Genome-Wide Identification and Phylogenetic Characterization of the FTIP Gene Family in Maize (Zea mays)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis of ZmFTIP Family Members
2.2. Reconstruction of the Phylogenetic Tree for ZmFTIPs in Maize
2.3. Plant Materials and Growth
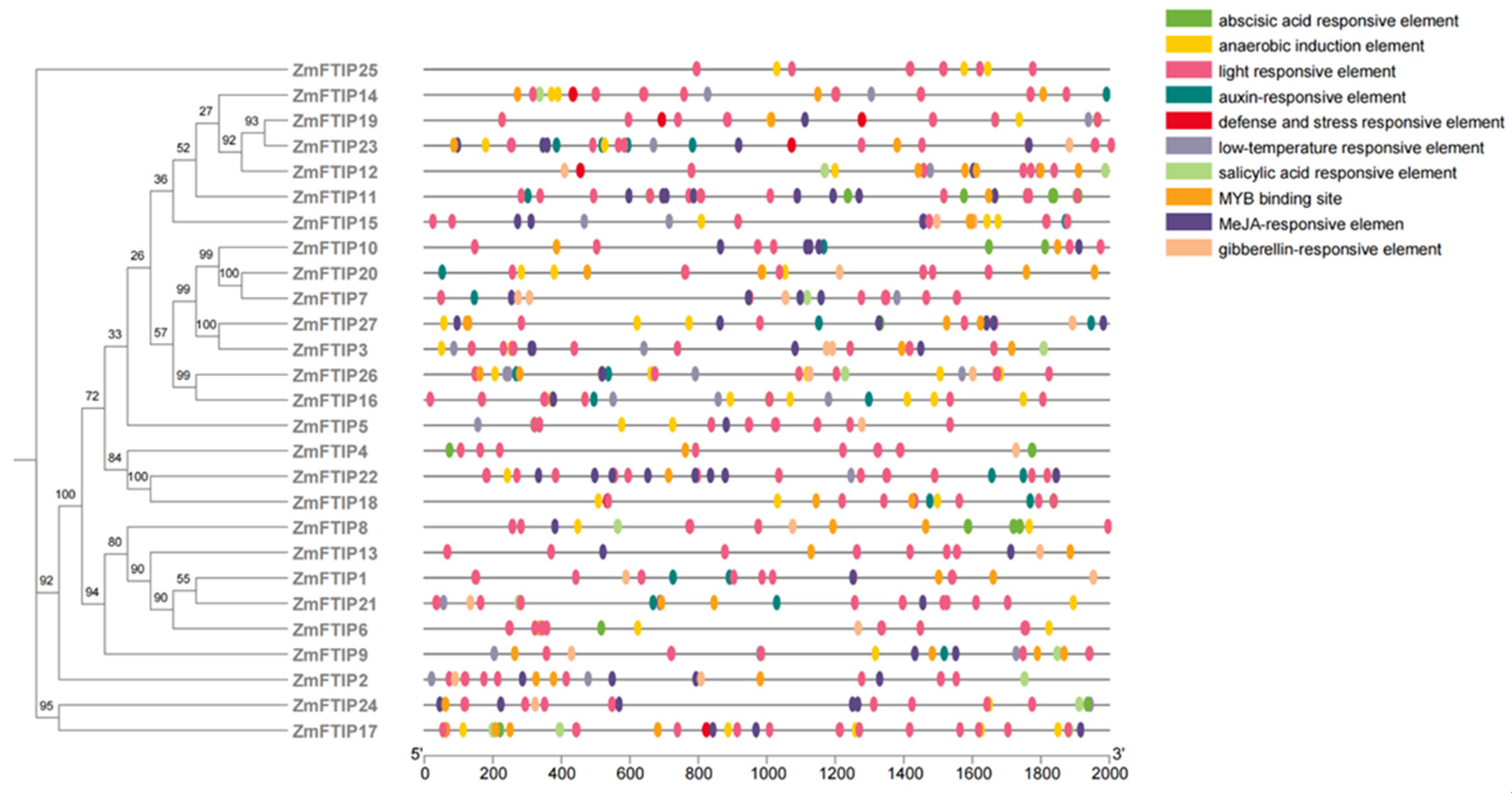
2.4. Cis-Regulatory Element Analysis of ZmFTIPs
2.5. RNA-Seq Database
2.6. Synteny Analysis
2.7. RNA Extraction, Reverse Transcription, and RT-PCR Analysis
3. Results
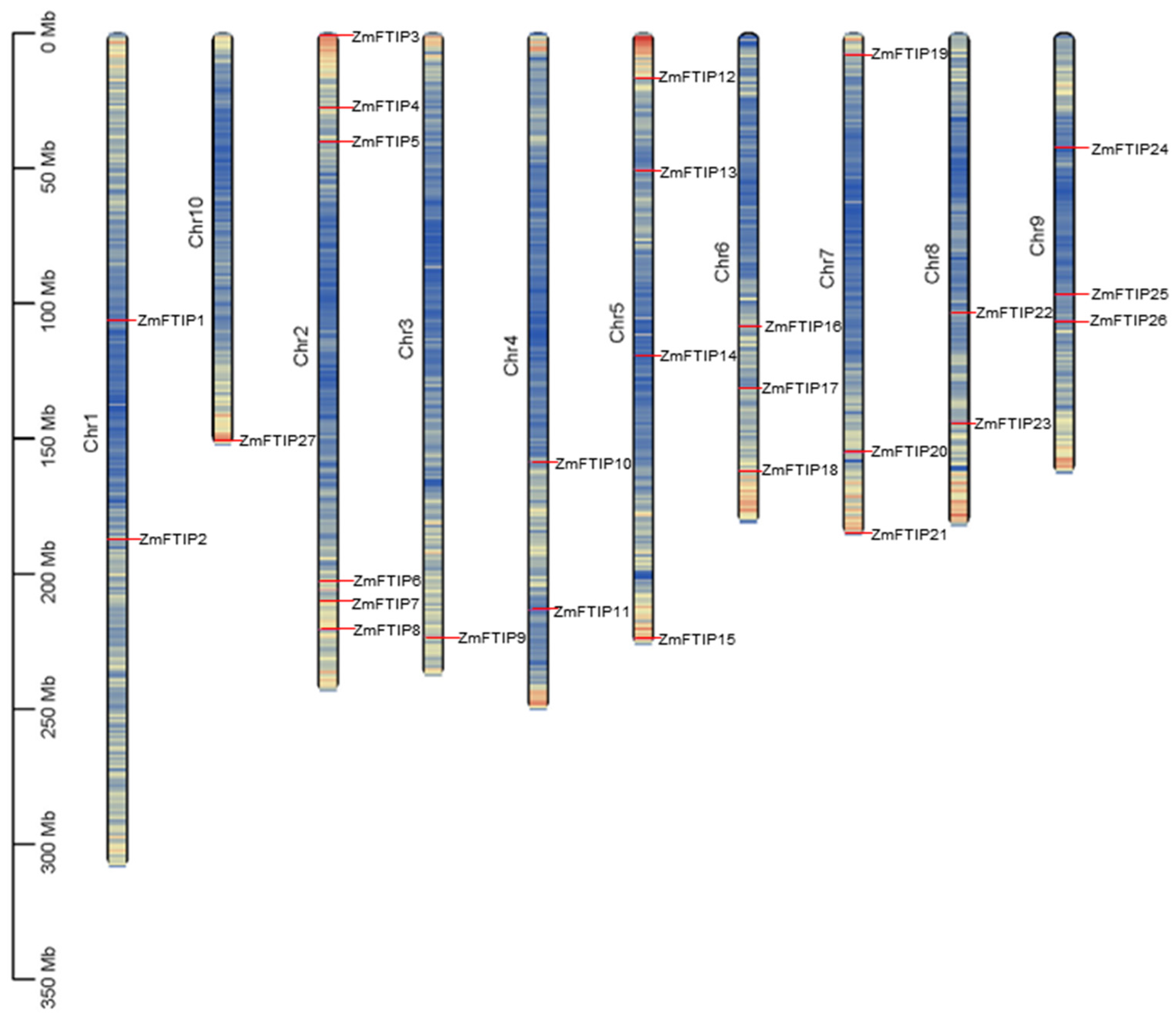
3.1. Identification of ZmFTIP Genes in Maize
3.2. Cis-Element Analysis of the ZmFTIP Genes in Maize
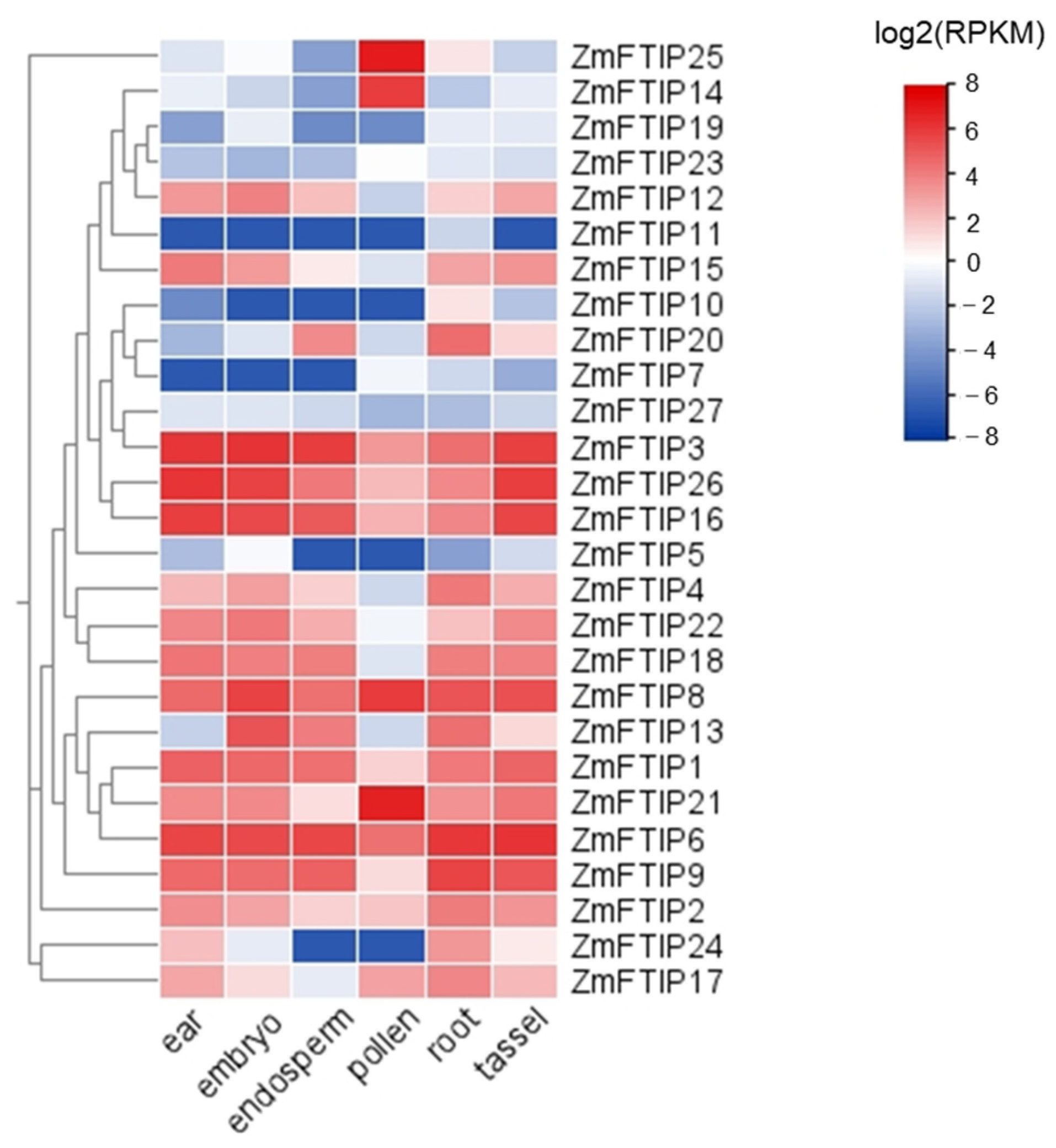
3.3. Analysis of the Expression of ZmFTIPs in Different Organs
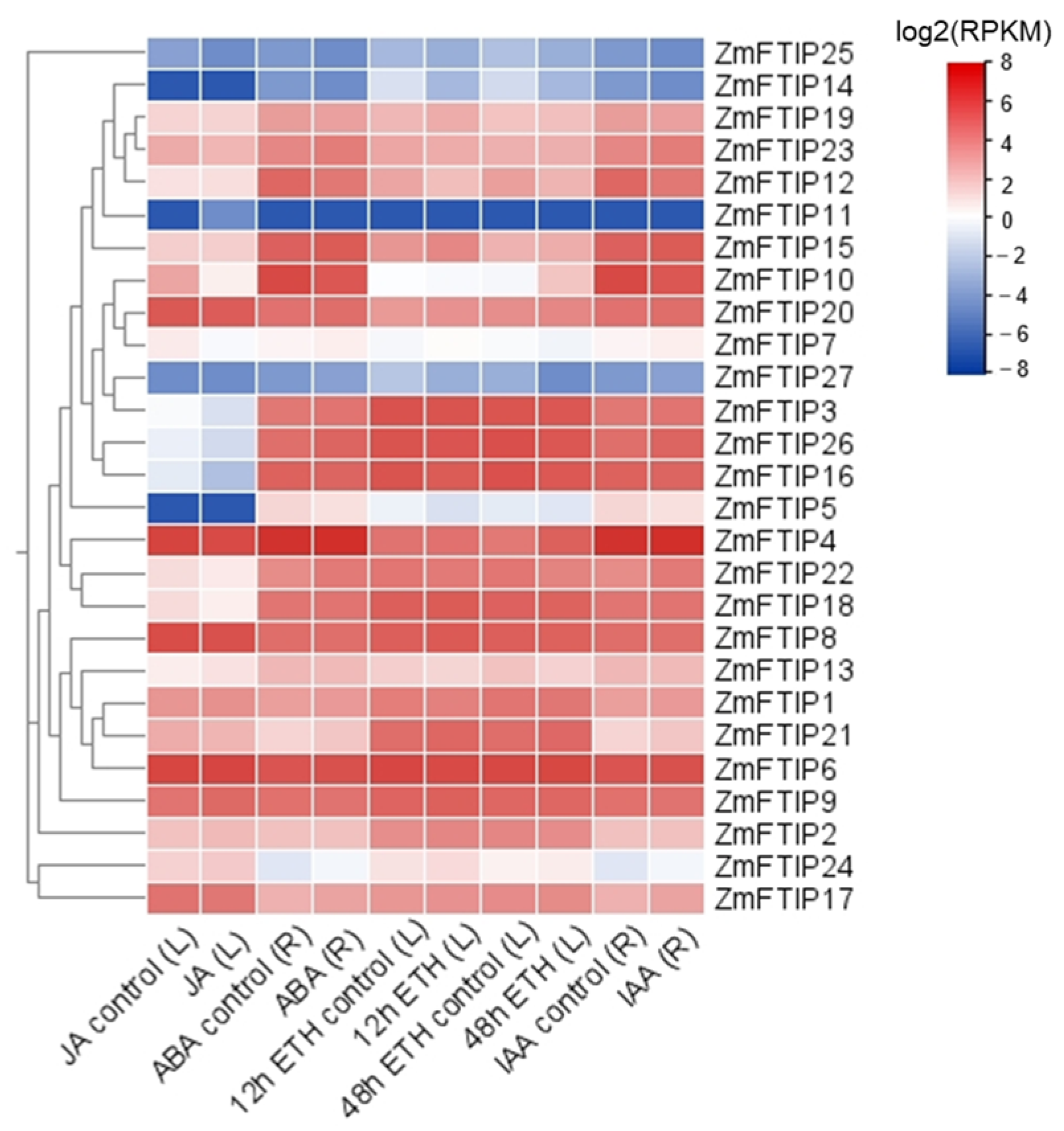
3.4. Analysis of ZmFTIPs Expression in Response to Various Hormones
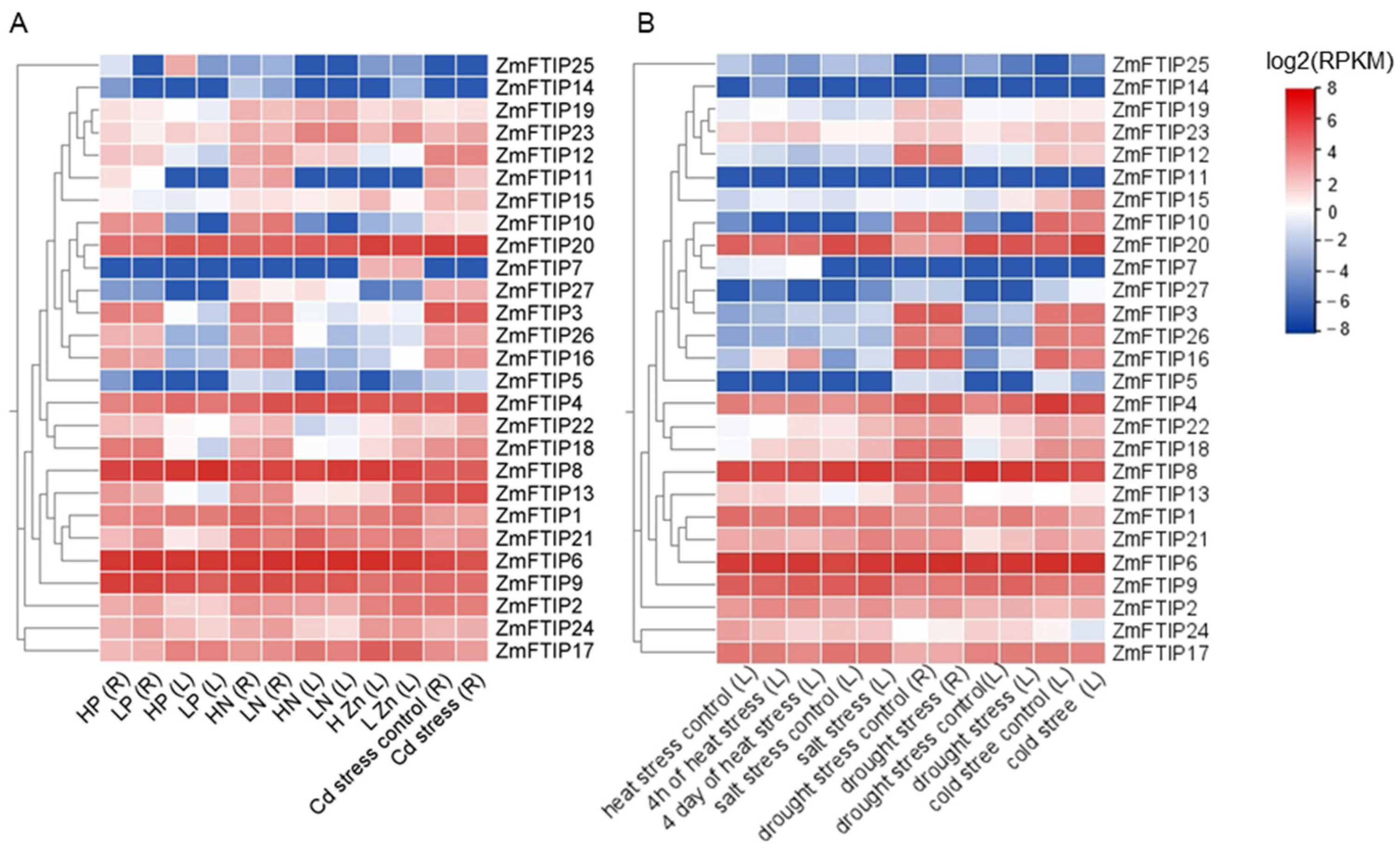
3.5. Comprehensive Analysis of ZmFTIPs Expression Profiles Under Diverse Stress Conditions
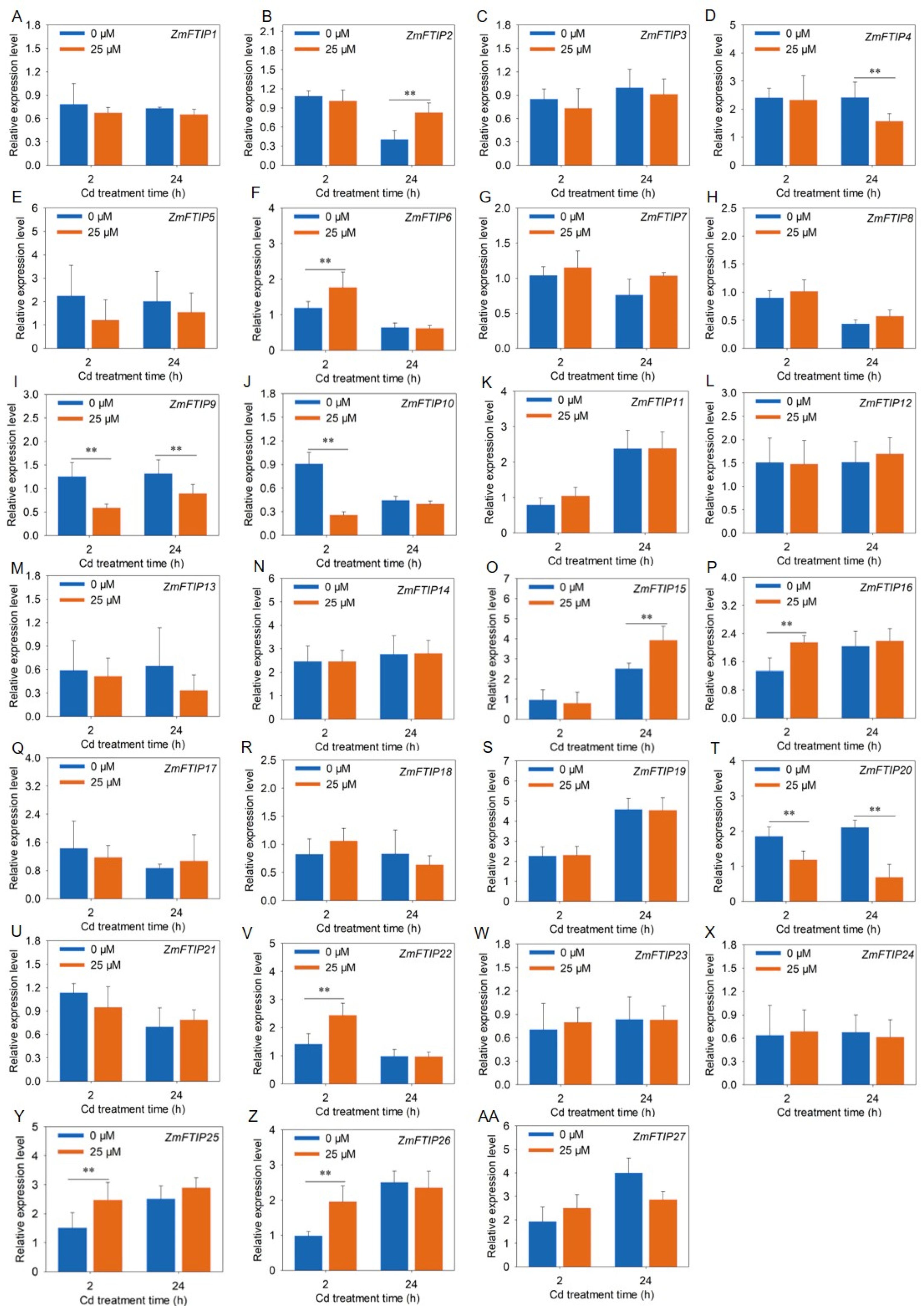
3.6. RT-PCR Analysis of the Expression of ZmFTIPs
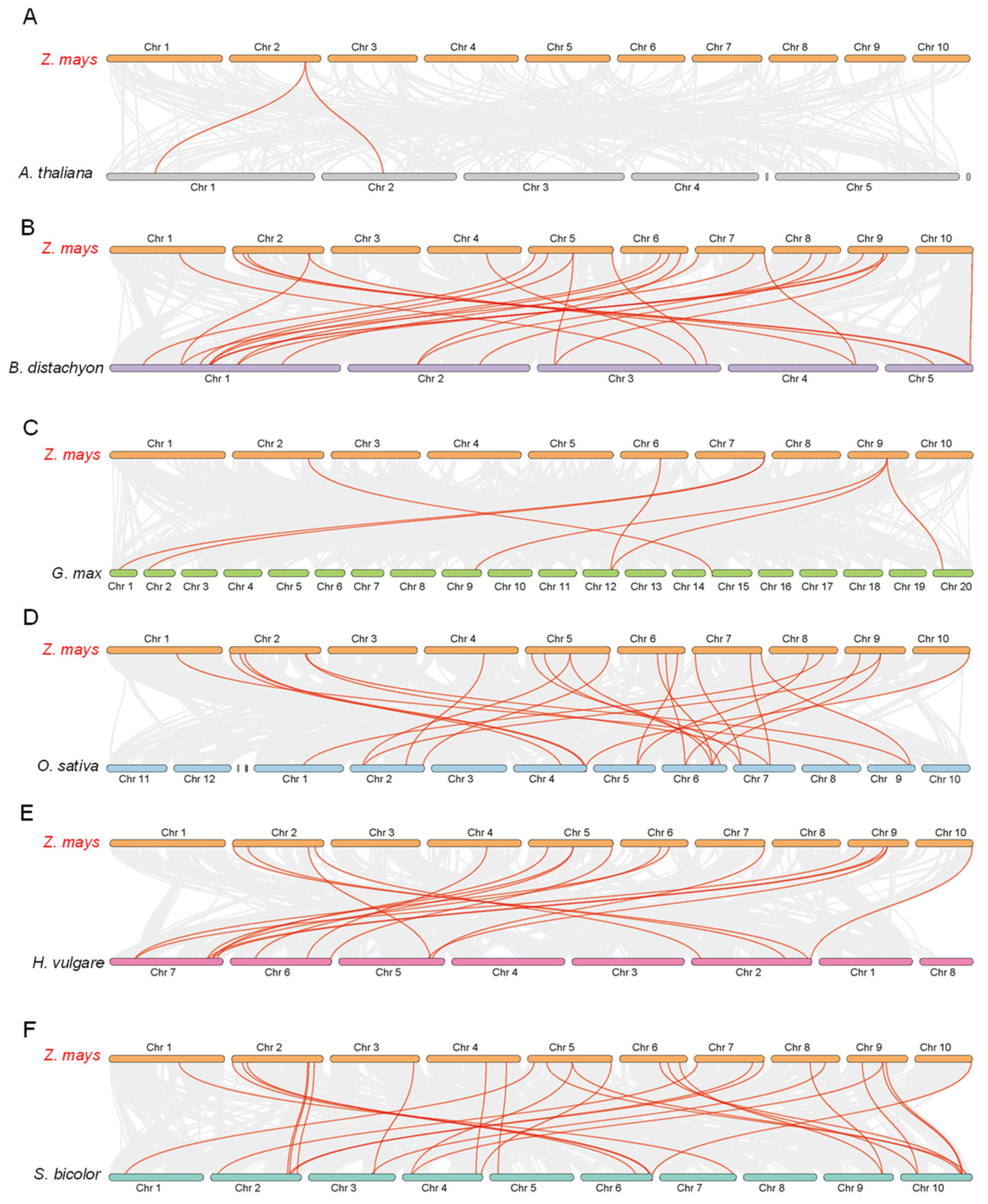
3.7. Synteny Analysis of ZmFTIPs Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, G.; Gao, H.; Yang, T. Exploring the role of the plant actin cytoskeleton: From signaling to cellular functions. Int. J. Mol. Sci. 2023, 24, 15480. [Google Scholar] [CrossRef]
- Song, X.; Ju, Y.; Chen, L.; Zhang, W. Strategies and tools to construct stable and efficient artificial coculture systems as biosynthetic platforms for biomass conversion. Biotechnol. Biofuels Bioprod. 2024, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, M.; Neves, J.; Cardoso, T.; Pissarra, J.; Pereira, S.; Pereira, C. Coping with Abiotic Stress in Plants-An Endomembrane Trafficking Perspective. Plants. 2022, 11, 338. [Google Scholar] [CrossRef]
- Xuan, L.; Li, J.; Jiang, Y.; Shi, M.; Zhu, Y.; Bao, X.; Gong, Q.; Xue, H.-W.; Yu, H.; Liu, L. MCTP controls nucleocytoplasmic partitioning of AUXIN RESPONSE FACTORs during lateral root development. Dev. Cell 2024, 59, 3229–3244.e5. [Google Scholar] [CrossRef] [PubMed]
- Nalefski, E.A.; Falke, J.J. The C2 domain calcium-binding motif: Structural and functional diversity. Protein Sci. 1996, 5, 2375–2390. [Google Scholar] [CrossRef]
- Cho, W.; Stahelin, R.V. Membrane binding and subcellular targeting of C2 domains. Biochim. Biophys. Acta 2006, 1761, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Fulton, L.; Batoux, M.; Vaddepalli, P.; Yadav, R.K.; Busch, W.; Andersen, S.U.; Jeong, S.; Lohmann, J.U.; Schneitz, K. DETORQUEO, QUIRKY, and ZERZAUST represent novel components involved in organ development mediated by the receptor-like kinase STRUBBELIG in Arabidopsis thaliana. PLoS Genet. 2009, 5, e1000355. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Hou, X.; Xi, W.; Shen, L.; Tao, Z.; Wang, Y.; Yu, H. FTIP1 is an essential regulator required for florigen transport. PLoS Biol. 2012, 10, e1001313. [Google Scholar] [CrossRef]
- Chen, X.; Leśniewska, B.; Boikine, R.; Yun, N.; Mody, T.A.; Vaddepalli, P.; Schneitz, K. Arabidopsis MCTP family member QUIRKY regulates the formation of the STRUBBELIG receptor kinase complex. Plant Physiol. 2023, 193, 2538–2554. [Google Scholar] [CrossRef]
- Brault, M.L.; Petit, J.D.; Immel, F.; Nicolas, W.J.; Glavier, M.; Brocard, L.; Gaston, A.; Fouché, M.; Hawkins, T.J.; Crowet, J.; et al. Multiple C2 domains and transmembrane region proteins (MCTPs) tether membranes at plasmodesmata. EMBO Rep. 2019, 20, e47182. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Liang, Z.; Yu, H. Characterization of Multiple C2 Domain and Transmembrane Region Proteins in Arabidopsis. Plant Physiol. 2018, 176, 2119–2132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yan, B.; Hu, Y.; Cui, Z.; Wang, X. Genome-wide identification and phylogenetic analysis of rice FTIP gene family. Genomics. 2020, 112, 3803–3814. [Google Scholar] [CrossRef]
- Song, S.; Chen, Y.; Liu, L.; Wang, Y.; Bao, S.; Zhou, X.; Teo, Z.W.N.; Mao, C.; Gan, Y.; Yu, H. OsFTIP1-Mediated Regulation of Florigen Transport in Rice Is Negatively Regulated by the Ubiquitin-Like Domain Kinase OsUbDKγ4. Plant Cell 2017, 29, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Chen, Y.; Liu, L.; See, Y.H.B.; Mao, C.; Gan, Y.; Yu, H. OsFTIP7 determines auxin-mediated anther dehiscence in rice. Nat. Plants 2018, 4, 495–504. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, J.; Zhang, L.; Qi, H.; Yang, L.; Wang, H.; Wang, J.; Wang, Y.; Du, H.; Tao, Z.; et al. Nuclear translocation of OsMFT1 that is impeded by OsFTIP1 promotes drought tolerance in rice. Mol. Plant 2021, 14, 1297–1311. [Google Scholar] [CrossRef]
- Hu, Q.; Zeng, M.; Wang, M.; Huang, X.; Li, J.; Feng, C.; Xuan, L.; Liu, L.; Huang, G. Family-Wide Evaluation of Multiple C2 Domain and Transmembrane Region Protein in Gossypium hirsutum. Front. Plant Sci. 2021, 12, 767667. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, Q.; Chen, L.; Long, Y.; Song, N.; Jiang, H.; Si, W. Characterization and phylogenetic analysis of multiple C2 domain and transmembrane region proteins in maize. BMC Plant Biol. 2022, 22, 388. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; McCubbin, T.J.; Bihmidine, S.; Julius, B.T.; Baker, R.F.; Schauflinger, M.; Weil, C.; Springer, N.; Chomet, P.; Wagner, R.; et al. Maize Carbohydrate Partitioning Defective33 Encodes an MCTP Protein and Functions in Sucrose Export from Leaves. Mol. Plant 2019, 12, 1278–1293. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H.B. Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Li, F.; Mai, C.; Liu, Y.; Deng, Y.; Wu, L.; Zheng, X.; He, H.; Huang, Y.; Luo, Z.; Wang, J. Soybean PHR1-regulated low phosphorus-responsive GmRALF22 promotes phosphate uptake by stimulating the expression of GmPTs. Plant Sci. 2024, 348, 112211. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Yu, Y.; Feng, L.; Jia, J.; Liu, B.; Li, B.; Guo, H.; Zhai, J. A comprehensive online database for exploring ~20,000 public Arabidopsis RNA-seq libraries. Mol. Plant 2020, 13, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, Y.; Liu, Y.; Mai, C.; Xu, Y.; Wu, J.; Zheng, X.; Liang, C.; Wang, J. Arabidopsis transcription factor WRKY45 confers cadmium tolerance via activating PCS1 and PCS2 expression. J. Hazard. Mater. 2023, 460, 132496. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Pan, X.; Jing, Y.; Zhao, Y.; Duan, Y.; Yang, J.; Wang, B.; Liu, Y.; Shen, R.; Cao, Y.; et al. ZmSPL10/14/26 are required for epidermal hair cell fate specification on maize leaf. New Phytol. 2021, 230, 1533–1549. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Q.; Yu, H.; Li, Q. Comparative Analysis of Promoter Activity in Crassostrea gigas Embryos: Implications for Bivalve Gene Editing. Mar. Biotechnol. 2024, 27, 20. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Kong, X.; Li, C.; Zhang, F.; Yu, Q.; Gao, S.; Zhang, M.; Tian, H.; Zhang, J.; Yuan, X.; Ding, Z. Ethylene promotes cadmium-induced root growth inhibition through EIN3 controlled XTH33 and LSU1 expression in Arabidopsis. Plant Cell Environ. 2018, 41, 2449–2462. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Yang, L.; Fan, C.; Hu, J.; Zheng, Y.; Wang, Z.; Liu, Y.; Xiao, X.; Yang, L.; Lei, T.; et al. Abscisic acid (ABA) alleviates cadmium toxicity by enhancing the adsorption of cadmium to root cell walls and inducing antioxidant defense system of Cosmos bipinnatus. Ecotoxicol. Environ. Saf. 2023, 261, 115101. [Google Scholar] [CrossRef]
- Yang, S.; Wu, P.; Jeyakumar, P.; Wang, H.; Zheng, X.; Liu, W.; Wang, L.; Li, X.; Ru, S. Technical solutions for minimizing wheat grain cadmium: A field study in North China. Sci. Total Environ. 2022, 818, 151791. [Google Scholar] [CrossRef]
- Wu, X.; Chen, L.; Lin, X.; Chen, X.; Han, C.; Tian, F.; Wan, X.; Liu, Q.; He, F.; Chen, L.; et al. Integrating physiological and transcriptome analyses clarified the molecular regulation mechanism of PyWRKY48 in poplar under cadmium stress. Int. J. Biol. Macromol. 2023, 238, 124072. [Google Scholar] [CrossRef] [PubMed]
- Maule, A.J.; Benitez-Alfonso, Y.; Faulkner, C. Plasmodesmata-membrane tunnels with attitude. Curr. Opin. Plant Biol. 2011, 14, 683–690. [Google Scholar] [CrossRef]
- Hao, P.; Wang, H.; Ma, L.; Wu, A.; Chen, P.; Cheng, S.; Wei, H.; Yu, S. Genome-wide identification and characterization of multiple C2 domains and transmembrane region proteins in Gossypium hirsutum. Research Square. 2020, 21, 445. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Nawaz, R.; Ahmad, S.; Irshad, M.A.; Irfan, A.; Qayyum, M.M.N.; Almaary, K.S.; Afzaal, M.; Ahmed, Z.; Bourhia, M. Effect of daily thinning on biweekly increment of growth and yield of maize (Zea mays L.) in mountainous agroecosystem. BMC Plant Biol. 2025, 25, 428. [Google Scholar]
- Wang, J.; Guo, J.; Li, G.; Lu, W.; Lu, D. Effects of different fertilizer combinations on the yield and nitrogen use efficiency of summer maize in Jiangsu Province, China. Sci. Rep. 2025, 15, 12458. [Google Scholar] [CrossRef]
- Liu, S.; Lei, X.; Gou, W.; Xiong, C.; Min, W.; Kong, D.; Wang, X.; Liu, T.; Ling, Y.; Ma, X.; et al. Genome-wide identification, characterization and expression analysis of key gene families in RNA silencing in centipede grass. BMC Genom. 2024, 25, 1139. [Google Scholar] [CrossRef]
- Ezoe, A.; Seki, M. Exploring the complexity of genome size reduction in angiosperms. Plant Mol. Biol. 2024, 114, 121. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Shi, Y.; Wang, H.; Zhang, Y.; Luo, F.; Li, Z.; Tian, Y.; Lu, X.; Pei, Z. Genome-wide identification and expression analysis of SMALL AUXIN UP RNA (SAUR) genes in rice (Oryza sativa). Plant Signal. Behav. 2024, 19, 2391658. [Google Scholar] [CrossRef]
- Peng, Y.; Guo, X.; Fan, Y.; Liu, H.; Sun, L.; Liu, D.; Li, H.; Wang, X.; Guo, H.; Lu, H. Identifying a cis-element in PtoCP1 promoter for efficiently controlling constitutive gene expression in Populus tomentosa. PeerJ 2024, 12, e18292. [Google Scholar] [CrossRef]
- Sghaier, N.; Ben Ayed, R.; Ben Marzoug, R.; Rebai, A. Dempster-Shafer Theory for the Prediction of Auxin-Response Elements (AuxREs) in Plant Genomes. Biomed Res. Int. 2018, 2018, 3837060. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, R.; Peng, Y.; Chen, J.; Zhu, X.; Xie, K.; Su, Q.; Huang, R.; Zhan, S.; Peng, D.; et al. Bioinformatic assessment and expression profiles of the AP2/ERF superfamily in the Melastoma dodecandrum genome. Int. J. Mol. Sci. 2023, 24, 16362. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Sui, X.; Xiao, J.; Tang, W.; Song, X.; Xu, Z.; Wang, D.; Xie, M.; Sun, B.; Tang, Y.; et al. ERD14 regulation by the HY5- or HY5-MED2 module mediates the cold signal transduction of asparagus bean. Plant J. 2025, 121, e17172. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhou, L.; Sheng, A.; Lin, L.; Liu, H. Comparative transcriptome analysis on drought stress-induced floral formation of Curcuma kwangsiensis. Plant Signal. Behav. 2022, 17, 2114642. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, G.; Li, F.; Chen, J.; Wu, Z.; Wang, T.; Ding, H.; Zhang, Z.; Qiu, F. Genome-Wide Identification and Phylogenetic Characterization of the FTIP Gene Family in Maize (Zea mays). Genes 2025, 16, 539. https://doi.org/10.3390/genes16050539
Lv G, Li F, Chen J, Wu Z, Wang T, Ding H, Zhang Z, Qiu F. Genome-Wide Identification and Phylogenetic Characterization of the FTIP Gene Family in Maize (Zea mays). Genes. 2025; 16(5):539. https://doi.org/10.3390/genes16050539
Chicago/Turabian StyleLv, Guihua, Fangjian Li, Jianjian Chen, Zhenxing Wu, Tingzhen Wang, Haiping Ding, Zhiming Zhang, and Fazhan Qiu. 2025. "Genome-Wide Identification and Phylogenetic Characterization of the FTIP Gene Family in Maize (Zea mays)" Genes 16, no. 5: 539. https://doi.org/10.3390/genes16050539
APA StyleLv, G., Li, F., Chen, J., Wu, Z., Wang, T., Ding, H., Zhang, Z., & Qiu, F. (2025). Genome-Wide Identification and Phylogenetic Characterization of the FTIP Gene Family in Maize (Zea mays). Genes, 16(5), 539. https://doi.org/10.3390/genes16050539





