Local Adaptation Shapes Phenotypic and Genetic Diversity in Zygophyllum loczyi
Abstract
1. Introduction
2. Materials and Methods
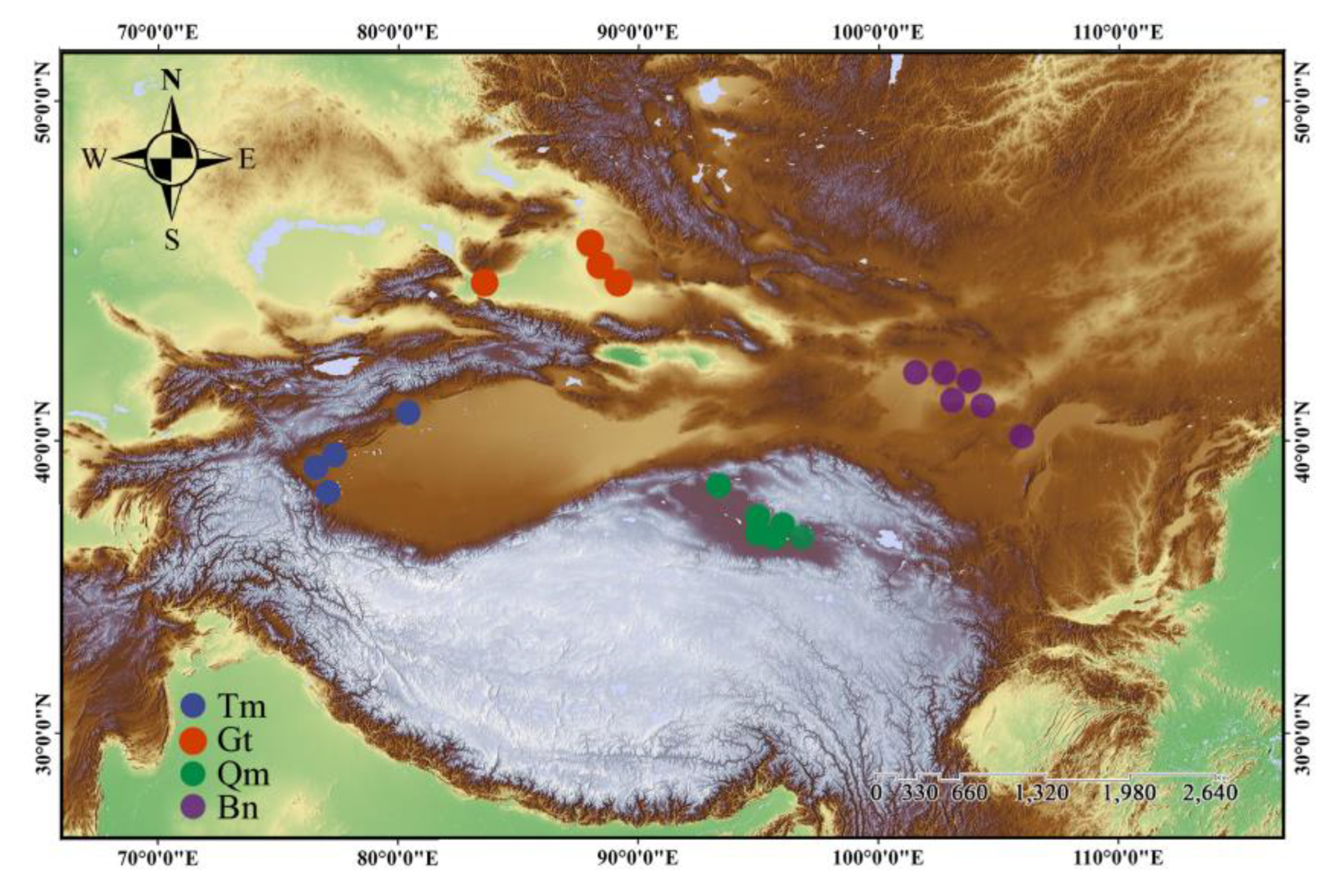
2.1. Field Sampling Design
2.2. Phenotypic Trait Measurements
2.3. Environmental Data Collection
2.4. SNP Genotyping and Sequencing
2.5. Data Processing and Statistical Analysis
3. Results
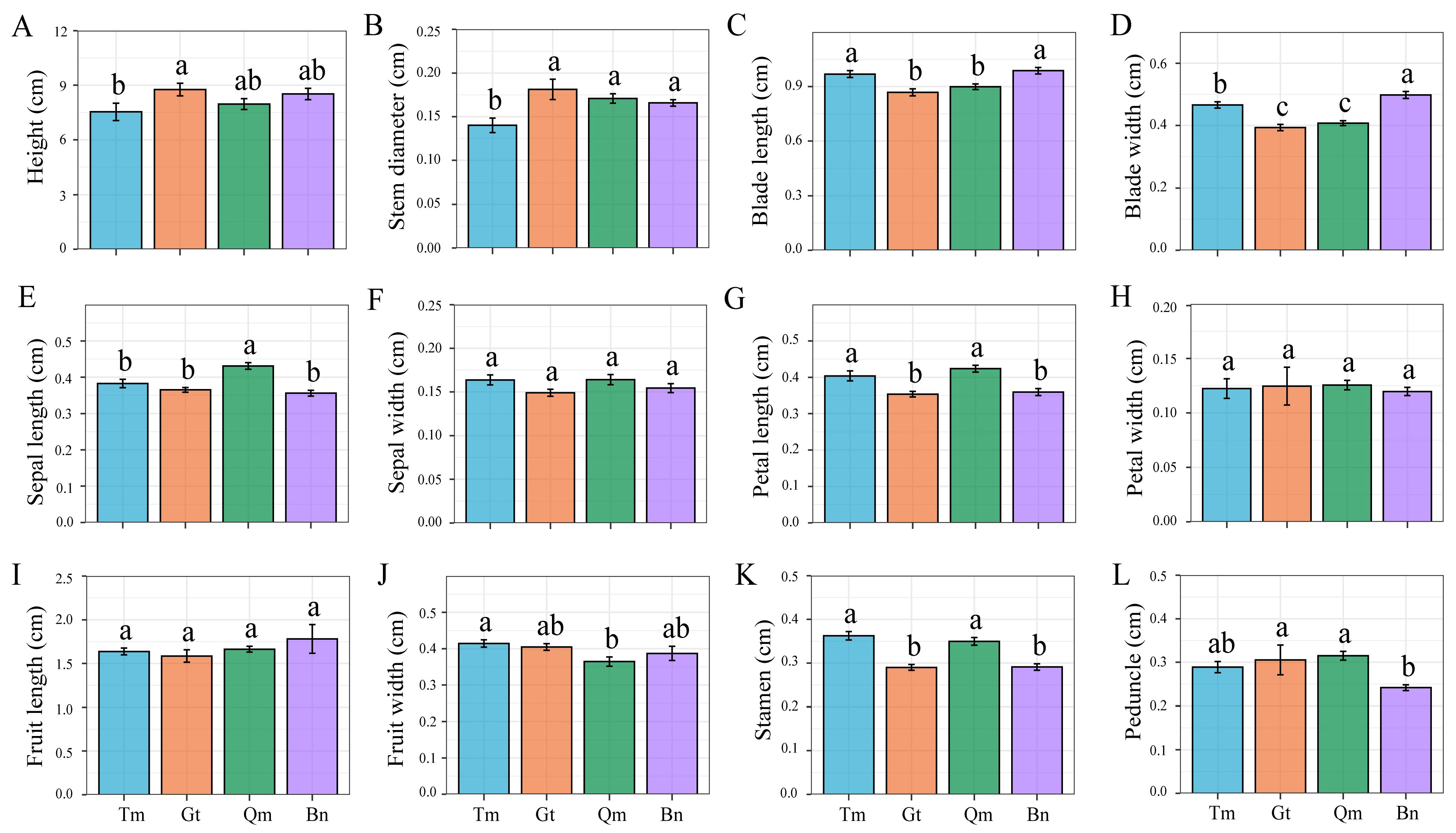
3.1. Analysis of Phenotypic Differences Among Desert Populations
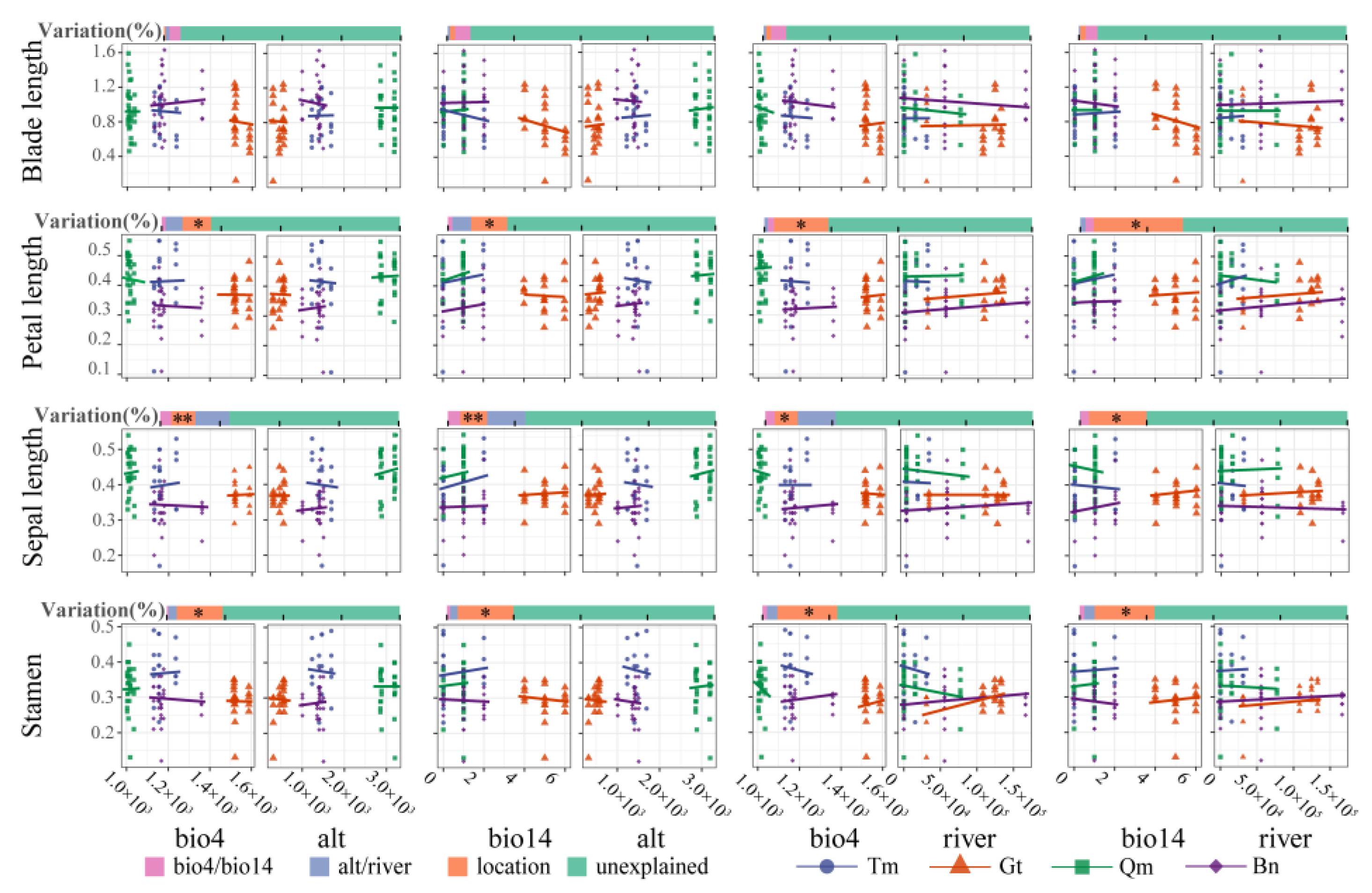
3.2. Screening of Environmental Factors and Phenotypic Traits
3.3. Modeling Environment–Phenotype Relationships
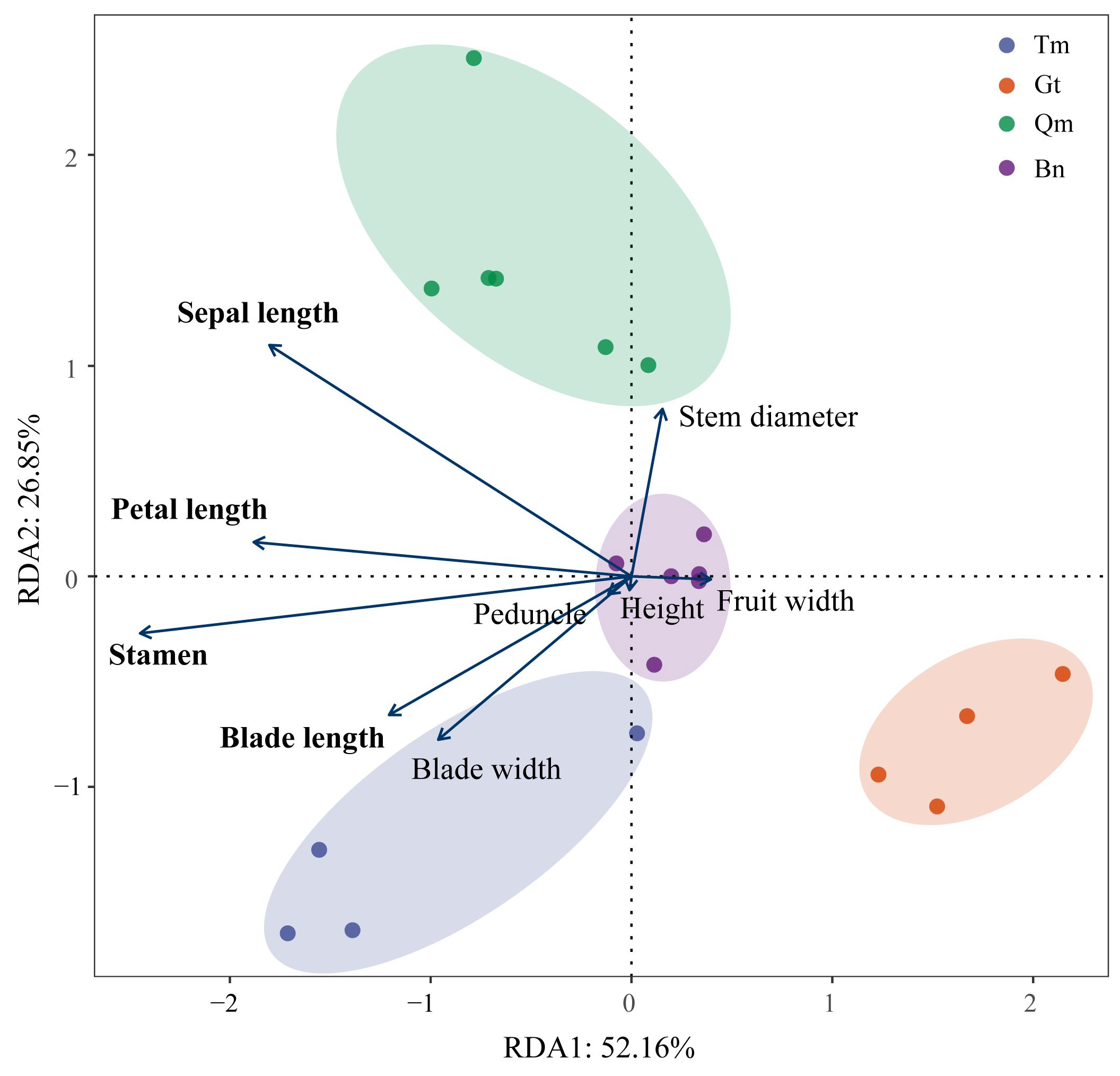
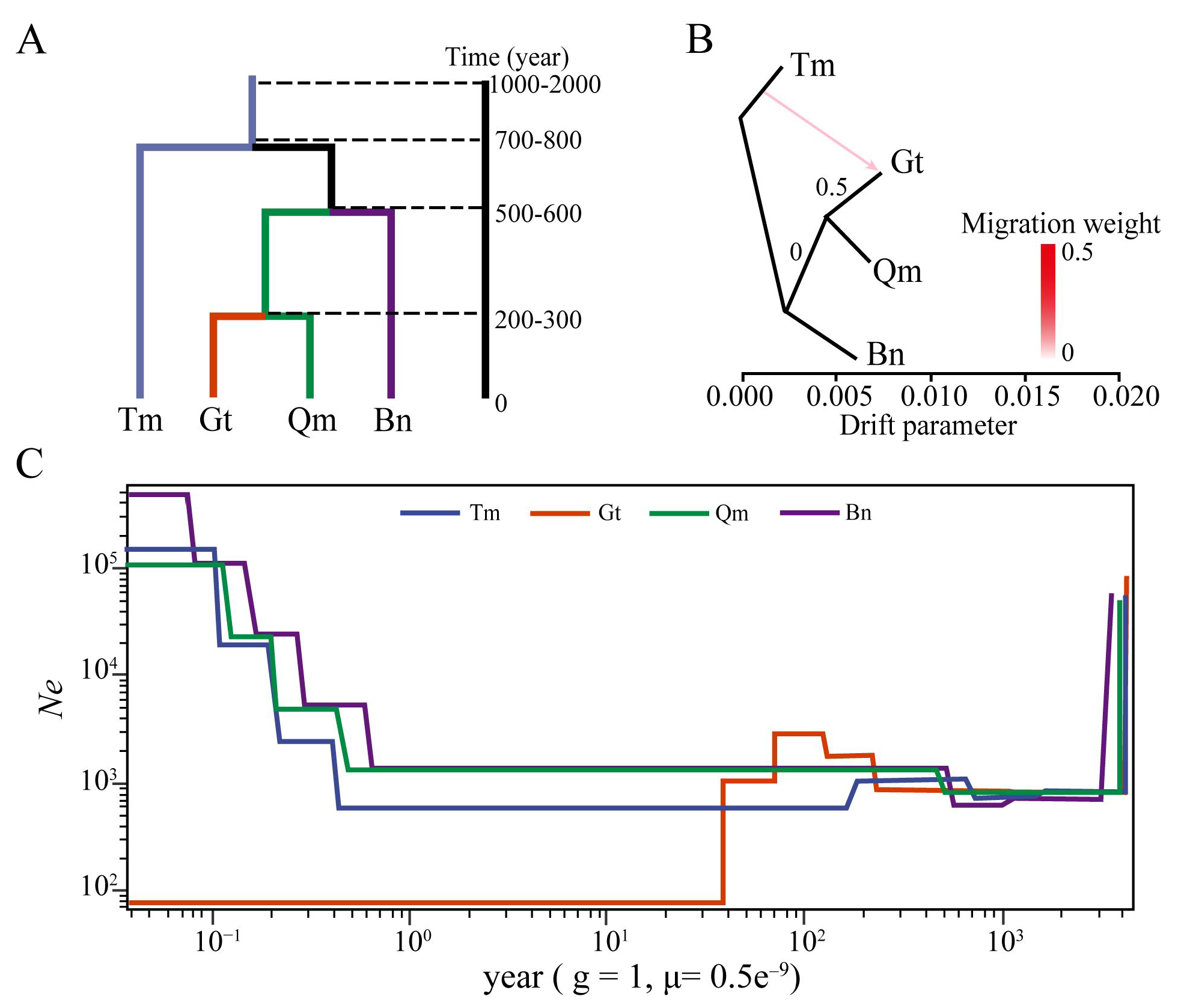
3.4. Genetic Diversity Analysis of Different Desert Populations
4. Discussion
4.1. Phenotypic Differentiation and Variation in Z. loczyi Populations
4.2. Response of Phenotypic Traits to Environmental Factors
4.3. Phenotypic Validation of SNP Genetic Differentiation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Liu, X. Phenotypic variation in Phoebe bournei Populations Preserved in the Primary Distribution Area. J. For. Res. 2018, 29, 35–44. [Google Scholar] [CrossRef]
- Rui, Z.; Chunce, G. Developmental Repatterning and Biodiversity. Biodivers. Sci. 2014, 22, 66. [Google Scholar] [CrossRef]
- Pigliucci, M.; Murren, C.J. Phenotypic Plasticity and Evolution by Genetic Assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef] [PubMed]
- Kawecki, T.J.; Ebert, D. Conceptual Issues in Local Adaptation. Ecol. Lett. 2004, 7, 1225–1241. [Google Scholar] [CrossRef]
- Inostroza, L.; Espinoza, S. Phenotypic Diversity and Productivity of Medicago sativa Subspecies from Drought-Prone Environments in Mediterranean Type Climates. Plants 2021, 10, 862. [Google Scholar] [CrossRef]
- Coleman, J.S.; McConnaughay, K.D.M. Interpreting Phenotypic Variation in Plants. Trends Ecol. Evol. 1994, 9, 187–191. [Google Scholar] [CrossRef]
- Van Kleunen, M.; Fischer, M. Constraints on the Evolution of Adaptive Phenotypic Plasticity in Plants. New Phytol. 2005, 166, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, R.A.E.; Nikoloski, Z. Genetic Basis of Plasticity in Plants. Exp. Bot. 2019, 70, 739–745. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J. Morphological Variation in Cynodon dactylon (L.) Pers., and Its Relationship with the Environment along a Longitudinal Gradient. Hereditas 2020, 157, 4. [Google Scholar] [CrossRef]
- Hughes, A.R.; Inouye, B.D. Ecological Consequences of Genetic Diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef]
- Wang, X.; Wang, D. Identification of the Complete Chloroplast Genome of Malus zhaojiaoensis Jiang and Its Comparison and Evolutionary Analysis with Other Malus Species. Genes 2022, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Bontrager, M.; Usui, T. Adaptation across Geographic Ranges Is Consistent with Strong Selection in Marginal Climates and Legacies of Range Expansion. Evolution 2021, 75, 1316–1333. [Google Scholar] [CrossRef] [PubMed]
- Tigano, A.; Friesen, V.L. Genomics of Local Adaptation with Gene Flow. Mol. Ecol. 2016, 25, 2144–2164. [Google Scholar] [CrossRef] [PubMed]
- Last, L.; Lüscher, G. Indicators for Genetic and Phenotypic Diversity of Dactylis glomerata in Swiss Permanent Grassland. Ecol. Indic. 2014, 38, 181–191. [Google Scholar] [CrossRef]
- Cavender-Bares, J.; Pahlich, A. Molecular, Morphological, and Ecological Niche Differentiation of Sympatric Sister Oak Species, Quercus virginiana and Q. geminata (Fagaceae). Am. J. Bot. 2009, 96, 1690–1702. [Google Scholar] [CrossRef]
- Lassois, L.; Denancé, C. Genetic Diversity, Population Structure, Parentage Analysis, and Construction of Core Collections in the French Apple Germplasm Based on SSR Markers. Plant Mol. Biol. Report. 2016, 34, 827–844. [Google Scholar] [CrossRef]
- Huang, C.-L.; Chen, J.-H. Disentangling the Effects of Isolation-by-Distance and Isolation-by-Environment on Genetic Differentiation among Rhododendron Lineages in the Subgenus Tsutsusi. Tree Genet. Genomes 2016, 12, 53. [Google Scholar] [CrossRef]
- Meng, H.-H.; Zhang, M.-L. Diversification of Plant Species in Arid Northwest China: Species-Level Phylogeographical History of Lagochilus Bunge ex Bentham (Lamiaceae). Mol. Phylogenet. Evol. 2013, 68, 398–409. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Q. Regional-Scale Differentiation and Phylogeography of a Desert Plant Allium mongolicum (Liliaceae) Inferred from Chloroplast DNA Sequence Variation. Plant Syst. Evol. 2017, 303, 451–466. [Google Scholar] [CrossRef]
- Savi, T.; Bertuzzi, S. Drought-induced Xylem Cavitation and Hydraulic Deterioration: Risk Factors for Urban Trees under Climate Change? New Phytol. 2015, 205, 1106–1116. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K. Impacts of Climate Change and Human Activities on Vegetation Cover in Hilly Southern China. Ecol. Eng. 2015, 81, 451–461. [Google Scholar] [CrossRef]
- Bally, J.; Nakasugi, K. The Extremophile Nicotiana benthamiana Has Traded Viral Defence for Early Vigour. Nat. Plants 2015, 1, 15165. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.K.; Shi, C. Pearl Millet Genome Sequence Provides a Resource to Improve Agronomic Traits in Arid Environments. Nat. Biotechnol. 2017, 35, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.-L.; Wang, M.-M. Phylogeographic Evidence for a Link of Species Divergence of Ephedra in the Qinghai-Tibetan Plateau and Adjacent Regions to the Miocene Asian Aridification. PLoS ONE 2013, 8, e56243. [Google Scholar] [CrossRef]
- Wu, S.-D.; Lin, L. Evolution of Asian Interior Arid-Zone Biota: Evidence from the Diversification of Asian Zygophyllum (Zygophyllaceae). PLoS ONE 2015, 10, e0138697. [Google Scholar] [CrossRef]
- Anderegg, L.; Loy, X. Aridity Drives Coordinated Trait Shifts but not Decreased Trait Variance Across the Geographic Range of Eight Australian Trees. New Phytol. 2021, 229, 1375–1387. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Kang, H.Z. Pattern of Leaf Vein Density and Climate Relationship of Quercus variabilis Populations Remains Unchanged with Environmental Changes. Trees 2011, 26, 597–607. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Alia, R. Geographical Variation in Growth form Traits in Quercus suber and its Relation to Population Evolutionary History. Evol. Ecol. 2014, 28, 55–68. [Google Scholar] [CrossRef]
- Solé-Medina, A.; Robledo-Arnuncio, J.J. Multi-trait Genetic Variation in Resource-use Strategies and Phenotypic Plasticity Correlates with Local Climate across the Range of a Mediterranean Oak (Quercus faginea). New Phytol. 2022, 234, 462–478. [Google Scholar] [CrossRef]
- Bellstedt, D.U.; Van Zyl, L. Phylogenetic Relationships, Character Evolution and Biogeography of Southern African Members of Zygophyllum (Zygophyllaceae) Based on Three Plastid Regions. Mol. Phylogenet. Evol. 2008, 47, 932–949. [Google Scholar] [CrossRef]
- Savolainen, O.; Lascoux, M. Ecological Genomics of Local Adaptation. Nat. Rev. Genet. 2013, 14, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.-K.; Shi, J. Biomass and reproductive allocation characteristics of Zygophyllum population in Ili River Valley area. Arid Land Geogr. 2013, 36, 475–481. [Google Scholar] [CrossRef]
- Moles, A.T.; Perkins, S.E. Which Is a Better Predictor of Plant Traits: Temperature or Precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Allen, G.C.; Flores-Vergara, M.A. A Modified Protocol for Rapid DNA Isolation from Plant Tissues using Cetyltrimethylammonium Bromide. Nat. Protoc. 2006, 1, 2320–2325. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S. Comparative Chloroplast Genomics and Phylogenetic Analysis of Zygophyllum (Zygophyllaceae) of China. Front. Plant Sci. 2021, 12, 723622. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A. The Variant Call Format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Banks, E. A Framework for Variation Discovery and Genotyping using Next-generation DNA Sequencing Data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Santos Del Blanco, L. Adaptation of Mediterranean Forest Species to Climate: Lessons from Common Garden Experiments. J. Ecol. 2022, 110, 1022–1042. [Google Scholar] [CrossRef]
- Terhorst, J.; Kamm, J.A. Robust and Scalable Inference of Population History from Hundreds of Unphased Whole Genomes. Nat. Genet. 2017, 49, 303–309. [Google Scholar] [CrossRef]
- Sakhale, S.A.; Yadav, S. Genome-Wide Association Analysis for Emergence of Deeply Sown Rice (Oryza sativa) Reveals Novel Aus-Specific Phytohormone Candidate Genes for Adaptation to Dry-Direct Seeding in the Field. Front. Plant Sci. 2023, 14, 1172816. [Google Scholar] [CrossRef] [PubMed]
- Leiblein-Wild, M.C.; Tackenberg, O. Phenotypic Variation of 38 European Ambrosia artemisiifolia Populations Measured in a Common Garden Experiment. Biol. Invasions 2014, 16, 2003–2015. [Google Scholar] [CrossRef]
- Westerband, A.C.; Funk, J.L. Intraspecific Trait Variation in Plants: A Renewed Focus on Its Role in Ecological Processes. Ann. Bot. 2021, 127, 397–410. [Google Scholar] [CrossRef]
- Muellner-Riehl, A.N. Mountains as Evolutionary Arenas: Patterns, Emerging Approaches, Paradigm Shifts, and Their Implications for Plant Phylogeographic Research in the Tibeto-Himalayan Region. Front. Plant Sci. 2019, 10, 195. [Google Scholar] [CrossRef]
- Bourne, E.C.; Bocedi, G. Between Migration Load and Evolutionary Rescue: Dispersal, Adaptation and the Response of Spatially Structured Populations to Environmental Change. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132795. [Google Scholar] [CrossRef] [PubMed]
- Maestre, F.T.; Quero, J.L. Plant Species Richness and Ecosystem Multifunctionality in Global Drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yang, X. Geochemical Compositions of Soluble Salts in Aeolian Sands from the Taklamakan and Badanjilin Deserts in Northern China, and Their Influencing Factors and Environmental Implications. Environ. Earth Sci. 2012, 66, 337–353. [Google Scholar] [CrossRef]
- Conover, D.O.; Duffy, T.A. The Covariance between Genetic and Environmental Influences across Ecological Gradients: Reassessing the Evolutionary Significance of Countergradient and Cogradient Variation. Ann. N. Y. Acad. Sci. 2009, 1168, 100–129. [Google Scholar] [CrossRef]
- Slatkin, M. Gene Flow and the Geographic Structure of Natural Populations. Science 1987, 236, 787–792. [Google Scholar] [CrossRef]
- Wang, I.J. Examining the Full Effects of Landscape Heterogeneity on Spatial Genetic Variation: A Multiple Matrix Regression Approach for Quantifying Geographic and Ecological Isolation: Special Section. Evolution 2013, 67, 3403–3411. [Google Scholar] [CrossRef]
- Zeng, Y.-F.; Zhang, J.-G. Phylogeographic Patterns of the Desert Poplar in Northwest China Shaped by Both Geology and Climatic Oscillations. BMC Evol. Biol. 2018, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, S.; Otto, S.P. Establishment and maintenance of adaptive genetic divergence under migration, selection, and drift. Evolution 2011, 65, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Pujol, B.; Zhou, S.-R. Reduced Inbreeding Depression after Species Range Expansion. Proc. Natl. Acad. Sci. USA 2009, 106, 15379–15383. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.-G.; Zhang, X.-Q. Genetic Diversity Analysis and Transferability of Cereal EST-SSR Markers to Orchardgrass (Dactylis glomerata L.). Biochem. Syst. Ecol. 2010, 38, 740–749. [Google Scholar] [CrossRef]

| Phenotypic Traits | Coefficient of Phenotypic Variation Among Populations (%) | |||||
|---|---|---|---|---|---|---|
| Same Desert | Different Deserts | p-Value | ||||
| Tm | Gt | Qm | Bn | |||
| Height | 6.63 | 3.50 | 3.32 | 4.66 | 5.82 | >0.05 |
| Stem diameter | 6.16 | 3.02 | 2.03 | 7.51 | 9.20 | <0.05 |
| Blade length | 2.01 | 1.60 | 1.63 | 2.65 | 5.26 | <0.001 |
| Blade width | 2.26 | 1.80 | 2.02 | 3.01 | 9.66 | <0.001 |
| Sepal length | 4.99 | 1.71 | 1.89 | 2.05 | 7.52 | <0.01 |
| Sepal width | 5.80 | 2.88 | 2.81 | 3.13 | 4.06 | >0.05 |
| Petal length | 5.50 | 2.05 | 2.61 | 2.64 | 7.97 | <0.01 |
| Petal width | 12.01 | 2.78 | 2.65 | 16.74 | 1.85 | >0.05 |
| Peduncle | 7.18 | 2.58 | 2.29 | 13.67 | 9.80 | >0.05 |
| Stamen | 4.36 | 2.04 | 2.15 | 2.79 | 10.24 | <0.001 |
| Fruit length | 2.88 | 1.75 | 9.06 | 4.94 | 4.30 | >0.05 |
| Fruit width | 2.96 | 2.98 | 4.95 | 2.56 | 4.82 | >0.05 |
| Cohen’s d | Q_st | F_st | |
|---|---|---|---|
| Tm_vs_Gt | 0.34 | 0.38 | 0.06 |
| Gt_vs_Qm | 0.37 | 0.39 | 0.04 |
| Qm_vs_Bn | 0.50 | 0.37 | 0.02 |
| Tm_vs_Qm | 0.34 | 0.33 | 0.07 |
| Gt_vs_Bn | 0.19 | 0.39 | 0.04 |
| Tm_vs_Bn | 0.36 | 0.36 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-C.; Wu, D.-Y.; Li, X.-R.; Lu, J.-Y.; Wang, S.-M.; Ma, Q.; Liu, H.-S.; Wang, X.-Y.; Liu, J.-D.; Zhang, D.-Y. Local Adaptation Shapes Phenotypic and Genetic Diversity in Zygophyllum loczyi. Genes 2025, 16, 729. https://doi.org/10.3390/genes16070729
Wang J-C, Wu D-Y, Li X-R, Lu J-Y, Wang S-M, Ma Q, Liu H-S, Wang X-Y, Liu J-D, Zhang D-Y. Local Adaptation Shapes Phenotypic and Genetic Diversity in Zygophyllum loczyi. Genes. 2025; 16(7):729. https://doi.org/10.3390/genes16070729
Chicago/Turabian StyleWang, Jan-Cheng, De-Yan Wu, Xue-Rong Li, Jia-Yi Lu, Suo-Min Wang, Qing Ma, Hai-Shuang Liu, Xi-Yong Wang, Jing-Dian Liu, and Dao-Yuan Zhang. 2025. "Local Adaptation Shapes Phenotypic and Genetic Diversity in Zygophyllum loczyi" Genes 16, no. 7: 729. https://doi.org/10.3390/genes16070729
APA StyleWang, J.-C., Wu, D.-Y., Li, X.-R., Lu, J.-Y., Wang, S.-M., Ma, Q., Liu, H.-S., Wang, X.-Y., Liu, J.-D., & Zhang, D.-Y. (2025). Local Adaptation Shapes Phenotypic and Genetic Diversity in Zygophyllum loczyi. Genes, 16(7), 729. https://doi.org/10.3390/genes16070729






