Clinicopathological Characteristics of Ovarian and Breast Cancer in PALB2, RAD51C, and RAD51D Germline Pathogenic Variant Carriers
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Study Population
2.3. Study Design
2.4. Data Collection
2.5. Variable Definition and Statistical Analysis
3. Results
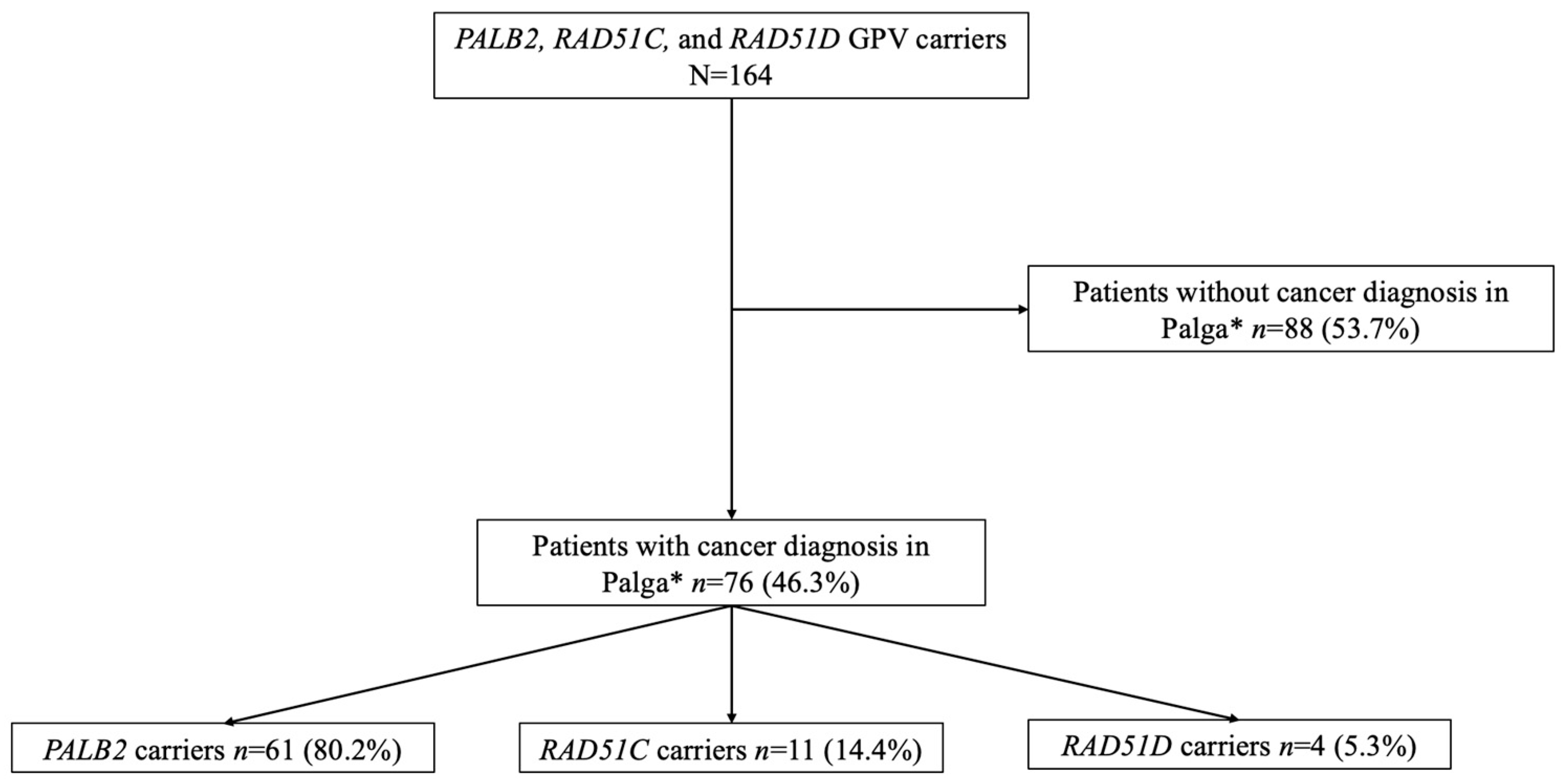
3.1. Patient Characteristics
3.2. Breast Cancer
3.3. Ovarian Cancer
4. Discussion
Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GPV | germline pathogenic variant |
| BC | breast cancer |
| OC | ovarian cancer |
| NCR | national cancer registry |
| RRSO | risk-reducing salpingo-oophorectomy |
| UMCG | University Medical Center Groningen |
| PV | pathogenic variant |
| METc | Medical Ethics Review Board |
| HR | hormone receptor |
| NST | no special type |
References
- Russo, A.; Calò, V.; Bruno, L.; Rizzo, S.; Bazan, V.; Di Fede, G. Hereditary Ovarian Cancer. Crit. Rev. Oncol. Hematol. 2009, 69, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R. Hereditary Breast and Ovarian Cancer (HBOC): Review of Its Molecular Characteristics, Screening, Treatment, and Prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Paul, S. The Breast Cancer Susceptibility Genes (BRCA) in Breast and Ovarian Cancers. Front. Biosci. Landmark Ed. 2014, 19, 605–618. [Google Scholar] [CrossRef]
- Varol, U.; Kucukzeybek, Y.; Alacacioglu, A.; Somali, I.; Altun, Z.; Aktas, S.; Oktay Tarhan, M. BRCA Genes: BRCA1 and BRCA2. J. Balk. Union Oncol. 2018, 23, 862–866. [Google Scholar]
- Yang, X.; Song, H.; Leslie, G.; Engel, C.; Hahnen, E.; Auber, B.; Horváth, J.; Kast, K.; Niederacher, D.; Turnbull, C.; et al. Ovarian and Breast Cancer Risks Associated with Pathogenic Variants in RAD51C and RAD51D. J. Natl. Cancer Inst. 2020, 112, 1242–1250. [Google Scholar] [CrossRef]
- Breast Cancer Association Consortium; Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Luccarini, C.; Wahlström, C.; Pooley, K.A.; Parsons, M.T.; Fortuno, C.; et al. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef]
- Potapova, A.; Hoffman, A.M.; Godwin, A.K.; Al-Saleem, T.; Cairns, P. Promoter Hypermethylation of the PALB2 Susceptibility Gene in Inherited and Sporadic Breast and Ovarian Cancer. Cancer Res. 2008, 68, 998–1002. [Google Scholar] [CrossRef]
- Pylkäs, K.; Erkko, H.; Nikkilä, J.; Sólyom, S.; Winqvist, R. Analysis of Large Deletions in BRCA1, BRCA2, and PALB2 Genes in Finnish Breast and Ovarian Cancer Families. BMC Cancer 2008, 8, 146. [Google Scholar] [CrossRef]
- Meindl, A.; Hellebrand, H.; Wiek, C.; Erven, V.; Wappenschmidt, B.; Niederacher, D.; Freund, M.; Lichtner, P.; Hartmann, L.; Schaal, H.; et al. Germline Mutations in Breast and Ovarian Cancer Pedigrees Establish RAD51C as a Human Cancer Susceptibility Gene. Nat. Genet. 2010, 42, 410–414. [Google Scholar] [CrossRef]
- Loveday, C.; Turnbull, C.; Ramsay, E.; Hughes, D.; Ruark, E.; Frankum, J.R.; Bowden, G.; Kalmyrzaev, B.; Warren-Perry, M.; Snape, K.; et al. Germline Mutations in RAD51D Confer Susceptibility to Ovarian Cancer. Nat. Genet. 2011, 43, 879–882. [Google Scholar] [CrossRef]
- Kanker in de Familie, PALB2. Available online: https://kankerindefamilie.nl/kanker-in-de-familie/erfelijke-aanleg-borstkanker/palb2/#:~:text=Een%20afwijking%20(mutatie)%20in%20het,in%20het%20PALB2%2Dgen%20gevonden (accessed on 16 April 2025).
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer Risks Associated with Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. 2020, 38, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Shimelis, H.; LaDuca, H.; Hu, C.; Hart, S.N.; Na, J.; Thomas, A.; Akinhanmi, M.; Moore, R.M.; Brauch, H.; Cox, A.; et al. Triple-Negative Breast Cancer Risk Genes Identified by Multigene Hereditary Cancer Panel Testing. J. Natl. Cancer Inst. 2018, 110, 855–862. [Google Scholar] [CrossRef]
- Breast Cancer Association Consortium; Mavaddat, N.; Dorling, L.; Carvalho, S.; Allen, J.; González-Neira, A.; Keeman, R.; Bolla, M.K.; Dennis, J.; Wang, Q.; et al. Pathology of Tumors Associated With Pathogenic Germline Variants in 9 Breast Cancer Susceptibility Genes. JAMA Oncol. 2022, 8, e216744. [Google Scholar] [CrossRef] [PubMed]
- Richtlijnendatabase. Screening Buiten Het BVO. Available online: https://richtlijnendatabase.nl/richtlijn/borstkanker/screening/screening_buiten_het_bvo/screening_buiten_het_bvo.html (accessed on 8 January 2025).
- Ramus, S.J.; Song, H.; Dicks, E.; Tyrer, J.P.; Rosenthal, A.N.; Intermaggio, M.P.; Fraser, L.; Gentry-Maharaj, A.; Hayward, J.; Philpott, S.; et al. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women with Ovarian Cancer. J. Natl. Cancer Inst. 2015, 107, djv214. [Google Scholar] [CrossRef] [PubMed]
- Van der Velde, N.M.; Mourits, M.J.; Arts, H.J.; de Vries, J.; Leegte, B.K.; Dijkhuis, G.; Oosterwijk, J.C.; de Bock, G.H. Time to Stop Ovarian Cancer Screening in BRCA1/2 Mutation Carriers? Int. J. Cancer 2009, 124, 919–923. [Google Scholar] [CrossRef]
- Richtlijnendatabase. Erfelijk en Familiair Ovariumcarcinoom. Available online: https://richtlijnendatabase.nl/richtlijn/erfelijk_en_familiair_ovariumcarcinoom/erfelijk_en_familiair_ovariumcarcinoom_algemeen.html. (accessed on 8 January 2025).
- VKGN. [Erfelijke en Familiare Tumoren, Richtlijnen Voor Diagnostiek en Preventie]. VKGN. Available online: https://vkgn.stoet.nl/index.php?article_id=69 (accessed on 17 March 2025).
- Lee, A.; Mavaddat, N.; Wilcox, A.N.; Cunningham, A.P.; Carver, T.; Hartley, S.; Babb de Villiers, C.; Izquierdo, A.; Simard, J.; Schmidt, M.K.; et al. BOADICEA: A Comprehensive Breast Cancer Risk Prediction Model Incorporating Genetic and Nongenetic Risk Factors. Genet. Med. 2019, 21, 1708–1718. [Google Scholar] [CrossRef]
- Carver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Archer, S.; Babb de Villiers, C.; Roberts, J.; Ruston, R.; Walter, F.M.; Tischkowitz, M.; et al. CanRisk Tool—A Web Interface for the Prediction of Breast and Ovarian Cancer Risk and the Likelihood of Carrying Genetic Pathogenic Variants. Cancer Epidemiol. Biomark. Prev. 2021, 30, 469–473. [Google Scholar] [CrossRef]
- Archer, S.; Babb de Villiers, C.; Scheibl, F.; Carver, T.; Hartley, S.; Lee, A.; Cunningham, A.P.; Easton, D.F.; McIntosh, J.G.; Emery, J.; et al. Evaluating Clinician Acceptability of the Prototype CanRisk Tool for Predicting Risk of Breast and Ovarian Cancer: A Multi-Methods Study. PLoS ONE 2020, 15, e0229999. [Google Scholar] [CrossRef]
- NKR Cijfers. Incidentie per Jaar. Available online: https://nkr-cijfers.iknl.nl/viewer/incidentie-per-jaar?language=nl_NL&viewerId=3b55d752-ce1a-4809-89d9-8421febdb1f4 (accessed on 7 February 2025).
- Casparie, M.; Tiebosch, A.T.; Burger, G.; Blauwgeers, H.; van de Pol, A.; van Krieken, J.H.; Meijer, G.A. Pathology Databanking and Biobanking in The Netherlands, a Central Role for PALGA, the Nationwide Histopathology and Cytopathology Data Network and Archive. Cell. Oncol. 2007, 29, 19–24. [Google Scholar] [CrossRef]
- Antoniou, A.C.; Casadei, S.; Heikkinen, T.; Barrowdale, D.; Pylkäs, K.; Roberts, J.; Lee, A.; Subramanian, D.; De Leeneer, K.; Fostira, F.; et al. Breast-Cancer Risk in Families with Mutations in PALB2. N. Engl. J. Med. 2014, 371, 497–506. [Google Scholar] [CrossRef]
- Tibiletti, M.G.; Carnevali, I.; Facchi, S.; Libera, L.; Chiappa, C.; Sessa, F.; Rosa, S.; Rovera, F. PALB2 Analysis in the Diagnostic Process of Breast Cancer: An Italian Monocentric Experience. Tumori J. 2024, 24, 3008916241290738. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, P.; Cai, Q.; Chen, L.; Wang, Y.; Cai, W.; Qiu, Y.; Liu, S.; Guo, W.; Chen, M.; et al. Spectrum and Characteristics of Germline PALB2 Pathogenic Variants in 1556 Early-Onset Breast Cancer Patients in China. J. Cancer Res. Clin. Oncol. 2024, 150, 322. [Google Scholar] [CrossRef] [PubMed]
- Kansu, B.; Gardner, J.; Price-Tate, R.; Murch, O.; Murray, A. BRCA Gene Testing in Women with High-Grade Serous Ovarian Carcinoma. J. Obstet. Gynaecol. 2021, 41, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Dicks, E.; Ramus, S.J.; Tyrer, J.P.; Intermaggio, M.P.; Hayward, J.; Edlund, C.K.; Conti, D.; Harrington, P.; Fraser, L.; et al. Contribution of Germline Mutations in the RAD51B, RAD51C, and RAD51D Genes to Ovarian Cancer in the Population. J. Clin. Oncol. 2015, 33, 2901–2907. [Google Scholar] [CrossRef]
- Labidi-Galy, S.I.; Papp, E.; Hallberg, D.; Niknafs, N.; Adleff, V.; Noe, M.; Bhattacharya, R.; Novak, M.; Jones, S.; Phallen, J.; et al. High Grade Serous Ovarian Carcinomas Originate in the Fallopian Tube. Nat. Commun. 2017, 8, 1093. [Google Scholar] [CrossRef]
| Characteristics | Total N = 164 |
|---|---|
| Age at the time of genetic testing in years (median (IQR)) | 54.0 (40–65) |
| Type of mutation | |
| PALB2 | 125 (76.2%) |
| RAD51C | 30 (18.3%) |
| RAD51D | 9 (5.5%) |
| Women with a diagnosis of primary cancer ** | 76 (%) |
| Breast | 53 (69.7%) |
| Ovarian | 5 (6.6%) |
| Breast and ovarian | 1 (1.3%) |
| Other | 17 (22.4%) |
| Second diagnosis/Recurrence | 12 |
| Breast | 11 |
| Ovarian | 1 |
| Sequence of genetic testing and cancer diagnosis | 76 (%) |
| Cancer diagnosed before genetic testing | 65 (85.5%) |
| Cancer diagnosed after genetic testing | 9 (11.8%) |
| Unknown *** | 2 (2.6%) |
| PALB2 | RAD51C | RAD51D | NCR Data | |
|---|---|---|---|---|
| Breast cancer (n) | 50 | 3 * | 1 | |
| Age at the time of diagnosis (median (IQR)) | 52 (42–61.5) | 71 (n.a.) ** | 43 (n.a.) ** | 62 (51–72) |
| Histological subtype | ||||
| Invasive carcinoma NST | 45 (90.0%) | 1 (33.3%) | 0 | 79.7% |
| Invasive lobular carcinoma | 4 (8.0%) | 1 (33.3%) | 1 (100%) | 13.0% |
| Other subtypes | 1 (2.0%) | 1 (33.3%) | 0 | 7.3% |
| Tumor grade | ||||
| 1 | 5 (12.8%) | 2 (100%) | 0 | - |
| 2 | 21 (53.8%) | 0 | 0 | - |
| 3 | 13 (33.3%) | 0 | 1 (100%) | - |
| Not reported | 11 (22.0%) | 1 (33.3%) | 0 | - |
| Tumor diameter at the time of diagnosis (median (IQR)) | 15.0 mm (11.0–18.0) | 20.5 mm (n.a.) ** | Unknown | - |
| Hormone receptor status | ||||
| HR + | 32 (72.7%) | 3 (100%) | 0 | 81.7% |
| HER2 + | 3 (7.7%) | 0 | 0 | 13.0% |
| Triple negative | 9 (18.0%) | 0 | 1 (100%) | 11.1% |
| Unknown | 4 (8.0%) | 0 | 0 | |
| Ovarian cancer (n) | 0 | 4 * | 2 | |
| Age at the time of diagnosis (median (IQR)) | - | 66 (61–69.5) | 56 (n.a.) ** | 67 (59–76) |
| Histological subtype | ||||
| High-grade serous | - | 3 (75.0%) | 2 (100%) | 51.5% |
| Low-grade serous | - | - | - | 4.9% |
| Endometroid | - | - | - | 5.9% |
| Clear cell | - | - | - | 5.2% |
| Mucinous | - | - | - | 5.7% |
| Carcinosarcoma | - | - | - | 2.0% |
| Other | - | 1 (25.0%) | - | 24.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spijkervet, J.-R.J.A.H.; Lanjouw, L.; Berger, L.P.V.; Dorrius, M.D.; van der Vegt, B.; de Bock, G.H. Clinicopathological Characteristics of Ovarian and Breast Cancer in PALB2, RAD51C, and RAD51D Germline Pathogenic Variant Carriers. Genes 2025, 16, 556. https://doi.org/10.3390/genes16050556
Spijkervet J-RJAH, Lanjouw L, Berger LPV, Dorrius MD, van der Vegt B, de Bock GH. Clinicopathological Characteristics of Ovarian and Breast Cancer in PALB2, RAD51C, and RAD51D Germline Pathogenic Variant Carriers. Genes. 2025; 16(5):556. https://doi.org/10.3390/genes16050556
Chicago/Turabian StyleSpijkervet, Jella-Rike J. A. H., L. Lanjouw, L. P. V. Berger, M. D. Dorrius, B. van der Vegt, and G. H. de Bock. 2025. "Clinicopathological Characteristics of Ovarian and Breast Cancer in PALB2, RAD51C, and RAD51D Germline Pathogenic Variant Carriers" Genes 16, no. 5: 556. https://doi.org/10.3390/genes16050556
APA StyleSpijkervet, J.-R. J. A. H., Lanjouw, L., Berger, L. P. V., Dorrius, M. D., van der Vegt, B., & de Bock, G. H. (2025). Clinicopathological Characteristics of Ovarian and Breast Cancer in PALB2, RAD51C, and RAD51D Germline Pathogenic Variant Carriers. Genes, 16(5), 556. https://doi.org/10.3390/genes16050556







