Development and Application of a TaqMan-Based qPCR Assay for Detecting ENTV-2 in Goats
Abstract
:1. Background
2. Materials and Methods
2.1. DNA and RNA Extraction
2.2. Primers and Probe Design
2.3. Generation of DNA and RNA Standards
2.4. Development of RT-qPCR and qPCR Methods
2.5. Specificity and Sensitivity Analysis of ENTV-2 RT-qPCR and qPCR Assay
2.6. Analysis of Clinical Samples Using the ENTV-2 RT-qPCR Assay
2.7. Whole-Genome Sequencing and Genomic Analysis
3. Results
3.1. The High Specificity, Sensitivity, Repeatability, and Practicability of ENTV-2 RT-qPCR and qPCR Methods
3.2. Detection and Analysis of Clinical Samples
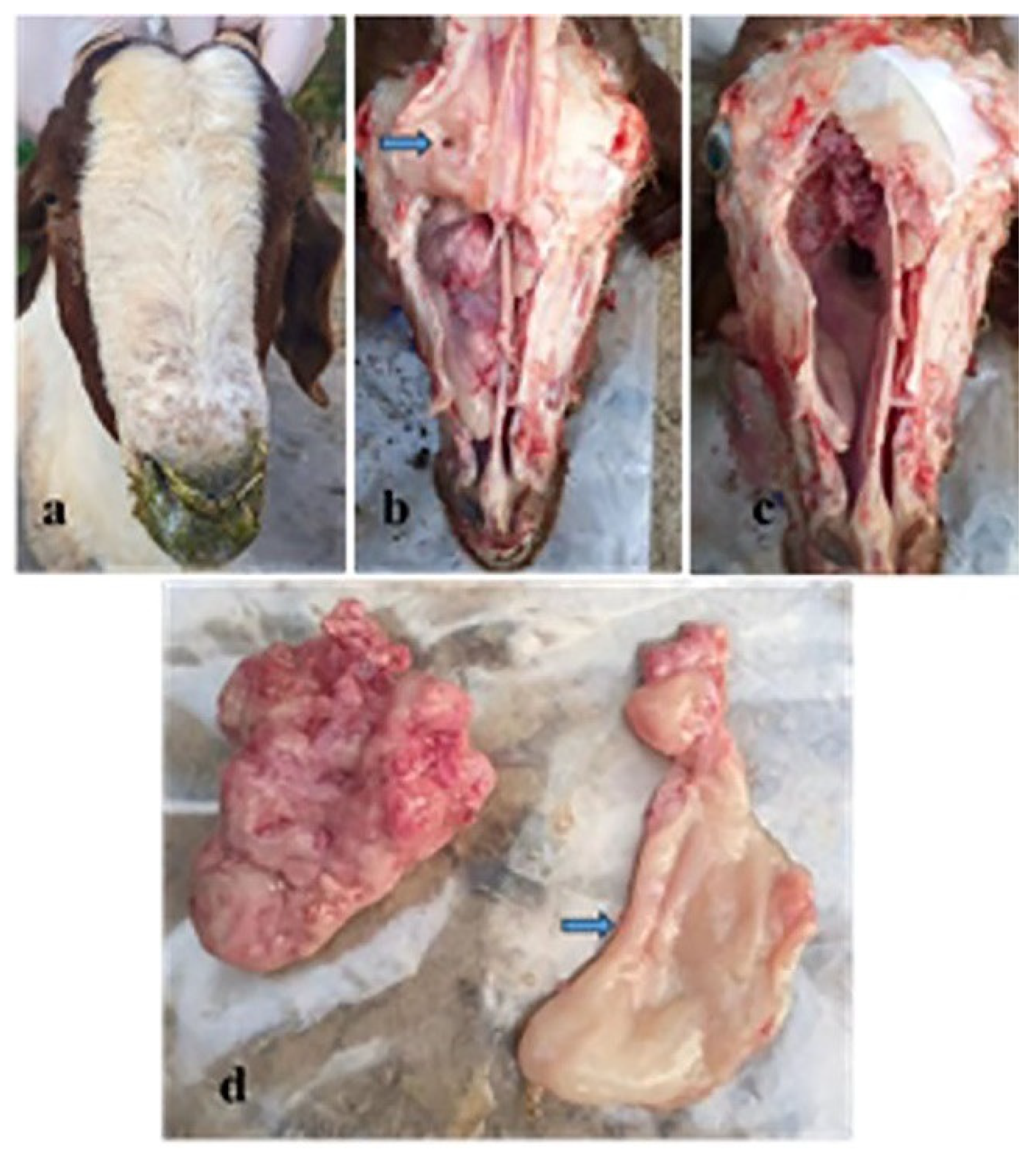
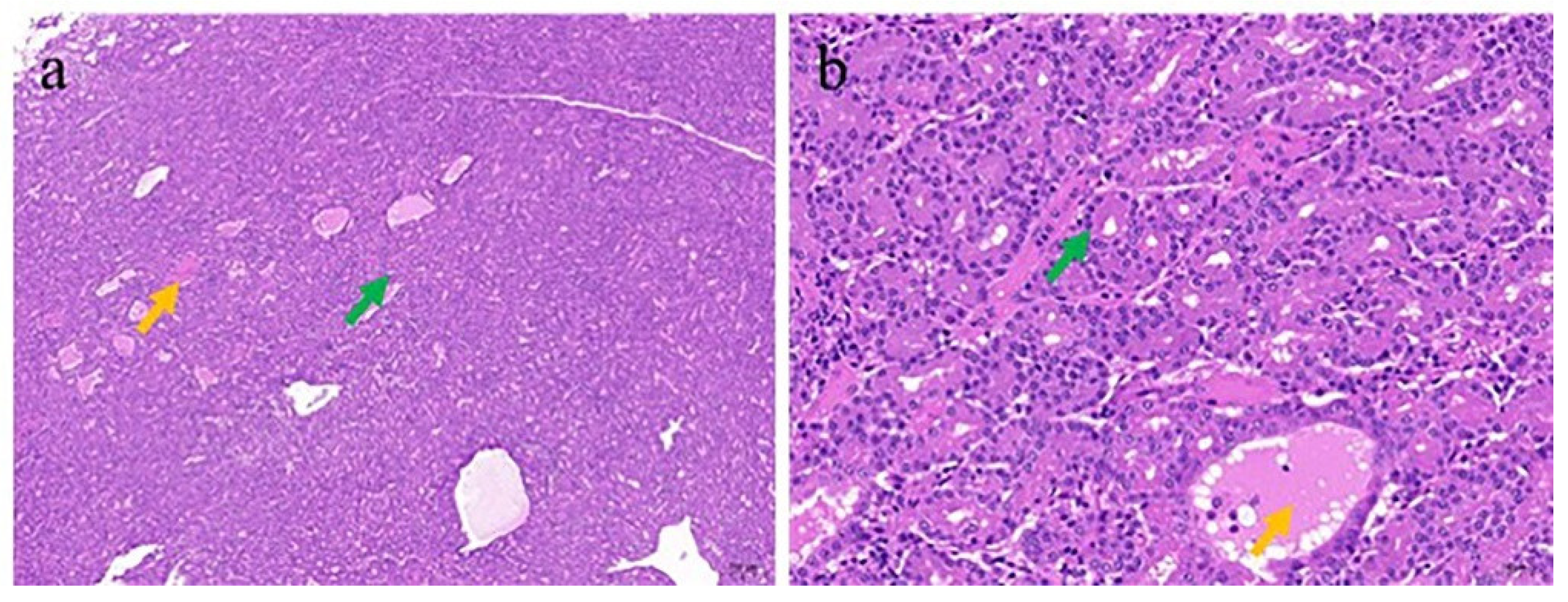
3.3. Pathologic and Electron Microscopic Findings of the ENA
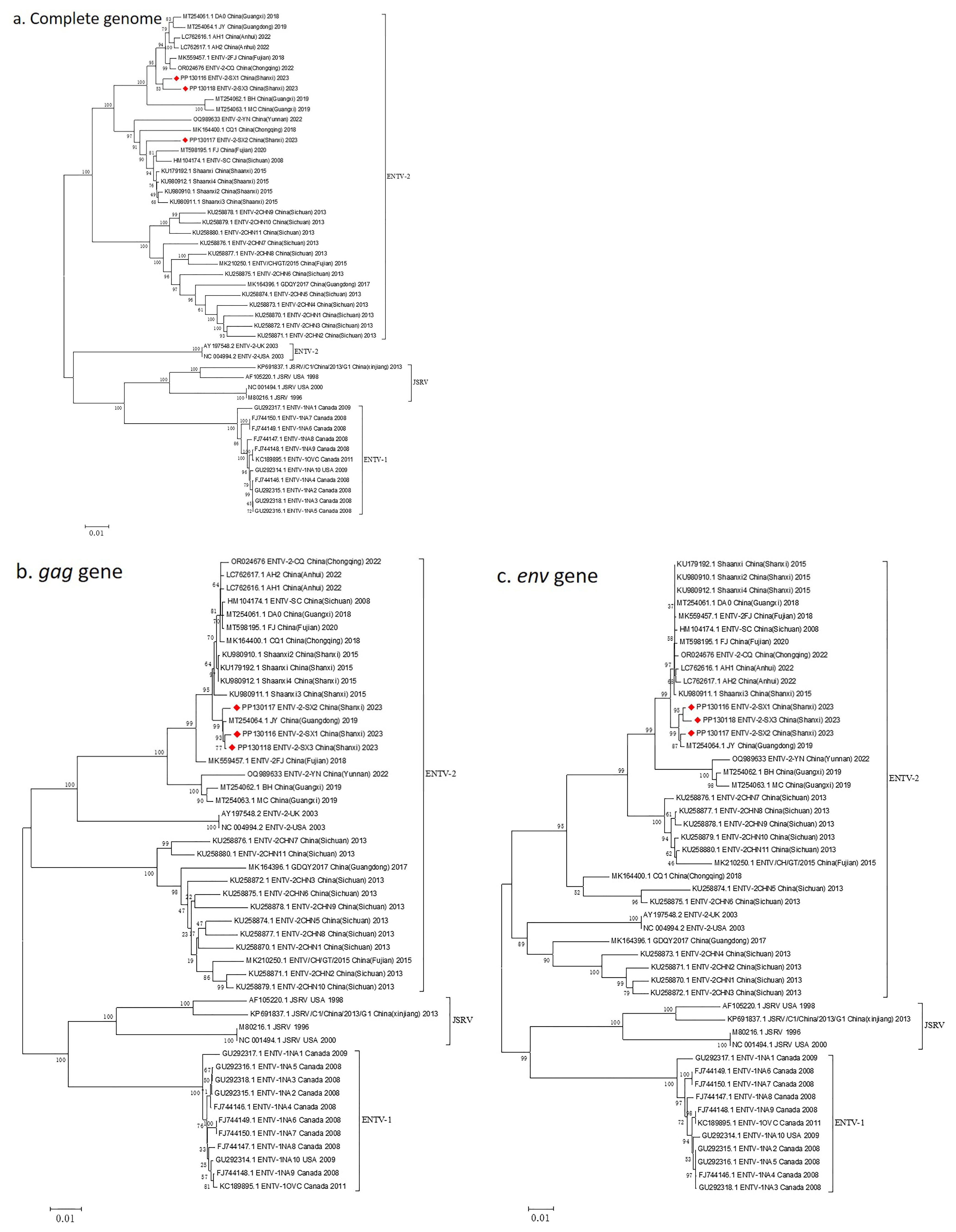
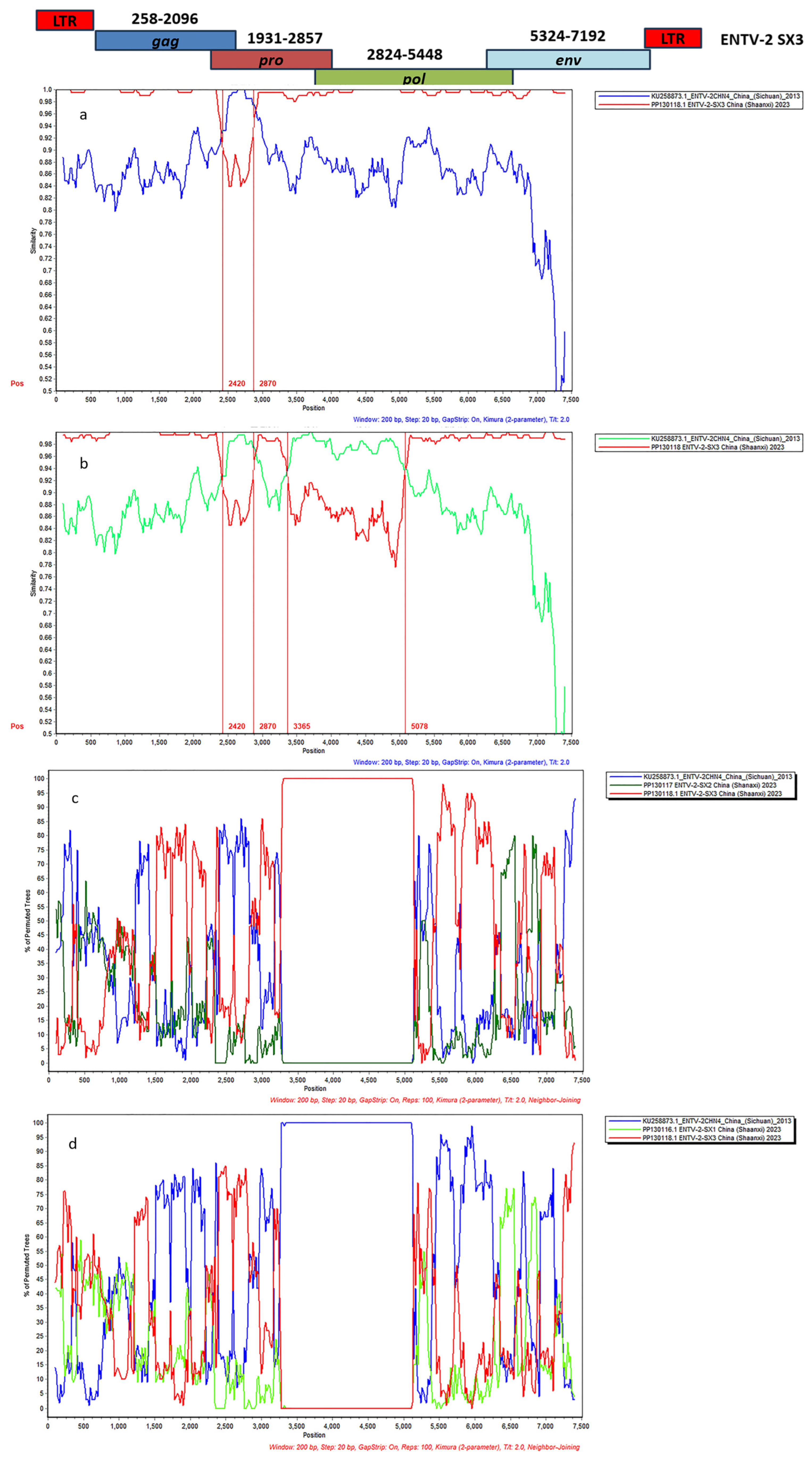
3.4. Complete Genome Characterization of ENTV-2 and Phylogenetic Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortin, A.; Cousens, C.; Minguijon, E.; Pascual, Z.; Villarreal, M.P.; Sharp, J.M.; Heras, M.L. Characterization of enzootic nasal tumour virus of goats: Complete sequence and tissue distribution. J. Gen. Virol. 2003, 84 Pt 8, 2245–2252. [Google Scholar] [CrossRef]
- Heras, M.D.L.; Ortín, A.; Cousens, C.; Minguijón, E.; Sharp, J. Enzootic nasal adenocarcinoma of sheep and goats. Curr. Top. Microbiol. Immunol. 2003, 275, 201–223. [Google Scholar] [PubMed]
- Svara, T.; Gombac, M.; Vrecl, M.; Juntes, P.; Kostanjsek, R.; Pogacnik, A.; Pogacnik, M. Enzootic Nasal Adenocarcinoma of Sheep in Slovenia. J. Vet. Med. Ser. A 2006, 53, 26–29. [Google Scholar] [CrossRef]
- Cousens, C.; Minguijon, E.; Dalziel, R.G.; Ortin, A.; Garcia, M.; Park, J.; Gonzalez, L.; Sharp, J.M.; de las Heras, M. Complete Sequence of Enzootic Nasal Tumor Virus, a Retrovirus Associated with Transmissible Intranasal Tumors of Sheep. J. Virol. 1999, 73, 3986–3993. [Google Scholar] [CrossRef]
- Cumer, T.; Pompanon, F.; Boyer, F. Old origin of a protective endogenous retrovirus (enJSRV) in the Ovis genus. Heredity 2018, 122, 187–194. [Google Scholar] [CrossRef] [PubMed]
- De las Heras, M.; Garcia de Jalon, J.A.; Sharp, J.M. Pathology of Enzootic Intranasal Tumor in Thirty-eight Goats. Vet. Pathol. 1991, 28, 474–481. [Google Scholar] [CrossRef]
- De las Heras, M.; García de Jalón, J.A.; Minguijón, E.; Gray, E.W.; Dewar, P.; Sharp, J.M. Experimental Transmission of Enzootic Intranasal Tumors of Goats. Vet. Pathol. 1995, 32, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Apostolidi, E.D.; Psalla, D.; Chassalevris, T.; Chaintoutis, S.C.; Giadinis, N.D.; Psychas, V.; Dovas, C.I. Development of real-time PCR-based methods for the detection of enzootic nasal tumor virus 2 in goats. Arch. Virol. 2019, 164, 707–716. [Google Scholar] [CrossRef]
- Wang, B.; Ye, N.; Cao, S.-j.; Wen, X.-t.; Huang, Y.; Yan, Q.-G. Identification of novel and differentially expressed MicroRNAs in goat enzootic nasal adenocarcinoma. BMC Genom. 2016, 17, 896. [Google Scholar] [CrossRef]
- Sid, N.; Belalmi, N.E.H.; Benhamza, L.; Ouhida, S.; Zebiri, M.E.; Aydoğan, A.; Leroux, C. First case report of enzootic nasal adenocarcinoma in “Ouled Djellal” ewe in Algeria. Open Vet. J. 2018, 8, 9–12. [Google Scholar] [CrossRef]
- de Cecco, B.S.; Lorenzett, M.P.; Henker, L.C.; Weber, M.N.; Moséna, A.C.S.; Baumbach, L.; Canal, C.W.; Driemeier, D.; Pavarini, S.P.; Sonne, L. Detection of enzootic nasal tumor virus (ENTV) in a sheep flock in southern Brazil. Trop. Anim. Health Prod. 2019, 51, 2095–2098. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, N.M.; Yousef, S.G.; Youssef, C.R.B.; Goyal, S.M. Molecular detection of mixed infection with peste des petits ruminants and retroviruses in Egyptian sheep and goats. Trop. Anim. Health Prod. 2023, 55, 102. [Google Scholar] [CrossRef]
- Ye, C.; Huang, Q.; Chen, T.; Jiang, J.; Hou, F.; Xu, D.; Peng, Y.; Fang, R.; Chen, J. First detection and genotypic analysis of goat enzootic nasal tumor virus 2 in Chongqing, China. Arch. Virol. 2019, 164, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Cao, X.; Wu, J.; Liu, X.; Mao, S.; Yuan, L.; Shang, Y. Detection and analysis of enzootic nasal tumor virus 2 in China. J. Vet. Diagn. Investig. 2025, 37, 244–251. [Google Scholar] [CrossRef]
- Zhai, S.L.; Lv, D.H.; Xu, Z.H.; Yu, J.S.; Wen, X.H.; Zhang, H.; Chen, Q.L.; Jia, C.L.; Zhou, X.R.; Zhai, Q.; et al. A Novel Enzootic Nasal Tumor Virus Circulating in Goats from Southern China. Viruses 2019, 11, 956. [Google Scholar] [CrossRef]
- Lee, C.; Kim, J.; Shin, S.G.; Hwang, S. Absolute and relative qPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 2006, 123, 273–280. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Li, R.; Liu, L.; Yuan, W. Rapid and sensitive detection of canine distemper virus by real-time reverse transcription recombinase polymerase amplification. BMC Vet. Res. 2017, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.R.; Linnerth-Petrik, N.M.; Yu, D.L.; Foster, R.A.; Menzies, P.I.; Diaz-Méndez, A.; Chalmers, H.J.; Wootton, S.K. Experimental transmission of enzootic nasal adenocarcinoma in sheep. Vet. Res. 2013, 44, 66. [Google Scholar] [CrossRef]
- Müllhaupt, G.; Enzler-Tschudy, A.; Horg, K.; Bubendorf, L.; Pratsinis, M.; Schmid, H.-P.; Abt, D. Informative value of histological assessment of tissue acquired during aquablation of the prostate. World J. Urol. 2020, 39, 2043–2047. [Google Scholar] [CrossRef]
- Macías Sánchez, K.L.; González Martínez, H.D.R.; Carrera Cerritos, R.; Martínez Espinosa, J.C. In Vitro Evaluation of the Antifungal Effect of AgNPs on Fusarium oxysporum f. sp. lycopersici. Nanomaterials 2023, 13, 1274. [Google Scholar] [CrossRef]
- Perez Contreras, A.; van der Meer, F.; Checkley, S.; Joseph, T.; King, R.; Ravi, M.; Peters, D.; Fonseca, K.; Gagnon, C.A.; Provost, C.; et al. Analysis of Whole-Genome Sequences of Infectious laryngotracheitis Virus Isolates from Poultry Flocks in Canada: Evidence of Recombination. Viruses 2020, 12, 1302. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Q.; Wang, J.; Zhou, M.; Fu, M.; Xu, X. Full-length genome sequence analysis of enzootic nasal tumor virus isolated from goats in China. Virol. J. 2017, 14, 141. [Google Scholar] [CrossRef]
- Palmarini, M.; Sharp, J.M.; de las Heras, M.; Fan, H. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 1999, 73, 6964–6972. [Google Scholar] [CrossRef]
- Cuevas-Romero, S.; Blomström, A.-L.; Alvarado, A.; Hernández-Jauregui, P.; Rivera-Benitez, F.; Ramírez-Mendoza, H.; Berg, M. Development of a real-time RT-PCR method for detection of porcine rubulavirus (PoRV-LPMV). J. Virol. Methods 2013, 189, 1–6. [Google Scholar] [CrossRef]
- Nandhini, G.; Sujatha, S.; Jain, N.; Dhodapkar, R.; Kadhiravan, T.; Krishnamurthy, S. Poor performance characteristics of conventional PCR in detection of respiratory syncytial virus-experience of a tertiary care centre in Southern India. Indian J. Med. Microbiol. 2015, 33, 274–276. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Du, Y.; Gan, L.; Mohsin, M.A.; He, B.X. Development of a SYBR Green-based real-time quantitative polymerase chain reaction assay to detect enzootic nasal tumor virus in goats. Can. J. Vet. Res. 2021, 85, 145–150. [Google Scholar]
- Huang, Q.; Ye, C.; Chen, T.; Jiang, J.; Peng, Y.; Chen, J.; Fang, R. EvaGreen-based real-time PCR assay for sensitive detection of enzootic nasal tumor virus 2. Mol. Cell. Probes 2019, 44, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Heras, M.D.L.; Jalón, J.A.G.d.; Balaguer, L.; Badiola, J.J. Retrovirus-like particles in enzootic intranasal tumours in Spanish goats. Vet. Rec. 1988, 123, 135. [Google Scholar] [CrossRef]
- Incili, C.A.; Eroksuz, Y.; Timurkan, M.O.; Karabulut, B.; Yerlikaya, Z.; Eroksuz, H. Simultaneous Occurrence of Nasal Carcinoma Induced by Enzootic Nasal Tumour Virus-2 (ENTV-2) and Border Disease Virus in Three Goats. Vet. Med. Sci. 2025, 11, e70325. [Google Scholar] [CrossRef]
- Heras, M.d.l.; Minguijon, E.; Ferrer, L.M.; Ortin, A.; Dewar, P.; Cebrian, L.M.; Pascual, Z.; Garcia, L.; Garcia de Jalon, J.A.; Sharp, J.M. Naturally occurring enzootic nasal tumor of sheep in Spain: Pathology and associated retrovirus. Eur. J. Vet. Pathol. 1998, 4, 11–15. [Google Scholar]
- McKinnon, A.O.; Thorsen, J.; Hayes, M.A.; Misener, C.R. Enzootic nasal adenocarcinoma of sheep in Canada. Can. Vet. J. = La. Rev. Vet. Can. 1982, 23, 88–94. [Google Scholar]
- Li, Y.; Niu, J.; Liu, Y.; Dai, Y.; Ni, H.; Wang, J.; Fang, R.; Ye, C. Genomic Sequencing and Analysis of Enzootic Nasal Tumor Virus Type 2 Provides Evidence for Recombination within the Prevalent Chinese Strains. Vet. Sci. 2024, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Horváth, K.B.; Boros, Á.; Kálmán, E.; Pankovics, P.; Delwart, E.; Reuter, G. Characterization of an integrated, endogenous mouse mammary tumor virus-like (MMTV) betaretrovirus genome in a black Syrian hamster (Mesocricetus auratus). Infect. Genet. Evol. 2019, 75, 103995. [Google Scholar] [CrossRef] [PubMed]

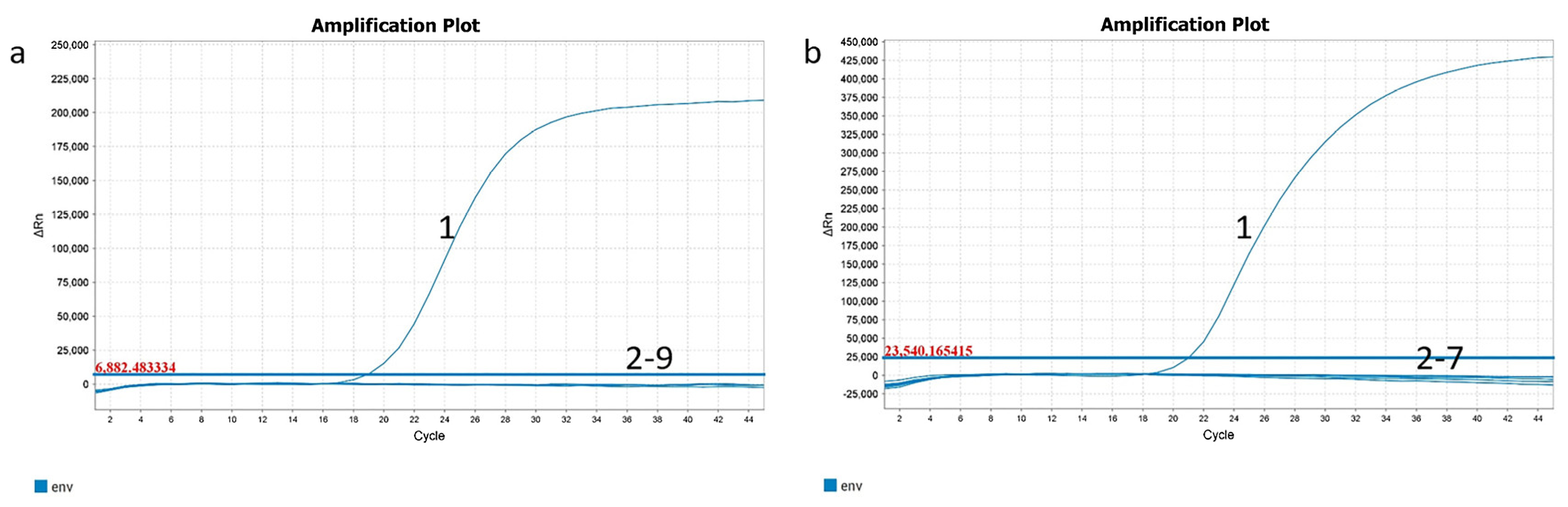
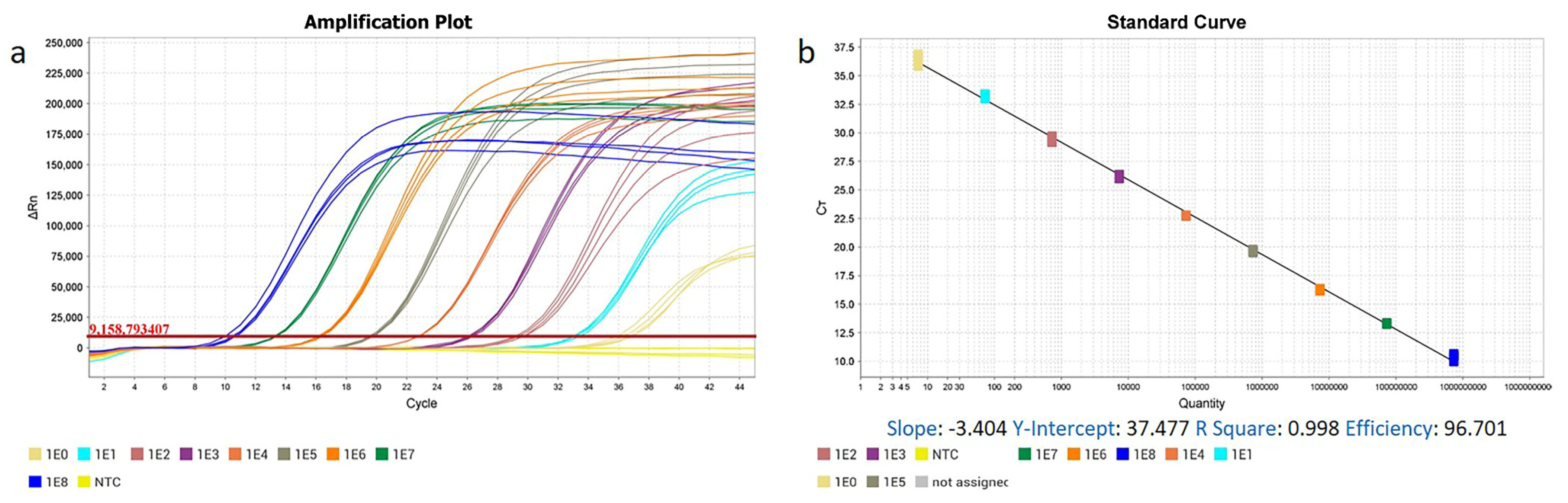
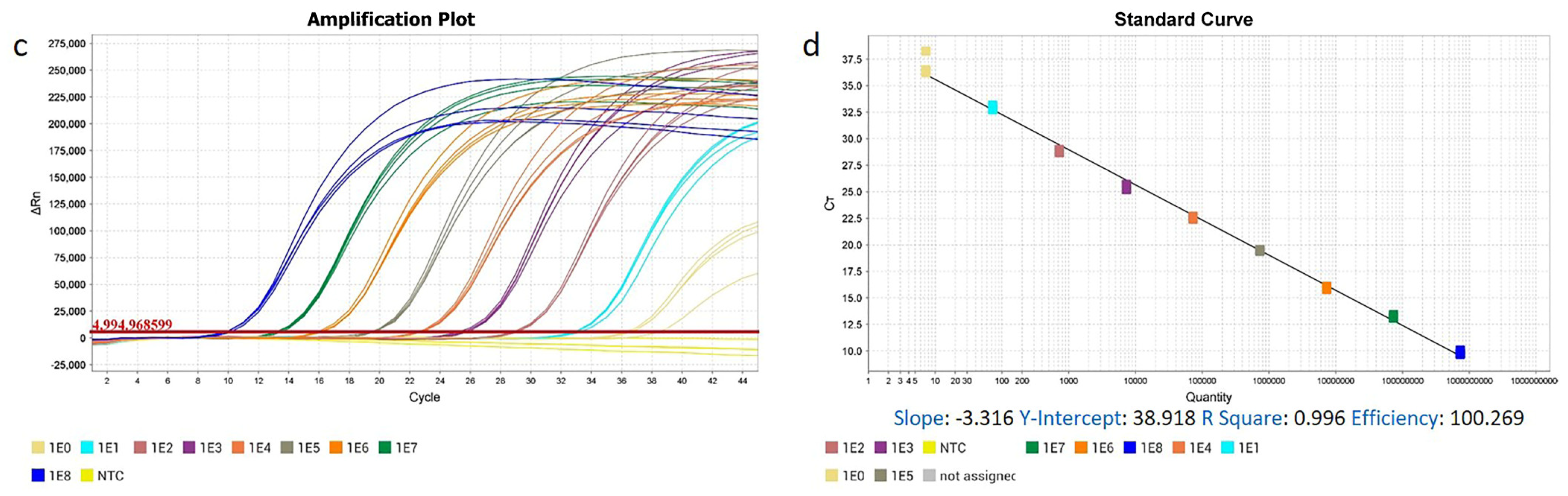
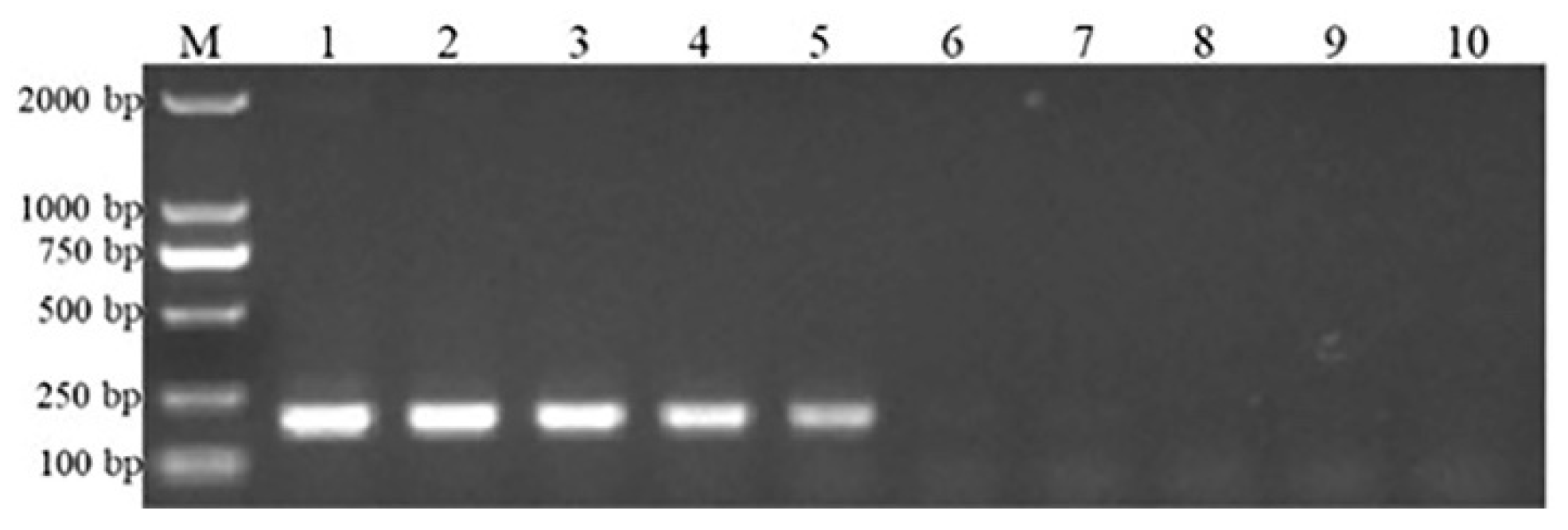

| Type | Copy Number | Ct (Mean ± S.D.) | CV% | Type | Copy Number | Ct (Mean ± S.D.) | CV% |
|---|---|---|---|---|---|---|---|
| Standard RNA a | 2.73 × 108 | 8.986 ± 0.058 | 0.946 | Standard DNA b | 7.28 × 108 | 9.813 ± 0.184 | 1.875 |
| 2.73 × 107 | 12.208 ± 0.012 | 0.098 | 7.28 × 107 | 13.212 ± 0.147 | 1.113 | ||
| 2.73 × 106 | 15.467 ± 0.042 | 0.268 | 7.28 × 106 | 15.989 ± 0.143 | 0.894 | ||
| 2.73 × 105 | 18.904 ± 0.312 | 1.650 | 7.28 × 105 | 19.478 ± 0.069 | 0.354 | ||
| 2.73 × 104 | 22.288 ± 0.081 | 0.363 | 7.28 × 104 | 22.587 ± 0.118 | 0.522 | ||
| 2.73 × 103 | 25.398 ± 0.950 | 3.740 | 7.28 × 103 | 25.503 ± 0.229 | 0.898 | ||
| 2.73 × 102 | 29.224 ± 0.281 | 0.962 | 7.28 × 102 | 28.862 ± 0.131 | 0.454 | ||
| 2.73 × 101 | 32.896 ± 0.110 | 0.334 | 7.28 × 101 | 32.877 ± 0.193 | 0.587 | ||
| 2.73 × 100 | 36.023 ± 0.450 | 1.249 | 7.28 × 100 | 36.855 ± 0.949 | 2.575 | ||
| NC | None | None | NC | None | None |
| Age | Sex | Number of Samples | Number of Positive Samples (%) |
|---|---|---|---|
| 8 years old | ♀ | 1 | 0 (0%) |
| 7 years old | ♀ | 11 | 0 (0%) |
| 6 years old | ♂ | 1 | 0 (0%) |
| ♀ | 29 | 1 (3.4%) | |
| 5 years old | ♂ | 1 | 0 (0%) |
| ♀ | 38 | 1 (2.6%) | |
| 4 years old | ♂ | 4 | 0 (0%) |
| ♀ | 18 | 0 (0%) | |
| 3 years old | ♂ | 7 | 0 (0%) |
| ♀ | 57 | 7 (12.3%) | |
| 2 years old | ♂ | 24 | 1 (4.2%) |
| ♀ | 91 | 17 (18.5%) | |
| 1 year old | ♂ | 14 | 0 (0%) |
| ♀ | 36 | 0 (0%) | |
| 11 months old | ♂ | 93 | 17 (18.3%) |
| ♀ | 133 | 14 (10.5%) |
| Sample | Goat 1 | Goat 2 | Goat 3 |
|---|---|---|---|
| Nasal swab | 3.82 × 105 | 1.57 × 105 | 1.08 × 105 |
| Tumor | 2.26 × 107 | 7.34 × 107 | 1.90 × 107 |
| Lymph node | 1.76 × 101 | 2.93 × 102 | 2.96 × 101 |
| Trachea | 4.94 × 104 | 4.87 × 104 | 4.77 × 104 |
| Heart | 4.59 × 101 | 1.01 × 102 | 9.34 × 101 |
| Liver | 9.86 × 101 | 2.15 × 102 | 1.16 × 102 |
| Spleen | 2.76 × 101 | 4.72 × 101 | 5.08 × 101 |
| Lung | 5.98 × 101 | 4.43 × 102 | 5.26 × 101 |
| Kidney | None | None | None |
| Blood | 9.11 × 100 | 1.46 × 101 | 2.46 × 101 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Yin, H.; Cao, X.; Lan, X.; Wu, J.; He, J.; Yuan, L.; Shang, Y. Development and Application of a TaqMan-Based qPCR Assay for Detecting ENTV-2 in Goats. Genes 2025, 16, 529. https://doi.org/10.3390/genes16050529
Li P, Yin H, Cao X, Lan X, Wu J, He J, Yuan L, Shang Y. Development and Application of a TaqMan-Based qPCR Assay for Detecting ENTV-2 in Goats. Genes. 2025; 16(5):529. https://doi.org/10.3390/genes16050529
Chicago/Turabian StyleLi, Pengfei, Haike Yin, Xiaoan Cao, Xi Lan, Jinyan Wu, Jijun He, Ligang Yuan, and Youjun Shang. 2025. "Development and Application of a TaqMan-Based qPCR Assay for Detecting ENTV-2 in Goats" Genes 16, no. 5: 529. https://doi.org/10.3390/genes16050529
APA StyleLi, P., Yin, H., Cao, X., Lan, X., Wu, J., He, J., Yuan, L., & Shang, Y. (2025). Development and Application of a TaqMan-Based qPCR Assay for Detecting ENTV-2 in Goats. Genes, 16(5), 529. https://doi.org/10.3390/genes16050529






