Automated Quantitative Immunofluorescence Microscopy Approach for Diagnosis of Hereditary Thrombopathies: A Proof of Concept Using Bernard–Soulier Syndrome and Glanzmann Thrombasthenia
Abstract
:1. Introduction
2. Materials and Methods
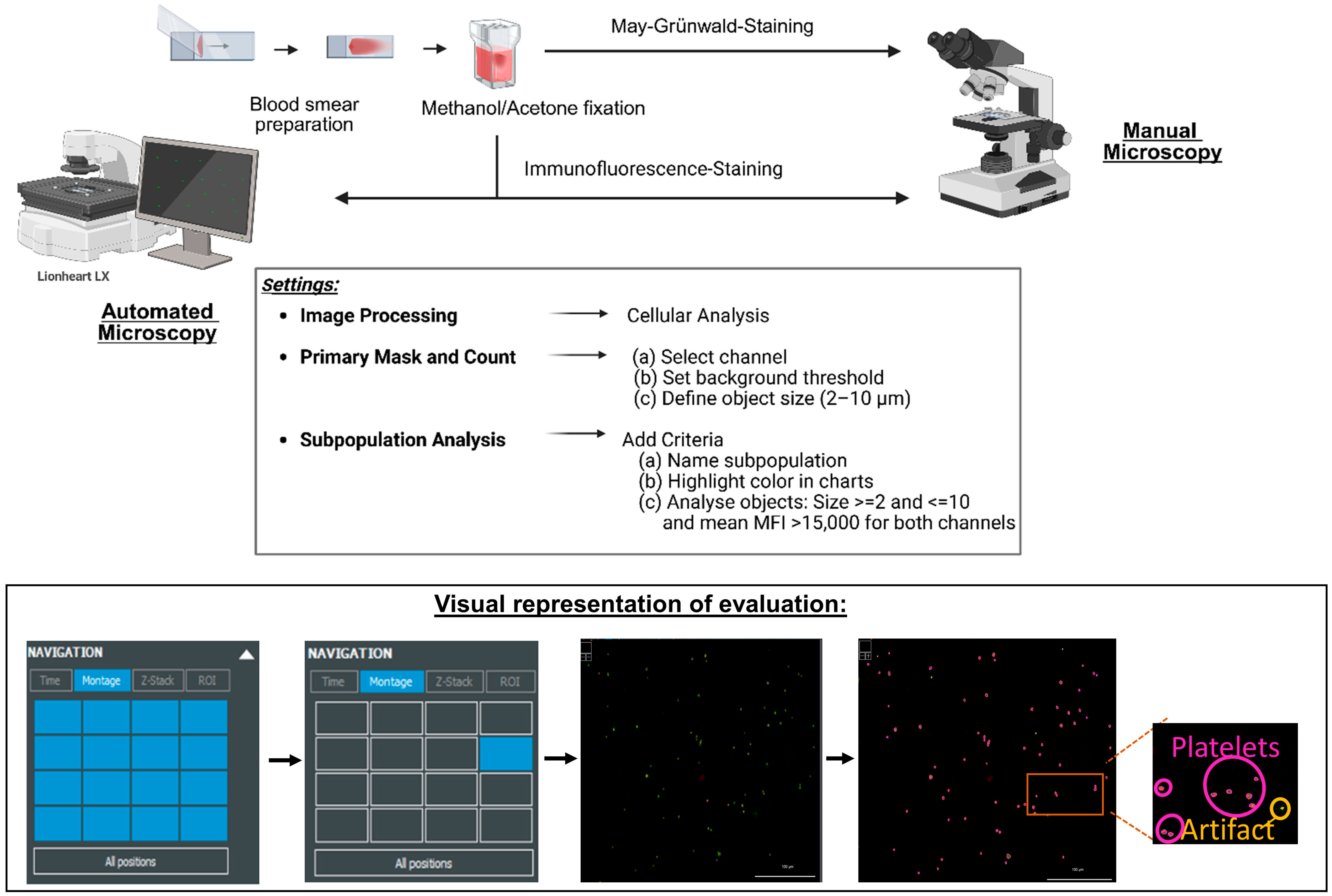
2.1. Collection and Preparation of Blood Samples for Analysis
2.2. Image Acquisition and Analysis
3. Results
3.1. Assessing Pre-Analytical Influences and Assay Variability on MFI Values
3.1.1. Influence of Blood Smear Preparation Timing and Anticoagulant Selection on Result Accuracy
3.1.2. Inter-Assay Evaluation
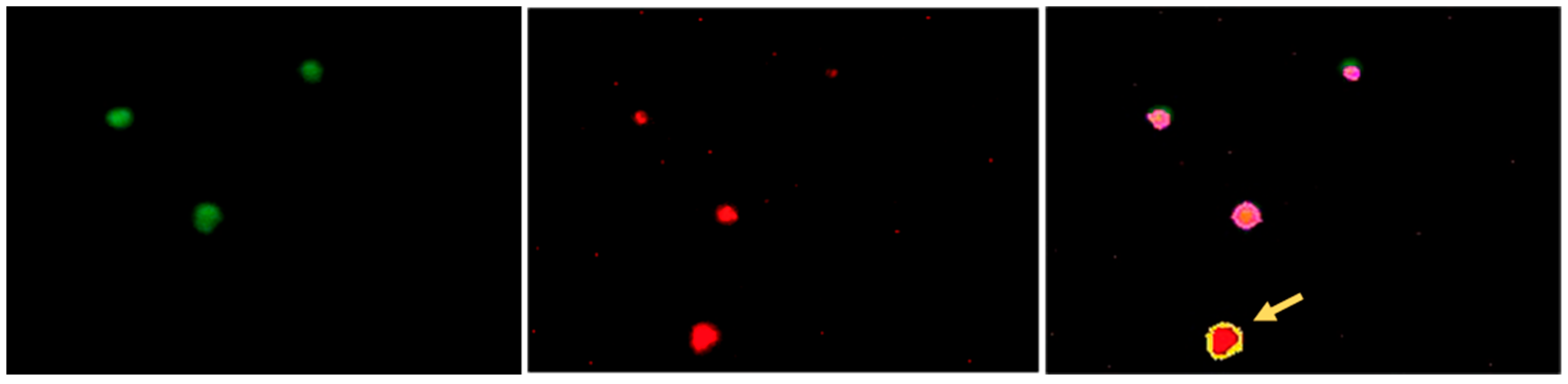
3.2. Establishing the Parameters for Automated Assessment of the Platelets
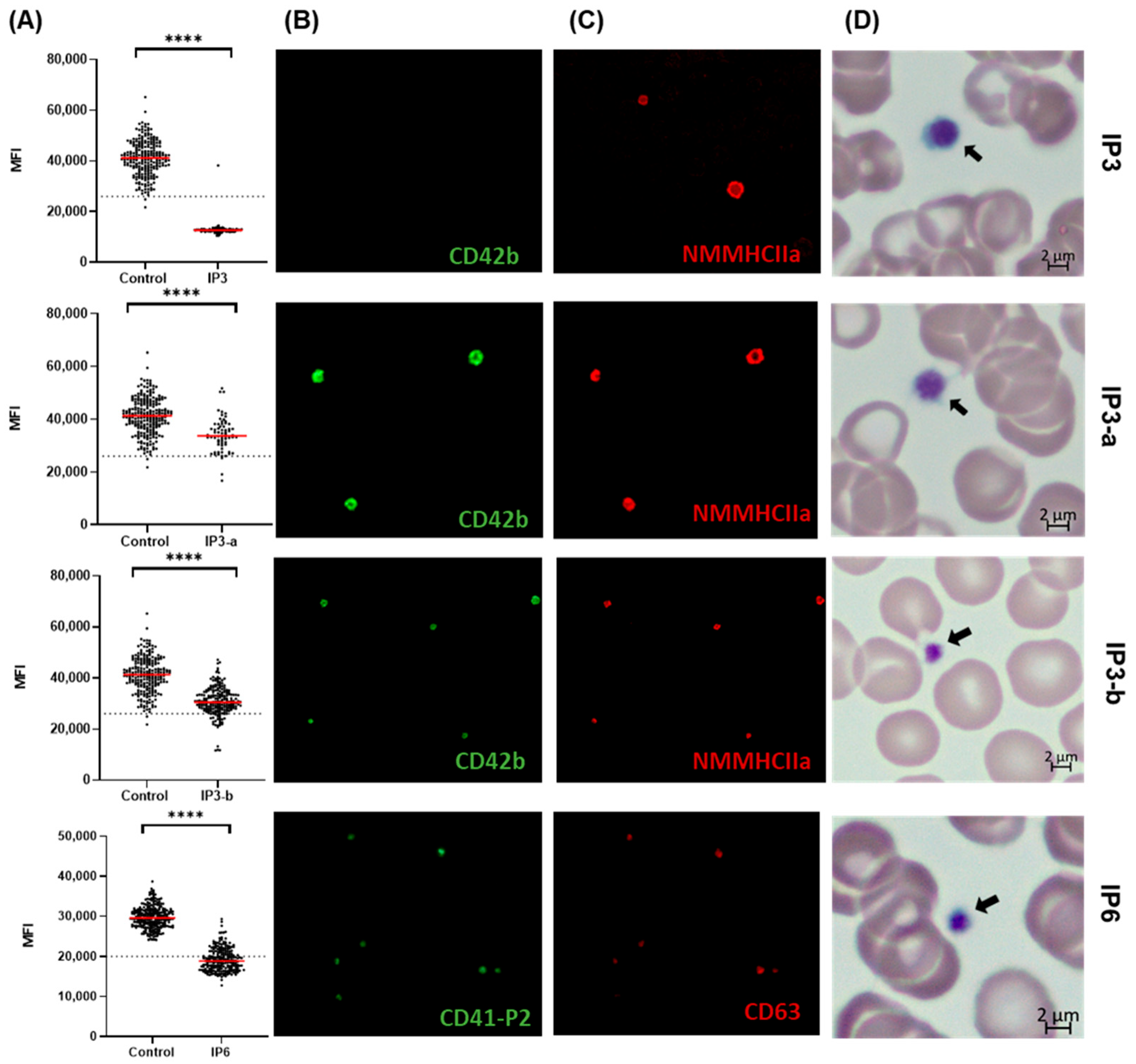
3.3. Quantitative Image-Based Confirmation of BSS and GT Diagnosis on IF-Stained Blood Smears
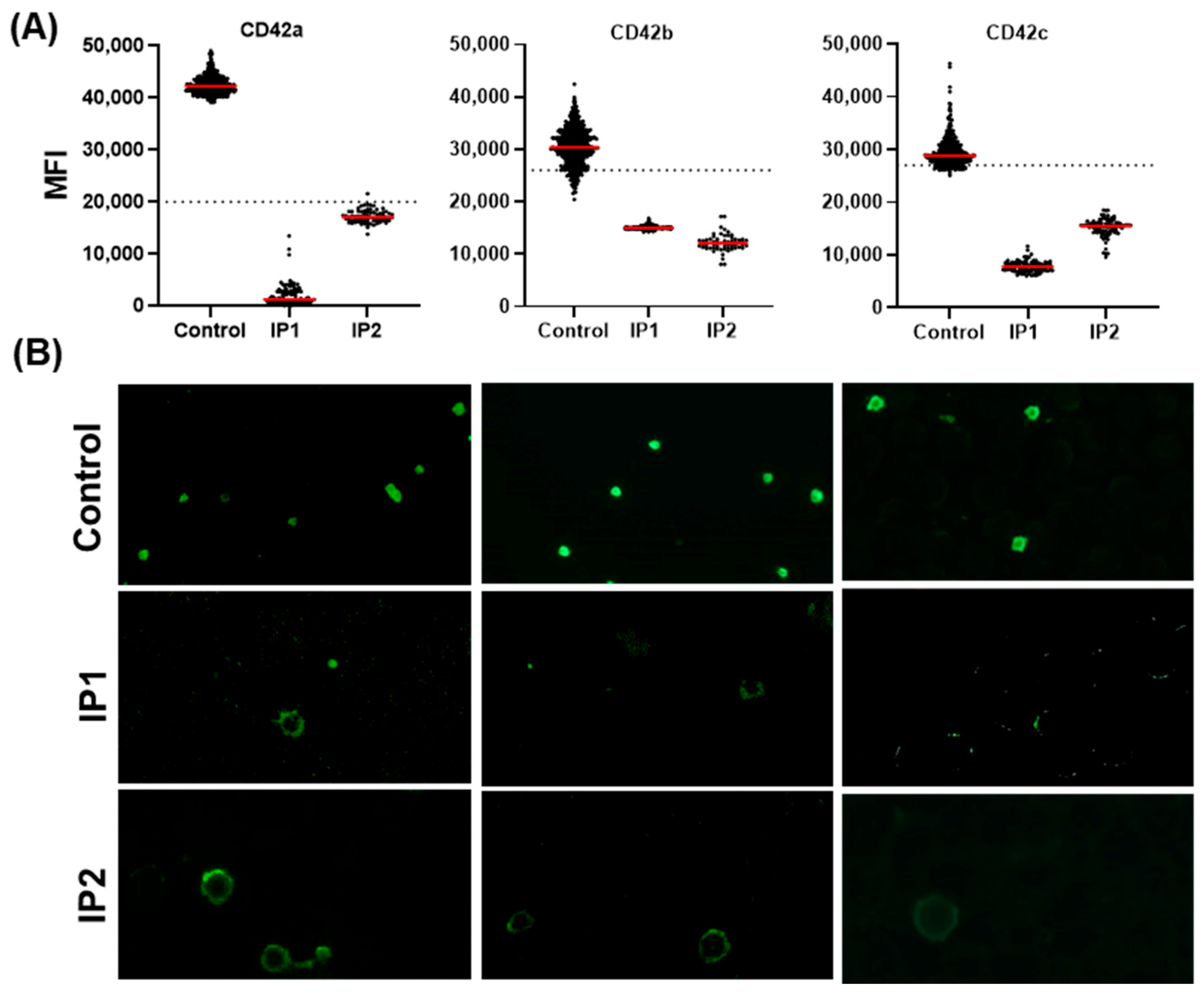
3.3.1. Validation of the Automated IF Approach for the Diagnosis of BSS
3.3.2. Validation-Automated IF Approach for Diagnosis of GT
3.3.3. Evaluating Quantitative Defects of BSS and GT Using the Established Automated IF Approach
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ADP | Adenosine diphosphate |
| BSS | Bernard–Soulier Syndrome |
| CD | Cluster of differentiation (used to identify cell surface molecules, e.g., CD41, CD42a) |
| CV | Coefficient of variation |
| DAPI | 4′,6-diamidino-2-phenylindole (DNA fluorescent stain) |
| DNA | Deoxyribonucleic acid |
| EDTA | Ethylenediaminetetraacetic acid (anticoagulant) |
| FACS | Fluorescence-activated cell sorting (flow cytometry technique) |
| GP1BA | Gene encoding GPIbα |
| GP1BB | Gene encoding GPIbb |
| GP9 | Gene encoding GPIX |
| GPIb/IX/V | Glycoprotein Ib–IX–V Complex |
| GPIbb | Glycoprotein Ib beta |
| GPIbα | Glycoprotein Ib alpha |
| GPIIb/IIIa | Glycoprotein IIb–IIIa complex (αIIbβ3 integrin) |
| GPIX | Glycoprotein IX |
| GT | Glanzmann thrombasthenia |
| HGVS | Human Genome Variation Society |
| HLA | Human leukocyte antigen |
| HPA | Human platelet antigen |
| IF | Immunofluorescence |
| IPDs | Inherited platelet disorders |
| IPF | Immature platelet fraction |
| iPFDs | Inherited platelet function disorders |
| ITGA2B | Integrin subunit alpha 2b (gene encoding GPIIb) |
| ITGB3 | Integrin subunit beta 3 (gene encoding GPIIIa) |
| MFI | Mean fluorescence intensity |
| MPV | Mean platelet volume |
| SD | Standard deviation |
| VUS | Variant of unknown significance |
References
- Nurden, A.T.; Nurden, P. Inherited thrombocytopenias: History, advances and perspectives. Haematologica 2020, 105, 2004–2019. [Google Scholar] [CrossRef] [PubMed]
- Nurden, P.; Stritt, S.; Favier, R.; Nurden, A.T. Inherited platelet diseases with normal platelet count: Phenotypes, genotypes and diagnostic strategy. Haematologica 2021, 106, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Boeckelmann, D.; Glonnegger, H.; Sandrock-Lang, K.; Zieger, B. Pathogenic Aspects of Inherited Platelet Disorders. Hamostaseologie 2021, 41, 460–468. [Google Scholar] [CrossRef]
- Bongiovanni, D.; Han, J.; Klug, M.; Kirmes, K.; Viggiani, G.; von Scheidt, M.; Schreiner, N.; Condorelli, G.; Laugwitz, K.-L.; Bernlochner, I. Role of Reticulated Platelets in Cardiovascular Disease. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 527–539. [Google Scholar] [CrossRef]
- Wang, L.; Tang, C. Targeting Platelet in Atherosclerosis Plaque Formation: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 9760. [Google Scholar] [CrossRef]
- Huilcaman, R.; Venturini, W.; Fuenzalida, L.; Cayo, A.; Segovia, R.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Platelets, a Key Cell in Inflammation and Atherosclerosis Progression. Cells 2022, 11, 1014. [Google Scholar] [CrossRef]
- Aronson, D.; Rayfield, E.J. How hyperglycemia promotes atherosclerosis: Molecular mechanisms. Cardiovasc. Diabetol. 2002, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Y.; Xing, R.; Cui, X.; Xiao, Y.; Xie, L.; You, P.; Wang, T.; Zeng, L.; Peng, W.; et al. Hyperlipidemia induces typical atherosclerosis development in Ldlr and Apoe deficient rats. Atherosclerosis 2018, 271, 26–35. [Google Scholar] [CrossRef]
- Jurk, K.; Shiravand, Y. Platelet Phenotyping and Function Testing in Thrombocytopenia. J. Clin. Med. 2021, 10, 1114. [Google Scholar] [CrossRef]
- Kim, B. Diagnostic workup of inherited platelet disorders. Blood Res. 2022, 57, 11–19. [Google Scholar] [CrossRef]
- Hoepner, G.; Althaus, K.; Müller, J.; Zieger, B.; Pavlova, A.; Boeckelmann, D.; Knöfler, R.; Bugert, P.; Kehrel, B.; Streif, W.; et al. The Diagnostic Assessment of Inherited Platelet Function Defects—Part 1: An Overview of the Diagnostic Approach and Laboratory Methods. Hamostaseologie 2025. [Google Scholar] [CrossRef] [PubMed]
- Althaus, K.; Hoepner, G.; Zieger, B.; Prüller, F.; Pavlova, A.; Boeckelmann, D.; Birschmann, I.; Müller, J.; Rühl, H.; Sachs, U.; et al. The Diagnostic Assessment of Platelet Function Defects—Part 2: Update on Platelet Disorders. Hamostaseologie 2025. [Google Scholar] [CrossRef] [PubMed]
- Bernard, J.; Soulier, J.P. Sur une nouvelle variété de dystrophie thrombocytairehémoŗragipare congénitale. Sem. Hop. 1948, 24, 3217–3223. [Google Scholar]
- Stevens, R.F.; Meyer, S. Fanconi and Glanzmann: The men and their works. Br. J. Haematol. 2002, 119, 901–904. [Google Scholar] [CrossRef]
- Duncan, A.; Kellum, A.; Peltier, S.; Cooper, D.L.; Saad, H. Disease Burden in Patients with Glanzmann’s Thrombasthenia: Perspectives from the Glanzmann’s Thrombasthenia Patient/Caregiver Questionnaire. J. Blood Med. 2020, 11, 289–295. [Google Scholar] [CrossRef]
- Lanza, F. Bernard-Soulier syndrome (hemorrhagiparous thrombocytic dystrophy). Orphanet J. Rare Dis. 2006, 1, 46. [Google Scholar] [CrossRef]
- Savoia, A.; Pastore, A.; de Rocco, D.; Civaschi, E.; Di Stazio, M.; Bottega, R.; Melazzini, F.; Bozzi, V.; Pecci, A.; Magrin, S.; et al. Clinical and genetic aspects of Bernard-Soulier syndrome: Searching for genotype/phenotype correlations. Haematologica 2011, 96, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Minkov, M.; Zeitlhofer, P.; Zoubek, A.; Kager, L.; Panzer, S.; Haas, O.A. Novel Compound Heterozygous Mutations in Two Families with Bernard-Soulier Syndrome. Front. Pediatr. 2020, 8, 589812. [Google Scholar] [CrossRef]
- Ware, J.; Russell, S.R.; Vicente, V.; Scharf, R.E.; Tomer, A.; McMillan, R.; Ruggeri, Z.M. Nonsense mutation in the glycoprotein Ib alpha coding sequence associated with Bernard-Soulier syndrome. Proc. Natl. Acad. Sci. USA 1990, 87, 2026–2030. [Google Scholar] [CrossRef]
- Boeckelmann, D.; Hengartner, H.; Greinacher, A.; Nowak-Göttl, U.; Sachs, U.J.; Peter, K.; Sandrock-Lang, K.; Zieger, B. Patients with Bernard-Soulier syndrome and different severity of the bleeding phenotype. Blood Cells Mol. Dis. 2017, 67, 69–74. [Google Scholar] [CrossRef]
- Barozzi, S.; Bozzi, V.; de Rocco, D.; Giangregorio, T.; Noris, P.; Savoia, A.; Pecci, A. A Novel Mutation in GP1BB Reveals the Role of the Cytoplasmic Domain of GPIbβ in the Pathophysiology of Bernard-Soulier Syndrome and GPIb-IX Complex Assembly. Int. J. Mol. Sci. 2021, 22, 10190. [Google Scholar] [CrossRef] [PubMed]
- Botero, J.P.; Lee, K.; Branchford, B.R.; Bray, P.F.; Freson, K.; Lambert, M.P.; Luo, M.; Mohan, S.; Ross, J.E.; Bergmeier, W.; et al. Glanzmann thrombasthenia: Genetic basis and clinical correlates. Haematologica 2020, 105, 888–894. [Google Scholar] [CrossRef]
- Huang, J.; Li, X.; Shi, X.; Zhu, M.; Wang, J.; Huang, S.; Huang, X.; Wang, H.; Li, L.; Deng, H.; et al. Platelet integrin αIIbβ3: Signal transduction, regulation, and its therapeutic targeting. J. Hematol. Oncol. 2019, 12, 26. [Google Scholar] [CrossRef]
- George, J.N.; Caen, J.P.; Nurden, A.T. Glanzmann’s thrombasthenia: The spectrum of clinical disease. Blood 1990, 75, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Zaninetti, C.; Leinøe, E.; Lozano, M.L.; Rossing, M.; Bastida, J.M.; Zetterberg, E.; Rivera, J.; Greinacher, A. Validation of immunofluorescence analysis of blood smears in patients with inherited platelet disorders. J. Thromb. Haemost. 2023, 21, 1010–1019. [Google Scholar] [CrossRef]
- Greinacher, A.; Pecci, A.; Kunishima, S.; Althaus, K.; Nurden, P.; Balduini, C.L.; Bakchoul, T. Diagnosis of inherited platelet disorders on a blood smear: A tool to facilitate worldwide diagnosis of platelet disorders. J. Thromb. Haemost. 2017, 15, 1511–1521. [Google Scholar] [CrossRef]
- Simundic, A.-M.; Bölenius, K.; Cadamuro, J.; Church, S.; Cornes, M.P.; van Dongen-Lases, E.C.; Eker, P.; Erdeljanovic, T.; Grankvist, K.; Guimaraes, J.T.; et al. Joint EFLM-COLABIOCLI Recommendation for venous blood sampling. Clin. Chem. Lab. Med. 2018, 56, 2015–2038. [Google Scholar] [CrossRef] [PubMed]
- Binder, T.; Diem, H.; Fuchs, R.; Gutensohn, K.; Nebe, T. Pappenheim -Färbung: Beschreibung einer hämatologischen Standardfärbung—Geschichte, Chemie, Durchführung, Artefakte und Problemlösungen/ Pappenheim Stain: Description of a hematological standard stain—History, chemistry, procedure, artifacts and problem solutions. Laboratoriumsmedizin 2012, 36, 293–309. [Google Scholar] [CrossRef]
- Amoruso, M.; Alberio, L.; Nagy, M. EDTA-related degranulation mimicking Storage Pool Disease. Am. J. Hematol. 2018, 93, 1192–1193. [Google Scholar] [CrossRef]
- Scholz, R.; Sigel, C.E.; Roggenbuck, J.; Zaninetti, C.; Wesche, J.; Fuhrmann, J.; Kaderali, L.; Hiemann, R.; Roggenbuck, D.; Greinacher, A. Semiautomatic assessment of immunofluorescence microscopy on blood smears in inherited platelet disorders using artificial intelligence: A proof of concept. In Proceedings of the GTH Congress 2024—68th Annual Meeting of the Society of Thrombosis and Haemostasis Research—Building Bridges in Coagulation, Vienna, Austria, 27 February–1 March 2024; Georg Thieme: Stuttgart, Germany, 2024. [Google Scholar]
- Spurgeon, B.E.J.; Linden, M.D.; Michelson, A.D.; Frelinger, A.L. Immunophenotypic Analysis of Platelets by Flow Cytometry. Curr. Protoc. 2021, 1, e178. [Google Scholar] [CrossRef]
- Krause, K.A.; Graham, B.C. StatPearls: Glanzmann Thrombasthenia; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kappelmayer, J.; Nagy, B.; Miszti-Blasius, K.; Hevessy, Z.; Setiadi, H. The emerging value of P-selectin as a disease marker. Clin. Chem. Lab. Med. 2004, 42, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Frelinger, A.L.; Rivera, J.; Connor, D.E.; Freson, K.; Greinacher, A.; Harrison, P.; Kunishima, S.; Lordkipanidzé, M.; Michelson, A.D.; Ramström, S.; et al. Consensus recommendations on flow cytometry for the assessment of inherited and acquired disorders of platelet number and function: Communication from the ISTH SSC Subcommittee on Platelet Physiology. J. Thromb. Haemost. 2021, 19, 3193–3202. [Google Scholar] [CrossRef] [PubMed]
- de Bitencourt, E.D.D.S.; Voegeli, C.F.; Onzi, G.D.S.; Boscato, S.C.; Ghem, C.; Munhoz, T. Validation of the Sysmex sp-1000i automated slide preparer-stainer in a clinical laboratory. Rev. Bras. Hematol. Hemoter. 2013, 35, 404–408. [Google Scholar] [CrossRef] [PubMed]

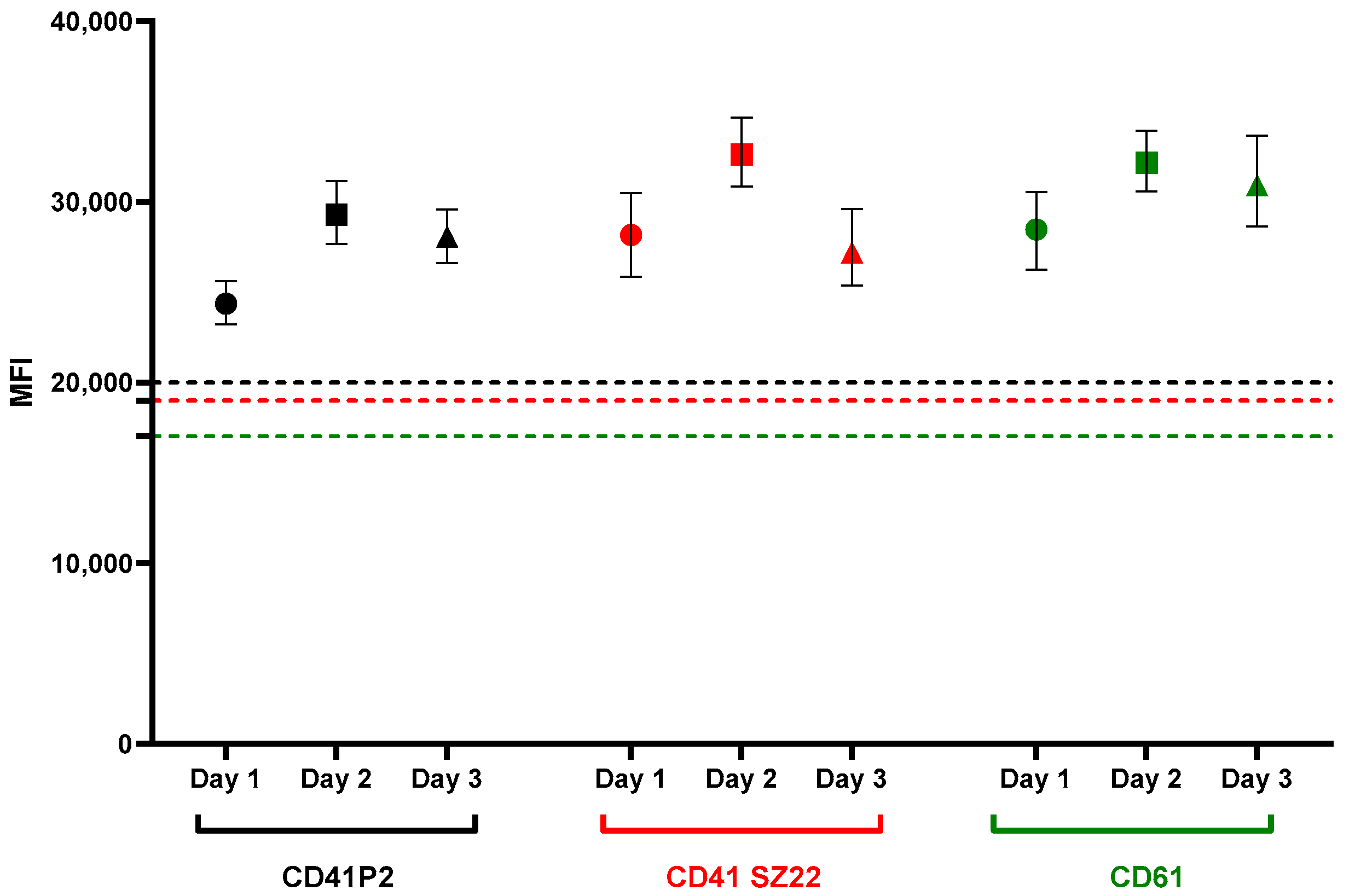

| Antibody | Day 1 | Day 2 | Day 3 | Inter-Assay CV | ||
|---|---|---|---|---|---|---|
| MFI 1 | Mean MFI | St DV 2 | Inter CV% | |||
| CD41 P2 | 24,431 | 29,485 | 28,577 | 27,498 | 2694 | 9.8 |
| CD41 SZ22 | 28,461 | 32,885 | 27,690 | 29,679 | 2803 | 9.4 |
| CD61 | 28,516 | 32,118 | 30,969 | 30,534 | 1840 | 6 |
| IPD | Bernard–Soulier Syndrome | Glanzmann Thrombasthenia | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | IP1 | IP2 | IP3 | IP3-a | IP3-b | IP4 | IP5 | IP6 | |
| Platelet count {150–370} × 103/µL | 78 | 3 | 48 | 153 | 224 | 207 | 291 | 306 | |
| MPV {8.5–11.5} fL | - | - | - | 13.5 | 10.9 | 12.3 | 11.2 | 10.0 | |
| Genetic defect | Gene | GP9 | GP9 | GP9 | GP9 | GP9 | ITGB3 | ITGB3 | ITGA2B |
| HGVS c. | c.[70T>C]; [70T>C] | c.[70T>C];[ 182A>G] | c.[70T>G]; [182A>G] | c.[182A>G];[=] | c.[70T>G];[=] | c.[353T>A]; [353T>A] | c.[118C>T]; [941A>C] | c.[957T>A]; 2326_2331dupGAGGCC | |
| HGVS p. | p.(Cys24Arg) | p.(Cys24Arg; Asn61Ser) | p.(Cys24Arg; Asn61Ser) | p.(Asn61Ser) | p.(Cys24Arg) | p.(Leu118His) | p.(Gln40Ter; Asp314Ala) | p.(Y319Ter; p.Glu776_Ala777dup) | |
| Zygosity | homozygous | compound heterozygous | compound heterozygous | heterozygous | heterozygous | homozygous | compound heterozygous | compound heterozygous | |
| Variant | missense | missense | missense | missense | missense | missense | nonsense, missense | nonsense, in-frame duplication | |
| Flow Cytometry | CD41 P2 | N | N | N | N | n.d. | P | P | P |
| CD41 SZ22 | N | N | N | N | n.d. | P | P | P | |
| CD42b | P | P | P | P | n.d. | N | N | N | |
| Automated IF Microscopy | CD41 P2 | N | N | n.d. | n.d. | n.d. | P | P | P |
| CD41 SZ22 | N | N | n.d. | n.d. | n.d. | P | P | n.d. | |
| CD61 | N | N | n.d. | n.d. | n.d. | P | P | n.d. | |
| CD42a | P | P | n.d. | n.d. | n.d. | N | N | n.d. | |
| CD42b | P | P | P | P | P | N | N | n.d. | |
| CD42c | P | P | n.d. | n.d. | n.d. | N | N | n.d. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loos, K.; Al-Rifai, R.; Ohlenforst, S.; Klein, C.; Oldenburg, J.; Pavlova, A.; Pezeshkpoor, B. Automated Quantitative Immunofluorescence Microscopy Approach for Diagnosis of Hereditary Thrombopathies: A Proof of Concept Using Bernard–Soulier Syndrome and Glanzmann Thrombasthenia. Genes 2025, 16, 621. https://doi.org/10.3390/genes16060621
Loos K, Al-Rifai R, Ohlenforst S, Klein C, Oldenburg J, Pavlova A, Pezeshkpoor B. Automated Quantitative Immunofluorescence Microscopy Approach for Diagnosis of Hereditary Thrombopathies: A Proof of Concept Using Bernard–Soulier Syndrome and Glanzmann Thrombasthenia. Genes. 2025; 16(6):621. https://doi.org/10.3390/genes16060621
Chicago/Turabian StyleLoos, Kevin, Rawya Al-Rifai, Sandra Ohlenforst, Claudia Klein, Johannes Oldenburg, Anna Pavlova, and Behnaz Pezeshkpoor. 2025. "Automated Quantitative Immunofluorescence Microscopy Approach for Diagnosis of Hereditary Thrombopathies: A Proof of Concept Using Bernard–Soulier Syndrome and Glanzmann Thrombasthenia" Genes 16, no. 6: 621. https://doi.org/10.3390/genes16060621
APA StyleLoos, K., Al-Rifai, R., Ohlenforst, S., Klein, C., Oldenburg, J., Pavlova, A., & Pezeshkpoor, B. (2025). Automated Quantitative Immunofluorescence Microscopy Approach for Diagnosis of Hereditary Thrombopathies: A Proof of Concept Using Bernard–Soulier Syndrome and Glanzmann Thrombasthenia. Genes, 16(6), 621. https://doi.org/10.3390/genes16060621







