SNP Analysis Reveals Novel Insights into the Genetic Diversity of Colombian Vaccinium meridionale
Abstract
1. Introduction
2. Materials and Methods
2.1. V. meridionale Plant Material Collection
2.2. V. meridionale DNA Extraction
2.3. Library Preparation and Genotyping by Sequencing (GBS)
2.4. SNP Characterization
2.5. Population Structure Analysis and Genetic Diversity Parameters
3. Results
3.1. SNP Genotyping and Characterization
3.2. Population Structure Analysis
3.3. Genetic Diversity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chamorro, F.J.; Nates-Parra, G. Biología floral y reproductiva de Vaccinium meridionale (Ericaceae) en los Andes orientales de Colombia. Rev. Biol. Trop. 2015, 63, 1197–1212. [Google Scholar] [CrossRef]
- de Valencia, M.L.C.; de Lozano, N.B. Anatomia del fruto del “Agraz” Vaccinium meridionale Swartz. Acta Biol. Colomb. 1995, 2, 159–172. [Google Scholar]
- Redpath, L.E.; Aryal, R.; Lynch, N.; Spencer, J.A.; Hulse-Kemp, A.M.; Ballington, J.R.; Green, J.; Bassil, N.; Hummer, K.; Ranney, T. Nuclear DNA Contents and Ploidy Levels of North American Vaccinium Species and Interspecific Hybrids. Sci. Hortic. 2022, 297, 110955. [Google Scholar] [CrossRef]
- Magnitskiy, S. Native Plants from the Genus Vaccinium in Colombia and Their Potential Uses. A Review. Rev. Colomb. Cienc. Hortícolas 2023, 17, e15503. [Google Scholar] [CrossRef]
- Abreu, O.A.; Barreto, G.; Prieto, S. Vaccinium (Ericaceae): Ethnobotany and Pharmacological Potentials. Emir. J. Food Agric. 2014, 26, 577. [Google Scholar] [CrossRef]
- Galvis-Pérez, Y.; Marín-Echeverri, C.; Franco Escobar, C.P.; Aristizábal, J.C.; Fernández, M.-L.; Barona-Acevedo, J. Comparative Evaluation of the Effects of Consumption of Colombian Agraz (Vaccinium meridionale Swartz) on Insulin Resistance, Antioxidant Capacity, and Markers of Oxidation and Inflammation, Between Men and Women with Metabolic Syndrome. BioResearch Open Access 2020, 9, 247–254. [Google Scholar] [CrossRef]
- Quevedo-Rubiano, S.; Aranda-Camacho, Y.; Ligarreto-Moreno, G.A.; Magnitskiy, S. Characterization of the Localized Agri-Food System (SYAL) for the Andean Blueberry (Vaccinium meridionale Swartz) in the Boyaca Department, Colombia. Rev. Colomb. Cienc. Hortícolas 2021, 15, e11593. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Lorenzo, J.M.; Zamuz, S.; Valdés, M.E.; Moreno, D.; Balcázar, M.C.G.; Fernández-Arias, J.M.; Reyes, J.F.; Franco, D. The Antioxidant Effect of Colombian Berry (Vaccinium meridionale Sw.) Extracts to Prevent Lipid Oxidation during Pork Patties Shelf-Life. Antioxidants 2021, 10, 1290. [Google Scholar] [CrossRef]
- Magnitskiy, S.V.; Ligarreto, G.A. El Efecto Del Nitrato de Potasio, Del Ácido Giberélico y Del Ácido Indolacético Sobre La Germinación de Semillas de Agraz (Vaccinium meridionale Swartz). Rev. Colomb. Cienc. Hortícolas 2011, 1, 137–141. [Google Scholar] [CrossRef]
- Rache Cardenal, L.Y.; Pacheco Maldonado, J.C. Propagación in vitro de plantas adultas de Vaccinium meridionale (Ericaceae). Acta Bot. Bras. 2010, 24, 1086–1095. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Polashock, J.J.; Rowland, L.J.; Ogden, E.; Luteyn, J.L. Fertile Intersectional Hybrids of 4x Andean Blueberry (Vaccinium meridionale) and 2x Lingonberry (V. Vitis-Idaea). HortScience 2022, 57, 525–531. [Google Scholar] [CrossRef]
- Celis, M.E.M.; Franco Tobón, Y.N.; Agudelo, C.; Arango, S.S.; Rojano, B. Andean Berry (Vaccinium meridionale Swartz). In Fruit and Vegetable Phytochemicals: Chemestry and Human Health, 2nd ed.; Yahia, E.M., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 869–882. ISBN 978-1-119-15804-2. [Google Scholar]
- SiB Colombia Catálogo de La Biodiversidad de Colombia. Available online: https://colecciones.biodiversidad.co/ (accessed on 23 March 2023).
- Aguilar Garavito, M.; Ramírez, W. Restauración Ecológica de los Páramos de Colombia: Transformación y Herramientas para su Transformación, 1st ed.; Cabrera, M., Ed.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH): Bogotá, Colombia, 2014; Volume 1, ISBN 978-958-8343-98-3. [Google Scholar]
- Madriñán, S.; Cortés, A.J.; Richardson, J.E. Páramo Is the World’s Fastest Evolving and Coolest Biodiversity Hotspot. Front. Genet. 2013, 4, 192. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Trujillo, L.; Alzate Agudelo, G.; Echeverry Gómez, A.; Toro Murillo, J.; Villegas Londoño, C. Conozcamos y Usemos El Mortiño; Corantioquia: Medellín, Colombia, 2009. [Google Scholar]
- Hernández, M.I.; Lobo, M.; Medina, C.I.; Cartagena, J.R.; Delgado, O.A. Comportamiento de La Germinación y Categorización de La Latencia En Semillas de Mortiño (Vaccinium meridionale Swartz). Agron. Colomb. 2009, 27, 15–23. [Google Scholar]
- Ehlenfeldt, M.K.; Luteyn, J.L. Fertile Intersectional F1 Hybrids of 4x Vaccinium meridionale (Section Pyxothamnus) and Highbush Blueberry, V. corymbosum (Section Cyanococcus). HortScience 2021, 56, 318–323. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Polashock, J.J.; Vorsa, N.; Zalapa, J.; De La Torre, F.; Luteyn, J.L. Fertile Intersectional F1 Hybrids of 4x Andean Blueberry (Vaccinium meridionale) and 4x American Cranberry (Vaccinium macrocarpon). HortScience 2023, 58, 234–239. [Google Scholar] [CrossRef]
- Covarrubias-Pazaran, G.; Diaz-Garcia, L.; Schlautman, B.; Deutsch, J.; Salazar, W.; Hernandez-Ochoa, M.; Grygleski, E.; Steffan, S.; Iorizzo, M.; Polashock, J.; et al. Exploiting Genotyping by Sequencing to Characterize the Genomic Structure of the American Cranberry through High-Density Linkage Mapping. BMC Genom. 2016, 17, 451. [Google Scholar] [CrossRef]
- Campa, A.; Ferreira, J.J. Genetic Diversity Assessed by Genotyping by Sequencing (GBS) and for Phenological Traits in Blueberry Cultivars. PLoS ONE 2018, 13, e0206361. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A Robust, Simple Genotyping-by-Sequencing (GBS) Approach for High Diversity Species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef]
- Mammadov, J.; Aggarwal, R.; Buyyarapu, R.; Kumpatla, S. SNP Markers and Their Impact on Plant Breeding. Int. J. Plant Genom. 2012, 2012, 728398. [Google Scholar] [CrossRef]
- Nyirahabimana, F.; Shimira, F.; Zahid, G.; Solmaz, I. Recent Status of Genotyping by Sequencing (GBS) Technology in Cucumber (Cucumis sativus L.): A Review. Mol. Biol. Rep. 2022, 49, 5547–5554. [Google Scholar] [CrossRef]
- Rasheed, A.; Hao, Y.; Xia, X.; Khan, A.; Xu, Y.; Varshney, R.K.; He, Z. Crop Breeding Chips and Genotyping Platforms: Progress, Challenges, and Perspectives. Mol. Plant 2017, 10, 1047–1064. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.I.; Lobo Arias, M.; Patiño, M.d.P.; Ligarreto, G.A.; Delgado, O.A.; Lopera, S.A.; Toro, J.L. Variabilidad Morfológica en Agraz o Mortiño (Vaccinium meridionale Swartz) en la Zona Altoandina de Colombia; Universidad Nacional de Colombia: Bogotá, Colombia, 2009. [Google Scholar]
- Medina Cano, C.I.; Ligarreto Moreno, G.A.; Vargas Arcila, M.O. Proposal of Descriptors to Study the Variability of Vaccinium meridionale Swartz. Rev. Fac. Nac. Agron. Medellín 2023, 76, 10445–10455. [Google Scholar] [CrossRef]
- Azmat, M.A.; Khan, I.A.; Cheema, H.M.N.; Rajwana, I.A.; Khan, A.S.; Khan, A.A. Extraction of DNA Suitable for PCR Applications from Mature Leaves of Mangifera indica L. J. Zhejiang Univ. Sci. B 2012, 13, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Inglis, P.W.; Pappas, M.D.C.R.; Resende, L.V.; Grattapaglia, D. Fast and Inexpensive Protocols for Consistent Extraction of High Quality DNA and RNA from Challenging Plant and Fungal Samples for High-Throughput SNP Genotyping and Sequencing Applications. PLoS ONE 2018, 13, e0206085. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for Association Mapping of Complex Traits in Diverse Samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef]
- Glaubitz, J.C.; Casstevens, T.M.; Lu, F.; Harriman, J.; Elshire, R.J.; Sun, Q.; Buckler, E.S. TASSEL-GBS: A High Capacity Genotyping by Sequencing Analysis Pipeline. PLoS ONE 2014, 9, e90346. [Google Scholar] [CrossRef] [PubMed]
- Colle, M.; Leisner, C.P.; Wai, C.M.; Ou, S.; Bird, K.A.; Wang, J.; Wisecaver, J.H.; Yocca, A.E.; Alger, E.I.; Tang, H.; et al. Haplotype-Phased Genome and Evolution of Phytonutrient Pathways of Tetraploid Blueberry. GigaScience 2019, 8, giz012. [Google Scholar] [CrossRef]
- Zhidkin, R.R.; Matveeva, T.V. Phylogeny Problems of the Genus Vaccinium L. and Ways to Solve Them. Ecol. Genet. 2022, 20, 151–164. [Google Scholar] [CrossRef]
- Powell, E.A.; Kron, K.A. Hawaiian Blueberries and Their Relatives—A Phylogenetic Analysis of Vaccinium Sections Macropelma, Myrtillus, and Hemimyrtillus (Ericaceae). Syst. Bot. 2002, 27, 768–779. [Google Scholar]
- Kulkarni, K.P.; Vorsa, N.; Natarajan, P.; Elavarthi, S.; Iorizzo, M.; Reddy, U.K.; Melmaiee, K. Admixture Analysis Using Genotyping-by-Sequencing Reveals Genetic Relatedness and Parental Lineage Distribution in Highbush Blueberry Genotypes and Cross Derivatives. Int. J. Mol. Sci. 2020, 22, 163. [Google Scholar] [CrossRef]
- Manzanero, B.R.; Kulkarni, K.P.; Vorsa, N.; Reddy, U.K.; Natarajan, P.; Elavarthi, S.; Iorizzo, M.; Melmaiee, K. Genomic and Evolutionary Relationships among Wild and Cultivated Blueberry Species. BMC Plant Biol. 2023, 23, 126. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Huber, W.; Pagès, H.; Aboyoun, P.; Carlson, M.; Gentleman, R.; Morgan, M.T.; Carey, V.J. Software for Computing and Annotating Genomic Ranges. PLoS Comput. Biol. 2013, 9, e1003118. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.; Gentleman, R.; Carey, V. Rtracklayer: An R Package for Interfacing with Genome Browsers. Bioinformatics 2009, 25, 1841–1842. [Google Scholar] [CrossRef]
- Obenchain, V.; Lawrence, M.; Carey, V.; Gogarten, S.; Shannon, P.; Morgan, M. VariantAnnotation : A Bioconductor Package for Exploration and Annotation of Genetic Variants. Bioinformatics 2014, 30, 2076–2078. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, H.; Tang, Z.; Xu, J.; Yin, D.; Zhang, Z.; Yuan, X.; Zhu, M.; Zhao, S.; Li, X.; et al. rMVP: A Memory-Efficient, Visualization-Enhanced, and Parallel-Accelerated Tool for Genome-Wide Association Study. Genom. Proteom. Bioinform. 2020, 19, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Frichot, E.; François, O. LEA: An R Package for Landscape and Ecological Association Studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Goudet, J.; Jombart, T.; Goudet, M.J. Package ‘Hierfstat’. Estimation and Tests of Hierarchical F-Statistics. 2015. Available online: https://cloud.r-project.org/ (accessed on 13 October 2022).
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Lam, T.T.-Y. Ggtree: An R Package for Visualization and Annotation of Phylogenetic Trees with Their Covariates and Other Associated Data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar] [CrossRef]
- VanRaden, P.M. Efficient Methods to Compute Genomic Predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z. GAPIT Version 3: An Interactive Analytical Tool for Genomic Association and Prediction. Genom. Proteom. Bioinform. 2022, 20, 557–560. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps; CRAN: Vienna, Austria. 2019. Available online: https://cran.r-project.org/web/packages/pheatmap/pheatmap.pdf (accessed on 12 February 2025).
- Ford-Lloyd, B.V.; Schmidt, M.; Armstrong, S.J.; Barazani, O.; Engels, J.; Hadas, R.; Hammer, K.; Kell, S.P.; Kang, D.; Khoshbakht, K.; et al. Crop Wild Relatives—Undervalued, Underutilized and Under Threat? BioScience 2011, 61, 559–565. [Google Scholar] [CrossRef]
- Jinyuan, S.; Yu, Y.; Chong, L.; Dan, L.; Du Fang, K. Informing Conservation Strategies with Genetic Diversity in Wild Plant with Extremely Small Populations: A Review on Gymnosperms. Biodivers. Sci. 2020, 28, 376–384. [Google Scholar] [CrossRef]
- Ligarreto, G.A.; del Pilar Patiño, M.; Magnitskiy, S.V. Phenotypic Plasticity of Vaccinium meridionale(Ericaceae) in Wild Populations of Mountain Forests in Colombia. Rev. Biol. Trop. 2011, 59, 569–583. [Google Scholar]
- Zapata, I.C.; Sepúlveda-Valencia, U.; Rojano, B.A. Efecto Del Tiempo de Almacenamiento Sobre Las Propiedades Fisicoquímicas, Probióticas y Antioxidantes de Yogurt Saborizado con Mortiño (Vaccinium meridionale Sw). Inf. Tecnol. 2015, 26, 17–28. [Google Scholar] [CrossRef]
- Quintero-Quiroz, J.; Galvis-Pérez, Y.; Galeano-Vásquez, S.; Marín-Echeverri, C.; Franco-Escobar, C.; Ciro-Gómez, G.; Núñez-Rangel, V.; Aristizábal-Rivera, J.C.; Barona-Acevedo, J. Physico-Chemical Characterization and Antioxidant Capacity of the Colombian Berry (Vaccinium meridionale Swartz) with a High-Polyphenol Content: Potential Effects in People with Metabolic Syndrome. Food Sci. Technol. 2019, 39, 573–582. [Google Scholar] [CrossRef]
- Alam, Z.; Roncal, J.; Peña-Castillo, L. Genetic Variation Associated with Healthy Traits and Environmental Conditions in Vaccinium Vitis-Idaea. BMC Genom. 2018, 19, 4. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Benevenuto, J.; Oliveira, I.D.B.; Cellon, C.; Olmstead, J.; Kirst, M.; Resende, M.F.R.; Munoz, P. Insights Into the Genetic Basis of Blueberry Fruit-Related Traits Using Diploid and Polyploid Models in a GWAS Context. Front. Ecol. Evol. 2018, 6, 107. [Google Scholar] [CrossRef]
- Ferrão, L.F.V.; Johnson, T.S.; Benevenuto, J.; Edger, P.P.; Colquhoun, T.A.; Munoz, P.R. Genome-wide Association of Volatiles Reveals Candidate Loci for Blueberry Flavor. New Phytol. 2020, 226, 1725–1737. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, K.; Yamane, H.; Nishiyama, S.; Ebihara, S.; Matsuzaki, R.; Shoji, M.; Tao, R. Insights into the Physiological and Molecular Mechanisms Underlying Highbush Blueberry Fruit Growth Affected by the Pollen Source. Hortic. J. 2022, 91, 140–151. [Google Scholar] [CrossRef]
- Rodriguez-Bonilla, L.; Williams, K.A.; Rodríguez Bonilla, F.; Matusinec, D.; Maule, A.; Coe, K.; Wiesman, E.; Diaz-Garcia, L.; Zalapa, J. The Genetic Diversity of Cranberry Crop Wild Relatives, Vaccinium Macrocarpon Aiton and V. oxycoccos L., in the US, with Special Emphasis on National Forests. Plants 2020, 9, 1446. [Google Scholar] [CrossRef]
- Hokanson, K.; Hancock, J. Levels of Allozymic Diversity in Diploid and Tetraploid Vaccinium sect. Cyanococcus (Blueberries). Can. J. Plant Sci. 1998, 78, 327–332. [Google Scholar] [CrossRef]
- Vega-Polo, P.; Cobo, M.M.; Argudo, A.; Gutierrez, B.; Rowntree, J.; Torres, M.d.L. Characterizing the Genetic Diversity of the Andean Blueberry (Vaccinium floribundum Kunth.) across the Ecuadorian Highlands. PLoS ONE 2020, 15, e0243420. [Google Scholar] [CrossRef] [PubMed]
- Guarnizo, C.E.; Paz, A.; Muñoz-Ortiz, A.; Flechas, S.V.; Méndez-Narváez, J.; Crawford, A.J. DNA Barcoding Survey of Anurans across the Eastern Cordillera of Colombia and the Impact of the Andes on Cryptic Diversity. PLoS ONE 2015, 10, e0127312. [Google Scholar] [CrossRef] [PubMed]
- Pennington, R.T.; Lavin, M.; Särkinen, T.; Lewis, G.P.; Klitgaard, B.B.; Hughes, C.E. Contrasting Plant Diversification Histories within the Andean Biodiversity Hotspot. Proc. Natl. Acad. Sci. USA 2010, 107, 13783–13787. [Google Scholar] [CrossRef] [PubMed]
- Sanín, M.J.; Zapata, P.; Pintaud, J.-C.; Galeano, G.; Bohórquez, A.; Tohme, J.; Hansen, M.M. Up and Down the Blind Alley: Population Divergence with Scant Gene Flow in an Endangered Tropical Lineage of Andean Palms (Ceroxylon quindiuense Clade: Ceroxyloideae). J. Hered. 2017, 108, 288–298. [Google Scholar] [CrossRef]
- Trénel, P.; Hansen, M.M.; Normand, S.; Borchsenius, F. Landscape Genetics, Historical Isolation and cross-Andean Gene Flow in the Wax Palm, Ceroxylon echinulatum (Arecaceae). Mol. Ecol. 2008, 17, 3528–3540. [Google Scholar] [CrossRef]
- Heiser, C.B. Aspects of Unconscious Selection and the Evolution of Domesticated Plants. Euphytica 1988, 37, 77–81. [Google Scholar] [CrossRef]
- Zohary, D. Unconscious Selection and the Evolution of Domesticated Plants. Econ. Bot. 2004, 58, 5–10. [Google Scholar] [CrossRef]
- Naflath, T.; Rajendra Prasad, S.; Ravikumar, R.L. Population Structure and Genetic Diversity Characterization of Soybean for Seed Longevity. PLoS ONE 2022, 17, e0278631. [Google Scholar] [CrossRef]
- Luteyn, J.L. Diversity, Adaptation, and Endemism in Neotropical Ericaceae: Biogeographical Patterns in the Vaccinieae. Bot. Rev. 2002, 68, 55–87. [Google Scholar] [CrossRef]
- Lobos, G.A.; Retamales, J.B.; Hancock, J.F.; Flore, J.A.; Romero-Bravo, S.; Del Pozo, A. Productivity and Fruit Quality of Vaccinium corymbosum cv. Elliott under Photo-Selective Shading Nets. Sci. Hortic. 2013, 153, 143–149. [Google Scholar] [CrossRef]
- Dyukaryeva, V.; Mallik, A.U. Shade Effect on Phenology, Fruit Yield, and Phenolic Content of Two Wild Blueberry Species in Northwestern Ontario, Canada. Plants 2023, 12, 4099. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, C.I.; Sánchez, N.Y.; Medina, C.I.; Lobo-Arias, M.; Galeano, P.L.; Mosquera, A.J.; Tamayo, A.; Lopera, Y.E.; Rojano, B.A.; Gaviria, C.A. Propiedades Antioxidantes de Los Frutos de Agraz o Mortiño (Vaccinium meridionale Swartz). In Perspectivas Del Cultivo de Agraz o Mortiño en la Zona Altoandina de Colombia; Gente Nueva Editorial: Bogotá, Colombia, 2009. [Google Scholar]
- Aguilar, C.M.; Patino, O.; González, E.A.; Nieto, M.E.; Torres, G.; Puyana, M. Preliminary Chemical Study and Evaluation of Antioxidant, Antifeedant and Toxic Activity of the Species Pernettya Prostrata (Ericaceae). Rev. Cuba. Plantas Med. 2014, 19, 138–150. [Google Scholar]
- Mezey, K. Pernettya prostrata: Var. pentlandii. Rev. Fac. Med. Vet. Zootec. 1943, 12, 40–58. [Google Scholar]
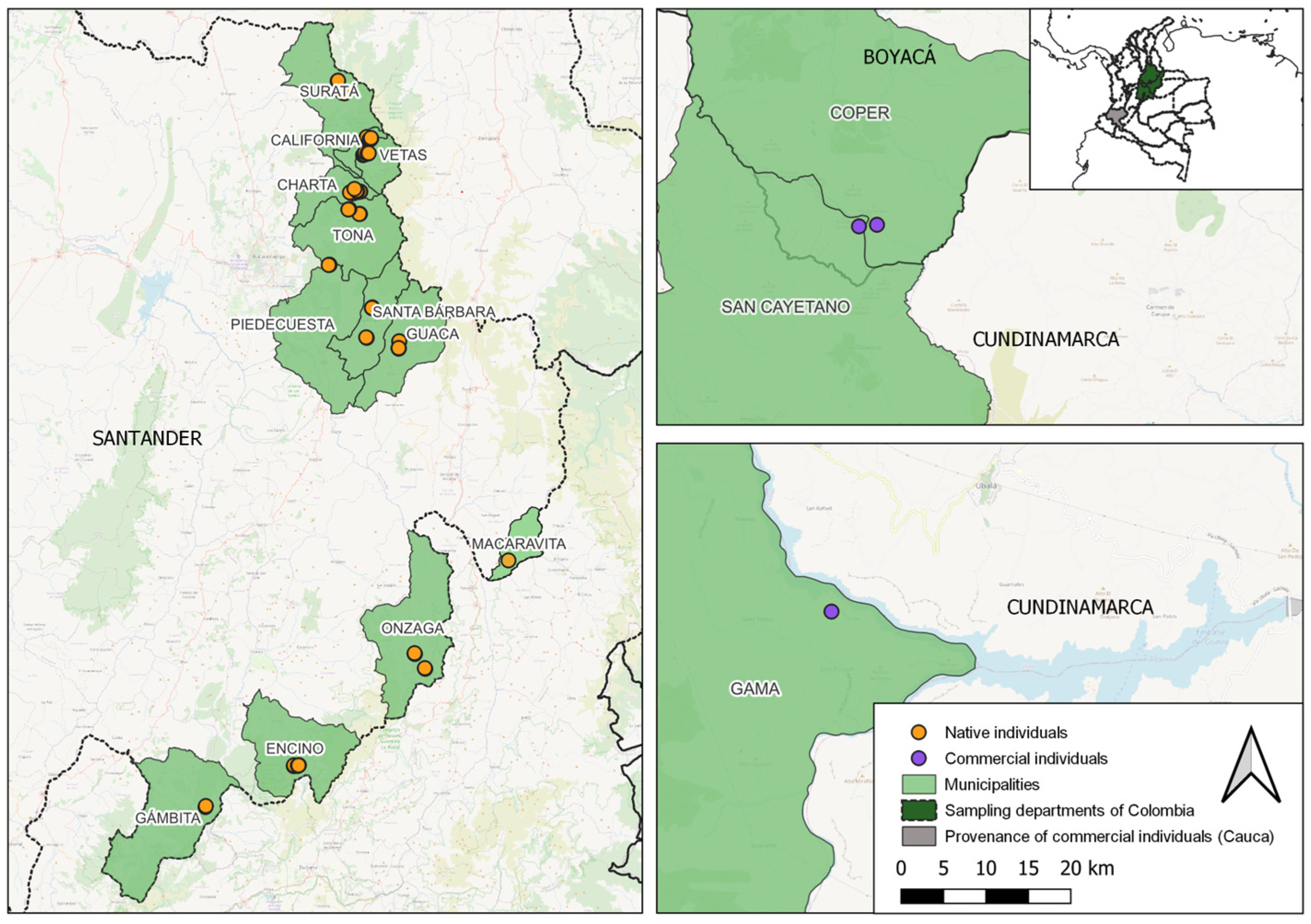
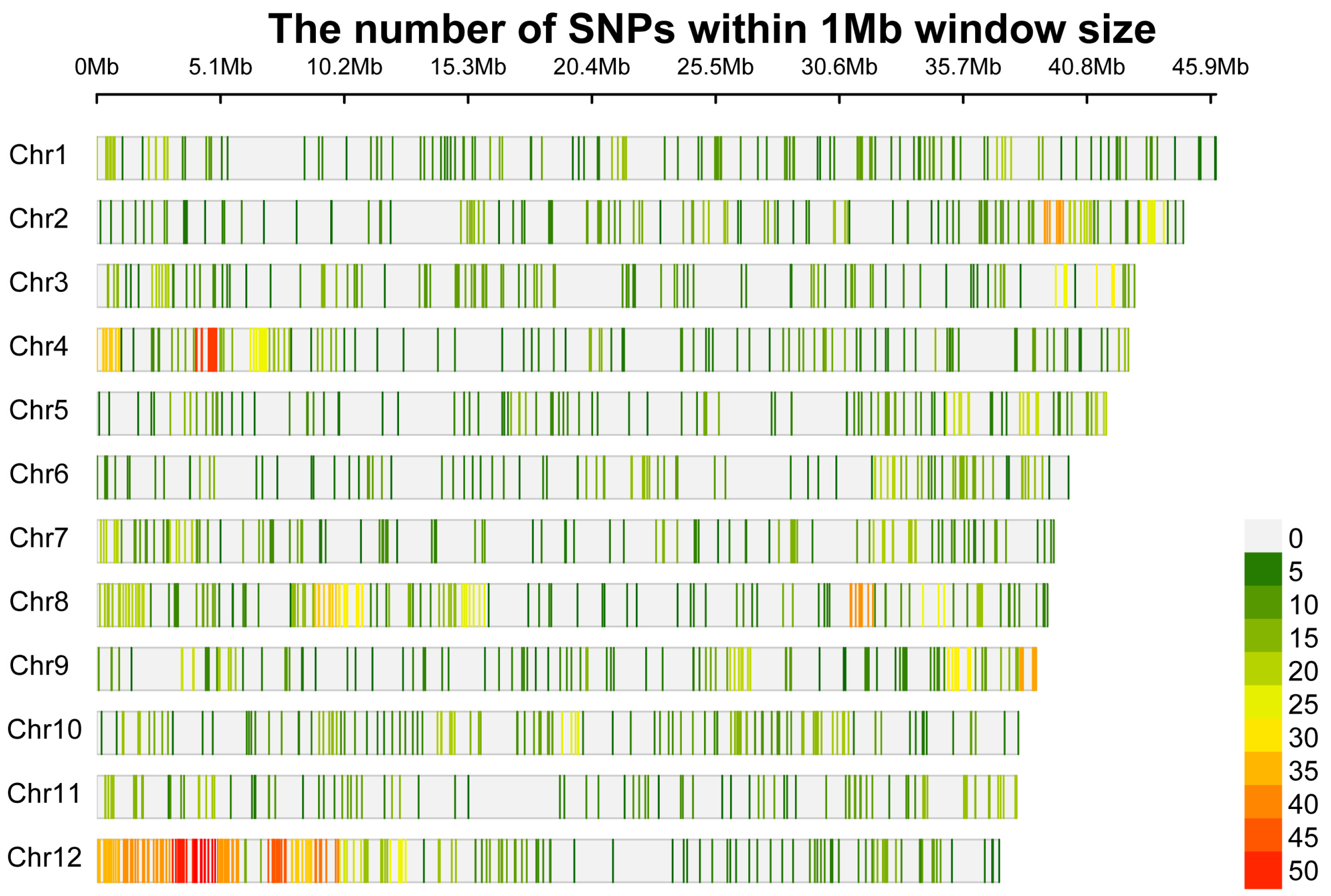

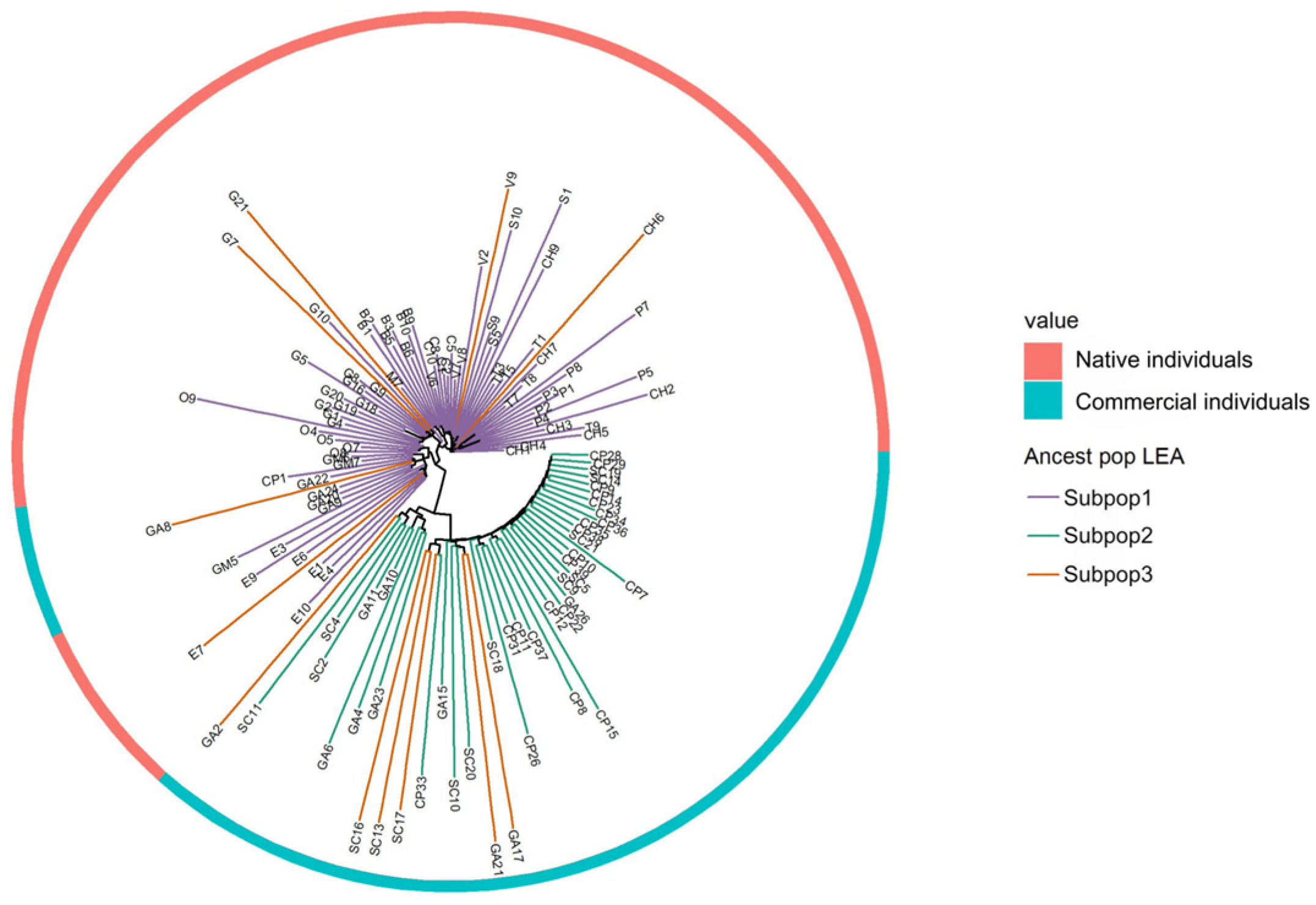
| Subpopulation | Number of Individuals | Ho | He | FIS | HT |
|---|---|---|---|---|---|
| Complete | 123 | 0.4653 | 0.3586 | −0.2975 | 0.3682 |
| Subpop1 | 72 | 0.5678 | 0.3654 | −0.554 | 0.3654 |
| Subpop2 | 39 | 0.5592 | 0.3586 | −0.559 | 0.3586 |
| Subpop3 | 12 | 0.2689 | 0.3602 | 0.253 | 0.3603 |
| Subpopulation Comparisons | FST | Nei |
|---|---|---|
| Subpop1 vs. Subpop2 | 0.060 | 0.036 |
| Subpop1 vs. Subpop3 | 0.050 | 0.029 |
| Subpop2 vs. Subpop3 | 0.053 | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sepúlveda, J.; Rondón González, F.; Soto Sedano, J.C.; Velasco, G.P.; Mosquera, T.; Delgado, M.C.; Ligarreto Moreno, G.A.; Magnitskiy, S.; Miranda, Y.; Garzón Gutiérrez, L.N. SNP Analysis Reveals Novel Insights into the Genetic Diversity of Colombian Vaccinium meridionale. Genes 2025, 16, 675. https://doi.org/10.3390/genes16060675
Sepúlveda J, Rondón González F, Soto Sedano JC, Velasco GP, Mosquera T, Delgado MC, Ligarreto Moreno GA, Magnitskiy S, Miranda Y, Garzón Gutiérrez LN. SNP Analysis Reveals Novel Insights into the Genetic Diversity of Colombian Vaccinium meridionale. Genes. 2025; 16(6):675. https://doi.org/10.3390/genes16060675
Chicago/Turabian StyleSepúlveda, John, Fernando Rondón González, Johana Carolina Soto Sedano, Ginna Patricia Velasco, Teresa Mosquera, María Cecilia Delgado, Gustavo Adolfo Ligarreto Moreno, Stanislav Magnitskiy, Yuranis Miranda, and Luz Nayibe Garzón Gutiérrez. 2025. "SNP Analysis Reveals Novel Insights into the Genetic Diversity of Colombian Vaccinium meridionale" Genes 16, no. 6: 675. https://doi.org/10.3390/genes16060675
APA StyleSepúlveda, J., Rondón González, F., Soto Sedano, J. C., Velasco, G. P., Mosquera, T., Delgado, M. C., Ligarreto Moreno, G. A., Magnitskiy, S., Miranda, Y., & Garzón Gutiérrez, L. N. (2025). SNP Analysis Reveals Novel Insights into the Genetic Diversity of Colombian Vaccinium meridionale. Genes, 16(6), 675. https://doi.org/10.3390/genes16060675





