AQP7-Mediated Mitochondrial Redox Homeostasis in Vitrified Oocytes: A Genetic Mechanism of PI3K/AKT Signaling Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Shelter
2.2. Chemicals and Antibodies
2.3. Oocyte Collection
2.4. Vitrification and Thawing of Oocytes
2.5. Oocyte in Vitro Maturation (IVM)
2.6. Immunohistochemical Staining (IHC) and Immunofluorescence (IF) Staining
2.7. Transmission Electron Microscopy (TEM)
2.8. Analysis of H2O2 and GSH Levels
2.9. Detection of the Mitochondrial Distribution
2.10. Determination of MMP
2.11. Determination of the Ca2+ Levels
2.12. Annexin V Staining
2.13. Real-Time Quantitative PCR (qRT-PCR)
2.14. Experimental Design
2.15. Statistical Analysis
3. Results
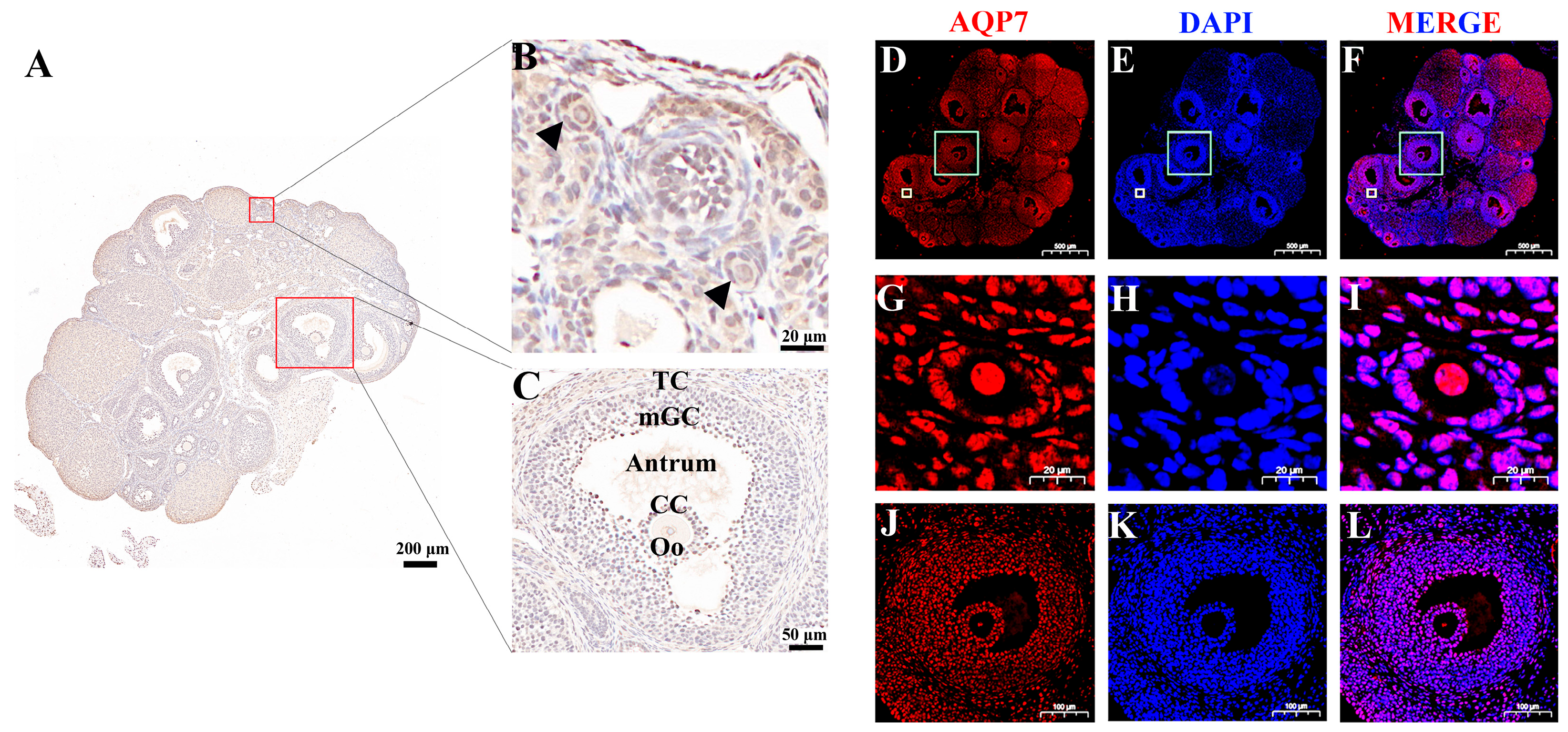
3.1. Abundance of Aquaporin 7 in Mouse Ovaries
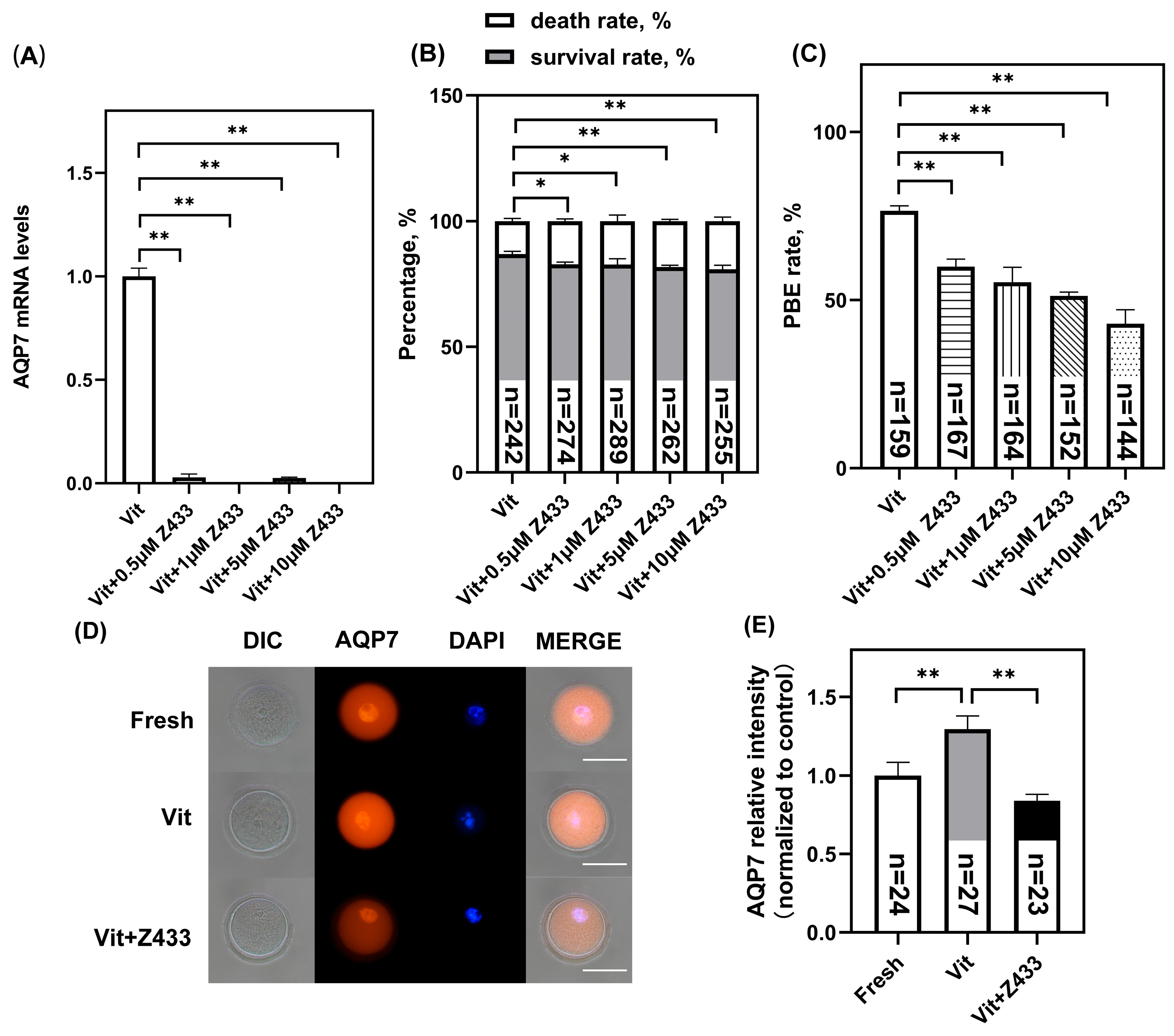
3.2. Effect of AQP7 on Oocyte Viability After Vitrification
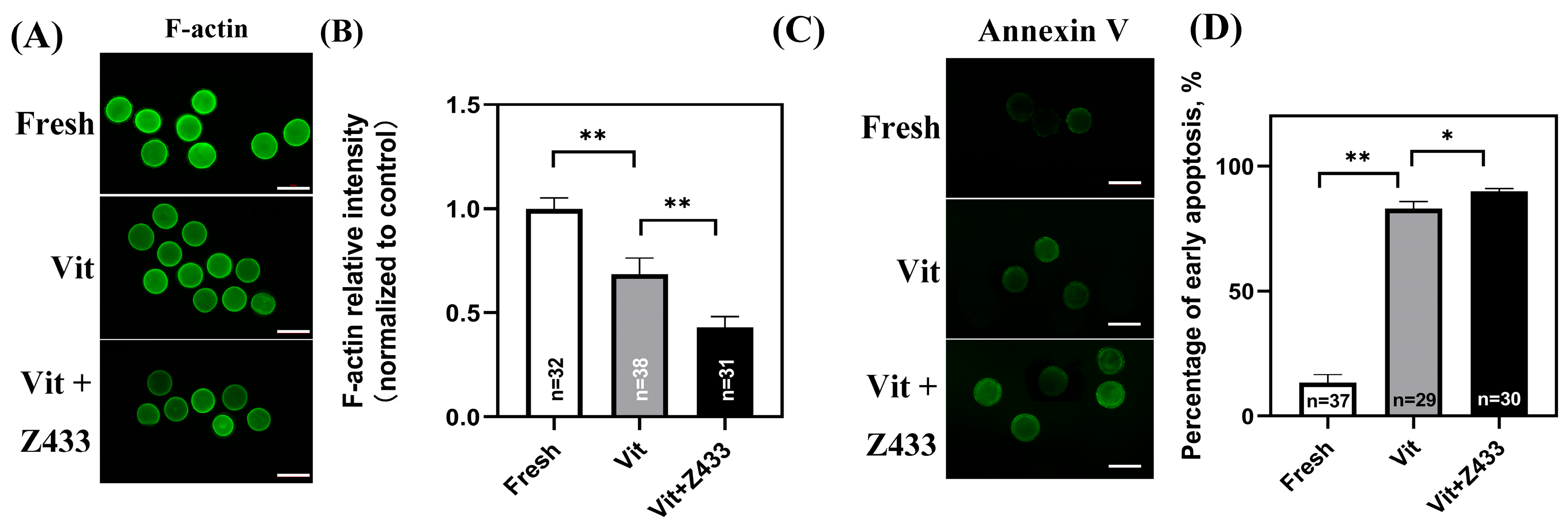
3.3. Effect of AQP7 on Vitrified Oocyte Meiosis and Apoptosis
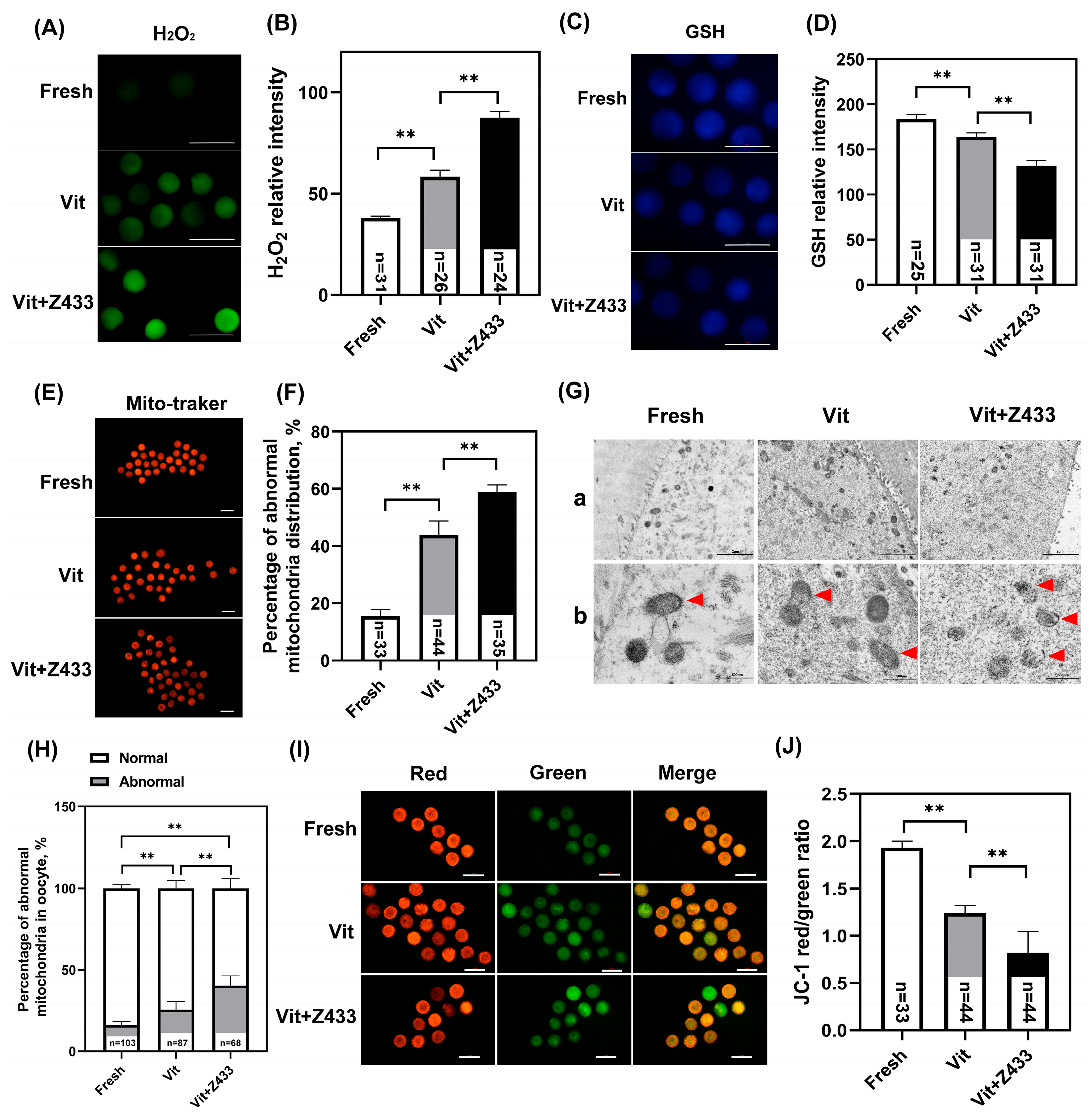
3.4. The Reduction in AQP7 Levels Affects Maturation Through Oxidative Stress Resulting from Damaged Mitochondria
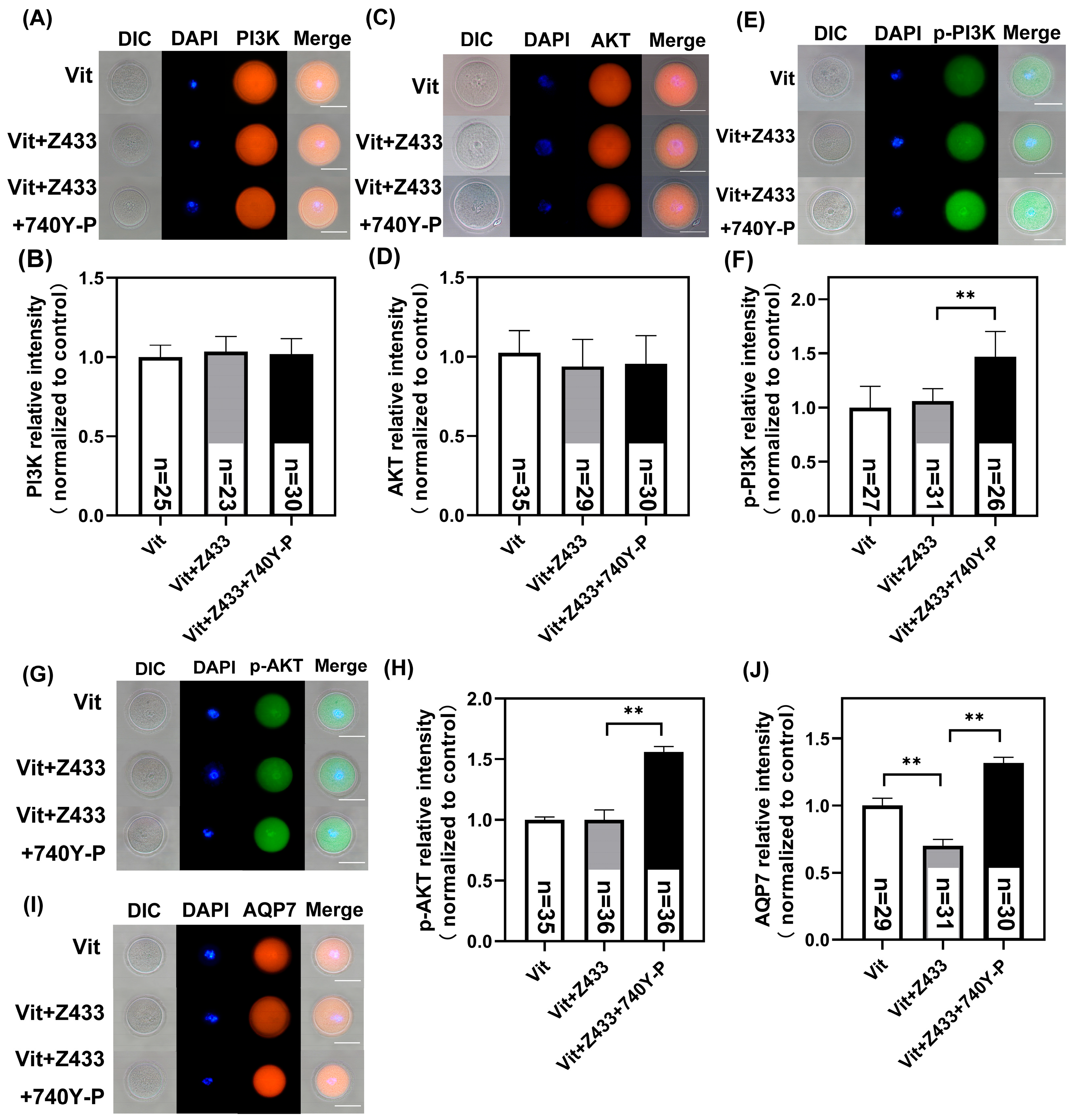
3.5. PI3K/AKT/AQP7 Pathway Alleviate the Effect of GV Oocyte Vitrification
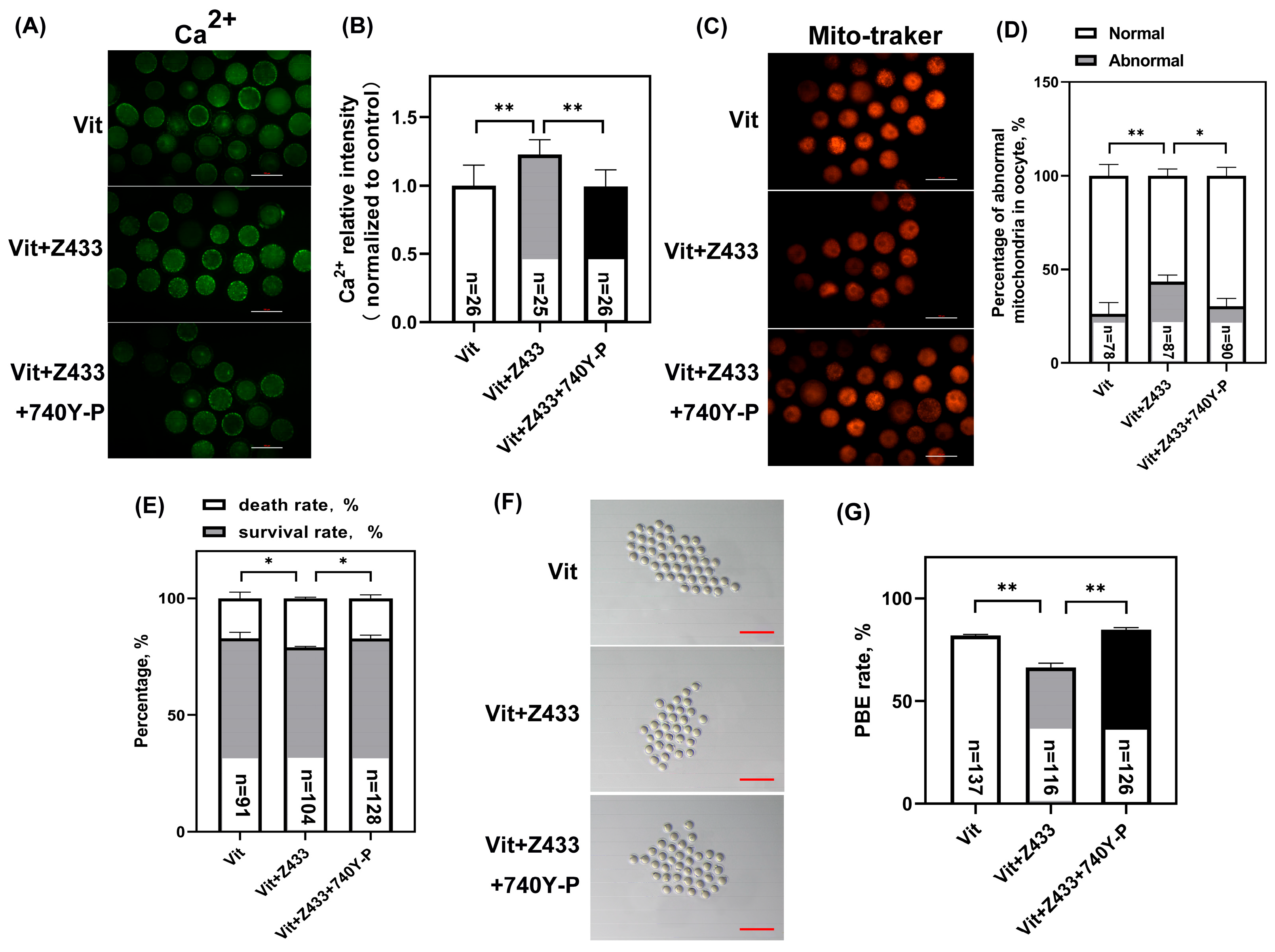
3.6. Activated PI3K/AKT Restores Calcium Homeostasis, Mitochondrial Distribution, and Maturation Failure by Raising AQP7 Levels
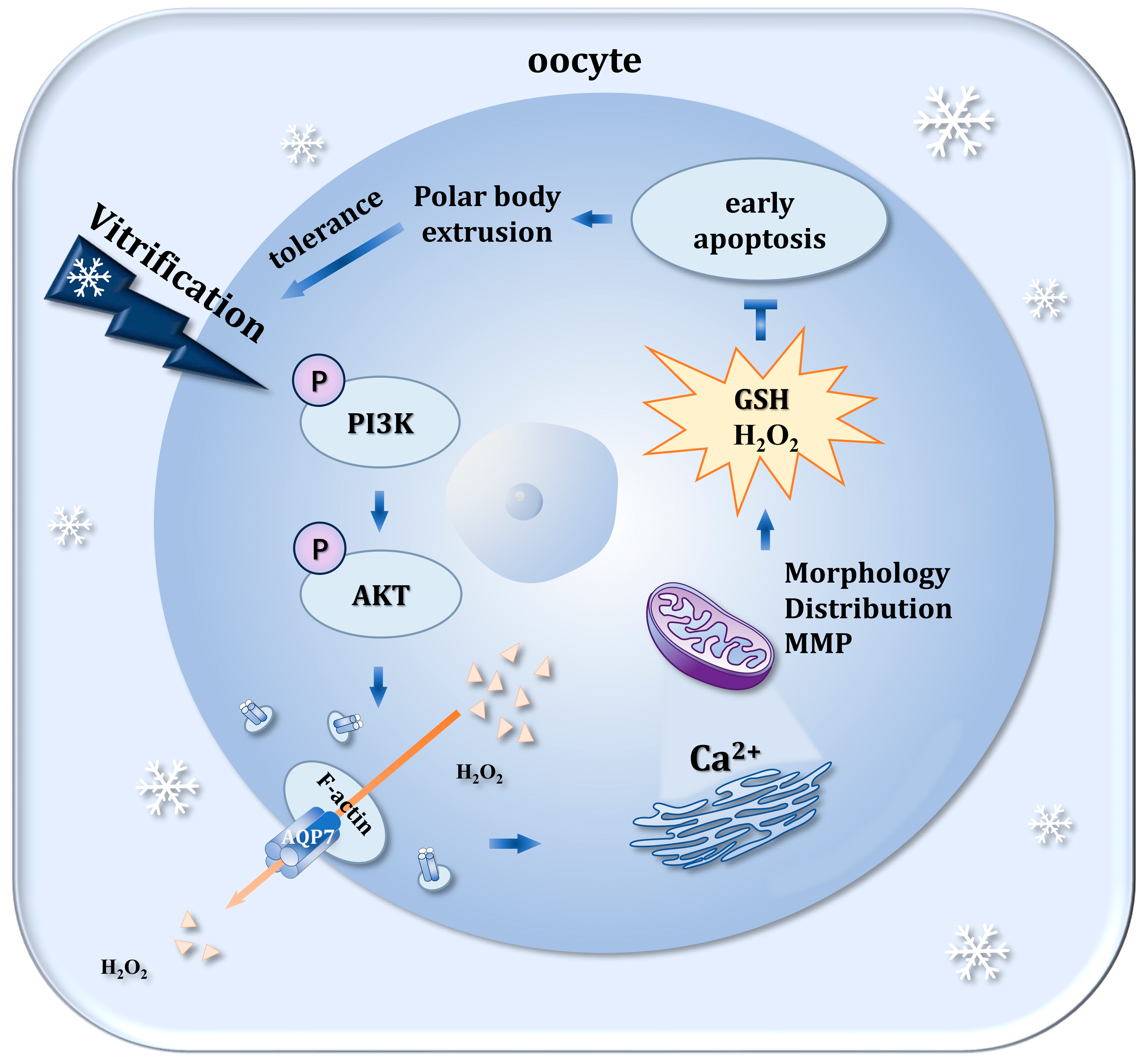
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Dong, H.; Cheng, M.; Wang, Q.; Jin, Y. Addition of cholesterol loaded cyclodextrin prior to GV-phase vitrification improves the quality of mature porcine oocytes in vitro. Cryobiology 2019, 90, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Cobo, A.; García-Velasco, J.A.; Remohí, J.; Pellicer, A. Oocyte vitrification for fertility preservation for both medical and nonmedical reasons. Fertil. Steril. 2021, 115, 1091–1101. [Google Scholar] [CrossRef]
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature 1985, 313, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Lechuga-Vieco, A.V.; Latorre-Pellicer, A.; Johnston, I.G.; Prota, G.; Gileadi, U.; Justo-Méndez, R.; Acín-Pérez, R.; Martínez-De-Mena, R.; Fernández-Toro, J.M.; Jimenez-Blasco, D.; et al. Cell identity and nucleo-mitochondrial genetic context modulate OXPHOS performance and determine somatic heteroplasmy dynamics. Sci. Adv. 2020, 6, eaba5345. [Google Scholar] [CrossRef]
- Rodríguez-Nuevo, A.; Torres-Sanchez, A.; Duran, J.M.; De Guirior, C.; Martínez-Zamora, M.A.; Böke, E. Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I. Nature 2022, 607, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. Commun. Free Radic. Res. 2009, 14, 102–108. [Google Scholar] [CrossRef]
- Len, J.S.; Koh, W.S.D.; Tan, S.X. The roles of reactive oxygen species and antioxidants in cryopreservation. Biosci. Rep. 2019, 39, BSR20191601. [Google Scholar] [CrossRef] [PubMed]
- Clérico, G.; Taminelli, G.; Veronesi, J.; Polola, J.; Pagura, N.; Pinto, C.; Sansinena, M. Mitochondrial function, blastocyst development and live foals born after ICSI of immature vitrified/warmed equine oocytes matured with or without melatonin. Theriogenology 2021, 160, 40–49. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, A.; Liu, Y.; Li, J.; Bai, J.; Hai, G.; Wang, J.; Liu, W.; Wan, P.; Fu, X. Hydroxyapatite nanoparticle improves ovine oocyte developmental capacity by alleviating oxidative stress in response to vitrification stimuli. Theriogenology 2024, 229, 88–99. [Google Scholar] [CrossRef]
- Ahola, S.; Langer, T. Ferroptosis in mitochondrial cardiomyopathy. Trends Cell Biol. 2024, 34, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Cao, B.; Qin, J.; Pan, B.; Qazi, I.H.; Ye, J.; Fang, Y.; Zhou, G. Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants. Cells 2022, 11, 3573. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhang, Y.; Liu, L.; Yang, Y.; Wang, Y.; Xu, B. Glycine and Melatonin Improve Preimplantation Development of Porcine Oocytes Vitrified at the Germinal Vesicle Stage. Front. Cell Dev. Biol. 2022, 10, 856486. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Liu, F.; Song, Y.; Liu, Y. Activated PI3K/AKT Signaling Pathway Associates with Oxidative Stress and Impaired Developmental Potential of Vitrified-Thawed Oocytes. Reprod. Sci. 2020, 27, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cheng, J.; Yao, Q.; Duan, J.; Chen, H.; Zhang, Z.; Yang, L.; Hua, R.; Li, Q. Isorhamnetin Improves Oocyte Maturation by Activating the Pi3k/Akt Signaling Pathway. Mol. Nutr. Food Res. 2024, 68, e2300904. [Google Scholar] [CrossRef]
- Li, X.; Duan, J.; Wang, S.; Cheng, J.; Chen, H.; Zhang, Z.; Yang, L.; Hua, R.; Li, Q. Isorhamnetin protects porcine oocytes from zearalenone-induced reproductive toxicity through the PI3K/Akt signaling pathway. J. Anim. Sci. Biotechnol. 2023, 14, 22. [Google Scholar] [CrossRef]
- Tan, Y.-J.; Zhang, X.-Y.; Ding, G.-L.; Li, R.; Wang, L.; Jin, L.; Lin, X.-H.; Gao, L.; Sheng, J.-Z.; Huang, H.-F. Aquaporin7 plays a crucial role in tolerance to hyperosmotic stress and in the survival of oocytes during cryopreservation. Sci. Rep. 2015, 5, 17741. [Google Scholar] [CrossRef]
- Bienert, G.P.; Møller, A.L.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Huang, P.; Åbacka, H.; Wilson, C.J.; Wind, M.L.; Rűtzler, M.; Hagström-Andersson, A.; Gourdon, P.; de Groot, B.L.; Venskutonytė, R.; Lindkvist-Petersson, K. Molecular basis for human aquaporin inhibition. Proc. Natl. Acad. Sci. USA 2024, 121, e2319682121. [Google Scholar] [CrossRef]
- Sonntag, Y.; Gena, P.; Maggio, A.; Singh, T.; Artner, I.; Oklinski, M.K.E.; Johanson, U.; Kjellbom, P.; Nieland, J.D.; Nielsen, S.; et al. Identification and characterization of potent and selective aquaporin-3 and aquaporin-7 inhibitors. J. Biol. Chem. 2019, 294, 7377–7387. [Google Scholar] [CrossRef]
- Khatun, H.; Yamanaka, K.I.; Sugimura, S. Antioxidant sericin averts the disruption of oocyte-follicular cell communication triggered by oxidative stress. Mol. Hum. Reprod. 2024, 30, gaae001. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.; Xiong, Y.; Ding, G.L.; Zhang, D.; Meng, Y.; Huang, H.F.; Sheng, J.Z. Cryoprotectants up-regulate expression of mouse oocyte AQP7, which facilitates water diffusion during cryopreservation. Fertil. Steril. 2013, 99, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Catalán, V.; Gómez-Ambrosi, J.; García-Navarro, S.; Rotellar, F.; Valentí, V.; Silva, C.; Gil, M.J.; Salvador, J.; Burrell, M.A.; et al. Insulin- and leptin-mediated control of aquaglyceroporins in human adipocytes and hepatocytes is mediated via the PI3K/Akt/mTOR signaling cascade. J. Clin. Endocrinol. Metab. 2011, 96, E586–E597. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, Y.; Li, X.; Zhu, J.; Zhang, W.; Lu, W.; Weng, Y.; Liu, J. 2,5-Hexanedione influences primordial follicular development in cultured neonatal mouse ovaries by interfering with the PI3K signaling pathway via miR-214-3p. Toxicol. Appl. Pharmacol. 2020, 409, 115335. [Google Scholar] [CrossRef]
- Iena, F.M.; Lebeck, J. Implications of Aquaglyceroporin 7 in Energy Metabolism. Int. J. Mol. Sci. 2018, 19, 154. [Google Scholar] [CrossRef]
- Song, W.-Y.; Wang, Y.; Hou, X.-M.; Tian, C.-C.; Wu, L.; Ma, X.-S.; Jin, H.-X.; Yao, G.-D.; Sun, Y.-P. Different expression and localization of aquaporin 7 and aquaporin 9 in granulosa cells, oocytes, and embryos of patients with polycystic ovary syndrome and the negatively correlated relationship with insulin regulation. Fertil. Steril. 2021, 115, 463–473. [Google Scholar] [CrossRef]
- Galli, M.; Hameed, A.; Żbikowski, A.; Zabielski, P. Aquaporins in insulin resistance and diabetes: More than channels! Redox Biol. 2021, 44, 102027. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.; Duarte, A.; Rodrigues, G.; Lima, L.; Silva, G.; Carvalho, A.; Brito, I.; da Maranguape, R.; Lobo, C.; Aragão, J.; et al. Steady-state level of messenger RNA and immunolocalization of aquaporins 3, 7, and 9 during in vitro growth of ovine preantral follicles. Theriogenology 2015, 84, 1–10. [Google Scholar] [CrossRef]
- Dutta, A.; Das, M. Deciphering the role of aquaporins in metabolic diseases: A mini review. Am. J. Med. Sci. 2022, 364, 148–162. [Google Scholar] [CrossRef]
- Grossman, H.; Har-Paz, E.; Gindi, N.; Levi, M.; Miller, I.; Nevo, N.; Galiani, D.; Dekel, N.; Shalgi, R. Regulation of GVBD in mouse oocytes by miR-125a-3p and Fyn kinase through modulation of actin filaments. Sci. Rep. 2017, 7, 2238. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, S.; Mogessie, B. Actin limits egg aneuploidies associated with female reproductive aging. Sci. Adv. 2023, 9, eadc9161. [Google Scholar] [CrossRef]
- Gao, L.; Hou, Y.; Zeng, S.; Li, J.; Zhu, S.; Fu, X. The Error-Prone Kinetochore-Microtubule Attachments During Meiosis I in Vitrified Oocytes. Front. Cell Dev. Biol. 2020, 8, 621. [Google Scholar] [CrossRef]
- López, A.; Ducolomb, Y.; Casas, E.; Retana-Márquez, S.; Betancourt, M.; Casillas, F. Effects of Porcine Immature Oocyte Vitrification on Actin Microfilament Distribution and Chromatin Integrity During Early Embryo Development in vitro. Front. Cell Dev. Biol. 2021, 9, 636765. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, K.; Giannini, F.; Lemonnier, T.; Mogessie, B. The prophase oocyte nucleus is a homeostatic G-actin buffer. J. Cell Sci. 2022, 135, jcs259807. [Google Scholar] [CrossRef]
- Casillas, F.; Betancourt, M.; Cuello, C.; Ducolomb, Y.; López, A.; Juárez-Rojas, L.; Retana-Márquez, S. An efficiency comparison of different in vitro fertilization methods: IVF, ICSI, and PICSI for embryo development to the blastocyst stage from vitrified porcine immature oocytes. Porc. Health Manag. 2018, 4, 16. [Google Scholar] [CrossRef]
- Nohales-Córcoles, M.; Sevillano-Almerich, G.; Di Emidio, G.; Tatone, C.; Cobo, A.; Dumollard, R.; De Los Santos Molina, M.J. Impact of vitrification on the mitochondrial activity and redox homeostasis of human oocyte. Hum. Reprod. 2016, 31, 1850–1858. [Google Scholar] [CrossRef]
- Xia, W.; Fu, X.; Zhou, G.; Xu, J.; Wang, L.; Du, M.; Yuan, D.; Yue, M.; Tian, J.; Zhu, S. Cytokeratin distribution and expression during the maturation of mouse germinal vesicle oocytes after vitrification. Cryobiology 2013, 66, 261–266. [Google Scholar] [CrossRef]
- Kierszenbaum, A.L.; Rivkin, E.; Tres, L.L. Cytoskeletal track selection during cargo transport in spermatids is relevant to male fertility. Spermatogenesis 2011, 1, 221–230. [Google Scholar] [CrossRef]
- Sasaki, S.; Yui, N.; Noda, Y. Actin directly interacts with different membrane channel proteins and influences channel activities: AQP2 as a model. Biochim. Biophys. Acta 2014, 1838, 514–520. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.; Zhu, C.-C.; Tang, F.; Cui, X.-S.; Kim, N.-H.; Sun, S.-C. Exposure to HT-2 toxin causes oxidative stress induced apoptosis/autophagy in porcine oocytes. Sci. Rep. 2016, 6, 33904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, C.; Wen, D.; Li, R.; Lu, S.; Xu, R.; Tang, Y.; Sun, Y.; Zhao, X.; Pan, M.; et al. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply. Redox Biol. 2022, 49, 102215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chang, H.; He, Q.; Xue, Y.; Zhang, K.; Kang, J.; Wang, Y.; Xu, Z.; Zhang, Y.; Quan, F. Effect of oocyte vitrification on glucose transport in mouse metaphase II oocytes. Reproducation 2021, 161, 549–559. [Google Scholar] [CrossRef]
- Wu, Z.; Pan, B.; Qazi, I.H.; Yang, H.; Guo, S.; Yang, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Han, H.; et al. Melatonin Improves In Vitro Development of Vitrified-Warmed Mouse Germinal Vesicle Oocytes Potentially via Modulation of Spindle Assembly Checkpoint-Related Genes. Cells 2019, 8, 1009. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3-mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef]
- Thiagarajah, J.R.; Chang, J.; Goettel, J.A.; Verkman, A.S.; Lencer, W.I. Aquaporin-3 mediates hydrogen peroxide-dependent responses to environmental stress in colonic epithelia. Proc. Natl. Acad. Sci. USA 2017, 114, 568–573. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, J.; Li, Y.; Hu, H.; Ye, Q.; Yang, C.; Yang, L.; Zhang, B.; Ma, T. Aquaporin-7 Facilitates Proliferation and Adipogenic Differentiation of Mouse Bone Marrow Mesenchymal Stem Cells by Regulating Hydrogen Peroxide Transport. Stem Cell Rev. Rep. 2023, 19, 2378–2390. [Google Scholar] [CrossRef]
- Ye, J.; Han, Y.; Chen, X.; Xie, J.; Liu, X.; Qiao, S.; Wang, C. L-carnitine attenuates H2O2-induced neuron apoptosis via inhibition of endoplasmic reticulum stress. Neurochem. Int. 2014, 78, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Gao, L.; Zhang, C.; Wang, Y.; Lan, H.; Chu, Q.; Li, S.; Zheng, X. Glycine Ameliorates Endoplasmic Reticulum Stress Induced by Thapsigargin in Porcine Oocytes. Front. Cell Dev. Biol. 2021, 9, 733860. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-M.; Wu, Y.-T.; Chen, L.; Tan, Z.-B.; Fan, H.-J.; Xie, L.-P.; Zhang, W.-T.; Chen, H.-M.; Li, J.; Liu, B.; et al. 3,5-Dicaffeoylquinic acid protects H9C2 cells against oxidative stress-induced apoptosis via activation of the PI3K/Akt signaling pathway. Food Nutr. Res. 2018, 62, 1423. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.G.; Wagner, A.J.; Conzen, S.D.; Jordán, J.; Bellacosa, A.; Tsichlis, P.N.; Hay, N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997, 11, 701–713. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Yang, M.; Yang, J.; Zhao, C.; Han, Y.; Zhao, H.; Jiang, N.; Wei, L.; Xiao, Y.; et al. PACS-2 Ameliorates Tubular Injury by Facilitating Endoplasmic Reticulum-Mitochondria Contact and Mitophagy in Diabetic Nephropathy. Diabetes 2022, 71, 1034–1050. [Google Scholar] [CrossRef]
- Orike, N.; Middleton, G.; Borthwick, E.; Buchman, V.; Cowen, T.; Davies, A. Role of PI 3-kinase, Akt and Bcl-2-related proteins in sustaining the survival of neurotrophic factor-independent adult sympathetic neurons. J. Cell Biol. 2001, 154, 995–1006. [Google Scholar] [CrossRef]
- Liu, M.; Mi, Y.-J.; Dai, J.; Charpigny, G. Aquaporin 7 is upregulated through the PI3K-Akt pathway and modulates decidualisation of endometrial stromal cells. Reprod. Fertil. Dev. 2023, 35, 669–675. [Google Scholar] [CrossRef]
- Mourelatou, R.; Kostopoulou, E.; Rojas-Gil, A.P.; Kehagias, I.; Linos, D.; Kalfarentzos, F.E.; Spiliotis, B.E. Decreased adipocyte glucose transporter 4 (GLUT4) and aquaglyceroporin-7 (AQP7) in adults with morbid obesity: Possible early markers of metabolic dysfunction. Hormones 2019, 18, 297–306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Xia, W.; Tao, C.; Fang, X.; Yu, Y.; Hu, J.; Tian, X.; Qin, T.; Yao, C.; Zhang, W.; et al. AQP7-Mediated Mitochondrial Redox Homeostasis in Vitrified Oocytes: A Genetic Mechanism of PI3K/AKT Signaling Regulation. Genes 2025, 16, 730. https://doi.org/10.3390/genes16070730
Qi Y, Xia W, Tao C, Fang X, Yu Y, Hu J, Tian X, Qin T, Yao C, Zhang W, et al. AQP7-Mediated Mitochondrial Redox Homeostasis in Vitrified Oocytes: A Genetic Mechanism of PI3K/AKT Signaling Regulation. Genes. 2025; 16(7):730. https://doi.org/10.3390/genes16070730
Chicago/Turabian StyleQi, Yatian, Wei Xia, Chenyu Tao, Xiaohuan Fang, Yang Yu, Jingwei Hu, Xiaofeng Tian, Tianmiao Qin, Congcong Yao, Wentao Zhang, and et al. 2025. "AQP7-Mediated Mitochondrial Redox Homeostasis in Vitrified Oocytes: A Genetic Mechanism of PI3K/AKT Signaling Regulation" Genes 16, no. 7: 730. https://doi.org/10.3390/genes16070730
APA StyleQi, Y., Xia, W., Tao, C., Fang, X., Yu, Y., Hu, J., Tian, X., Qin, T., Yao, C., Zhang, W., & Li, J. (2025). AQP7-Mediated Mitochondrial Redox Homeostasis in Vitrified Oocytes: A Genetic Mechanism of PI3K/AKT Signaling Regulation. Genes, 16(7), 730. https://doi.org/10.3390/genes16070730




