Transcriptomic Profiling of Paulownia fortunei (Seem.) Hemsl. Roots in Response to Chromium and Copper Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Total RNA Extraction, Reliability Assessment and RNA Sequence Analyses
2.3. Sequencing Data Analysis
2.4. Differential Expression Analysis, Functional Annotation and Enrichment Analysis
2.5. Weighted Gene Co-Expression Network Analysis
2.6. Quantitative Real-Time Polymerase Chain Reaction
2.7. Data Processing
3. Results
3.1. Accumulation of Cr and Cu in Different Tissue Types of Paulownia Seedlings
3.2. RNA-Seq Data Quality and Statistics
3.3. Differentially Expressed Genes
3.4. GO and KEGG Analysis of Differentially Expressed Genes
3.5. Expression Profiles of Putative Transporters Response to Cr and Cu
3.6. Co-Expression Network Analysis
3.7. Quantitative Real-Time PCR Analysis of Selected Genes Under Cr and Cu Stress
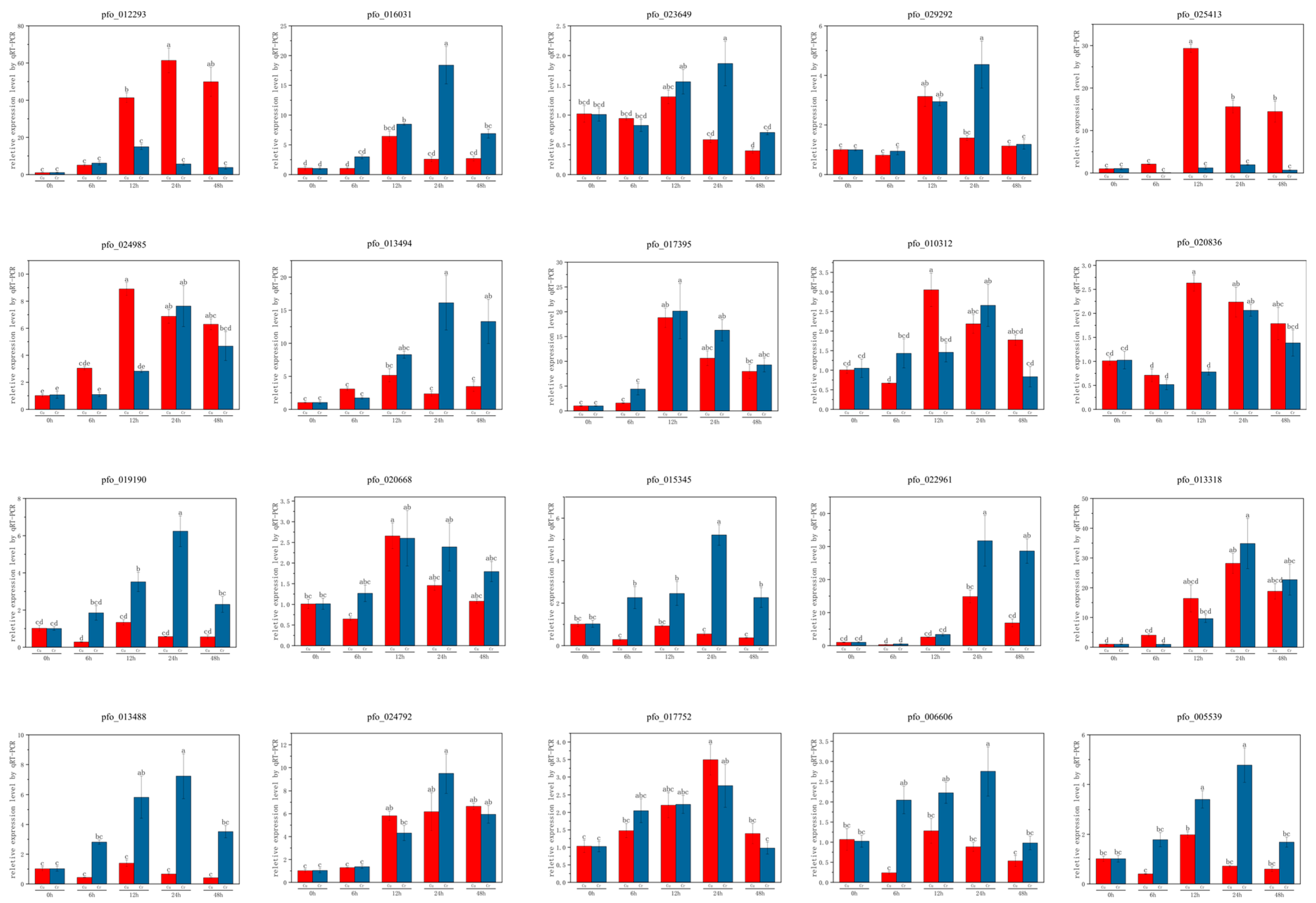
3.7.1. Genes Associated with Detoxification and Stress Response
3.7.2. Genes Related to Signaling and Structural Integrity
4. Discussion
4.1. Heavy Metal Tolerance and Remediation Potential of P. fortunei
4.2. Differentially Expressed Genes in Response to Cr and Cu
4.3. Transporter Protein
4.4. Co-Expression Network
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, H.; Wang, J.; Wang, Y.; Yao, Y.; Liu, X.; Zhou, Y. Contamination Characteristics, Source Analysis, and Spatial Prediction of Soil Heavy Metal Concentrations on the Qinghai-Tibet Plateau. J. Soils Sediments 2023, 23, 2202–2215. [Google Scholar] [CrossRef]
- Luo, W.; Wei, P.; Zhang, Y.; Sun, C. Characterization and Source Analysis of Heavy Metal(Loid)s Pollution in Soil of an Industrial Park in Kunming, China. Appl. Sci. 2024, 14, 6547. [Google Scholar] [CrossRef]
- Gikas, P.; Romanos, P. Effects of Tri-Valent (Cr(III)) and Hexa-Valent (Cr(VI)) Chromium on the Growth of Activated sludge. J. Hazard. Mater. 2006, 133, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Liu, Y.; Li, H.; Wang, Q.; Huang, Z.; Liu, W.; Shi, P. Trivalent Chromium Supplementation Ameliorates Oleic Acid-Induced Hepatic Steatosis in Mice. Biol. Trace Elem. Res. 2018, 187, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health Hazards of Hexavalent Chromium (Cr (VI)) and Its Microbial Reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Q.; Li, L.; Wang, R.; Chen, Y.; Wang, X. Insights into the Evolution of Cr(VI) Species in Long-Term Hexavalent Chromium Contaminated Soil. Sci. Total Environ. 2023, 858, 160149. [Google Scholar] [CrossRef]
- Suzuki, T.; Kawai, K.; Moribe, M.; Niinae, M. Recovery of Cr as Cr(III) from Cr(VI)-Contaminated Kaolinite Clay by Electrokinetics coupled with a Permeable Reactive Barrier. J Hazard Mater 2014, 278, 297–303. [Google Scholar] [CrossRef]
- Xia, S.; Song, Z.; Jeyakumar, P.; Bolan, N.; Wang, H. Characteristics and Applications of Biochar for Remediating Cr(VI)-Contaminated Soils and Wastewater. Environ. Geochem. Health 2020, 42, 1543–1567. [Google Scholar] [CrossRef]
- Milosavljevic, J.S.; Serbula, S.M.; Cokesa, D.M.; Milanovic, D.B.; Radojevic, A.A.; Kalinovic, T.S.; Kalinovic, J.V. Soil Enzyme Activities under the Impact of Long-Term Pollution from Mining-Metallurgical Copper Production. Eur. J. Soil Biol. 2020, 101, 103232. [Google Scholar] [CrossRef]
- Lamb, D.T.; Ming, H.; Megharaj, M.; Naidu, R. Heavy Metal (Cu, Zn, Cd and Pb) Partitioning and Bioaccessibility in Uncontaminated and Long-Term Contaminated Soils. J. Hazard. Mater. 2009, 171, 1150–1158. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A Comparison of Technologies for Remediation of Heavy Metal Contaminated Soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Hseu, Z.-Y.; Hsi, H.-C. Influences of Thermal Decontamination on Mercury Removal, Soil Properties, and Repartitioning of Coexisting Heavy Metals. Chemosphere 2011, 84, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent Advances in Soil Remediation Technology for Heavy Metal Contaminated Sites: A Critical Review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Peng, Y.-P.; Chen, K.-F.; Chen, T.-Y.; Tang, C.-T. The Effect of Different in Situ Chemical Oxidation (ISCO) Technologies on the Survival of Indigenous Microbes and the Remediation of Petroleum Hydrocarbon-Contaminated Soil. Process Saf. Environ. Prot. 2022, 163, 105–115. [Google Scholar] [CrossRef]
- Li, H.-K.; Xu, D.-M.; Wang, J.-X.; Xu, Z.-L.; Fu, R.-B. The Occurrence of “Yellowing” Phenomenon and Its Main Driving Factors after the Remediation of Chromium (Cr)-Contaminated Soils: A Literature Review. J. Hazard. Mater. 2023, 457, 131698. [Google Scholar] [CrossRef]
- Lone, M.I.; He, Z.; Stoffella, P.J.; Yang, X. Phytoremediation of Heavy Metal Polluted Soils and Water: Progresses and Perspectives. J. Zhejiang Univ. Sci. B 2008, 9, 210–220. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Xie, Y.; Liu, F.X.; Wang, B.; Xu, F.; Xiao, K. In Situ Mycoremediation of Acid Rain and Heavy Metals Co-Contaminated Soil through Microbial Inoculation with Pleurotus Ostreatus. Sci. Total Environ. 2024, 912, 169020. [Google Scholar] [CrossRef]
- Khan, I.; Aftab, M.; Shakir, S.; Ali, M.; Qayyum, S.; Rehman, M.U.; Haleem, K.S.; Touseef, I. Mycoremediation of Heavy Metal (Cd and Cr)–Polluted Soil through Indigenous Metallotolerant Fungal Isolates. Environ. Monit. Assess. 2019, 191, 585. [Google Scholar] [CrossRef]
- Azab, E.; Hegazy, A.K. Monitoring the Efficiency of Rhazya Stricta L. Plants in Phytoremediation of Heavy Metal-Contaminated Soil. Plants 2020, 9, 1057. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Zhang, C.; Ke, S. Physiological Responses and Detoxific Mechanisms to Pb, Zn, Cu and Cd in Young Seedlings of Paulownia fortunei. J. Environ. Sci. 2010, 22, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Chen, Y.; Chen, M.; Wu, Y.; Su, R.; Du, L.; Liu, Z. Mushroom Residue Modification Enhances Phytoremediation Potential of Paulownia fortunei to Lead-Zinc Slag. Chemosphere 2020, 253, 126774. [Google Scholar] [CrossRef]
- Su, J.; Xian, K.; Fu, C.; He, J.; Liu, B.; Huang, N. Selection of Suitable Reference Genes in Paulownia fortunei (Seem.) Hemsl. under Different Tissues and Abiotic Stresses for qPCR Normalization. Czech J. Genet. Plant Breed. 2023, 59, 205–218. [Google Scholar] [CrossRef]
- Gill, R.A.; Ali, B.; Cui, P.; Shen, E.; Farooq, M.A.; Islam, F.; Ali, S.; Mao, B.; Zhou, W. Comparative Transcriptome Profiling of Two Brassica napus Cultivars under Chromium Toxicity and Its Alleviation by Reduced Glutathione. BMC Genom. 2016, 17, 885. [Google Scholar] [CrossRef]
- Li, L.; Zhang, K.; Gill, R.A.; Islam, F.; Farooq, M.A.; Wang, J.; Zhou, W. Ecotoxicological and Interactive Effects of Copper and Chromium on Physiochemical, Ultrastructural, and Molecular Profiling in Brassica napus L. BioMed Res. Int. 2018, 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ewing, B.; Green, P. Base-Calling of Automated Sequencer Traces Using Phred. II. Error Probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-Seq: Accounting for Selection Bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated Genome Annotation and Pathway Identification Using the KEGG Orthology (KO) as a Controlled Vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R Package for Weighted Correlation Network Analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Chitimus, D.; Nedeff, V.; Mosnegutu, E.; Barsan, N.; Irimia, O.; Nedeff, F. Studies on the Accumulation, Translocation, and Enrichment Capacity of Soils and the Plant Species Phragmites Australis (Common Reed) with Heavy Metals. Sustainability 2023, 15, 8729. [Google Scholar] [CrossRef]
- Hejna, M.; Moscatelli, A.; Stroppa, N.; Onelli, E.; Pilu, S.; Baldi, A.; Rossi, L. Bioaccumulation of Heavy Metals from Wastewater through a Typha latifolia and Thelypteris palustris Phytoremediation System. Chemosphere 2020, 241, 125018. [Google Scholar] [CrossRef]
- Shehata, S.M.; Badawy, R.K.; Aboulsoud, Y.I.E. Phytoremediation of Some Heavy Metals in Contaminated Soil. Bull. Natl. Res. Cent. 2019, 43, 189. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Du, L.; Zhang, M.; Han, L. Accumulation and Subcellular Distribution of Heavy Metal in Paulownia fortunei Cultivated in Lead-Zinc Slag Amended with Peat. Int. J. Phytoremediation 2019, 21, 1153–1160. [Google Scholar] [CrossRef]
- Jiang, Z.-F.; Huang, S.-Z.; Han, Y.-L.; Zhao, J.-Z.; Fu, J.-J. Physiological Response of Cu and Cu Mine Tailing Remediation of Paulownia fortunei (Seem) Hemsl. Ecotoxicology 2012, 21, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Basit, F.; Abbas, S.; Sheteiwy, M.S.; Bhat, J.A.; Alsahli, A.A.; Ahmad, P. Deciphering the Alleviation Potential of Nitric oxide, for Low Temperature and Chromium Stress via Maintaining Photosynthetic Capacity, Antioxidant Defence, and Redox Homeostasis in Rice (Oryza sativa). Plant Physiol. Biochem. 2024, 214, 108957. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, G.; Zhai, X.; Xu, P.; Ma, L.; Deng, M.; Zhao, Z.; Yang, H.; Dong, Y.; Shang, Z.; et al. Genomic Insights into the Fast Growth of Paulownias and the Formation of Paulownia Witches’ Broom. Mol. Plant 2021, 14, 1668–1682. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Pan, Y.; Yu, H.; Zhang, X.; Shen, Y.; Jiao, S.; Wu, K.; La, G.; Yuan, Y.; et al. Mechanisms of Cd and Cr Removal and Tolerance by Macrofungus Pleurotus Ostreatus HAU-2. J. Hazard. Mater. 2017, 330, 1–8. [Google Scholar] [CrossRef]
- Zhang, X.; St. Leger, R.J.; Fang, W. Pyruvate Accumulation Is the First Line of Cell Defense against Heat Stress in a Fungus. mBio 2017, 8, e01284-17. [Google Scholar] [CrossRef]
- Zlobin, I.E.; Kartashov, A.V.; Shpakovski, G.V. Different Roles of Glutathione in Copper and Zinc Chelation in Brassica Napus Roots. Plant Physiol. Biochem. 2017, 118, 333–341. [Google Scholar] [CrossRef]
- Kushwaha, B.K.; Singh, V.P. Glutathione and Hydrogen Sulfide Are Required for Sulfur-mediated Mitigation of Cr(VI) Toxicity in Tomato, Pea and Brinjal Seedlings. Physiol. Plant. 2019, 168, 406–421. [Google Scholar] [CrossRef]
- Janas, K.M.; Zielińska-Tomaszewska, J.; Rybaczek, D.; Maszewski, J.; Posmyk, M.M.; Amarowicz, R.; Kosińska, A. The Impact of Copper Ions on Growth, Lipid Peroxidation, and Phenolic Compound Accumulation and Localization in Lentil (Lens Culinaris Medic.) Seedlings. J. Plant Physiol. 2010, 167, 270–276. [Google Scholar] [CrossRef]
- Sandmann, G.; Böger, P. Copper-Mediated Lipid Peroxidation Processes in Photosynthetic Membranes. Plant Physiol. 1980, 66, 797–800. [Google Scholar] [CrossRef]
- Nagalakshmi, N.; Prasad, M.N.V. Responses of Glutathione Cycle Enzymes and Glutathione Metabolism to Copper Stress in Scenedesmus bijugatus. Plant Sci. Int. J. Exp. Plant Biol. 2001, 160, 291–299. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Mostofa, M.G.; Polash, M.A.S.; Mahamud, A.G.M.S.U.; Afrin, S.; Hossain, M.A.; Hossain, M.A.; Murata, Y.; Tran, L.-S.P. Comparative Effects of Ascobin and Glutathione on Copper Homeostasis and Oxidative Stress Metabolism in Mitigation of Copper Toxicity in Rice. Plant Biol. 2021, 23, 162–169. [Google Scholar] [CrossRef]
- An, Q.; Zheng, N.; Ji, Y.; Sun, S.; Wang, S.; Li, X.; Chen, C.; Li, N.; Pan, J. Exploration the Interaction of Cadmium and Copper Toxic Effects in Pakchoi (Brassica chinensis L.) Roots through Combinatorial Transcriptomic and Weighted Gene Co-Expression Network Analysis. J. Environ. Manag. 2024, 359, 120956. [Google Scholar] [CrossRef]
- Younis, M.E.; Tourky, S.M.N.; Elsharkawy, S.E.A. Symptomatic Parameters of Oxidative Stress and Antioxidant Defense System in Phaseolus vulgaris L. in Response to Copper or Cadmium Stress. South Afr. J. Bot. 2018, 117, 207–214. [Google Scholar] [CrossRef]
- Ferrari, M.; Cozza, R.; Marieschi, M.; Torelli, A. Role of Sulfate Transporters in Chromium Tolerance in Scenedesmus acutus M. (Sphaeropleales). Plants 2022, 11, 223. [Google Scholar] [CrossRef]
- Adhikari, A.; Roy, D.; Adhikari, S.; Saha, S.; Ghosh, P.K.; Shaw, A.K.; Hossain, Z. microRNAomic Profiling of Maize Root Reveals Multifaceted Mechanisms to Cope with Cr (VI) Stress. Plant Physiol. Biochem. 2023, 198, 107693. [Google Scholar] [CrossRef]
- Andrés-Bordería, A.; Andrés, F.; Garcia-Molina, A.; Perea-García, A.; Domingo, C.; Puig, S.; Peñarrubia, L. Copper and Ectopic Expression of the Arabidopsis Transport Protein COPT1 Alter Iron Homeostasis in Rice (Oryza sativa L.). Plant Mol. Biol. 2017, 95, 17–32. [Google Scholar] [CrossRef]
- Perea-García, A.; Garcia-Molina, A.; Andrés-Colás, N.; Vera-Sirera, F.; Pérez-Amador, M.A.; Puig, S.; Peñarrubia, L. Arabidopsis Copper Transport Protein COPT2 Participates in the Cross Talk between Iron Deficiency Responses and Low-Phosphate Signaling. Plant Physiol. 2013, 162, 180–194. [Google Scholar] [CrossRef]
- Nie, G.; Zhong, M.; Cai, J.; Yang, X.; Zhou, J.; Appiah, C.; Tang, M.; Wang, X.; Feng, G.; Huang, L.; et al. Transcriptome Characterization of Candidate Genes Related to Chromium Uptake, Transport and Accumulation in Miscanthus sinensis. Ecotoxicol. Environ. Saf. 2021, 221, 112445. [Google Scholar] [CrossRef]
- Feil, S.B.; Zuluaga, M.Y.A.; Cesco, S.; Pii, Y. Copper Toxicity Compromises Root Acquisition of Nitrate in the High Affinity Range. Front. Plant Sci. 2023, 13, 1034425. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, W.; Sun, S.; Yang, X.; Mao, H.; Sheng, L.; Chen, Z. Metagenomic Analysis Revealed N-Metabolizing Microbial Response of Iris Tectorum to Cr Stress after Colonization by Arbuscular mycorrhizal Fungi. Ecotoxicol. Environ. Saf. 2024, 273, 116157. [Google Scholar] [CrossRef]
- Shabbir, R.; Javed, T.; Hussain, S.; Ahmar, S.; Naz, M.; Zafar, H.; Pandey, S.; Chauhan, J.; Siddiqui, M.H.; Pinghua, C. Calcium Homeostasis and Potential Roles in Combatting Environmental Stresses in Plants. South Afr. J. Bot. 2022, 148, 683–693. [Google Scholar] [CrossRef]
- Wan, B.; Lin, Y.; Mou, T. Expression of Rice Ca\textsuperscript2+-dependent Protein Kinases (CDPKs) Genes under Different Environmental Stresses. FEBS Lett. 2007, 581, 1179–1189. [Google Scholar] [CrossRef]
- Kaszycki, P.; Dubicka-Lisowska, A.; Augustynowicz, J.; Piwowarczyk, B.; Wesołowski, W. Callitriche Cophocarpa (Water Starwort) Proteome under Chromate Stress: Evidence for Induction of a Quinone Reductase. Environ. Sci. Pollut. Res. 2018, 25, 8928–8942. [Google Scholar] [CrossRef]
- Althammer, M.; Regl, C.; Herburger, K.; Blöchl, C.; Voglas, E.; Huber, C.G.; Tenhaken, R. Overexpression of UDP-Sugar Pyrophosphorylase Leads to Higher Sensitivity towards Galactose, Providing New Insights into the Mechanisms of Galactose Toxicity in Plants. Plant J. Cell Mol. Biol. 2022, 109, 1416–1426. [Google Scholar] [CrossRef]
- Gu, S.; Lan, C.Q. Biosorption of Heavy Metal Ions by Green Alga Neochloris Oleoabundans: Effects of Metal Ion Properties and Cell Wall Structure. J. Hazard. Mater. 2021, 418, 126336. [Google Scholar] [CrossRef]
- Davis, T.A.; Llanes, F.; Volesky, B.; Mucci, A. Metal Selectivity of Sargassum Spp. and Their Alginates in Relation to Their α-L-Guluronic Acid Content and Conformation. Environ. Sci. Technol. 2003, 37, 261–267. [Google Scholar] [CrossRef]
- Konno, H.; Nakato, T.; Nakashima, S.; Katoh, K. Lygodium Japonicum Fern Accumulates Copper in the Cell Wall Pectin. J. Exp. Bot. 2005, 56, 1923–1931. [Google Scholar] [CrossRef]
- Ren, C.; Qi, Y.; Huang, G.; Yao, S.; You, J.; Hu, H. Contributions of Root Cell Wall Polysaccharides to Cu Sequestration in Castor (Ricinus communis L.) Exposed to Different Cu Stresses. J. Environ. Sci. 2020, 88, 209–216. [Google Scholar] [CrossRef]
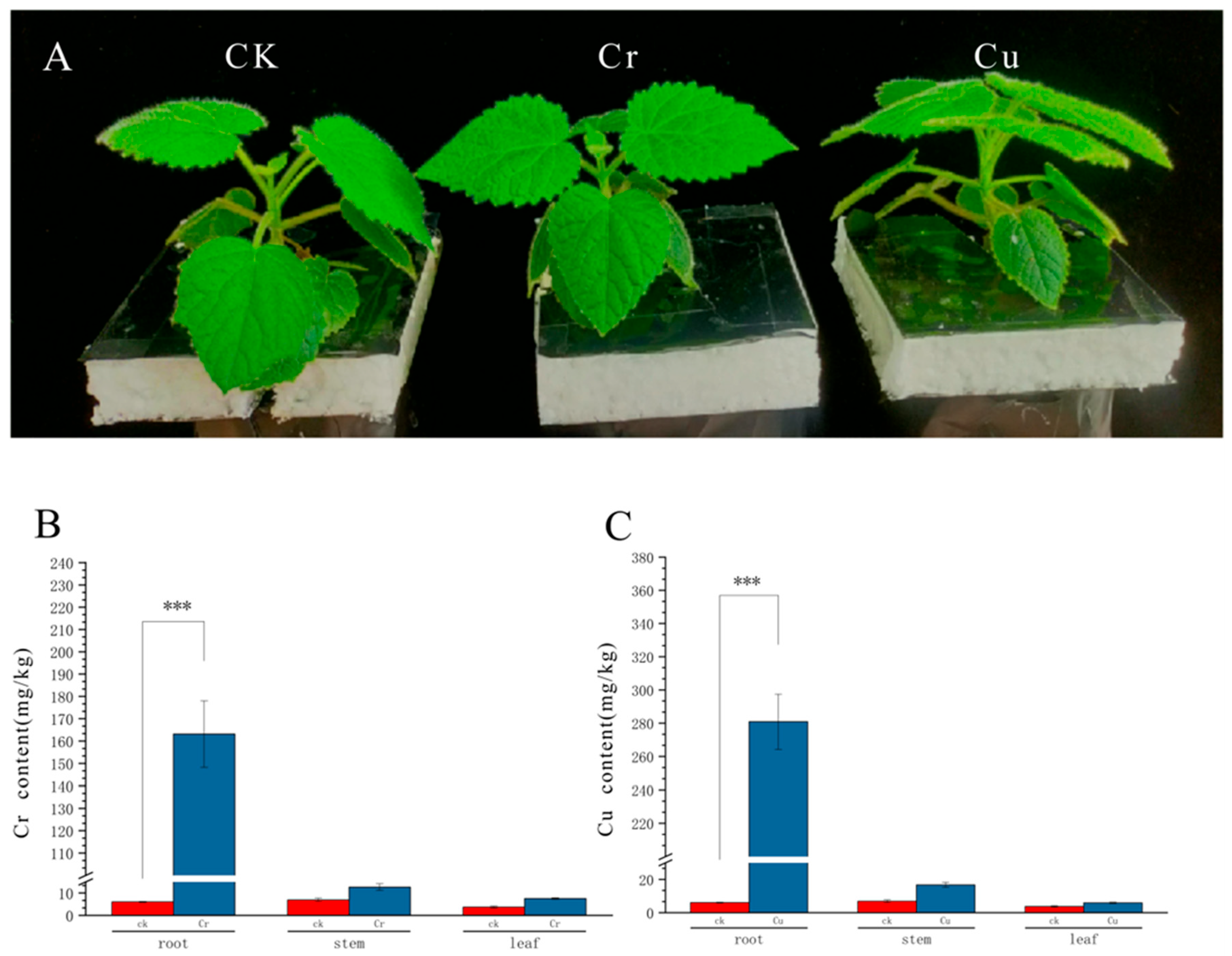
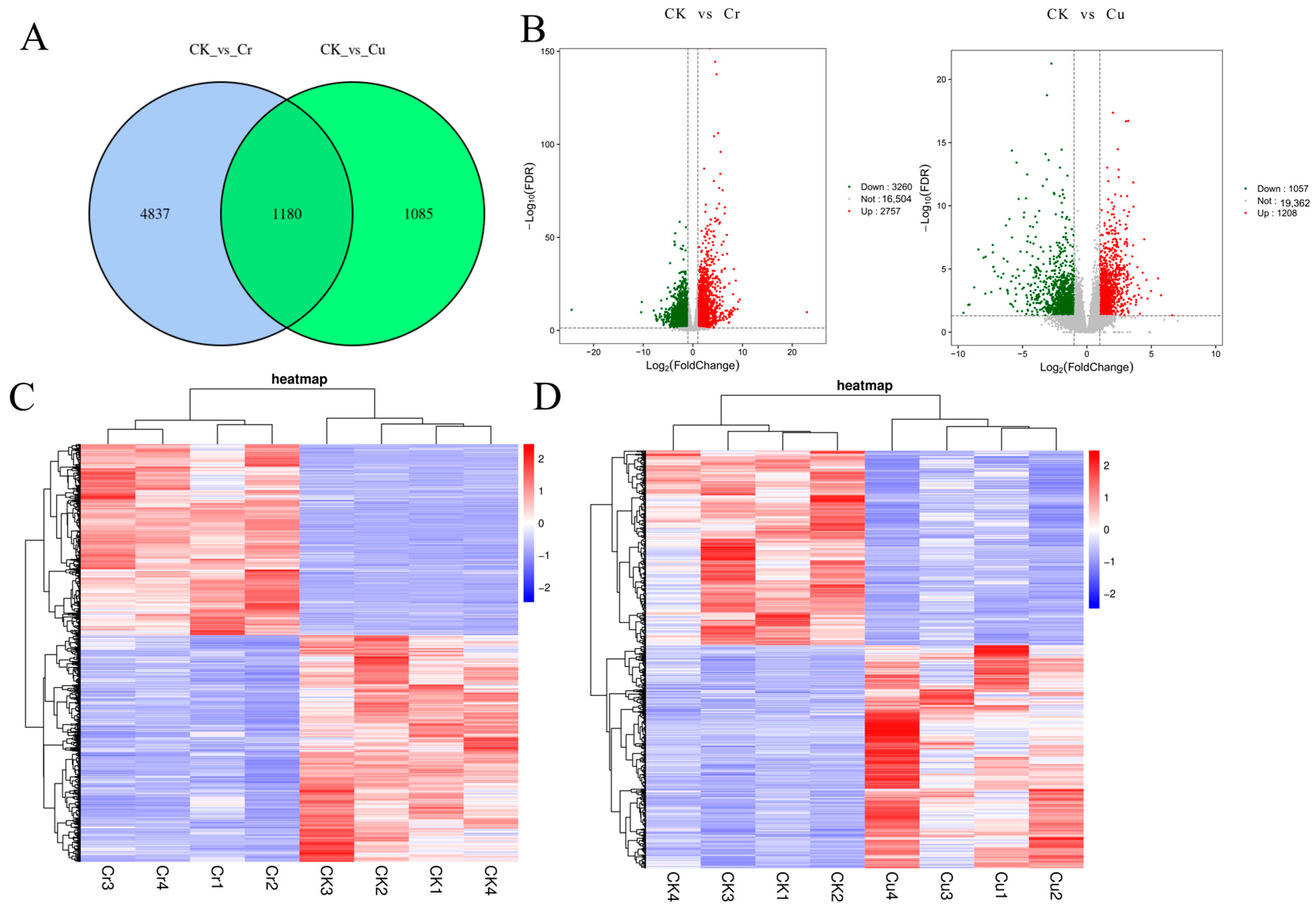
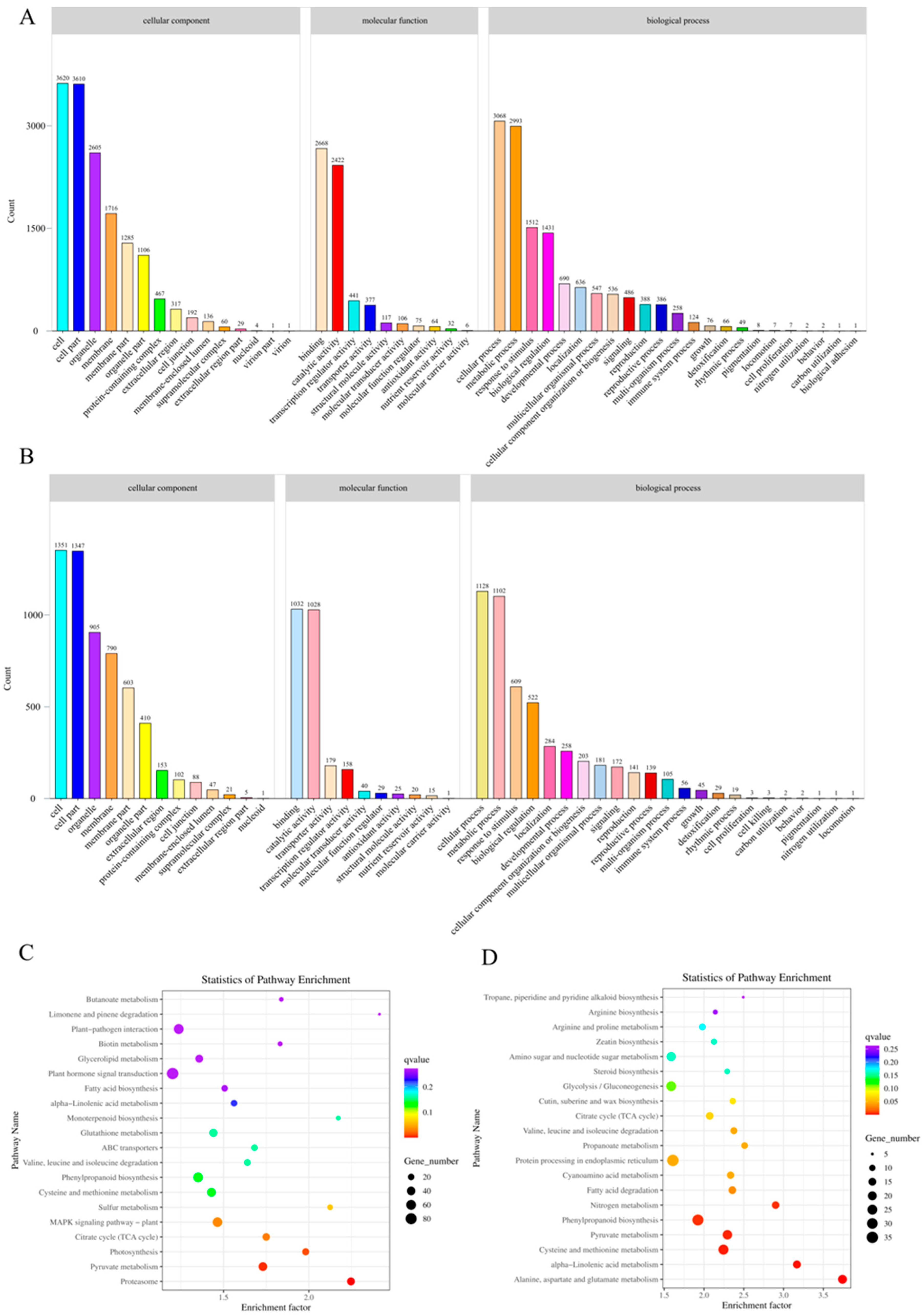


| Aspect of Response | Cr Stress Response | Cu Stress Response |
|---|---|---|
| Total DEGs | 6017 | 2265 |
| Unique DEGs | 4837 | 1085 |
| Key Enriched Pathways (KEGG) | Proteasome, Pyruvate metabolism, Sulfur metabolism, Glutathione metabolism | Alanine, Aspartate, Glutamate metabolism, α-Linolenic acid metabolism, Cysteine/Methionine metabolism |
| Prominent Molecular Mechanisms | Significant involvement of protein degradation, energy metabolism, and sulfur/glutathione pathways. | Stronger emphasis on specific amino acid metabolism precursors for GSH and potential membrane/cell wall related responses. |
| Transporter Families/Genes | Members from ABC, NRT, CTR, ST families showed differential expression; specific genes varied. | Members from ABC, ZIP, NRT, HMA, CTR, ST families showed differential expression; specific genes varied. |
| Representative Top Hub Genes | Pfo_030353(ARABIDILLO), Pfo_019190 (ATR/Flavodoxin), Pfo_020668 (CDPK) | Pfo_027922(CID), Pfo_014537 (KIN-14F), Pfo_010312(UDP-Gal transporter), Pfo_000197 (polygalacturonase) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Pan, X.; Xian, K.; Fu, C.; He, J.; Liu, B.; Sang, J.; Huang, N. Transcriptomic Profiling of Paulownia fortunei (Seem.) Hemsl. Roots in Response to Chromium and Copper Stress. Genes 2025, 16, 595. https://doi.org/10.3390/genes16050595
Su J, Pan X, Xian K, Fu C, He J, Liu B, Sang J, Huang N. Transcriptomic Profiling of Paulownia fortunei (Seem.) Hemsl. Roots in Response to Chromium and Copper Stress. Genes. 2025; 16(5):595. https://doi.org/10.3390/genes16050595
Chicago/Turabian StyleSu, Jiang, Xinfeng Pan, Kanghua Xian, Chuanming Fu, Jinxiang He, Baojun Liu, Jinhan Sang, and Ningzhen Huang. 2025. "Transcriptomic Profiling of Paulownia fortunei (Seem.) Hemsl. Roots in Response to Chromium and Copper Stress" Genes 16, no. 5: 595. https://doi.org/10.3390/genes16050595
APA StyleSu, J., Pan, X., Xian, K., Fu, C., He, J., Liu, B., Sang, J., & Huang, N. (2025). Transcriptomic Profiling of Paulownia fortunei (Seem.) Hemsl. Roots in Response to Chromium and Copper Stress. Genes, 16(5), 595. https://doi.org/10.3390/genes16050595






