequence comparisons were performed using the BLASTN and BLASTP tools available on the NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 8 May 2025). Biosynthetic gene cluster prediction was conducted using antiSMASH (https://antismash.secondarymetabolites.org/, accessed on 8 May 2025) [28]. Amino acid sequence analysis and metabolic pathway reconstruction were carried out using the KEGG database (Kyoto Encyclopedia of Genes and Genomes, https://www.genome.jp/kegg/, accessed on 8 May 2025) and GhostKOALA [29]. The pairwise alignment and phylogenetic tree construction of CYP450 gene products were performed using Clustal Omega (https://www.ebi.ac.uk/jdispatcher/msa/clustalo, accessed on 20 May 2025) [30], with tree visualization conducted in Jalview v. 2.11.4.1 [31]. The identification of genomic islands and the determination of GC content were performed using IslandViewer 4 [32] and GC Content Calculator (https://en.vectorbuilder.com/tool/gc-content-calculator.html, accessed on 17 June 2025), respectively.
2.4. PCR and Electrophoresis of Amplicons
The biomass of R. rhodochrous IEGM 107 was obtained after 24 h of cultivation in LB broth (Diaem, Moscow, Russia), and DNA was extracted using the ExtractDNA Blood Genomic DNA Isolation Kit (Evrogen, Moscow, Russia) following the manufacturer’s instructions. The concentration and purity of the extracted DNA were assessed using a Qubit™ Fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) with a QuDye dsDNA BR kit (Lumiprobe, Moscow, Russia) and a NanoPhotometer N50 (Implen, Munich, Germany), respectively.
The purified DNA was used as a template for PCR with qPCRmix-HS SYBR (Evrogen, Moscow, Russia) and specific primers [33] on a CFX Connect™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Species-specific primers targeting the 16S rRNA gene of R. rhodochrous were used as a positive control. The PCR cycling conditions were applied as previously described [33].
Amplicon presence and size were verified by horizontal electrophoresis on a 1.5% agarose gel in TBE buffer, stained with GelRed (Diaem, Moscow, Russia), and visualized using a Gel Doc XR+ system (Bio-Rad, Hercules, CA, USA). PCR products (5 μL) were mixed with 0.5 μL of 4X Blue Gel Loading Dye (Evrogen, Moscow, Russia) prior to loading. A DNA length marker ranging from 50 to 700 bp (Evrogen, Moscow, Russia) was included for size estimation. Electrophoresis was performed at 70 V for 40 min.
3. Results and Discussion
The assembled genome of R. rhodochrous IEGM 107 comprised 118 contigs, totaling 5.7 Mbp in length, with an N50 of 190,984 bp, a GC content of 67.8%, and an average sequencing coverage of 357×. Genome annotation identified 5555 coding sequences (CDSs) and 56 RNA genes (Table 1).

Table 1.
Genome features for R. rhodochrous IEGM 107 assembly (according to RAST).
Using phenotypic methods (Table S1, Supplementary Materials) [34] and an analysis of genomic characteristics (Table 2), the IEGM 107 strain was identified as belonging to the R. rhodochrous species (Figure 2). The resulting species and subspecies clusters are presented in Table S2 (Supplementary Materials). Eight distinct species clusters were identified, with all query strains assigned to specific clusters. Notably, the IEGM 107 strain was classified within one of the eight recognized subspecies clusters.

Table 2.
Overall genomic characteristics of R. rhodochrous IEGM 107 compared with type strains from TYGS database.
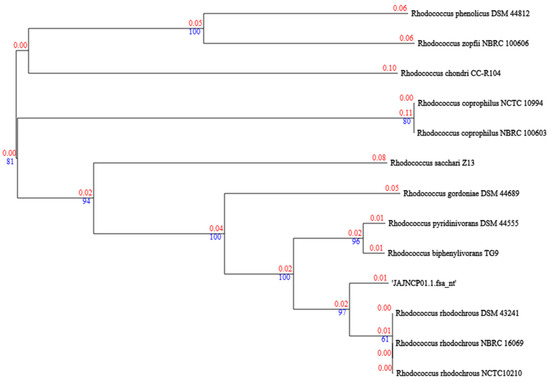
Figure 2.
Phylogenetic tree of genomes of R. rhodochrous IEGM 107 (JAJNCP01.1.fsa_nt) and type strains from TYGS database. Tree inferred with FastME 2.1.6.1 [21] from GBDP distances calculated from genome sequences. Branch lengths are scaled in terms of GBDP distance formula d5. Numbers above branches are GBDP pseudo-bootstrap support values > 60% from 100 replications, with average branch support of 84.2%. Tree was rooted at midpoint [22].
Figure 2.
Phylogenetic tree of genomes of R. rhodochrous IEGM 107 (JAJNCP01.1.fsa_nt) and type strains from TYGS database. Tree inferred with FastME 2.1.6.1 [21] from GBDP distances calculated from genome sequences. Branch lengths are scaled in terms of GBDP distance formula d5. Numbers above branches are GBDP pseudo-bootstrap support values > 60% from 100 replications, with average branch support of 84.2%. Tree was rooted at midpoint [22].
Among 5555 CDSs, genes of unknown and known functions were present in a 78:22 ratio. The functional category distribution of the known genes is shown in Figure 3. Notably, we identified genes encoding enzymes of the nonmevalonate pathway for isoprenoid biosynthesis, including EC 2.2.1.7, 1.1.1.267, 2.7.7.60, 2.7.1.148, 4.6.1.12, 1.17.7.1, and 1.17.1.2. Genome mining using AntiSMASH revealed biosynthetic gene clusters associated with terpene and terpene precursor biosynthesis (Table 3).

Table 3.
Biosynthetic gene clusters in the genome of R. rhodochrous IEGM 107.
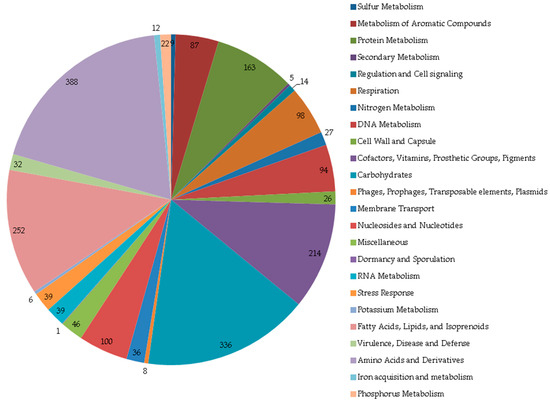
Figure 3.
Distribution of subsystem categories in genome of R. rhodochrous IEGM 107. Image obtained using SEED Viewer 2.0 [35].
Figure 3.
Distribution of subsystem categories in genome of R. rhodochrous IEGM 107. Image obtained using SEED Viewer 2.0 [35].
Additionally, biosynthetic gene clusters were identified for the synthesis of non-ribosomal peptide synthetases, β-lactones, ectoines, non-ribosomal peptide metallophores, non-α poly-amino acids, and type I polyketide synthases (Table 3). Within the genome of IEGM 107, 13 genes encoding non-ribosomal peptide synthetase modules for siderophore biosynthesis, 13 genes encoding polyketide synthase modules and related proteins, 1 gene for L-ectoine synthase, 4 genes for ectoine/hydroxyectoine ABC transporters, and 1 gene for ectoine hydroxylase were identified. Siderophores play a crucial role in regulating iron bioavailability, which is essential for iron-dependent enzymes including cytochrome P450 (CYP450) [36]. Polyketides and ectoines represent bioactive secondary metabolites of bacterial origin that show significant potential as therapeutic agents [37,38].
Representatives of Rhodococcus spp. are well known for their broad substrate specificity and ability to degrade a wide range of complex organic compounds [39]. A genomic analysis of the IEGM 107 strain identified 63 CDSs for monooxygenases, 33 for dioxygenases, 12 for hydroxylases, 7 for peroxidases, and 350 for dehydrogenases. Among the oxygenases, we detected genes encoding two alkane 1-monooxygenases, nine CYP450s, eight cyclohexanone monooxygenases, three 3-ketosteroid-9α-monooxygenases, two steroid C27-monooxygenases, two catechol 1,2-dioxygenases, three 4-hydroxyphenylpyruvate dioxygenases, two 2,3-dihydroxybiphenyl 1,2-dioxygenases, two benzoate 1,2-dioxygenases, five gentisate 1,2-dioxygenases, two protocatechuate 3,4-dioxygenases, and one lignostilbene-α,β-dioxygenase.
In addition, several genes related to sterol and steroid metabolism were identified, including six CDSs for cholesterol oxidases, one for lanosterol 14α-demethylase, four for 3-oxosteroid 1-dehydrogenases, one for 3α-hydroxysteroid dehydrogenase, one for steroid monooxygenase, two for steroid C27-monooxygenases, and three for 3-ketosteroid-9α-monooxygenases. Notably, we also identified a gene encoding limonene-1,2-epoxide hydrolase, a key enzyme in monoterpenoid metabolism. These findings highlight the extensive catalytic potential of the IEGM 107 strain.
Among the enzymes identified, CYP450s are of particular interest. CYP450 enzymes constitute a large and diverse superfamily of over 20,000 proteins that play key roles in the biosynthesis of complex natural products—including steroids, terpenes, alkaloids, flavonoids, and vitamins—as well as in drug metabolism and the degradation of environmental pollutants [40,41]. Since the 1950s, various fungal and bacterial strains harboring CYP450s have been utilized as biocatalysts in organic and medicinal chemistry applications [42].
Notably, the IEGM 107 strain catalyzes the same transformations of trans-carveol as R. erythropolis DCL14 (see Figure 1) [3,43]. While the carveol dehydrogenase from R. erythropolis DCL14 was identified by the authors, the complete protein sequence of carveol dehydrogenase is available in the UniProt database only for R. jostii RHA1 [44]. Therefore, we used this sequence for further comparative analysis. Regarding dehydroabietic acid transformations (see Figure 1), previous studies have characterized these reactions in Pseudomonas diterpeniphila A19-6a [45,46], with complete enzyme sequences available in UniProt. We conducted a search of these reference protein sequences in the R. rhodochrous IEGM 107 genome, and no high-identity matches were found. We therefore conclude that trans-carveol and dehydroabietic acid transformations in R. rhodochrous IEGM 107 are catalyzed by other functional genes and proteins.
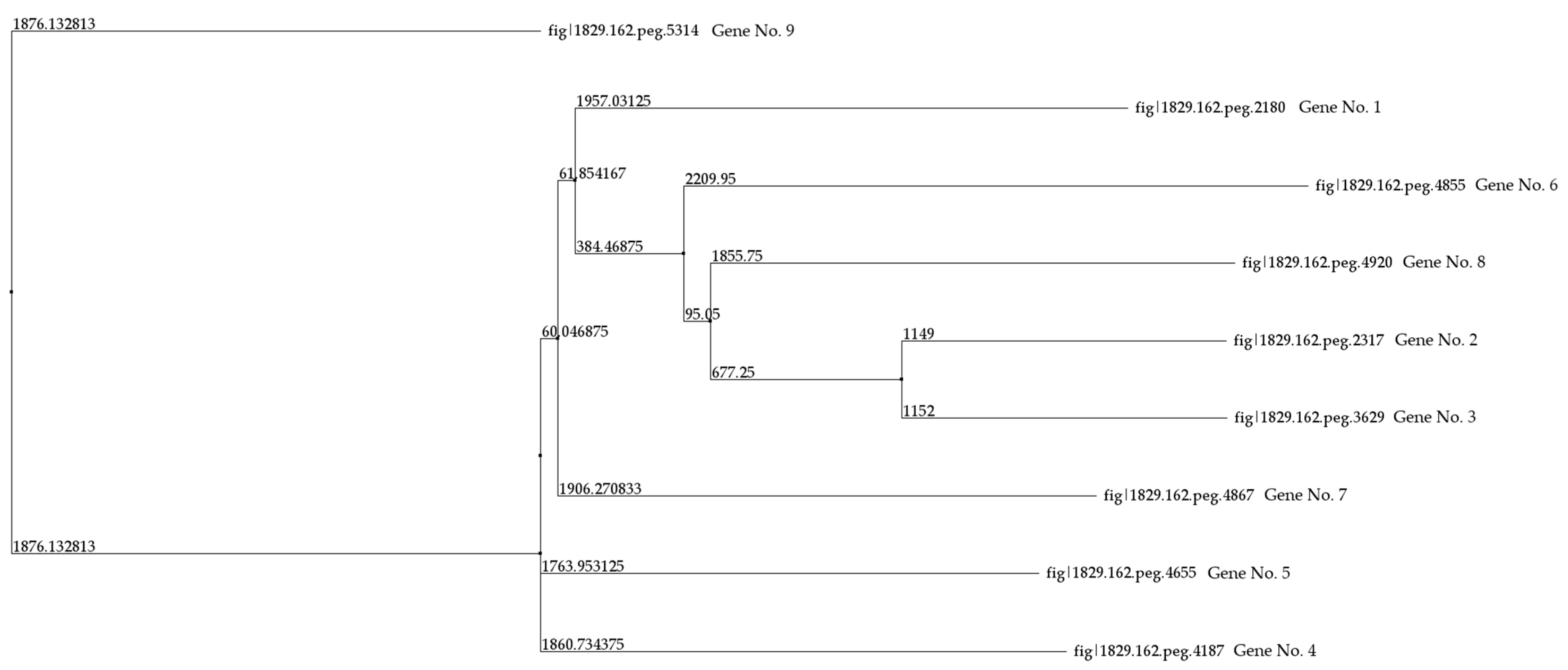
In the genome of R. rhodochrous IEGM 107, nine genes encoding CYP450 monooxygenases and hydroxylases were identified (Table 4). A comparative analysis of nucleotide and amino acid sequences, including pairwise alignments of the latter, revealed identity levels ranging from 7.68% to 33.48%. The highest amino acid sequence identity (63.78%) was observed between CYP450s 2 and 3. Using these pairwise alignment results, a phylogenetic tree was constructed (Figure 4).

Table 4.
Genes of R. rhodochrous IEGM 107 encoding CYP450.
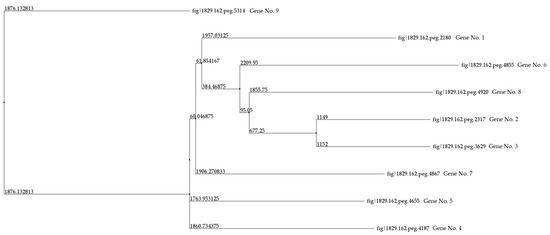
Figure 4.
A distance tree for the pairwise alignments of the amino acid sequences of the CYP450 of R. rhodochrous IEGM 107. The method used to construct the tree was Neighbor Joining. Protein IDs and gene numbers are the same as those in Table 4.
The genetic surroundings of CYP450 genes were analyzed (Table 5). In all cases, genes encoding transcriptional regulators were located either immediately upstream or in close proximity to the CYP450 genes. Furthermore, the potential for the horizontal gene transfer of the CYP450-coding genes in R. rhodochrous IEGM 107 was assessed. Mobile element proteins were detected adjacent to only three CYP450 genes, suggesting that horizontal gene transfer likely plays a limited role in the dissemination of CYP450 genes among Rhodococcus species.

Table 5.
Genetic surroundings of genes encoding CYP450 enzymes in R. rhodochrous IEGM 107.
To evaluate the uniqueness of the catalytic systems in R. rhodochrous IEGM 107, the sequences of identified genes were compared with those available in the NCBI database (Table 6). The comparison revealed that, with the exception of Genes 4 and 5, all genes are widely distributed among R. rhodochrous strains. Gene No. 4 is the least common, being present in only six strains, and is likely involved in complex or aromatic carbohydrate degradation. This functional inference is supported by the genomic context, which includes neighboring genes encoding 3-oxosteroid 1-dehydrogenase, isochorismatase, oxidoreductases, and a LysR family transcriptional regulator [47].

Table 6.
Homology of CYP450 genes among R. rhodochrous strains.
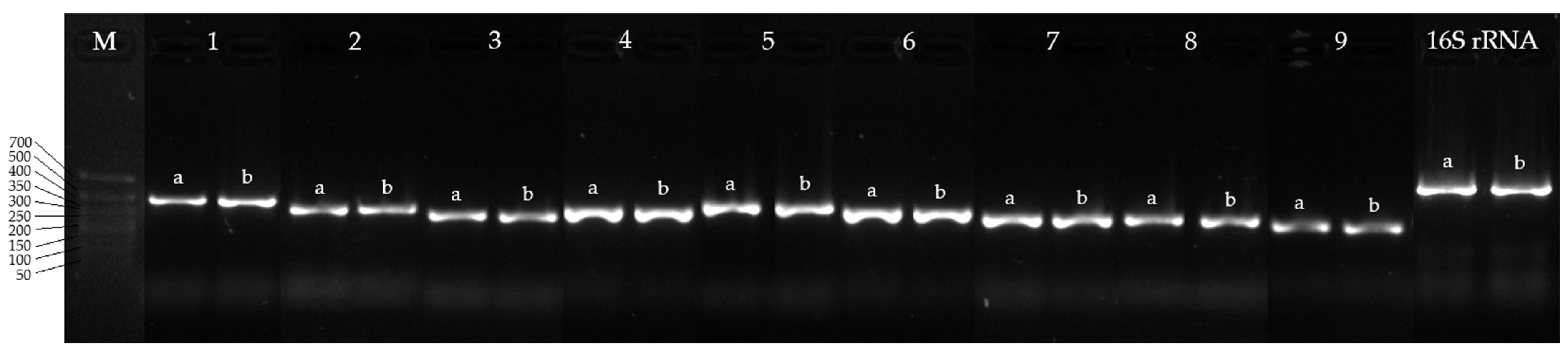
A comparative analysis of nucleotide and protein sequences (Table 6) demonstrated a high similarity between the CYP450s of IEGM 107 and those of IEGM 1362, the latter being well-characterized for its efficient biotransformation of the monoterpenoid (–)-isopulegol [48]. Given this sequence conservation, PCR was performed using primers previously designed for IEGM 1362 CYP450 genes [33]. The amplification results showed the specific detection of corresponding genes in the IEGM 107 genome, with no nonspecific products observed (Figure 5). These findings confirm that these primers can be effectively used in future studies aimed at identifying enzymes involved in terpenoid biotransformations.
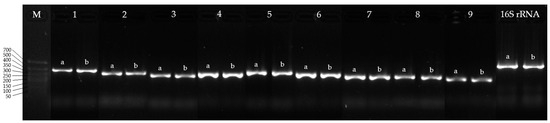
Figure 5.
Electropherogram of PCR products of R. rhodochrous IEGM 107 (a) and IEGM 1362 (b) with specific primers for CYP450 genes: M, DNA length marker from 700 to 50 bp; 16S rRNA, 16S rRNA gene of R. rhodochrous (positive control). Gene numbers (1–9) are same as gene numbers in Table 4.
Notably, CYP450 Gene 5, which shows similar nucleotide and amino acid sequences in nine and seven strains, respectively, exhibits 100% identity with CYP450 Gene 6 from the IEGM 1362 strain [33]. In the genomic context of Gene 5, genes encoding ferredoxin and ferredoxin reductase were also identified. Additionally, a mobile element protein located nearby may facilitate the dissemination of this CYP450 gene among R. rhodochrous strains (see Table 5). Moreover, this gene exhibits the lowest GC content (61%) among the identified CYP450 genes, compared to the 62–69% range observed for the others. Furthermore, genomic analysis revealed a genetic island containing both transposase and mobile element protein adjacent to Gene 5. These features support the potential for the horizontal transfer of this gene among Rhodococcus strains. These findings support evaluating the catalytic activity of the IEGM 107 strain toward (–)-isopulegol and other terpene substrates.
4. Conclusions
The obtained data advance our understanding of the molecular genetic basis underlying terpenoid transformation by Rhodococcus actinomycetes. Specifically, the identification and characterization of CYP450 enzymes and associated redox partners provide valuable insights into the enzymatic systems responsible for mono- and diterpenoid bioconversion. These findings establish a solid foundation for future investigations focused on gene expression profiling and functional validation, with the goal of identifying the key genes and enzymes involved in the highly efficient and selective biotransformation of terpenoid compounds. This research direction may ultimately contribute to the development of biocatalysts for industrial and pharmaceutical applications involving terpenoid modification.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/genes16070739/s1, Table S1: Physiological and biochemical features of R. rhodochrous IEGM 107; Table S2: Strains in the dataset and their type-based species and subspecies clustering.
Author Contributions
Conceptualization, I.B.I.; methodology, N.A.P.; investigation, P.Y.M. and N.A.P.; resources, I.B.I.; writing—original draft preparation, P.Y.M. and N.A.P.; writing—review and editing, I.B.I.; visualization, P.Y.M. and N.A.P.; supervision, I.B.I.; project administration, I.B.I.; funding acquisition, I.B.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant no. 24-14-20015.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The draft genome sequence data are available at NCBI under GenBank accession number JAJNCP000000000.1.
Acknowledgments
This work was carried out using equipment from The Core Facilities Centers “Regional Specialised Collection of Alkanotrophic Microorganisms” and “Research of Materials and Matter” at Perm Federal Research Center of the Ural Branch of the Russian Academy of Sciences. The draft genome of IEGM 107 was sequenced and assembled at CeGaT GmbH, Tuebingen, Germany (www.cegat.de).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CDS | Coding sequence |
| CYP450 | Cytochrome P450 |
| R. | Rhodococcus |
References
- Maltseva, P.Y.; Plotnitskaya, N.A.; Ivshina, I.B. Transformation of Terpenoids and Steroids Using Actinomycetes of the Genus Rhodococcus. Molecules 2024, 29, 3378. [Google Scholar] [CrossRef] [PubMed]
- Jakubovska, J.; Meškys, R. Characterization of 1,8-Cineole Degradation Encoding Operon from Rhodococcus sp. TMP1. Chemija 2016, 27, 84–91. [Google Scholar]
- van der Werf, M.J.; van der Ven, C.; Barbirato, F.; Eppink, M.H.M.; de Bont, J.A.M.; van Berkel, W.J.H. Stereoselective Carveol Dehydrogenase from Rhodococcus erythropolis DCL14. J. Biol. Chem. 1999, 274, 26296–26304. [Google Scholar] [CrossRef]
- van der Werf, M.J.; Overkamp, K.M.; de Bont, J.A.M. Limonene-1,2-Epoxide Hydrolase from Rhodococcus erythropolis DCL14 Belongs to a Novel Class of Epoxide Hydrolases. J. Bacteriol. 1998, 180, 5052–5057. [Google Scholar] [CrossRef]
- Giang, P.D.; Churchman, L.R.; Stok, J.E.; Soo, R.M.; De Voss, J.J. CYP108N12 Initiates p-Cymene Biodegradation in Rhodococcus globerulus. Arch. Biochem. Biophys. 2022, 730, 109410. [Google Scholar] [CrossRef]
- Giang, P.D.; Churchman, L.R.; Buczynski, J.B.; Bell, S.G.; Stok, J.E.; De Voss, J.J. CYP108N14: A Monoterpene Monooxygenase from Rhodococcus globerulus. Arch. Biochem. Biophys. 2024, 752, 109852. [Google Scholar] [CrossRef]
- Maltseva, P.Y.; Plotnitskaya, N.A.; Chudinova, A.A.; Ilyina, I.V.; Salakhutdinov, N.F.; Ivshina, I.B. Search for Rhodococcus Strains—Active Biotransformers of Monoterpenoids. Microb. Biotechnol. Fundam. Appl. Asp. Collect. Sci. Pap. 2024, 16, 351–360. (In Russian) [Google Scholar]
- Ivanova, K.M.; Grishko, V.V.; Ivshina, I.B. Highly Efficient Biodegradation of Ecotoxic Dehydroabietic Acid by Resting Cells of Rhodococcus rhodochrous IEGM 107. Microbiology 2022, 91, 364–377. [Google Scholar] [CrossRef]
- Zang, Y.; Sun, R.; Feng, R.; Zhu, H.; Li, X. Recent Advances of Terpenoids with Intriguing Chemical Skeletons and Biological Activities. Chin. J. Chem. 2025, 43, 443–469. [Google Scholar] [CrossRef]
- Rustamova, N.; Huang, G.; Isokov, M.; Movlanov, J.; Farid, R.; Buston, I.; Xiang, H.; Davranov, K.; Yili, A. Modification of Natural Compounds through Biotransformation Process by Microorganisms and Their Pharmacological Properties. Fitoterapia 2024, 179, 106227. [Google Scholar] [CrossRef]
- Aggarwal, K.K.; Khanuja, S.P.S.; Ahmad, A.; Santha Kumar, T.R.; Gupta, V.K.; Kumar, S. Antimicrobial Activity Profiles of the Two Enantiomers of Limonene and Carvone Isolated from the Oils of Mentha spicata and Anethum sowa. Flavour Fragr. J. 2002, 17, 59–63. [Google Scholar] [CrossRef]
- Yang, X.; Han, H.; Li, B.; Zhang, D.; Zhang, Z.; Xie, Y. Fumigant Toxicity and Physiological Effects of Spearmint (Mentha spicata, Lamiaceae) Essential Oil and Its Major Constituents against Reticulitermes Dabieshanensis. Ind. Crops Prod. 2021, 171, 113894. [Google Scholar] [CrossRef]
- Muftic, M. Essay on Antibiotic Properties of Abietyl Compounds. Q. J. Crude Drug Res. 1970, 10, 1301–1607. [Google Scholar] [CrossRef]
- Henriks, M.; Ekman, R.; Von Weissenberg, K. Bioassay of Some Resin and Fatty Acids with Fomes annosus. Acta Acad. Abo. 1979, 39, 1–7. [Google Scholar]
- Jiang, H.; Lei, R.; Ding, S.-W.; Zhu, S. Skewer: A Fast and Accurate Adapter Trimmer for next-Generation Sequencing Paired-End Reads. BMC Bioinform. 2014, 15, 182. [Google Scholar] [CrossRef]
- Andrews, S. FastQC A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 5 May 2025).
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.; Korobeynikov, A.; Lapidus, A.; Prjibelsky, A.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling genomes and mini-metagenomes from highly chimeric reads. In Research in Computational Molecular Biology, Proceedings of the 17th Annual International Conference, RECOMB 2013, Beijing, China, 7–10 April 2013; Springer: Berlin/Heidelberg, Germany, 2013; pp. 158–170. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Carbasse, J.S.; Peinado-Olarte, R.L.; Göker, M. TYGS and LPSN: A Database Tandem for Fast and Reliable Genome-Based Classification and Nomenclature of Prokaryotes. Nucleic Acids Res. 2022, 50, D801–D807. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.-P.; Göker, M. Genome Sequence-Based Species Delimitation with Confidence Intervals and Improved Distance Functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Lefort, V.; Desper, R.; Gascuel, O. FastME 2.0: A Comprehensive, Accurate, and Fast Distance-Based Phylogeny Inference Program. Mol. Biol. Evol. 2015, 32, 2798–2800. [Google Scholar] [CrossRef]
- Farris, J.S. Estimating Phylogenetic Trees from Distance Matrices. Am. Nat. 1972, 106, 645–668. [Google Scholar] [CrossRef]
- Kreft, Ł.; Botzki, A.; Coppens, F.; Vandepoele, K.; Van Bel, M. PhyD3: A Phylogenetic Tree Viewer with Extended phyloXML Support for Functional Genomics Data Visualization. Bioinformatics 2017, 33, 2946–2947. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Ha, S.; Lim, J.; Kwon, S.; Chun, J. A Large-Scale Evaluation of Algorithms to Calculate Average Nucleotide Identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid Annotations Using Subsystems Technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of Microbial Genomes Using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A Modular and Extensible Implementation of the RAST Algorithm for Building Custom Annotation Pipelines and Annotating Batches of Genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; Van Wezel, G.P.; Medema, M.H.; Weber, T. AntiSMASH 6.0: Improving Cluster Detection and Comparison Capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher Sequence Analysis Tools Framework in 2024. Nucleic Acids Res 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2--a Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Bertelli, C.; Laird, M.R.; Williams, K.P.; Fraser University Research Computing Group, S.; Lau, B.Y.; Hoad, G.; Winsor, G.L.; Brinkman, F.S. IslandViewer 4: Expanded Prediction of Genomic Islands for Larger-Scale Datasets. Nucleic Acids Res. 2017, 45, W30–W35. [Google Scholar] [CrossRef]
- Maltseva, P.Y.; Plotnitskaya, N.A.; Krivoruchko, A.V.; Beletskiy, A.V.; Rakitin, A.L.; Mardanov, A.V.; Ivshina, I.B. Bioinformatics Analysis of the Genome of Rhodococcus rhodochrous IEGM 1362, an (−)-Isopulegol Biotransformer. Genes 2024, 15, 992. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Goodfellow, M. Rhodococcus (Zopf 1891) Emend. Goodfellow, Alderson and Chun 1998a. In Bergey’s Manual of Systematics of Archaea and Bacteria; Springer: London, UK, 2012; pp. 1–50. [Google Scholar]
- Overbeek, R.; Begley, T.; Butler, R.M.; Choudhuri, J.V.; Chuang, H.-Y.; Cohoon, M.; de Crécy-Lagard, V.; Diaz, N.; Disz, T.; Edwards, R.; et al. The Subsystems Approach to Genome Annotation and Its Use in the Project to Annotate 1000 Genomes. Nucleic Acids Res. 2005, 33, 5691–5702. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Wei, X.; Wan, C.; Zhao, W.; Song, R.; Xin, S.; Song, K. Exploring the Biological Pathways of Siderophores and Their Multidisciplinary Applications: A Comprehensive Review. Molecules 2024, 29, 2318. [Google Scholar] [CrossRef]
- Baerson, S.R.; Rimando, A.M. A Plethora of Polyketides: Structures, Biological Activities, and Enzymes; ACS Symposium Series: Washington, DC, USA, 2007; pp. 2–14. [Google Scholar]
- Xingrong, L.; Gorish, B.M.T.; Qaria, M.A.; Hussain, A.; Abdelmula, W.I.Y.; Zhu, D. Unlocking Ectoine’s Postbiotic Therapeutic Promise: Mechanisms, Applications, and Future Directions. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef] [PubMed]
- Ivshina, I.; Bazhutin, G.; Tyumina, E. Rhodococcus Strains as a Good Biotool for Neutralizing Pharmaceutical Pollutants and Obtaining Therapeutically Valuable Products: Through the Past into the Future. Front. Microbiol. 2022, 13, 967127. [Google Scholar] [CrossRef]
- Janocha, S.; Schmitz, D.; Bernhardt, R. Terpene Hydroxylation with Microbial Cytochrome P450 Monooxygenases. Adv. Biochem. Eng. Biotechnol. 2015, 148, 215–250. [Google Scholar] [CrossRef]
- Cryle, M.J.; Stok, J.E.; De Voss, J.J. Reactions Catalyzed by Bacterial Cytochromes P450. Aust. J. Chem. 2003, 56, 749–762. [Google Scholar] [CrossRef]
- Smith, K.E.; Ahmed, F.; Antoniou, T. Microbial Transformations of Steroids. Biochem. Soc. Trans. 1993, 21, 1077–1080. [Google Scholar] [CrossRef]
- Tecelão, C.S.R.; Van Keulen, F.; Da Fonseca, M.M.R. Development of a Reaction System for the Selective Conversion of (–)-trans-Carveol to (–)-Carvone with Whole Cells of Rhodococcus erythropolis DCL14. J. Mol. Catal. B Enzym. 2001, 11, 719–724. [Google Scholar] [CrossRef]
- McLeod, M.P.; Warren, R.L.; Hsiao, W.W.L.; Araki, N.; Myhre, M.; Fernandes, C.; Miyazawa, D.; Wong, W.; Lillquist, A.L.; Wang, D.; et al. The Complete Genome of Rhodococcus sp. RHA1 Provides Insights into a Catabolic Powerhouse. Proc. Natl. Acad. Sci. USA. 2006, 103, 15582–15587. [Google Scholar] [CrossRef]
- Morgan, C.A.; Wyndham, R.C. Characterization of Tdt Genes for the Degradation of Tricyclic Diterpenes by Pseudomonas diterpeniphila A19-6a. Can. J. Microbiol. 2002, 48, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Martin, V.J.J.; Mohn, W.W. A Cytochrome P450 Involved in the Metabolism of Abietane Diterpenoids by Pseudomonas abietaniphila BKME-9. J. Bacteriol. 2004, 186, 3631–3639. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, S.E.; Oyston, P.C.F. Structure and Function of the LysR-Type Transcriptional Regulator (LTTR) Family Proteins. Microbiology 2008, 154, 3609–3623. [Google Scholar] [CrossRef] [PubMed]
- Ivshina, I.B.; Luchnikova, N.A.; Maltseva, P.Y.; Ilyina, I.V.; Volcho, K.P.; Gatilov, Y.V.; Korchagina, D.V.; Kostrikina, N.A.; Sorokin, V.V.; Mulyukin, A.L.; et al. Biotransformation of (–)-Isopulegol by Rhodococcus rhodochrous. Pharmaceuticals 2022, 15, 964. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).