Expression Dynamics and Estrogen Response of Estrogen Receptors in Duolang Sheep During Puberty
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care
2.2. Animals and Tissue Collection
2.3. Blood Sample Collection and Hormone Measurement
2.4. Granulosa Cell Culture
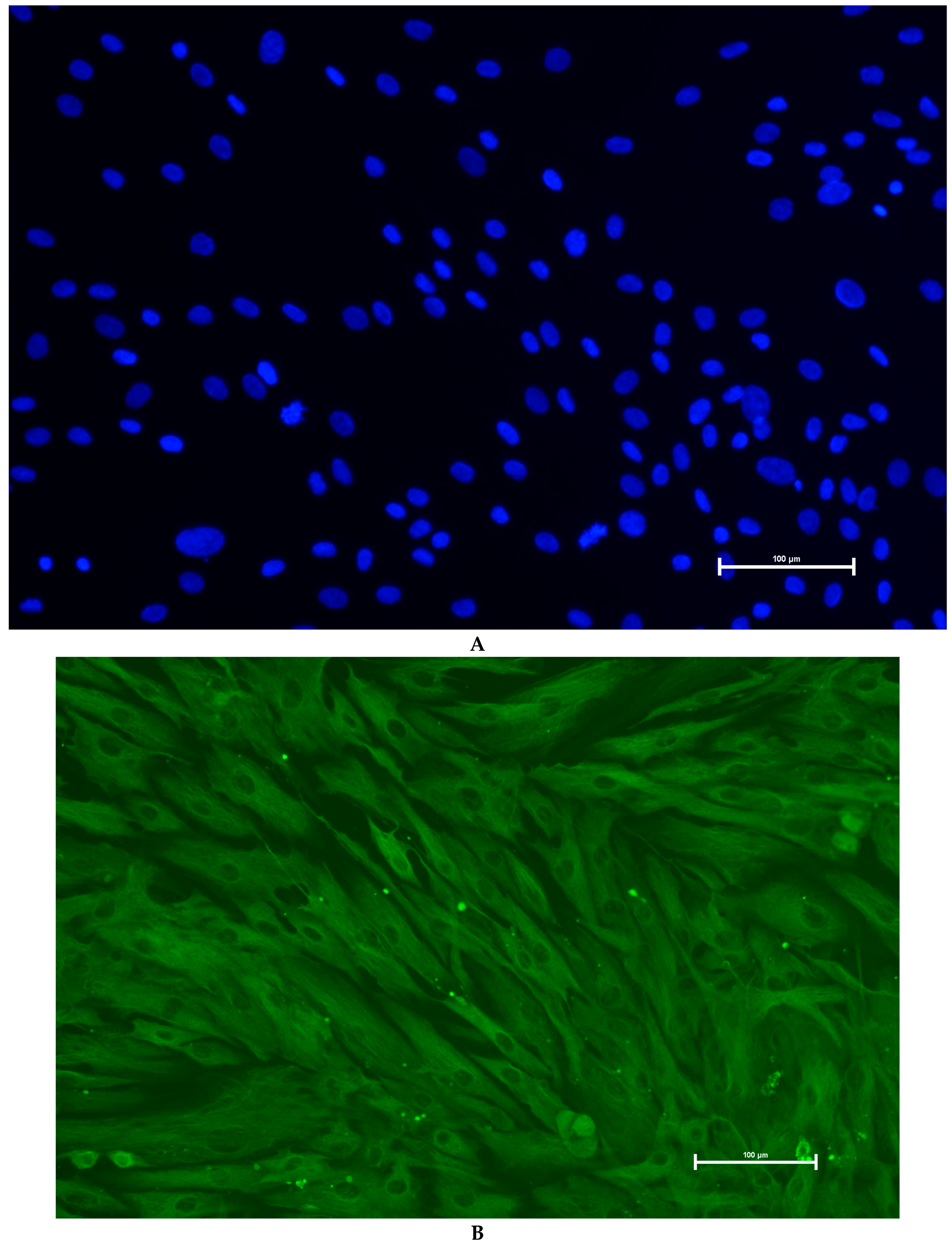
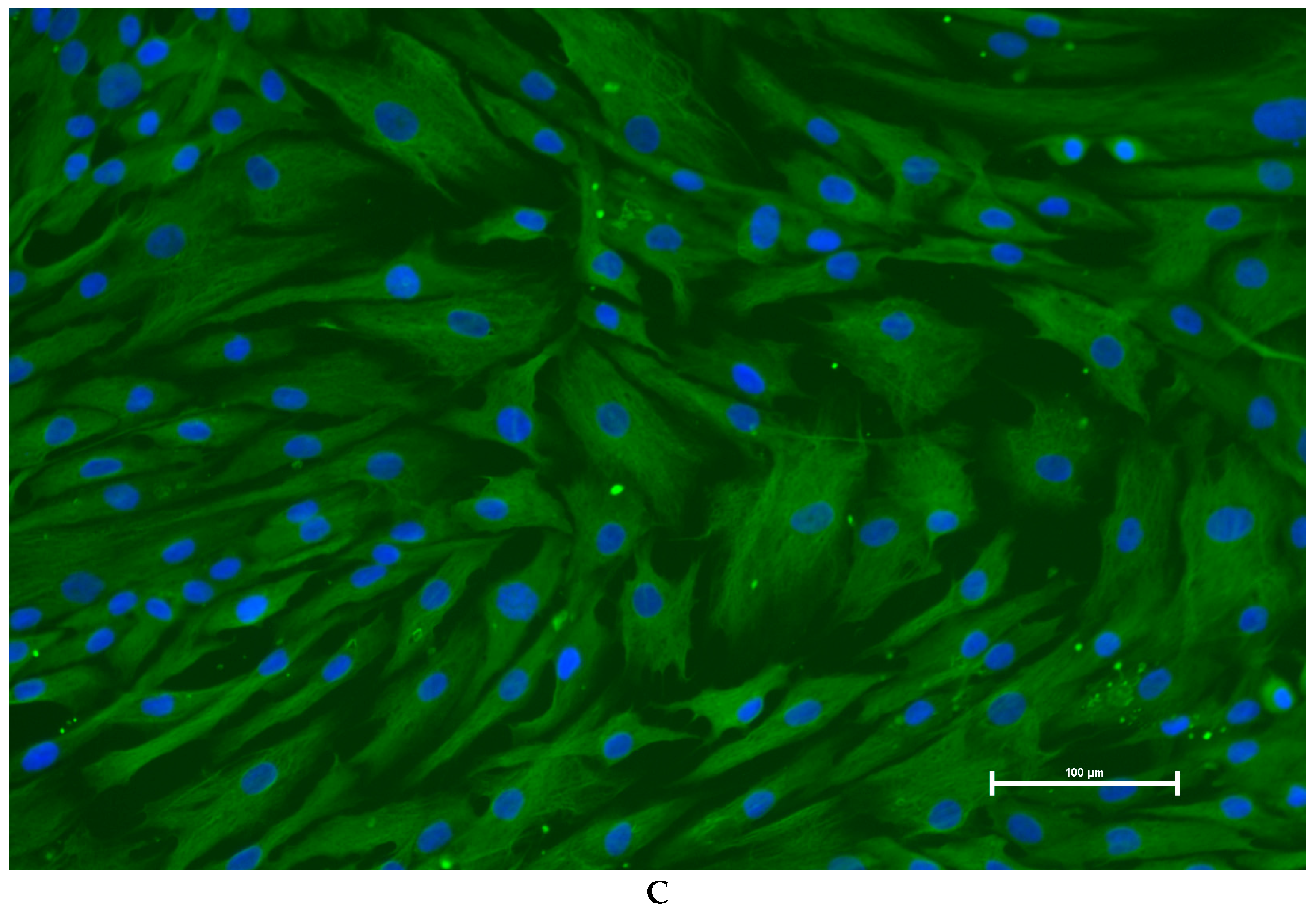
2.5. Granulosa Cell Identification
2.6. Total RNA Isolation and cDNA Synthesis
2.7. Quantitative Real-Time PCR and Cloning
2.8. Western Blot Analysis of ER Expression
2.9. Statistical Analysis
3. Results
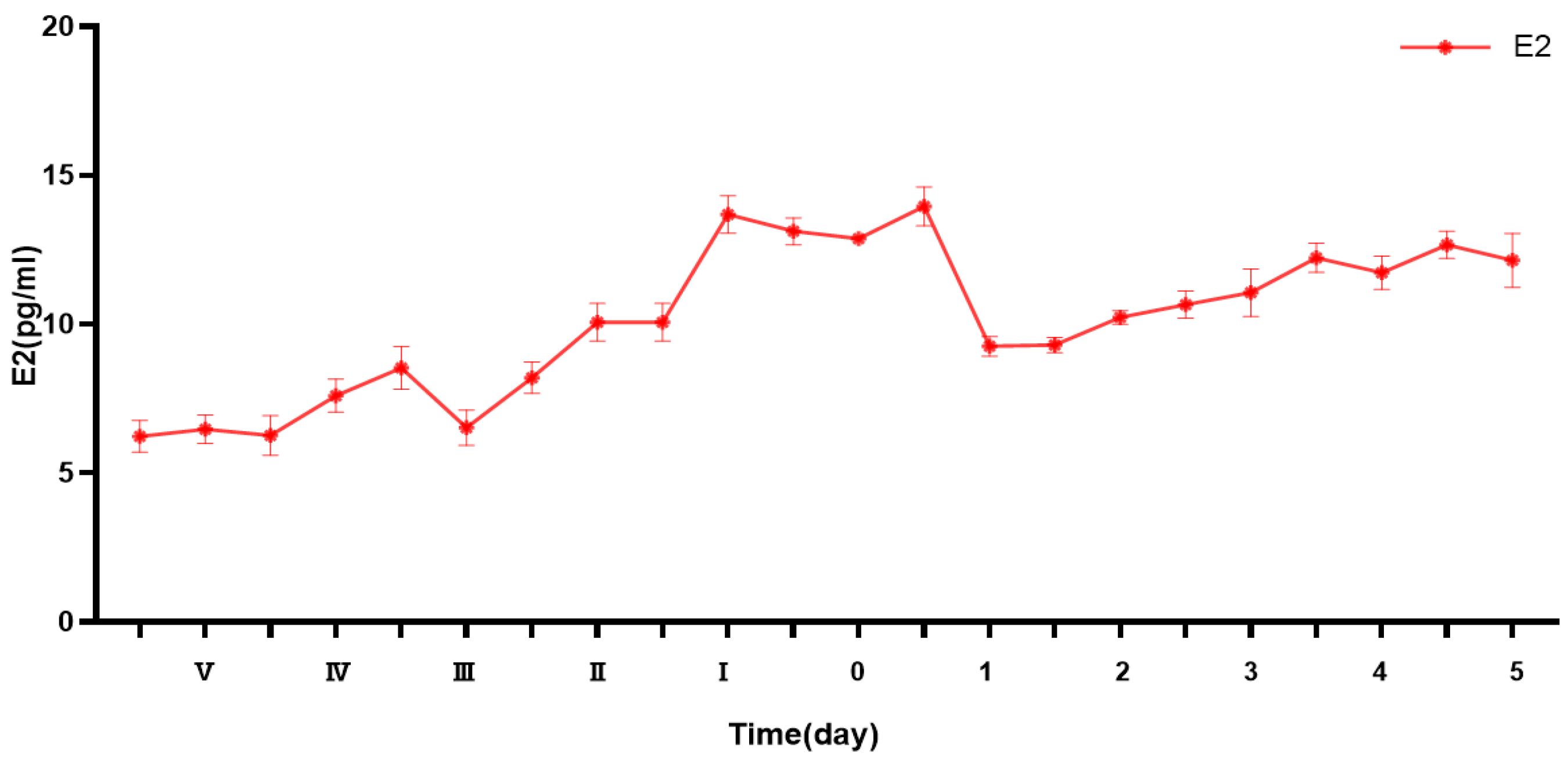
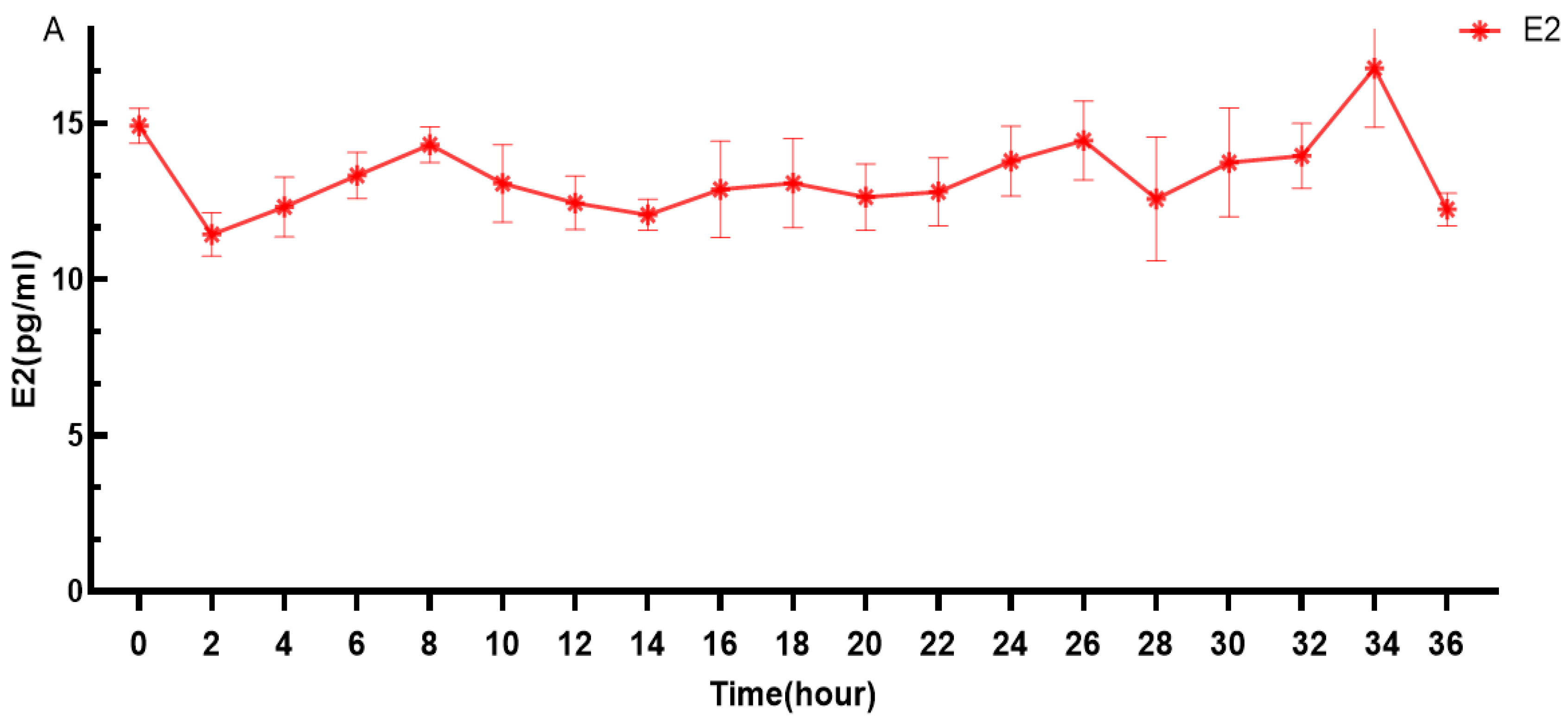
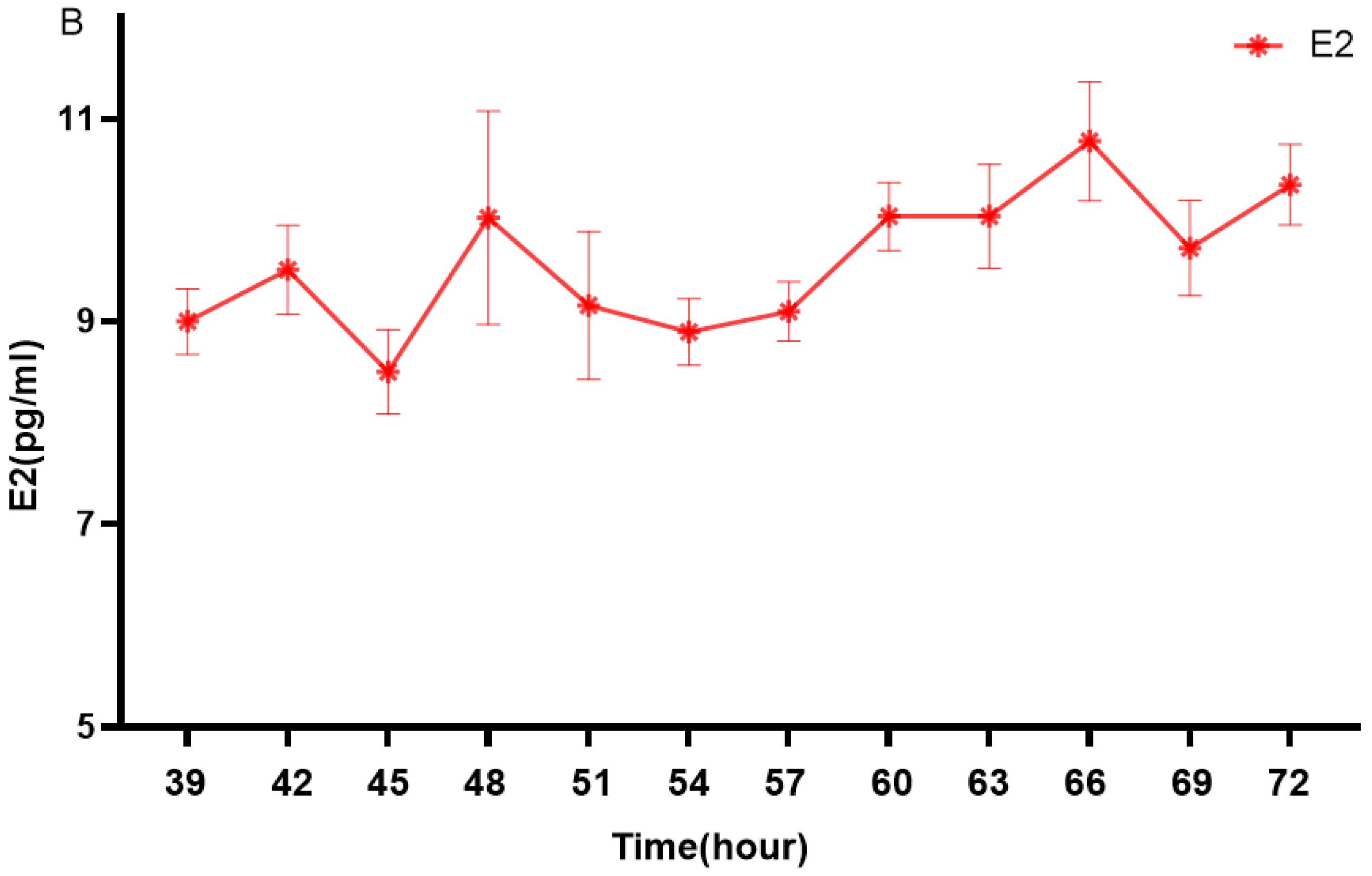
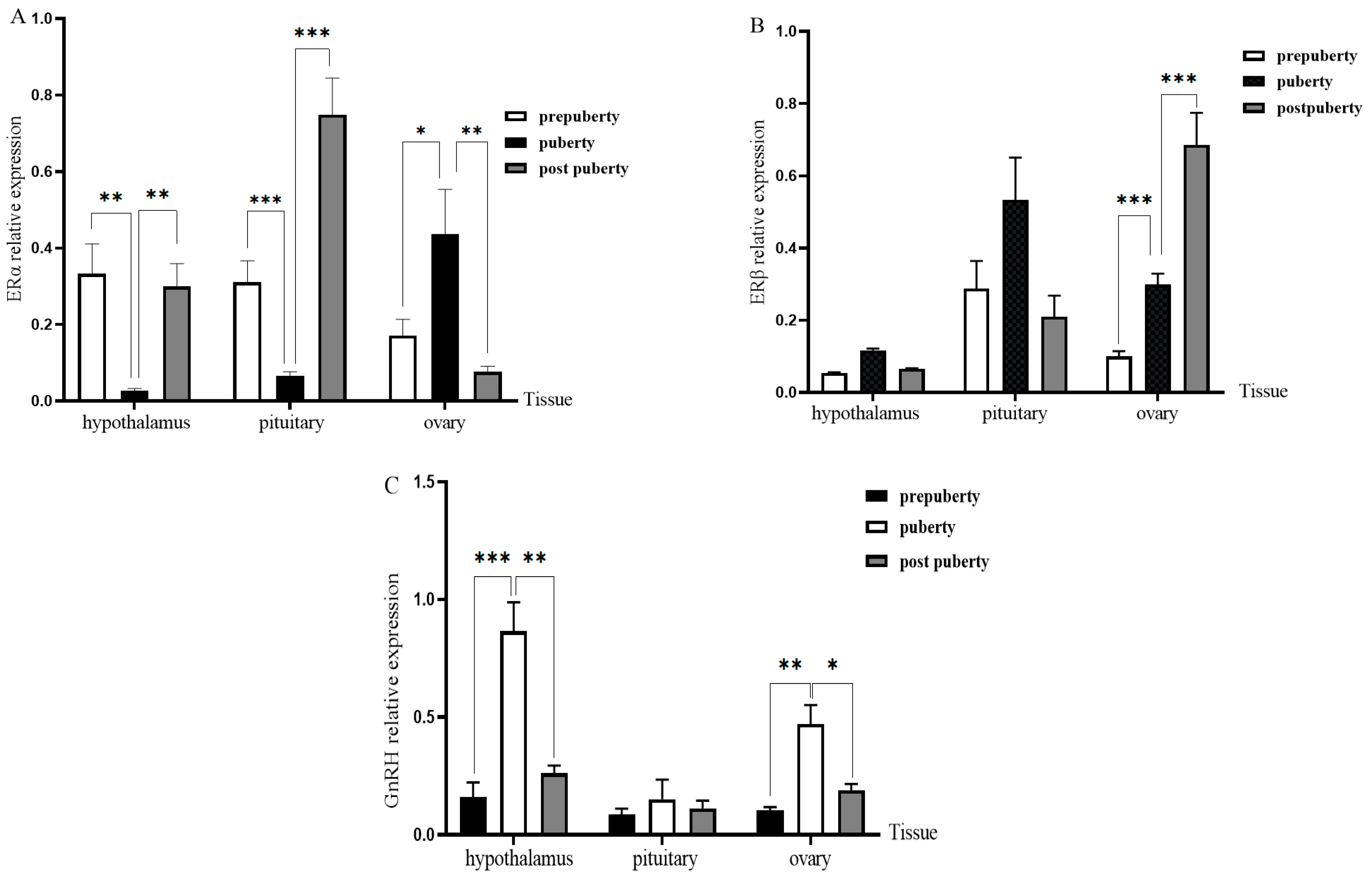
3.1. Changes in Estrogen Levels
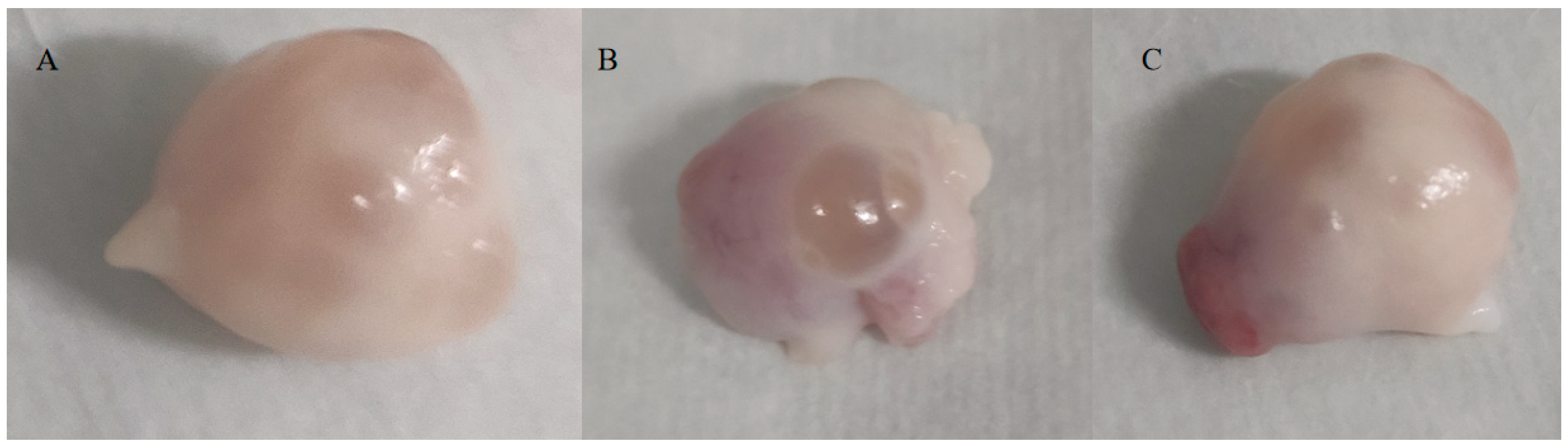
3.2. Ovarian Morphology Observations
3.3. Identification of Primary Granulosa Cells
3.4. Expression of ERs and PGR in Different Tissues and Periods of Duolang Sheep
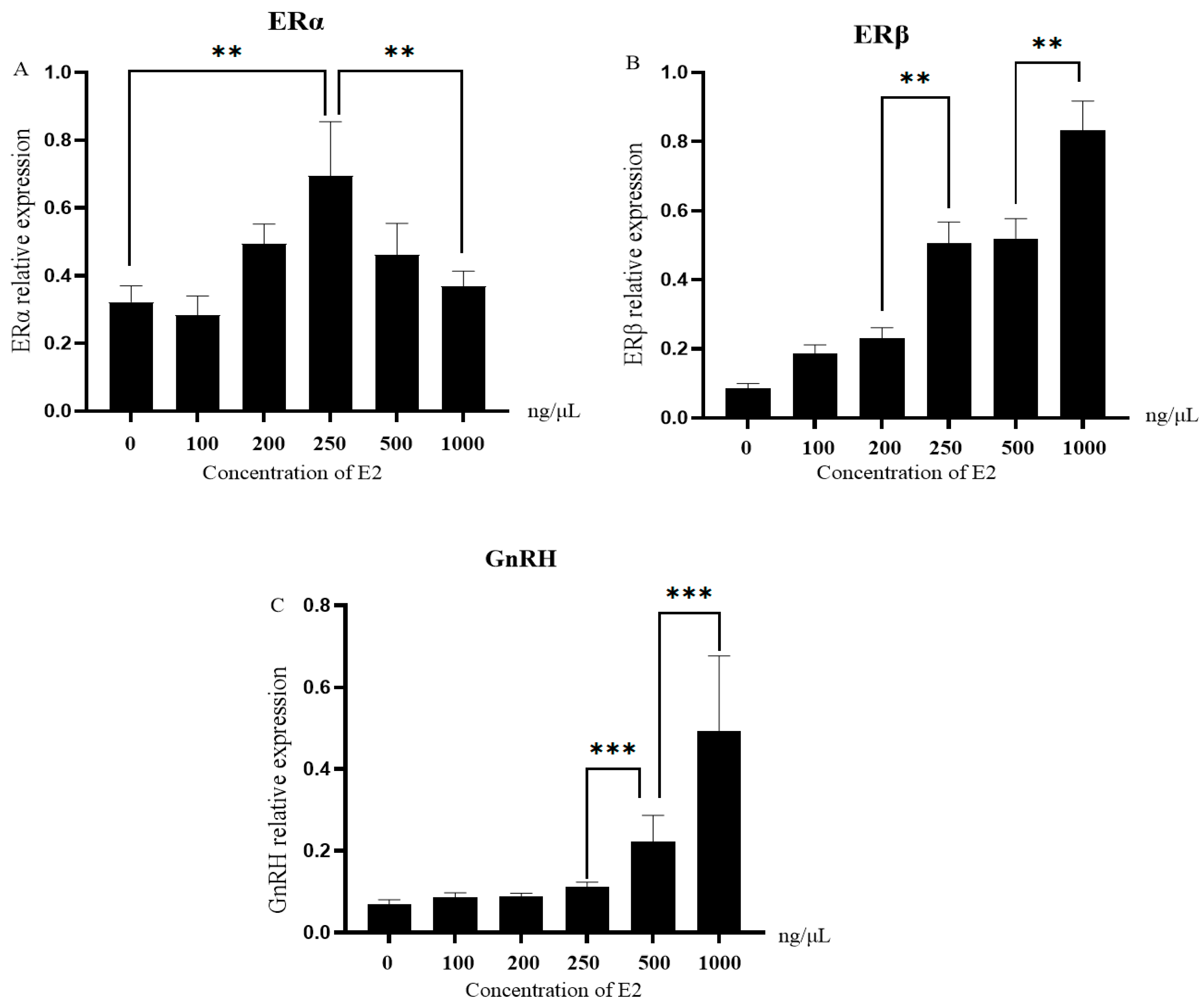
3.5. Expression of ERs and GnRH in Granulosa Cells Stimulated with Different Concentrations of E2
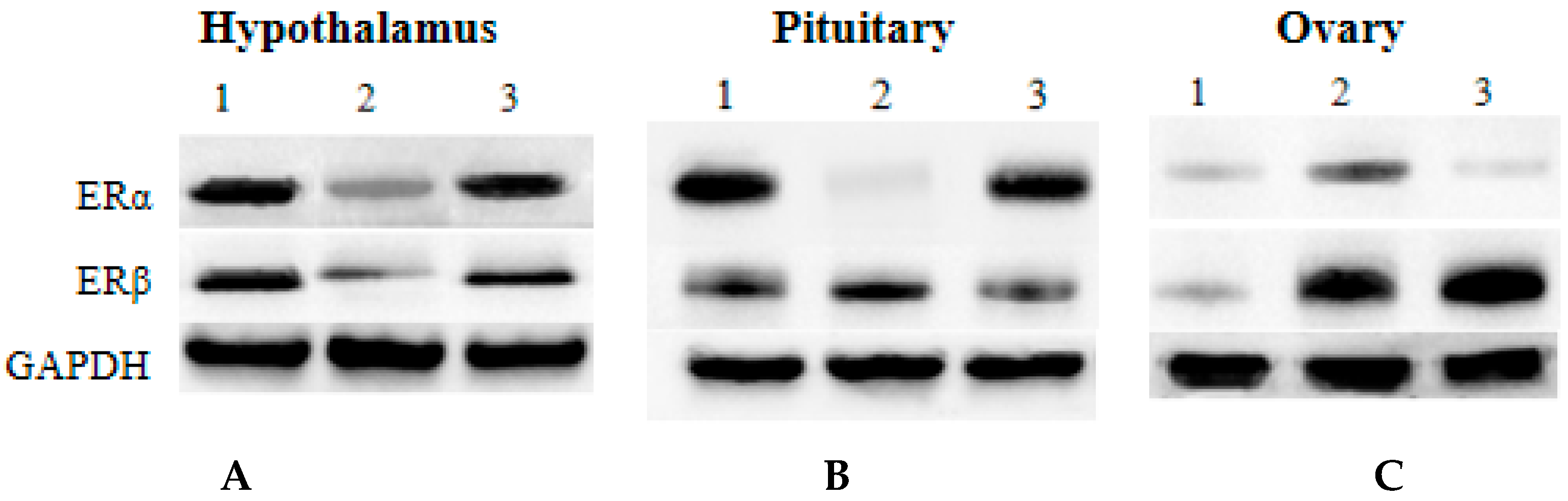
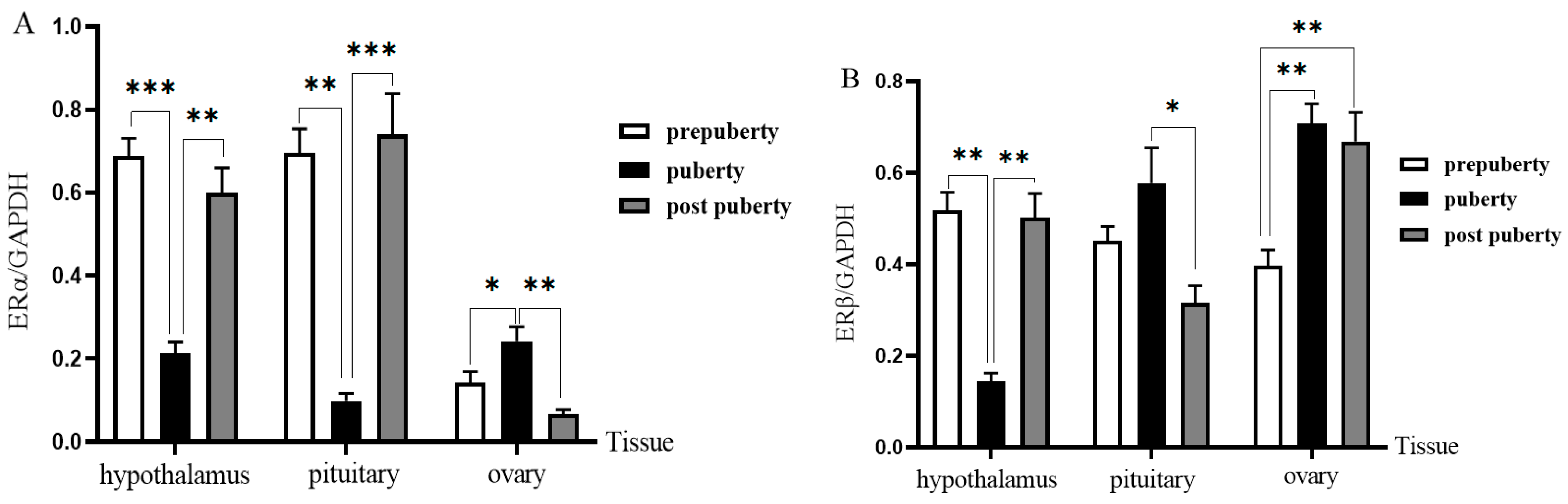
3.6. Western Blot Analysis
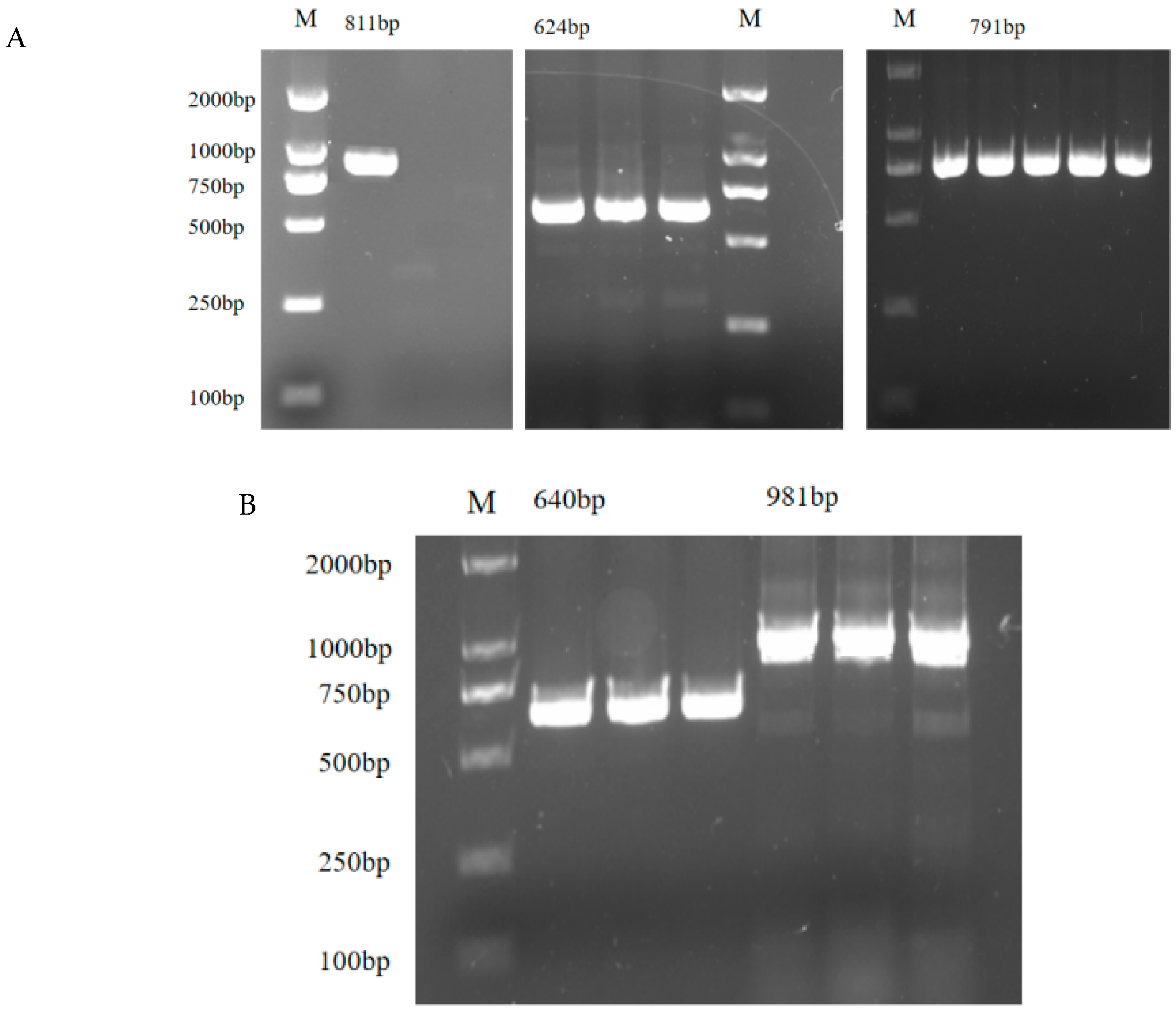
3.7. ERs Cloning
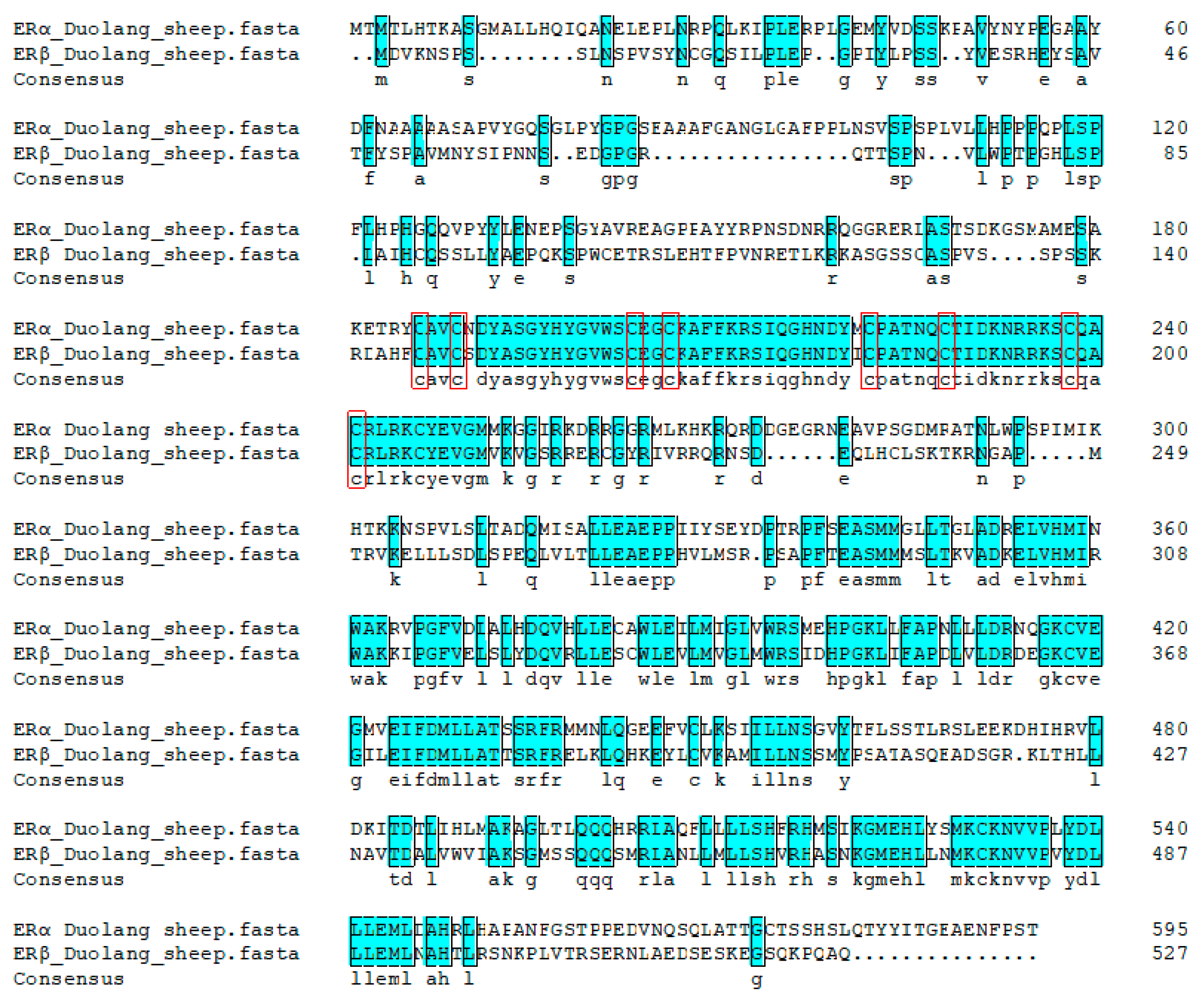
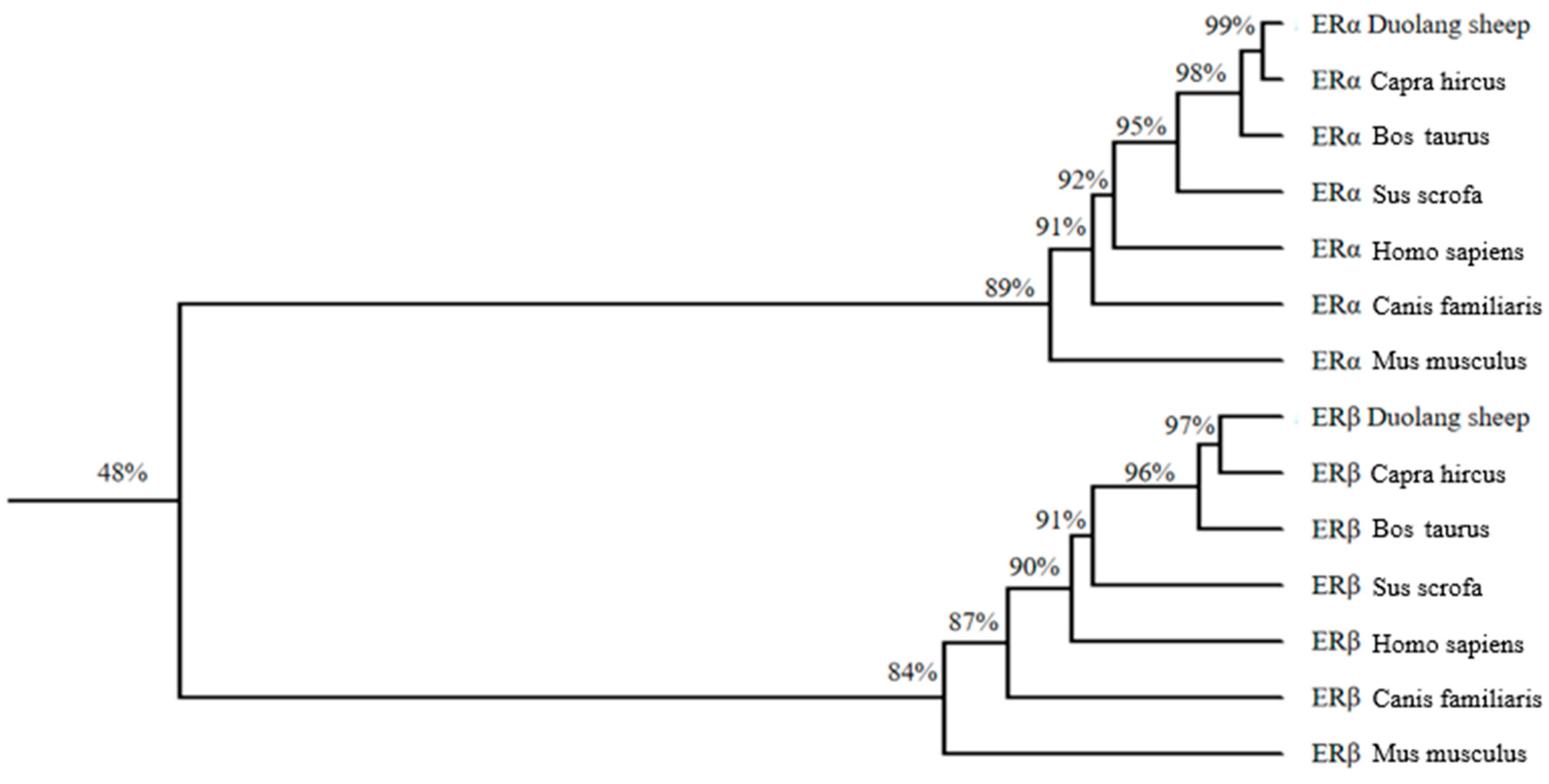
3.8. Homologous Sequence Analysis and Evolutionary Tree Construction
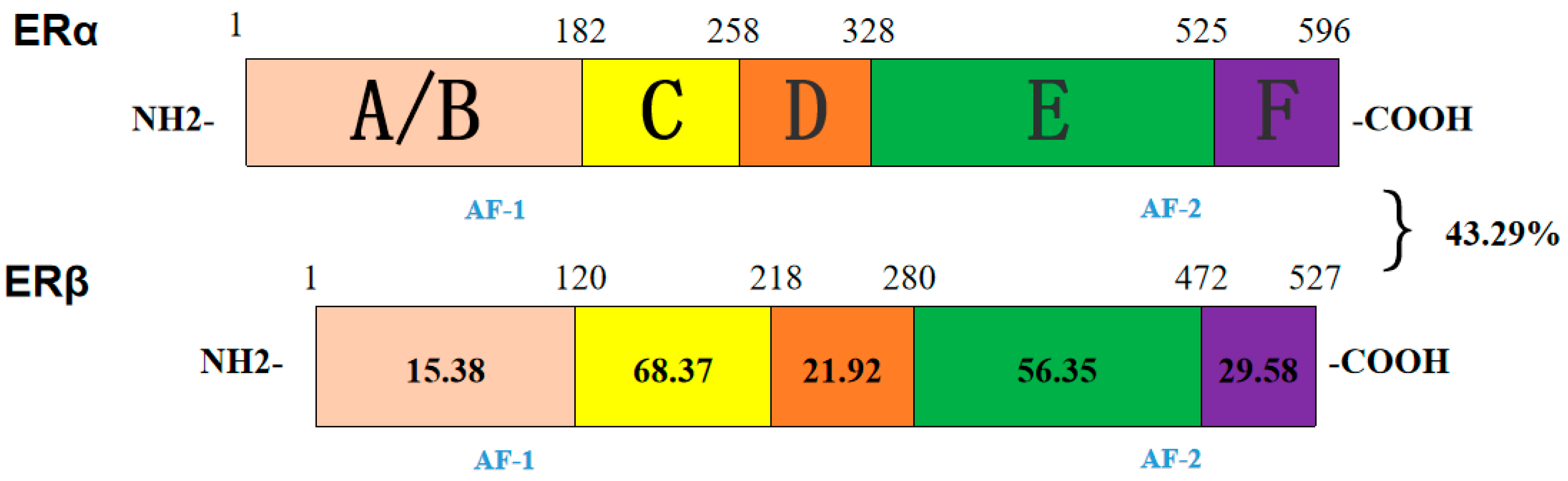
3.9. Bioinformatics Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ERs | Estrogen Receptors |
| DL | Duolong Sheep |
| GCs | Granulose Cells |
| qPCR | Quantitative Polymerase Chain Reaction |
| RT-PCR | Reverse-Transcription Polymerase Chain Reaction |
| ERα | Estrogen Receptors Alpha |
| ERβ | Estrogen Receptors Beta |
| GnRH | Follicle-Stimulating Hormone |
| HPO | Hypothalamus–Pituitary–Ovary |
| FSH | Follicle-Stimulating Hormone |
| LH | Luteinizing Hormone |
| DMEM | Dulbecco’s Modified Eagle Medium |
| PBS | Phosphate-Buffered Saline |
| RNA | Ribonucleic Acid |
| GADPH | Glyceraldehyde-3-Phosphate Dehydrogenase (housekeeping gene) |
| PVDF | Polyvinylidene Fluoride |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| SDS-PAGE | Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis |
| SEM | Standard Error of the Mean |
| P | Standard Error of the Mean |
| E2 | 17β-Estradiol (Estrogen) |
References
- Spaziani, M.; Tarantino, C.; Tahani, N.; Gianfrilli, D.; Sbardella, E.; Lenzi, A.; Radicioni, A.F. Hypothalamo-Pituitary axis and puberty. Mol. Cell. Endocrinol. 2021, 520, 111094. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.R. The genetic basis of delayed puberty. Front. Endocrinol. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.T.; Clarke, I.J. Seasonal breeding as a neuroendocrine model for puberty in sheep. Mol. Cell. Endocrinol. 2010, 324, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mijiddorj, T.; Kanasaki, H.; Sukhbaatar, U.; Oride, A.; Hara, T.; Kyo, S. Mutual regulation by GnRH and kisspeptin of their receptor expression and its impact on the gene expression of gonadotropin subunits. Gen. Comp. Endocrinol. 2017, 246, 382–389. [Google Scholar] [CrossRef]
- Dubois, E.A.; Zandbergen, M.A.; Peute, J.; Th, H.G. Evolutionary development of three gonadotropin-releasing hormone (GnRH) systems in vertebrates. Brain Res. Bull. 2002, 57, 413–418. [Google Scholar] [CrossRef]
- Eyster, K.M. The estrogen receptors: An overview from different perspectives. In Estrogen Receptors: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1, pp. 1–10. [Google Scholar]
- Ruan, X.; Seeger, H.; Wallwiener, D.; Huober, J.; Mueck, A.O. The ratio of the estradiol metabolites 2-hydroxyestrone (2-OHE1) and 16α-hydroxyestrone (16-OHE1) may predict breast cancer risk in postmenopausal but not in premenopausal women: Two case–control studies. Arch. Gynecol. Obstet. 2015, 291, 1141–1146. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Fujimoto, J.; Hong, B.L.; Tamaya, T. Quantitative analysis of estrogen receptor proteins in rat ovary. J. Steroid Biochem. Mol. Biol. 2005, 94, 83–91. [Google Scholar] [CrossRef]
- Patel, B.; Elguero, S.; Thakore, S.; Dahoud, W.; Bedaiwy, M.; Mesiano, S. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 2015, 21, 155–173. [Google Scholar]
- Duan, H.; Xiao, L.; Hu, J.; Zhang, Y.; Zhao, X.; Ge, W.; Jiang, Y.; Song, L.; Yang, S.; Luo, W. Expression of oestrogen receptor, androgen receptor and progesterone nuclear receptor in sheep uterus during the oestrous cycle. Reprod. Domest. Anim. 2019, 54, 1305–1312. [Google Scholar] [CrossRef]
- Schaub, C.E.; Gersting, J.A.; Keller-Wood, M.; Wood, C.E. Development of ER-α and ER-β expression in the developing ovine brain and pituitary. Gene Expr. Patterns 2008, 8, 457–463. [Google Scholar] [CrossRef]
- Xing, F.; Zhang, C.; Kong, Z. Cloning and expression of lin-28 homolog B gene in the onset of puberty in Duolang sheep. Asian-Australas. J. Anim. Sci. 2018, 32, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Li, X.; Zhang, Y.; Xing, F. Expression and functional analysis of GnRH at the onset of puberty in sheep. Arch. Anim. Breed. 2022, 65, 249–257. [Google Scholar] [CrossRef]
- Brélivet, Y.; Rochel, N.; Moras, D. Structural analysis of nuclear receptors: From isolated domains to integral proteins. Mol. Cell. Endocrinol. 2012, 348, 466–473. [Google Scholar] [CrossRef]
- Tora, L.; White, J.; Brou, C.; Tasset, D.; Webster, N.; Scheer, E.; Chambon, P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell 1989, 59, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Hilser, V.J.; Thompson, E.B. Structural dynamics, intrinsic disorder, and allostery in nuclear receptors as transcription factors. J. Biol. Chem. 2011, 286, 39675–39682. [Google Scholar] [CrossRef]
- Kim, M.Y.; Woo, E.M.; Chong, Y.T.; Homenko, D.R.; Kraus, W.L. Acetylation of estrogen receptor α by p300 at lysines 266 and 268 enhances the deoxyribonucleic acid binding and transactivation activities of the receptor. Mol. Endocrinol. 2006, 20, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Arao, Y.; Hamilton, K.J.; Coons, L.A.; Korach, K.S. Estrogen receptor α L543A, L544A mutation changes antagonists to agonists, correlating with the ligand binding domain dimerization associated with DNA binding activity. J. Biol. Chem. 2013, 288, 21105–21116. [Google Scholar] [CrossRef]
- Basappa, B.; Chumadathil Pookunoth, B.; Shinduvalli Kempasiddegowda, M.; Knchugarakoppal Subbegowda, R.; Lobie, P.E.; Pandey, V. Novel biphenyl amines inhibit oestrogen receptor (ER)-α in ER-positive mammary carcinoma cells. Molecules 2021, 26, 783. [Google Scholar] [CrossRef]
- Ottobre, J.S.; Lewis, G.S.; Thayne, W.V.; Inskeep, E.K. Mechanism by Which Progesterone Shortens the Estrous Cycle of the Ewe. Biol. Reprod. 1980, 23, 1046–1053. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, Q.; Lei, X.; Wen, Y.; Yang, Y.L.; Zhang, R.; Hu, M.Y. Association of estrogen receptor gene polymorphisms with human precocious puberty: A systematic review and meta-analysis. Gynecol. Endocrinol. 2015, 31, 516–521. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, S.; Bai, S.; Huang, L.; Hou, Y. Postnatal ovarian development and its relationship with steroid hormone receptors in JiNing Grey goats. Anim. Reprod. Sci. 2015, 154, 39–47. [Google Scholar] [CrossRef] [PubMed]
- van Eerdenburg, F.J.; Daemen, I.A.; van der Beek, E.M.; van Leeuwen, F.W. Changes in estrogen-alpha receptor immunoreactivity during the estrous cycle in lactating dairy cattle. Brain Res. 2000, 880, 219–223. [Google Scholar] [CrossRef]
- Zhu, Y.; Ye, J.; Qin, P.; Yan, X.; Gong, X.; Li, X.; Liu, Y.; Li, Y.; Yu, T.; Zhang, Y.; et al. Analysis of serum reproductive hormones and ovarian genes in pubertal female goats. J. Ovarian Res. 2023, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Govers, L.C.; Phillips, T.R.; Mattiske, D.M.; Rashoo, N.; Black, J.R.; Sinclair, A.; Baskin, L.S.; Risbridger, G.P.; Pask, A.J. A critical role for estrogen signaling in penis development. FASEB J. 2019, 33, 10383–10393. [Google Scholar] [CrossRef]
- Yart, L.; Finot, L.; Marnet, P.G.; Dessauge, F. Suppression of ovarian secretions before puberty strongly affects mammogenesis in the goat. J. Dairy Res. 2012, 79, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Porteous, R.; Herbison, A.E. Genetic deletion of Esr1 in the mouse preoptic area disrupts the LH surge and estrous cyclicity. Endocrinology 2019, 160, 1821–1829. [Google Scholar] [CrossRef]
- Lang-Muritano, M.; Sproll, P.; Wyss, S.; Kolly, A.; Hürlimann, R.; Konrad, D.; Biason-Lauber, A. Early-onset complete ovarian failure and lack of puberty in a woman with mutated estrogen receptor β (ESR2). J. Clin. Endocrinol. Metab. 2018, 103, 3748–3756. [Google Scholar] [CrossRef]
- Kim, S.; Pyun, J.A.; Kang, H.; Kim, J.; Cha, D.H.; Kwack, K. Epistasis between CYP19A1 and ESR1 polymorphisms is associated with premature ovarian failure. Fertil. Steril. 2011, 95, 353–356. [Google Scholar] [CrossRef]
- Glidewell-Kenney, C.; Hurley, L.A.; Pfaff, L.; Weiss, J.; Levine, J.E.; Jameson, J.L. Nonclassical estrogen receptor α signaling mediates negative feedback in the female mouse reproductive axis. Proc. Natl. Acad. Sci. USA 2007, 104, 8173–8177. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) | Product Size (bp) |
|---|---|---|---|
| ERα | CCCTCCACGATCAAGTCC | AGGTTGGGAGCAAATAGGA | 112 |
| ERβ | TCTGGTCTGGGTGATTGC | TGTTCCATGCCCTTGTTA | 117 |
| GnRH | ATTGTTCACCAGTCCCATTC | TTCCTCTGCCCAGTTTCC | 83 |
| ACTB | CCAACCGTGAGAAGATGACC | CAGAGGCGTACAGGGACAG | 96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Shahbaz, G.M.; Sun, H.; Zhang, J.; Li, W.; Gu, R.; Xing, F. Expression Dynamics and Estrogen Response of Estrogen Receptors in Duolang Sheep During Puberty. Genes 2025, 16, 731. https://doi.org/10.3390/genes16070731
Zhu L, Shahbaz GM, Sun H, Zhang J, Li W, Gu R, Xing F. Expression Dynamics and Estrogen Response of Estrogen Receptors in Duolang Sheep During Puberty. Genes. 2025; 16(7):731. https://doi.org/10.3390/genes16070731
Chicago/Turabian StyleZhu, Lexiao, Gul Muhammad Shahbaz, Huiping Sun, Jihu Zhang, Wei Li, Ruohuai Gu, and Feng Xing. 2025. "Expression Dynamics and Estrogen Response of Estrogen Receptors in Duolang Sheep During Puberty" Genes 16, no. 7: 731. https://doi.org/10.3390/genes16070731
APA StyleZhu, L., Shahbaz, G. M., Sun, H., Zhang, J., Li, W., Gu, R., & Xing, F. (2025). Expression Dynamics and Estrogen Response of Estrogen Receptors in Duolang Sheep During Puberty. Genes, 16(7), 731. https://doi.org/10.3390/genes16070731








