Human CD36: Gene Regulation, Protein Function, and Its Role in Atherosclerosis Pathogenesis
Abstract
1. Introduction
1.1. The Formation of Atherosclerotic Changes in Vessels
1.2. The Function of Scavenger Receptors, Including CD36
2. Human CD36 Gene
2.1. Structure of CD36 Gene
2.2. CD36 Gene Alternative Splicing
2.3. Regulation of CD36 Gene Expression
2.4. CD36 Gene Mutations
2.5. Metabolic Consequences of CD36 Mutations
3. Human CD36 Protein
3.1. Structure of CD36 Protein
3.2. CD36 Function
3.3. CD36 Signalling and Regulation of the Paracellular Pathway
4. CD36 Function in Heart
4.1. Control of Energy Metabolite Access
4.2. LCFA Binding
4.3. CD36-Mediated Ca2+-Dependent Platelet Activation After Myocardial Infarction
5. CD36 and Coronary Artery Disease (CAD)
6. sCD36, Receptor Soluble in Plasma
7. Perspectives in Diseases
Funding
Conflicts of Interest
References
- Stone, P.H.; Libby, P.; Boden, W.E. Fundamental Pathobiology of Coronary Atherosclerosis and Clinical Implications for Chronic Ischemic Heart Disease Management-The Plaque Hypothesis: A Narrative Review. JAMA Cardiol. 2023, 8, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Osterud, B.; Bjorklid, E. Role of monocytes in atherogenesis. Physiol. Rev. 2003, 83, 1069–1112. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Cui, Z.Y.; Huang, X.F.; Zhang, D.D.; Guo, R.J.; Han, M. Inflammation and atherosclerosis: Signaling pathways and therapeutic intervention. Signal Transduct. Target. Ther. 2022, 7, 131. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—From experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef]
- Doran, A.C. Inflammation Resolution: Implications for Atherosclerosis. Circ. Res. 2022, 130, 130–148. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Lin, L.; Mantzoros, C.S.; Bahijri, S.; Tuomilehto, J.; Ren, J. Inflammation in atherosclerosis: Pathophysiology and mechanisms. Cell Death Dis. 2024, 15, 817. [Google Scholar] [CrossRef]
- Ryu, S.; Howland, A.; Song, B.; Youn, C.; Song, P.I. Scavenger Receptor Class A to E Involved in Various Cancers. Chonnam Med. J. 2020, 56, 1–5. [Google Scholar] [CrossRef]
- Mineo, C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2020, 116, 1254–1274. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Dai, Y.; Mineo, C. Novel Functions of Endothelial Scavenger Receptor Class B Type I. Curr. Atheroscler. Rep. 2021, 23, 6. [Google Scholar] [CrossRef]
- Akhmedov, A.; Sawamura, T.; Chen, C.H.; Kraler, S.; Vdovenko, D.; Lüscher, T.F. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1): A crucial driver of atherosclerotic cardiovascular disease. Eur. Heart J. 2021, 42, 1797–1807. [Google Scholar] [CrossRef]
- Sweety Trivedi, S.; Chakravarty, A. Neurological Complications of Malaria. Curr. Neurol. Neurosci. Rep. 2022, 22, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.; DeNichilo, M.; King, D.P.; Cockshell, M.P.; Ebert, B.; Dale, B.; Ebert, L.M.; Woods, A.; Bonder, C.S. CD36 promotes vasculogenic mimicry in melanoma by mediating adhesion to the extracellular matrix. BMC Cancer 2021, 21, 765. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.; Metwally, N.G.; Allweier, J.; Cronshagen, J.; Del Pilar, M.; Tauler, M.; Murk, A.; Roth, L.K.; Torabi, H.; Wu, Y.; et al. CD36-A Host Receptor Necessary for Malaria Parasites to Establish and Maintain Infection. Microorganisms 2022, 10, 2356. [Google Scholar] [CrossRef]
- Available online: https://www.ncbi.nlm.nih.gov/gene/948/#gene-expression (accessed on 30 April 2025).
- Armesilla, A.L.; Vega, M.A. Structural organization of the gene for human CD36 glycoprotein. J. Biol. Chem. 1994, 269, 18985–18991. [Google Scholar] [CrossRef] [PubMed]
- Sato, O.; Takanashi, N.; Motojima, K. Third promoter and differential regulation of mouse and human fatty acid translocase/CD36 genes. Mol. Cell Biochem. 2007, 299, 37–43. [Google Scholar] [CrossRef]
- Andersen, M.; Lenhard, B.; Whatling, C.; Eriksson, P.; Abumrad, N.A. Alternative promoter usage of the membrane glycoprotein CD36. BMC Mol. Biol. 2006, 7, 8. [Google Scholar] [CrossRef][Green Version]
- Liang, C.P.; Han, S.; Okamoto, H.; Carnemolla, R.; Tabas, I.; Accili, D.; Tall, A.R. Increased CD36 protein as a response to defective insulin signaling in macrophages. J. Clin. Investig. 2004, 113, 764–773. [Google Scholar] [CrossRef]
- Nicholson, A.C. Expression of CD36 in macrophages and atherosclerosis: The role of lipid regulation of PPARgamma signaling. Trends Cardiovasc. Med. 2004, 14, 8–12. [Google Scholar] [CrossRef]
- Singh, A.; Chaudhary, R. Potentials of peroxisome proliferator-activated receptor (PPAR) α, β/delta, and γ: An in-depth and comprehensive review of their molecular mechanisms, cellular Signalling, immune responses and therapeutic implications in multiple diseases. Int. Immunopharmacol. 2025, 155, 114616. [Google Scholar] [CrossRef]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors with functions in the vascular wall. Z. Kardiol. 2001, 90 (Suppl. S3), 125–132. [Google Scholar] [CrossRef]
- Bastie, C.C.; Hajri, T.; Drover, V.A.; Grimaldi, P.A.; Abumrad, N.A. CD36 in myocytes channels fatty acids to a lipase-accessible triglyceride pool that is related to cell lipid and insulin responsiveness. Diabetes 2004, 53, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhou, X.; Yokoyama, T.; Hajjar, D.P.; Gotto, A.M.; Nicholson, A.C. Pitavastatin downregulates expression of the macrophage type B scavenger receptor, CD36. Circulation 2004, 17, 790–796. [Google Scholar] [CrossRef]
- Rubic, T.; Trottmann, M.; Lorenz, R.L. Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin. Biochem. Pharmacol. 2004, 67, 411–419. [Google Scholar] [CrossRef]
- Ruiz-Velasco, N.; Dominguez, A.; Vega, M.A. Statins upregulate CD36 expression in human monocytes, an effect strengthened when combined with PPAR-g ligands. Putative contribution of Rho GTPases in statins–induced CD36 expresssion. Biochem. Pharmacol. 2004, 67, 303–313. [Google Scholar] [CrossRef]
- Yamamoto, N.; Akamatsu, N.; Sakuraba, H.; Yamazaki, H.; Tanoue, K. Platelet glycoprotein IV (CD36) deficiency is associated with the absence (type I) or the presence (type II) of glycoprotein IV on monocytes. Blood 1994, 83, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Tamura, S.; Matsuno, K.; Nagasawa, A.; Hayasaka, K.; Shimizu, C.; Moriyama, T. Diverse CD36 expression among Japanese population: Defective CD36 mutations cause platelet and monocyte CD36 reductions in not only deficient but also normal phenotype subjects. Thromb. Res. 2015, 135, 951–957. [Google Scholar] [CrossRef]
- Okuyama, S.; Sumi, M.; Ishikawa, R.; Shishido, T.; Koyama, D.; Ueki, T.; Takahashi, D.; Kobayashi, H.; Kobayashi, H.; Tsuno, N.H. Successful allogeneic hematopoietic stem cell transplantation in a patient with type I CD36 deficiency: A case study and literature review. Int. J. Hematol. 2023, 118, 656–660. [Google Scholar] [CrossRef]
- Kuliczkowska-Płaksej, J.; Bednarek-Tupikowska, G.; Płaksej, R.; Filus, A. Scavenger receptor CD36: Its expression, regulation, and role in the pathogenesis of atherosclerosis. Part I. Postępy Hig. I Med. Doświadczalnej (Online) 2006, 60, 142–151. [Google Scholar]
- Liu, J.; Shao, Y.; Ding, H.; Deng, J.; Xu, X.; Wang, J.; Xia, W.; Santoso, S.; Ye, Y.; Fu, Y. Distribution of CD36 deficiency in different Chinese ethnic groups. Hum. Immunol. 2020, 81, 366–371. [Google Scholar] [CrossRef]
- Yanai, H.; Chiba, H.; Fujiwara, H.; Morimoto, M.; Abe, K.; Yoshida, S.; Takahashi, Y.; Fuda, H.; Hui, S.P.; Akita, H.; et al. Phenotype-genotype correlation in CD36 deficiency types I and II. Thromb. Haemost. 2000, 84, 436–441. [Google Scholar] [CrossRef]
- Flesch, B.K.; Scherer, V.; Opitz, A.; Ochmann, O.; Janson, A.; Steitz, M.; Zeiler, T. Platelet CD36 deficiency is present in 2.6% of Arabian individuals and can cause NAIT and platelet refractoriness. Transfusion 2021, 61, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Rac, M.; Safranow, K.; Poncyljusz, W. Molecular basis of human CD36 gene mutation. Mol. Med. 2007, 13, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Hajjar, D.P.; Tauras, J.M.; Feng, J.; Gotto, A.M.; Nicholson, A.C. Transforming growth factor-beta1 (TGF-beta1) and TGF-beta2 decrease expression of CD36, the type B scevenger, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-γ. J. Biol. Chem. 2000, 275, 1241–1246. [Google Scholar] [CrossRef]
- Kashiwagi, H.; Tomiyama, Y.; Nozaki, S.; Kiyoi, T.; Tadokoro, S.; Matsumoto, K.; Honda, S.; Kosugi, S.; Kurata, Y.; Matsuzawa, Y. Analyses of genetic abnormalities in type I CD36 deficiency in Japan: Identification and cell biological characterization of two novel mutations that cause CD36 deficiency in man. Hum. Genet. 2001, 108, 459–466. [Google Scholar] [CrossRef]
- Ma, X.; Bacci, S.; Mlynarski, W.; Gottardo, L.; Soccio, T.; Menzaghi, C.; Iori, E.; Lager, R.A.; Shroff, A.R.; Gervino, E.V.; et al. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum. Mol. Genet. 2004, 13, 2197–2205. [Google Scholar] [CrossRef]
- Hanawa, H.; Watanabe, K.; Nakamura, W.; Ogawa, Y.; Toba, K.; Fuse, I.; Kodama, M.; Kato, K.; Fuse, K.; Aizawa, Y. Identification of cryptic splice site, exon skipping, and novel point mutations in type I CD36 deficiency. J. Med. Genet. 2002, 39, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakata, T.; Oka, T.; Ogawa, T.; Okamoto, F.; Kusaka, Y.; Sohmiya, K.; Shimamoto, K.; Itakura, K. Defect in human myocardial long-chain fatty acid uptake is caused by FAT/CD36 mutations. J. Lipid Res. 2001, 42, 751–759. [Google Scholar] [CrossRef]
- Lyu, O.; Lin, Y.; Pan, Y.; Guan, X.; Ji, X.; Peng, M.; Li, Q.; Wang, Z.; Zhang, Z.; Luo, Z.; et al. The polymorphism analysis for CD36 among platelet donors. Sci. Rep. 2024, 14, 8534. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Hong, X.; Chen, S.; Ma, K.; Lan, X.; Ying, Y.; He, J.; Zhu, F.; Lv, H. Variants of CD36 gene and their association with CD36 protein expression in platelets. Blood Transfus. 2014, 12, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Meyre, D.; Andress, E.J.; Sharma, T.; Snippe, M.; Asif, H.; Maharaj, A.; Vatin, V.; Gaget, S.; Besnard, F.; Choquet, H.; et al. Contribution of rare coding mutations in CD36 to type 2 diabetes and cardio-metabolic complications. Sci. Rep. 2019, 9, 17123. [Google Scholar] [CrossRef]
- Rac, M.E. CD36 receptor mutations and atherosclerosis. Prog. Med. Res. 2005, 3, 9. [Google Scholar]
- Bonen, A.; Campbell, S.E.; Benton, C.R.; Chabowski, A.; Coort, S.L.M.; Han, X.X.; Koonen, D.P.Y.; Glatz, J.F.C.; Luiken, J.J.F.P. Regulation of fatty acid transport by fatty acid translocase/CD36. Proc. Nutr. Soc. 2004, 63, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, D.; Huang, W.; Song, Y.; Ge, L.; Zhang, X.; Yang, L.; Lu, J.; Tu, X.; Chen, Q.; et al. Feedback regulation of coronary artery disease susceptibility gene ADTRP and LDL receptors LDLR/CD36/LOX-1 in endothelia cell functions involved in atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166130. [Google Scholar] [CrossRef]
- Nicholson, A.C.; Han, J.; Febbraio, M.; Silversterin, R.L.; Hajjar, D.P. Role of CD36, the macrophage class B scavenger receptor, in atherosclerosis. Ann. N. Y. Acad. Sci. 2001, 947, 224–228. [Google Scholar] [CrossRef]
- Tsubokawa, T.; Nakamura, M.; Miyazaki, E.; Kimura, Y.; Kashiwagi, Y.; Sato, T.; Kida, K. Perioperative Management of a Patient With CD36 Deficiency Undergoing Urgent Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2022, 8 Pt B, 3149–3151. [Google Scholar] [CrossRef]
- Kadlecova, M.; Cejka, J.; Zicha, J.; Kunes, J. Does CD36 gene play a key role in disturbed glucose and fatty acid metabolism in Prague hypertensive hypertriglyceridemic rats? Physiol. Res. 2004, 53, 265–271. [Google Scholar] [CrossRef]
- Kintaka, T.; Tanaka, T.; Imai, M.; Adachi, I.; Narabayashi, I.; Kitaura, Y. CD36 genotype and long-chain fatty acid uptake in the heart. Circ. J. 2002, 66, 819–825. [Google Scholar] [CrossRef][Green Version]
- Available online: https://www.ncbi.nlm.nih.gov/datasets/gene/id/948/products/ (accessed on 30 April 2025).
- Luiken, J.J.F.P.; Chanda, D.; Nabben, M.; Neumann, D.; Glatz, J.F.C. Post-translational modifications of CD36 (SR-B2): Implications for regulation of myocellular fatty acid uptake. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 2253–2258. [Google Scholar] [CrossRef]
- Karunakaran, U.; Elumalai, S.; Moon, S.J.; Won, K.C. CD36 Signal Transduction in Metabolic Diseases: Novel Insights and Therapeutic Targeting. Cells 2021, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Huang, H.Z.; Tan, L.T.; Wan, J.M.; Gui, H.B.; Zhao, X.Z.; Ruan, L.; Chen, X.; Du, X. CD36 Mediated Fatty Acid-Induced Podocyte Apoptosis via Oxidative Stress. PLoS ONE 2015, 10, e0127507. [Google Scholar] [CrossRef]
- Zaidi, N.E.; Shazali, N.A.H.; Leow, T.C.; Osman, M.A.; Ibrahim, K.; Cheng, W.H.; Lai, K.S.; Rahman, N.M.A.N.A. CD36-Fatty Acid-Mediated Metastasis via the Bidirectional Interactions of Cancer Cells and Macrophages. Cells 2022, 11, 3556. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.W.; Zuppe, H.T.; Kurokawa, M. The Role of CD36 in Cancer Progression and Its Value as a Therapeutic Target. Cells 2023, 12, 1605. [Google Scholar] [CrossRef]
- Akachar, J.; Etchebest, C.; El Jaoudi, R.; Ibrahimi, A. The computational analyses, molecular dynamics of fatty-acid transport mechanism to the CD36 receptor. Sci. Rep. 2021, 11, 23207. [Google Scholar] [CrossRef]
- Banesh, S.; Trivedi, V. Therapeutic Potentials of Scavenger Receptor CD36 Mediated Innate Immune Responses Against Infectious and Non-Infectious Diseases. Curr. Drug Discov. Technol. 2020, 17, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Kuda, O.; Samovski, D.; Abumrad, N.A. Structure-function of CD36 and importance of fatty acid signal transduction in fat metabolism. Annu. Rev. Nutr. 2014, 34, 281–303. [Google Scholar] [CrossRef]
- Hao, J.W.; Wang, J.; Guo, H.; Zhao, Y.Y.; Sun, H.H.; Li, Y.F.; Lai, X.Y.; Zhao, N.; Wang, X.; Xie, C.; et al. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 2020, 11, 4765. [Google Scholar] [CrossRef]
- Chu, L.Y.; Ramakrishnan, D.P.; Silverstein, R.L. Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 2013, 122, 1822–1832. [Google Scholar] [CrossRef]
- Ramos-Jiménez, A.; Zavala-Lira, R.A.; Moreno-Brito, V.; González-Rodríguez, E. FAT/CD36 Participation in Human Skeletal Muscle Lipid Metabolism: A Systematic Review. J. Clin. Med. 2022, 12, 318. [Google Scholar] [CrossRef]
- Bendas, G.; Schlesinger, M. The Role of CD36/GPIV in Platelet Biology. Semin. Thromb. Hemost. 2024, 50, 224–235. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ashouri, R.; Fangman, M.; Mazur, A.; Garett, T.; Doré, S. Soluble Receptors Affecting Stroke Outcomes: Potential Biomarkers and Therapeutic Tools. Int. J. Mol. Sci. 2021, 22, 1108. [Google Scholar] [CrossRef]
- Zhao, L.; Varghese, Z.; Moorhead, J.F.; Chen, Y.; Ruan, X.Z. CD36 and lipid metabolism in the evolution of atherosclerosis. Br. Med. Bull. 2018, 126, 101–112. [Google Scholar] [CrossRef]
- Watanabe, T.; Sato, T. Roles of the kisspeptin/GPR54 system in pathomechanisms of atherosclerosis. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 889–895. [Google Scholar] [CrossRef]
- Febbraio, M.; Silverstein, R.L. CD36: Implications in cardiovascular disease. Int. J. Biochem. Cell Biol. 2007, 39, 2012–2030. [Google Scholar] [CrossRef]
- Korporaal, S.J.; van Eck, M.; Adelmeijer, J.; IJsseldijk, M.; Out, R.; Lisman, T.; Lenting, P.J.; van Berkel, T.J.; Akkerman, J.W. Platelet activation by oxidized low density lipoprotein is mediated by CD36 and scavenger receptor-A. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2476–2483. [Google Scholar] [CrossRef]
- Hoekstra, M. SR-BI as target in atherosclerosis and cardiovascular disease—A comprehensive appraisal of the cellular functions of SR BI in physiology and disease. Atherosclerosis 2017, 258, 153–161. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef]
- Samovski, D.; Jacome-Sosa, M.; Abumrad, N.A. Fatty Acid Transport and Signaling: Mechanisms and Physiological Implications. Annu. Rev. Physiol. 2023, 85, 317–337. [Google Scholar] [CrossRef]
- Zingg, J.M.; Vlad, A.; Ricciarelli, R. Oxidized LDLs as Signaling Molecules. Antioxidants 2021, 10, 1184. [Google Scholar] [CrossRef]
- Nergiz-Unal, R.; Lamers, M.M.E.; Van Kruchten, R.; Luiken, J.J.; Cosemans, J.M.E.M.; Glatz, J.F.C.; Kuijpers, M.J.E.; Heemskerk, J.W.M. Signaling role of CD36 in platelet activation and thrombus formation on immobilized thrombospondin or oxidized low-density lipoprotein. J. Thromb. Haemost. 2011, 9, 1835–1846. [Google Scholar] [CrossRef]
- Ricote, M.; Valledor, A.F.; Glass, C.K. Decoding transcriptional programs regulated by PPARs and LXRs in the macrophage: Effects on lipid homeostasis, inflammation, and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 230–239. [Google Scholar] [CrossRef]
- Maréchal, L.; Laviolette, M.; Rodrigue-Way, A.; Sow, B.; Brochu, M.; Caron, V.; Tremblay, A. The CD36-PPARγ pathway in metabolic disorders. Int. J. Mol. Sci. 2018, 19, 1529. [Google Scholar] [CrossRef]
- Raghavan, S.; Singh, N.K.; Gali, S.; Mani, A.M.; Rao, G.N. Protein kinase Cθvia activating transcription factor 2-mediated CD36 expression and foam cell formation of Ly6Chi cells contributes to atherosclerosis. Circulation 2018, 138, 2395–2412. [Google Scholar] [CrossRef]
- Ren, Q.; Xie, X.; Zhao, C.; Wen, Q.; Pan, R.; Du, Y. 2,2’,4,4’-Tetrabromodiphenyl Ether (PBDE 47) Selectively Stimulates Proatherogenic PPARγ Signatures in Human THP-1 Macrophages to Contribute to Foam Cell Formation. Chem. Res. Toxicol. 2022, 35, 1023–1035. [Google Scholar] [CrossRef]
- Li, H.; Herrmann, T.; Seeßle, J.; Liebisch, G.; Merle, U.; Stremmel, W.; Chamulitrat, W. Role of fatty acid transport protein 4 in metabolic tissues: Insights into obesity and fatty liver disease. Biosci. Rep. 2022, 42, BSR20211854. [Google Scholar] [CrossRef]
- Chen, K.; Febbraio, M.; Li, W.; Silverstein, R.L. A specific CD36-dependent signaling pathway is required for platelet activation by oxidized low-density lipoprotein. Circ. Res. 2008, 102, 1512–1519. [Google Scholar] [CrossRef]
- He, Q.; Chen, Y.; Wang, Z.; He, H.; Yu, P. Cellular Uptake, Metabolism and Sensing of Long-Chain Fatty Acids. Front. Biosci. 2023, 28, 10. [Google Scholar] [CrossRef]
- Ohm, R.G.; Mulumba, M.; Chingle, R.M.; Ahsanullah, M.; Zhang, J.; Chemtob, S.; Ong, H.; Lubell, W.D. Diversity-Oriented A3-Macrocyclization for Studying Influences of Ring-Size and Shape of Cyclic Peptides: CD36 Receptor Modulators. J. Med. Chem. 2021, 64, 9365–9380. [Google Scholar] [CrossRef]
- Luiken, J.J.F.P.; Nabben, M.; Neumann, D.; Glatz, J.F.C. Understanding the distinct subcellular trafficking of CD36 and GLUT4 during the development of myocardial insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165775. [Google Scholar] [CrossRef] [PubMed]
- Schianchi, F.; Glatz, J.F.C.; Gascon, A.N.; Nabben, M.; Neumann, D.; Luiken, J.J.F.P. Putative Role of Protein Palmitoylation in Cardiac Lipid-Induced Insulin Resistance. Int. J. Mol. Sci. 2020, 21, 9438. [Google Scholar] [CrossRef]
- Zuurbier, C.J.; Bertrand, L.; Beauloye, C.R.; Andreadou, I.; Ruiz-Meana, M.; Jespersen, N.R.; Kula-Alwar, D.; Prag, H.A.; Botker, H.E.; Dambrova, M.; et al. Cardiac metabolism as a driver and therapeutic target of myocardial infarction. J. Cell Mol. Med. 2020, 24, 5937–5954. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, A.Y.; Doman, E.R.; Sanchez, K.; Chilton, R.J. Impact of insulin resistance and microvascular ischemia on myocardial energy metabolism and cardiovascular function: Pathophysiology and therapeutic approaches. Cardiovasc. Endocrinol. Metab. 2025, 14, e00332. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L. Ischemic heart disease pathophysiology paradigms overview: From plaque activation to microvascular dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Nabben, M.; Luiken, J.J.F.P. CD36 (SR-B2) as master regulator of cellular fatty acid homeostasis. Curr. Opin. Lipidol. 2022, 33, 103–111. [Google Scholar] [CrossRef]
- Shu, H.; Peng, Y.; Hang, W.; Nie, J.; Zhou, N.; Wang, D.W. The role of CD36 in cardiovascular disease. Cardiovasc. Res. 2022, 118, 115–129. [Google Scholar] [CrossRef]
- Tian, K.; Xu, Y.; Sahebkar, A.; Xu, S. CD36 in Atherosclerosis: Pathophysiological Mechanisms and Therapeutic Implications. Curr. Atheroscler. Rep. 2020, 22, 59. [Google Scholar] [CrossRef]
- Samovski, D.; Sun, J.; Pietka, T.; Gross, R.W.; Eckel, R.H.; Su, X.; Stahl, F.D.; Abumrad, N.A. Regulation of AMPK activation by CD36 links fatty acid uptake to β-oxidation. Diabetes 2015, 64, 353–359. [Google Scholar] [CrossRef]
- Nakatani, K.; Watabe, T.; Masuda, D.; Imaizumi, M.; Shimosegawa, E.; Kobayashi, T.; Sairyo, M.; Zhu, Y.; Okada, T.; Kawase, R.; et al. Myocardial energy provision is preserved by increased utilization of glucose and ketone bodies in CD36 knockout mice. Metabolism 2015, 64, 1165–1174. [Google Scholar] [CrossRef]
- Mallick, R.; Basak, S.; Duttaroy, A.K. Fatty acids and evolving roles of their proteins in neurological, cardiovascular disorders and cancers. Prog. Lipid Res. 2021, 83, 101116. [Google Scholar] [CrossRef]
- Hung, J.; Scanlon, J.P.; Mahmoud, A.D.; Rodor, J.; Ballantyne, M.; Fontaine, M.A.C.; Temmerman, L.; Kaczynski, J.; Connor, K.L.; Bhushan, R.; et al. Novel plaque enriched long noncoding RNA in atherosclerotic macrophage regulation (PELATON). Arterioscler. Thromb. Vasc. Biol. 2020, 40, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Karsenberg, R.; Bianchi, F.; van den Bogaart, G. CD36 as a double-edged sword in cancer. Immunol. Lett. 2024, 265, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Dilmaghnai, N.A.; Shoorei, H.; Sharifi, G.; Mohaqiq, M.; Majidpoor, J.; Dinger, M.E.; Taheri, M.; Ghafouri-Fard, S. Non-coding RNAs modulate function of extracellular matrix proteins. Biomed. Pharmacother. 2021, 136, 111240. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.A.; Cabodevilla, A.G.; Samovski, D.; Pietka, T.; Basu, D.; Goldberg, I.J. Endothelial Cell Receptors in Tissue Lipid Uptake and Metabolism. Circ. Res. 2021, 128, 433–450. [Google Scholar] [CrossRef]
- Bharadwaj, K.G.; Hiyama, Y.; Hu, Y.; Huggins, L.A.; Ramakrishnan, R.; Abumrad, N.A.; Shulman, G.I.; Blaner, W.S.; Goldberg, I.J. Chylomicron- and VLDL-derived lipids enter the heart through different pathways: In vivo evidence for receptor- and non-receptor-mediated fatty acid uptake. J. Biol. Chem. 2010, 285, 37976–37986. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Cabodevilla, A.G.; Samovski, D.; Cifarelli, V.; Basu, D.; Abumrad, N.A. Lipolytic enzymes and free fatty acids at the endothelial interface. Atherosclerosis 2021, 329, 1–8. [Google Scholar] [CrossRef]
- Duncan, J.G.; Bharadwaj, K.G.; Fong, J.L.; Mitra, R.; Sambandam, N.; Courtois, M.R.; Lavine, K.J.; Goldberg, I.J.; Kelly, D.P. Rescue of cardiomyopathy in peroxisome proliferator-activated receptor-α transgenic mice by deletion of lipoprotein lipase identifies sources of cardiac lipids and peroxisome proliferator-activated receptor-α activators. Circulation 2010, 121, 426–435. [Google Scholar] [CrossRef]
- McTavish, P.V.; Mutch, D.M. Omega-3 fatty acid regulation of lipoprotein lipase and FAT/CD36 and its impact on white adipose tissue lipid uptake. Lipids Health Dis. 2024, 23, 386. [Google Scholar] [CrossRef]
- Dergunov, A.D.; Nosova, E.V.; Rozhkova, A.V.; Vinogradina, M.A.; Baserova, V.B.; Popov, M.A.; Limborska, S.A.; Dergunova, L.V. HDL Cholesterol-Associated Shifts in the Expression of Preselected Genes Reveal both Pro-Atherogenic and Atheroprotective Effects of HDL in Coronary Artery Disease. Front. Biosci. 2024, 29, 396. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Silverstein, R.L. CD36 and ERK5 link dyslipidemia to apoptotic like platelet procoagulant function. Curr. Opin. Hematol. 2019, 26, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Rać, M.E.; Suchy, J.; Kurzawski, G.; Safranow, K.; Jakubowska, K.; Olszewska, M.; Garanty-Bogacka, B.; Rać, M.; Poncyljusz, W.; Chlubek, D. Analysis of human CD36 gene sequence alterations in the oxidized low-density lipoprotein-binding region using denaturing high-performance liquid chromatography. Genet. Test. Mol. Biomark. 2010, 14, 551–557. [Google Scholar] [CrossRef]
- Rać, M.; Safranow, K.; Kurzawski, G.; Krzystolik, A.; Chlubek, D. Is CD36 gene polymorphism in region encoding lipid-binding domain associated with early onset CAD? Gene 2013, 530, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Love-Gregory, L.; Sherva, R.; Schappe, T.; Qi, J.S.; McCrea, J.; Klein, S.; Connelly, M.A.; Abumrad, N.A. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 2011, 20, 193–201. [Google Scholar] [CrossRef]
- Rać, M.E.; Suchy, J.; Kurzawski, G.; Kurlapska, A.; Safranow, K.; Rać, M.; Sagasz-Tysiewicz, D.; Krzystolik, A.; Poncyljusz, W.; Jakubowska, K.; et al. Polymorphism of the CD36 Gene and Cardiovascular Risk Factors in Patients with Coronary Artery Disease Manifested at a Young Age. Biochem. Genet. 2012, 50, 103–111. [Google Scholar] [CrossRef]
- Rać, M.; Kurzawski, G.; Safranow, K.; Rać, M.; Sagasz-Tysiewicz, D.; Krzystolik, A.; Poncyljusz, W.; Olszewska, M.; Dawid, G.; Chlubek, D. Association of CD36 gene polymorphisms with echo- and electrocardiographic parameters in patients with early onset coronary artery disease. Arch. Med. Sci. 2013, 9, 640–650. [Google Scholar] [CrossRef]
- Rać, M.; Safranow, K.; Rać, M.; Kurzawski, G.; Krzystolik, A.; Sagasz-Tysiewicz, D.; Jakubowska, K.; Poncyljusz, W.; Chlubek, D. CD36 gene is associated with thickness of atheromatous plaque and ankle-brachial index in patients with early coronary artery disease. Kardiol. Pol. 2012, 70, 918–923. [Google Scholar]
- Bartoszewicz, M.; Rać, M. Prognostic Value of the Selected Polymorphisms in the CD36 Gene in the Domain-Encoding Lipid-Binding Region at a 10-Year Follow-Up for Early-Onset CAD Patients. Biomedicines 2023, 11, 1332. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, J.; Zhang, C.; Li, X.; Meng, W.; Mo, X.; Zhang, Q.; Liu, Q.; Ren, K.; Du, R.; et al. Cell-derived microparticles in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Cell Physiol. Biochem. 2016, 39, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Handberg, A.; Højlund, K.; Gastaldelli, A.; Flyvbjerg, A.; Dekker, J.M.; Petrie, J.; Piatti, P.; Beck-Nielsen, H. RISC Investigators. Plasma sCD36 is associated with markers of atherosclerosis, insulin resistance and fatty liver in a nondiabetic healthy population. J. Intern. Med. 2012, 271, 294–304. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Aroner, S.; Overvad, K.; Cai, T.; Yang, M.; Tjønneland, A.; Handberg, A.; Jensen, M.K. Plasma CD36 and incident diabetes: A case-cohort study in danish men and women. Diabetes Metab. J. 2020, 44, 134–142. [Google Scholar] [CrossRef]
- Knøsgaard, L.; Kazankov, K.; Birkebæk, N.H.; Holland-Fischer, P.; Lange, A.; Solvig, J.; Hørlyck, A.; Kristensen, K.; Rittig, S.; Vilstrup, H.; et al. Reduced sCD36 following weight loss corresponds to improved insulin sensitivity, dyslipidemia and liver fat in obese children. Eur. J. Clin. Nutr. 2016, 70, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Krzystolik, A.; Dziedziejko, V.; Safranow, K.; Kurzawski, G.; Rać, M.; Sagasz-Tysiewicz, D.; Poncyljusz, W.; Jakubowska, K.; Chlubek, D.; Rać, M. Is plasma soluble CD36 associated with cardiovascular risk factors in early onset coronary artery disease patients? Scand. J. Clin. Lab. Invest. 2015, 75, 398–406. [Google Scholar] [CrossRef]
- Rać, M.; Krzystolik, A.; Rać, M.; Safranow, K.; Dziedziejko, V.; Goschorska, M.; Poncyljusz, W.; Chlubek, D. Is plasma-soluble CD36 associated with density of atheromatous plaque and ankle-brachial index in early onset coronary artery disease patients? Kardiol. Pol. 2016, 74, 570–575. [Google Scholar] [CrossRef]
- Rać, M.; Safranow, K.; Garanty-Bogacka, B.; Dziedziejko, V.; Kurzawski, G.; Goschorska, M.; Kuligowska, A.; Pauli, N.; Chlubek, D. CD36 gene polymorphism and plasma sCD36 as the risk factor in higher cholesterolemia. Arch. Pediatr. 2018, 25, 177–181. [Google Scholar] [CrossRef]
- Perumalsamy, S.; Huri, H.Z.; Abdullah, B.M.; Mazlan, O.; Ahmad, W.A.W.; Vethakkan, S.R.D.B. Genetic Markers of Insulin Resistance and Atherosclerosis in Type 2 Diabetes Mellitus Patients with Coronary Artery Disease. Metabolites 2023, 13, 427. [Google Scholar] [CrossRef]
- Nagao, M.; Esguerra, J.L.S.; Asai, A.; Ofori, J.K.; Edlund, A.; Wendt, A.; Sugihara, H.; Wollheim, C.B.; Oikawa, S.; Eliasson, L. Potential protection against type 2 diabetes in obesity through lower CD36 expression and improved exocytosis in β-cells. Diabetes 2020, 69, 1193–1205. [Google Scholar] [CrossRef]
- Dziedziejko, V.; Pauli, N.; Kuligowska, A.; Safranow, K.; Goschorska, M.; Chlubek, D.; Rać, M.E. Significant limitations associated with the analysis of human plasma soluble CD36 performed by ELISA. Pomeranian J. Life Sci. 2018, 64, 39–41. [Google Scholar] [CrossRef]
- Puchałowicz, K.; Rać, M. The Multifunctionality of CD36 in Diabetes Mellitus and Its Complications-Update in Pathogenesis, Treatment and Monitoring. Cells 2020, 9, 1877. [Google Scholar] [CrossRef]
- Varghese, D.S.; Ali, B.R. Pathological crosstalk between oxidized LDL and ER stress in human diseaseas: A comprehenisve review. Front. Cell Dev. Biol. 2021, 9, 674103. [Google Scholar] [CrossRef]
- Cammisotto, V.; Baratta, F.; Simeone, P.G.; Barale, C.; Lupia, E.; Galardo, G.; Santilli, F.; Russo, I.; Pignatelli, P. Proprotein Convertase Subtilisin Kexin Type 9 (PCSK9) Beyond Lipids: The Role in Oxidative Stress and Thrombosis. Antioxidants 2022, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Morotti, A.; Barale, C.; Melchionda, E.; Russo, I. Platelet Redox Imbalance in Hypercholesterolemia: A Big Problem for a Small Cell. Int. J. Mol. Sci. 2022, 23, 11446. [Google Scholar] [CrossRef]
- Garcia-Bonilla, L.; Racchumi, G.; Murphy, M.; Anrather, J.; Iadecola, C. Endothelial. CD36 contributes to postischemic brain injury by promoting neutrophil activation via CSF3. J. Neurosci. 2015, 35, 14783–14793. [Google Scholar] [CrossRef]
- Rada, P.; González-Rodríguez, A.; García-Monzón, C.; Valverde, A.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef]
- Vassiliou, E.; Farias-Pereira, R. Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity. Int. J. Mol. Sci. 2023, 24, 12032. [Google Scholar] [CrossRef]
- Tanase, C.; Enciu, A.M.; Codrici, E.; Popescu, I.D.; Dudau, M.; Dobri, A.M.; Pop, S.; Mihai, S.; Gheorghișan-Gălățeanu, A.A.; Hinescu, M.E. Fatty Acids, CD36, Thrombospondin-1, and CD47 in Glioblastoma: Together and/or Separately? Int. J. Mol. Sci. 2022, 23, 604. [Google Scholar] [CrossRef]
- Liao, X.; Yan, S.; Li, J.; Jiang, C.; Huang, S.; Liu, S.; Zou, X.; Zhang, G.; Zou, J.; Liu, Q. CD36 and Its Role in Regulating the Tumor Microenvironment. Curr. Oncol. 2022, 29, 8133–8145. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Su, M.; Lu, J.; Li, D.; Niu, X.; Wang, Y. CD36: The Bridge between Lipids and Tumors. Molecules 2024, 29, 531. [Google Scholar] [CrossRef]
- Wang, J.; Cao, H.; Yang, H.; Wang, N.; Weng, Y.; Luo, H. The function of CD36 in Mycobacterium tuberculosis infection. Front. Immunol. 2024, 15, 1413947. [Google Scholar] [CrossRef]
- Xia, L.; Zhou, Z.; Chen, X.; Luo, W.; Ding, L.; Xie, H.; Zhuang, W.; Ni, K.; Li, G. Ligand-dependent CD36 functions in cancer progression, metastasis, immune response, and drug resistance. Biomed. Pharmacother. 2023, 168, 115834. [Google Scholar] [CrossRef]
- Nagao, M.; Lagerstedt, J.O.; Eliasson, L. Secretory granule exocytosis and its amplification by cAMP in pancreatic β-cells. Diabetol. Int. 2022, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, L.; Qiu, Z.; Deng, W.; Wang, W. Key Molecules of Fatty Acid Metabolism in Gastric Cancer. Biomolecules 2022, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Yuasa-Kawase, M.; Masuda, D.; Yamashita, T.; Kawase, R.; Nakaoka, H.; Inagaki, M.; Nakatani, K.; Tsubakio-Yamamoto, K.; Ohama, T.; Matsuyama, A.; et al. Patients with CD36 deficiency are associated with enhanced atherosclerotic cardiovascular diseases. J. Atheroscler. Thromb. 2012, 19, 263–275. [Google Scholar] [CrossRef]
- Péč, M.J.; Benko, J.; Jurica, J.; Péčová, M.; Samec, M.; Hurtová, T.; Bolek, T.; Galajda, P.; Péč, M.; Samoš, M.; et al. The Anti-Thrombotic Effects of PCSK9 Inhibitors. Pharmaceuticals 2023, 16, 1197. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, J.; Chen, H.; Zhang, T.; He, D.; Luo, O.; Chi, J.; Hong, Z.; Liao, Y.; Zhang, S.; et al. PCSK9 Inhibition: From Current Advances to Evolving Future. Cells 2022, 11, 2972. [Google Scholar] [CrossRef]
- Liu, G.; Yu, X.; Cui, C.; Li, X.; Wang, T.; Palade, P.T.; Mehta, J.L.; Wang, X. The pleiotropic effects of PCSK9 in cardiovascular diseases beyond cholesterol metabolism. Acta Physiol. 2025, 241, e14272. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, L.; Xu, Y.; Yang, Y.; Wang, L.; Si, S.; Cho, S.; Hong, B. Salvianolic acid B inhibits macrophage uptake of modified low density lipoprotein (mLDL) in a scavenger receptor CD36-dependent manner. Atherosclerosis 2012, 223, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Javadifar, A.; Rastgoo, S.; Banach, M.; Jamialahmadi, T.; Johnston, T.P.; Sahebkar, A. Foam Cells as Therapeutic Targets in Atherosclerosis with a Focus on the Regulatory Roles of Non-Coding RNAs. Int. J. Mol. Sci. 2021, 22, 2529. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, X.; Hu, J.; Liu, X.; Guo, Z.; Wu, J.; Shao, Y.; Hao, M.; Zhang, S.; Hu, W.; et al. The translational potential of miR-26 in atherosclerosis and development of agents for its target genes ACC1/2, COL1A1, CPT1A, FBP1, DGAT2, and SMAD7. Cardiovasc. Diabetol. 2024, 23, 21. [Google Scholar] [CrossRef] [PubMed]
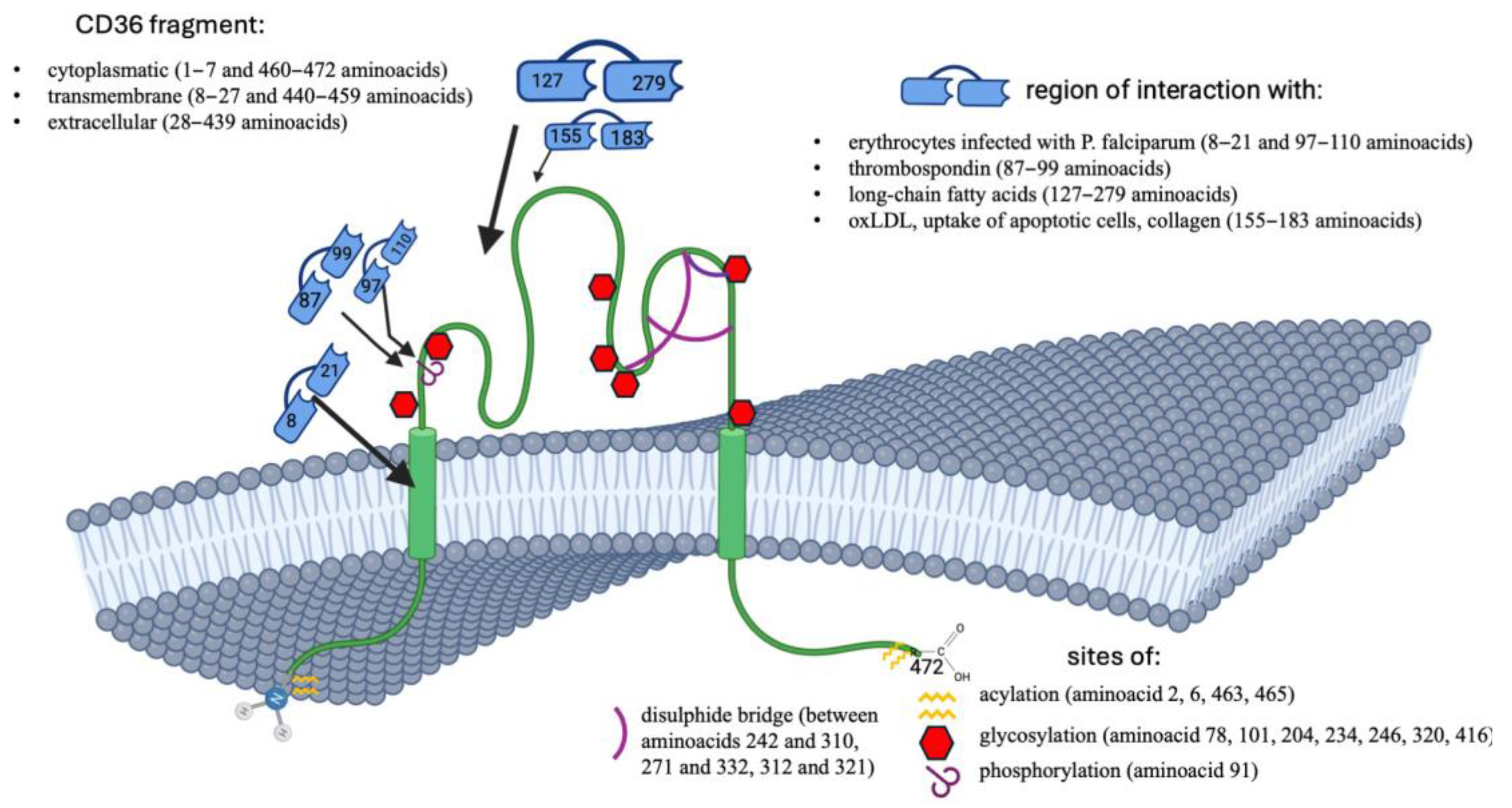
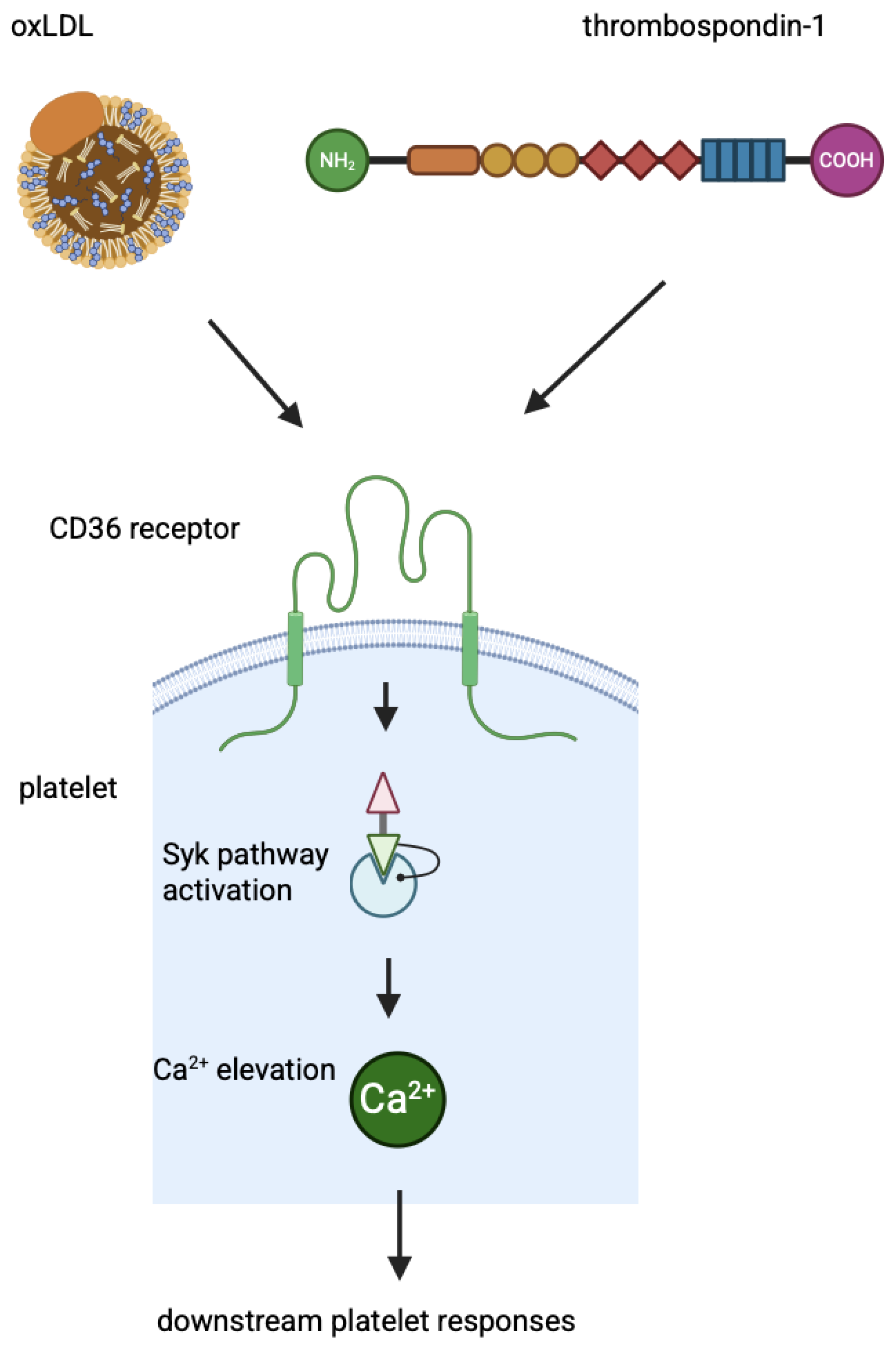
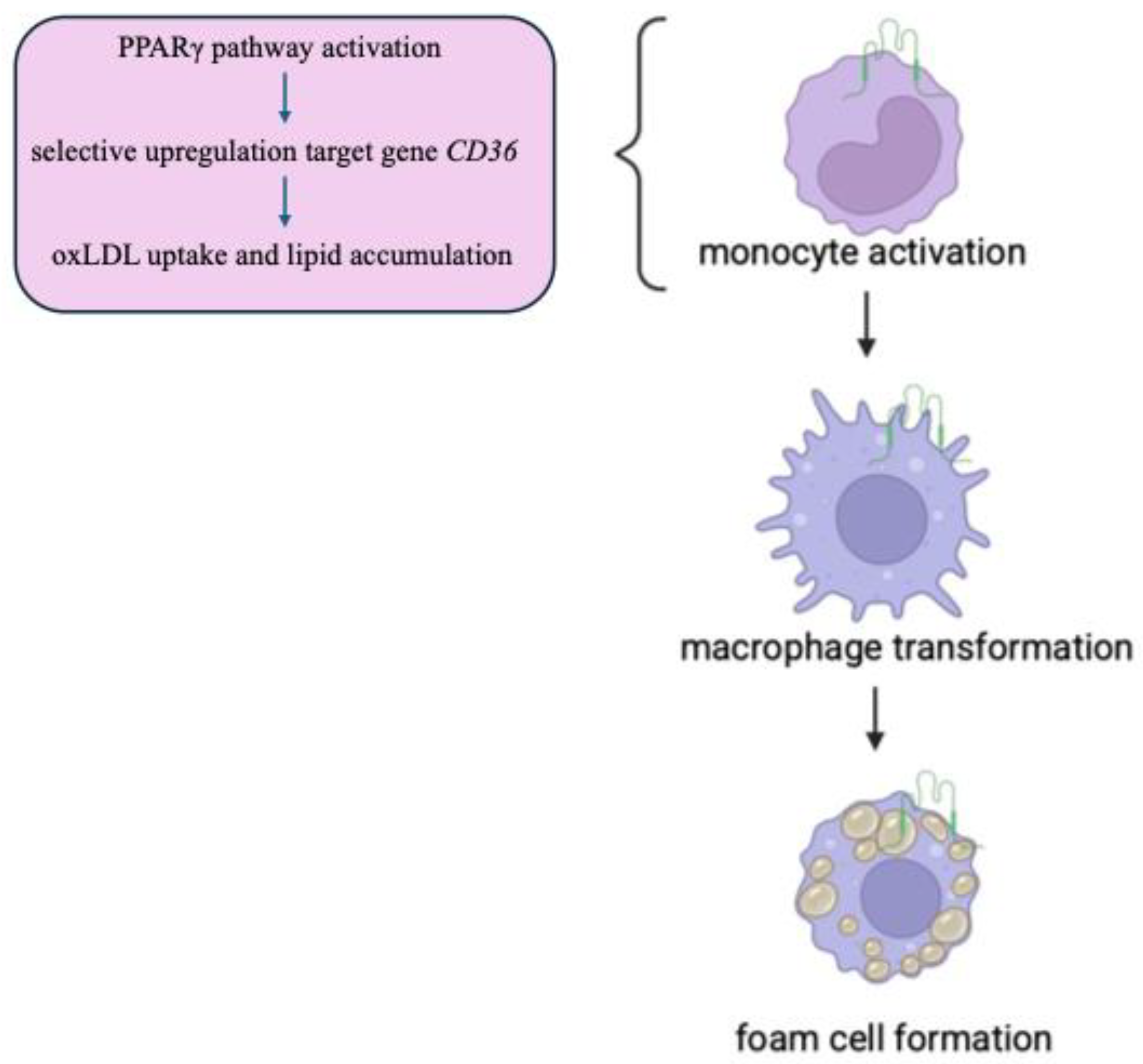
| Exon Number | Next Intron Length | mRNA Nucleotides | Amino Acids Encoded |
|---|---|---|---|
| 1 | 7341 | −289 to −184 | none |
| 2 | 470 | −183 to −90 | none |
| 3 | 9679 | −89 to +120 | 1 to 40 |
| 4 | 4362 | 121 to 281 | 41 to 94 |
| 5 | 1779 | 282 to 429 | 95 to 143 |
| 6 | 1236 | 430 to 609 | 144 to 203 |
| 7 | 1945 | 610 to 701 | 204 to 234 |
| 8 | 3463 | 702 to 748 | 235 to 250 |
| 9 | 954 | 749 to 818 | 251 to 273 |
| 10 | 757 | 819 to 1006 | 274 to 336 |
| 11 | 729 | 1007 to 1125 | 337 to 375 |
| 12 | 511 | 1126 to 1199 | 376 to 400 |
| 13 | 573 | 1200 to 1254 | 401 to 418 |
| 14 | 2236 | 1255 to 1419 | 419 to 472 |
| 15 | none | 1420 to 2044 | none |
| Exon | Rs Number | Nucleotide Change | Allele Frequency | Protein Change |
|---|---|---|---|---|
| int3/ex4 | rs1165943635 | 121–126delgCAAGTT | not found | del AA 41–42 frameshift with the stop codon |
| 4 | rs75326924 | C268T | 3.5% | Missense P90→S |
| 5 | rs572295823 | 329–330delAC | 1.2% | Frameshift at AA110 |
| 7 | Chr7: 80,664,415–80,664,421 | 619–624delACTGCA/insAAAAC | 1.8% | Frameshift at AA207 and a premature stop codon |
| 9 | Chr7: 80,669,953–80,670,022 | 749–818del 70 bp | not found | exon 9 skipping |
| 9 | rs142186404 | T760C | not found | Missense F253→L |
| 10 | rs70961716 | 949insA | 1% | Frameshift at AA317 |
| int12/ex13 | rs1261358979 | 1200_1202deltattacagAG | 1% | Exon 13 skipping |
| 13 | rs550565800 | 1228_1239 delATT GTG CCT ATT | 7.2% | Frameshift Mutation, 4 AA deletion |
| 13 | rs121918035 | A1237C | not found | I413→L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rac, M. Human CD36: Gene Regulation, Protein Function, and Its Role in Atherosclerosis Pathogenesis. Genes 2025, 16, 705. https://doi.org/10.3390/genes16060705
Rac M. Human CD36: Gene Regulation, Protein Function, and Its Role in Atherosclerosis Pathogenesis. Genes. 2025; 16(6):705. https://doi.org/10.3390/genes16060705
Chicago/Turabian StyleRac, Monika. 2025. "Human CD36: Gene Regulation, Protein Function, and Its Role in Atherosclerosis Pathogenesis" Genes 16, no. 6: 705. https://doi.org/10.3390/genes16060705
APA StyleRac, M. (2025). Human CD36: Gene Regulation, Protein Function, and Its Role in Atherosclerosis Pathogenesis. Genes, 16(6), 705. https://doi.org/10.3390/genes16060705





