Genotoxicity in Unconventional Mammalian Models of Wild, Urban, and Agricultural Ecosystems: A Systematic Review Under the One Health Approach
Abstract
1. Introduction
1.1. Mutagenesis and Genotoxicity Are Part of the Development of Cancer and Other Diseases
1.2. Test Guidelines for Genetic Toxicology (OECD)
2. Non-Conventional Mammals and Their Use in Genotoxicity Assays
2.1. Wildlife Non-Human Primates and Its Relation to Human and Ecosystem Health
2.2. Evaluating the Genotoxicity in Wildlife Species: The Non-Human Primates
2.3. Evaluating the Genotoxicity in Agro Ecosystems: The Cattle
2.3.1. Characteristics of the Agricultural Environment and the Meat Production System
2.3.2. Genotoxicity In Vitro Studies of Xenobiotics in Bovine Somatic Cells
2.3.3. Genotoxicity Studies in Bovine Cells of Chemicals Used in Food Production
2.3.4. Genotoxicity Studies in Bovines Used for Environmental Biomonitoring
2.4. Evaluating the Genotoxicity in Urban Life: The Dogs
3. In Vivo and In Vitro Tests, Selection of Doses and Concentrations to Be Used
4. The Present and Projections of Tests for Genetic Toxicology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Acronyms/Abbreviations
| BMCyt | Buccal Micronucleus Cytome assay |
| BNMN | Binucleated cells with micronuclei |
| CA | Chromosomal aberrations |
| CBMN | Cytokinesis Block Micronucleus assay |
| CIN | Chromosomal instability |
| COPD | Chronic nonspecific pulmonary disease |
| CP | Cyclophosphamide |
| CPF | Chlorpyrifos |
| CYP | Cypermethrin |
| DL-PCBs | Dioxin-like polychloro biphenyls |
| DL-PCDFs | Dioxin-like polychloro dibenzofurans |
| DNA-dsb | DNA double-strand breaks |
| DSB | Double strand breaks |
| EFSA | European Food Safety Authority |
| EPA | Environmental Protection Agency |
| FDA | Food and Drug Administration |
| FISH | Fluorescence in situ hybridization |
| ID | Index damage |
| IPCS | International Programme on Chemical Safety |
| MI | Mitotic index |
| MN | Micronuclei |
| MNE | Micronucleated normochromatic erythrocyte |
| MNU | N-Methyl Nitrosourea |
| MRLs | Maximum Residue Limits |
| MTZ | Metronidazole |
| NAMs | New Approach Methodologies |
| NBuds | Nuclear buds |
| NHPs | Non-human primates |
| NPBs | Nucleoplasmic bridges |
| NWM | New World Monkeys |
| OECD | Organization for Economic Cooperation and Development |
| ONZ | Ornidazole |
| OWM | Old World Monkeys |
| PCBs | Polychlorinated Biphenyls |
| PCDDs | Polychlorodibenzodioxins |
| PI | Proliferation Index |
| Ret | Reticulocytes |
| SCE | Sister Chromatid Exchange |
| SCGE | Single Cell Gel Electrophoresis |
| TG | Test Guideline for Genetic Toxicology |
| UDS | Unscheduled DNA Synthesis |
| VICH | International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products |
| WCP | Whole Chromosome Painting Probes |
References
- Dertinger, S.D.; Totsuka, Y.; Bielas, J.H.; Doherty, A.T.; Kleinjans, J.; Honma, M.; Marchetti, F.; Schuler, M.J.; Thybaud, V.; White, P. High information content assays for genetic toxicology testing: A report of the International Workshops on Genotoxicity Testing (IWGT). Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 847, 403022. [Google Scholar] [CrossRef] [PubMed]
- Gollapudi, B.B.; Krishna, G. Practical aspects of mutagenicity testing strategy: An industrial perspective. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2000, 455, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sundar, R.; Jain, M.R.; Valani, D. Mutagenicity testing: Regulatory guidelines and current needs. In Mutagenicity: Assays and Applications; Academic Press: Cambridge, MA, USA, 2018; pp. 191–228. [Google Scholar]
- Omori, T.; Hayashi, M. The assessment and communication of genotoxicity test results: Moving beyond binary. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2024, 893, 503722. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Baker, T.M.; Waise, S.; Tarabichi, M.; Van Loo, P. Aneuploidy and complex genomic rearrangements in cancer evolution. Nat. Cancer 2024, 5, 228–239. [Google Scholar] [CrossRef]
- Amoiridis, M.; Verigos, J.; Meaburn, K.; Gittens, W.H.; Ye, T.; Neale, M.J.; Soutoglou, E. Inhibition of topoisomerase 2 catalytic activity impacts the integrity of heterochromatin and repetitive DNA and leads to interlinks between clustered repeats. Nat. Commun. 2024, 15, 5727. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in Understanding Oxidative Stress, Aging, and Aging-Related Diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Brennan, P. Gene-environment interactions and aethiology of cancer: What does it mean and how can we measure it? Carcinogenesis 2002, 23, 381–387. [Google Scholar] [CrossRef]
- Cai, Y.; Song, W.; Li, J.; Jing, Y.; Liang, C.; Zhang, L.; Zhang, X.; Zhang, W.; Liu, B.; An, Y.; et al. The landscape of aging. Sci. China Life Sci. 2022, 65, 2354–2454. [Google Scholar]
- Slatter, M.A.; Gennery, A.R. Update on DNA-Double Strand Break Repair Defects in Combined Primary Immunodeficiency. Curr. Allergy Asthma Rep. 2020, 20, 57. [Google Scholar] [CrossRef]
- Alomair, A.; Alamri, A.; Shaik, J.; Aljafari, S.; Ba Abdullah, M.; Alanazi, M. Association between polymorphisms of the DNA repair genes RAD51 and OGG1 and risk of cardiovascular disease. Mol. Med. Rep. 2024, 29, 53. [Google Scholar] [CrossRef] [PubMed]
- Proukakis, C. Somatic mutations in neurodegeneration: An update. Neurobiol. Dis. 2020, 144, 105021. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, B.; Snyder, R.D.; Waters, M.D.; Benz, R.D.; Kemper, R.A.; Tice, R.R.; Richard, A.M. Genetic toxicology in the 21st century: Reflections and future directions. Environ. Mol. Mutagen. 2011, 52, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Lionetto, M.G.; Caricato, R.; Giordano, M.E. Pollution biomarkers in environmental and human biomonitoring. Open Biomark. J. 2019, 9, 1–9. [Google Scholar]
- Gantchev, J.; Ramchatesingh, B.; Berman-Rosa, M.; Sikorski, D.; Raveendra, K.; Amar, L.; Xu, H.H.; Villarreal, A.M.; Ordaz, D.J.G.; Litvinov, I.V. Tools used to assay genomic instability in cancers and cancer meiomitosis. J. Cell Commun. Signal. 2022, 16, 159–177. [Google Scholar] [CrossRef]
- OECD Organisation for Economic Co-operation and Development. Overview of the Set of OECD Genetic Toxicology Test Guidelines and Updates Performed in 2014–2015, 2nd ed.; OECD Series on Testing and Assessment, No. 238; OECD Publishing: Paris, France, 2017. [Google Scholar] [CrossRef]
- OECD Organisation for Economic Co-operation and Development. Test No. 490: In Vitro Mammalian Cell Gene Mutation Tests Using the Thymidine Kinase Gene, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2015. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 476: In Vitro Mammalian Cell Gene Mutation Tests Using the Hprt and Xprt Genes, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2015. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 473: In Vitro Mammalian Chromosomal Aberration Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2014. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 487: In Vitro Mammalian Cell Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2014. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 488: Transgenic Rodent Somatic and Germ Cell Gene Mutation Assays, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2013. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 474: Mammalian Erythrocyte Micronucleus Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2014. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 475: Mammalian Bone Marrow Chromosomal Aberration Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2014. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 478: Rodent Dominant Lethal Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2015. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 483: Mammalian Spermatogonial Chromosomal Aberration Test, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2015. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 485: Genetic Toxicology, Mouse Heritable Translocation Assay, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 1986. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 486: Unscheduled DNA Synthesis (UDS) Test with Mammalian Liver Cells In Vivo, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 1997. [Google Scholar]
- OECD Organisation for Economic Co-operation and Development. Test No. 489: In Vivo Mammalian Alkaline Comet Assay, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2014. [Google Scholar]
- Mohamed, S.A.K.S.; Sabita, U.; Rajendra, S.; Raman, D. Genotoxicity: Mechanisms, testing guidelines and methods. Glob. J. Pharm. Pharm. Sci. 2017, 1, 133–138. [Google Scholar]
- Yauk, C.L.; Aardema, M.J.; van Benthem, J.; Bishop, J.B.; Dearfield, K.L.; DeMarini, D.M.; Dubrova, Y.E.; Honma, M.; Lupski, J.R.; Marchetti, F.; et al. Approaches for identifying germ cell mutagens: Report of the 2013 IWGT workshop on germ cell assays. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015, 783, 36–54. [Google Scholar] [CrossRef]
- Wultsch, G.; Nersesyan, A.; Kundi, M.; Fenech, M.; Eibensteiner, F.; Mišík, M.; Knasmüller, S. Use of micronucleus cytome assays with buccal cells for the detection of genotoxic effects: A systematic review and meta-analysis of occupational exposures to metals. Mutat. Res. Rev. 2024, 794, 108510. [Google Scholar] [CrossRef]
- Ferré, D.M.; Carracedo, R.T.; Pedrosa, A.; Caliri, M.N.; Gorla, N.B.M. Domestic bovines as potential environmental bioindicators: Analysis of oral epithelium and application in the micronucleus assay. Rev. Vet. 2024, 35, 22–29. [Google Scholar] [CrossRef]
- Gorla, N.B.M. Los animales centinelas son mucho más que un faro vigía. In De Relaciones Tóxicas a un Vínculo Amoroso: El Ambiente, los Animales y Nosotros; Editorial de la Universidad Nacional de Cuyo, colección “Indagaciones”: Mendoza, Argentina, 2023; pp. 21–66. ISBN 978-950-39-0405-3. [Google Scholar]
- Reif, J.S. Animal sentinels for environmental and public health. Public Health Rep. 2011, 126 (Suppl. S1), 50–57. [Google Scholar] [CrossRef]
- Sexton, C.; Ruple, A. Canine sentinels and our shared exposome. Science 2024, 384, 1170–1172. [Google Scholar] [CrossRef]
- Ferré, D.M.; Gorla, N.B.M. De Relaciones Tóxicas a un Vínculo Amoroso: El Ambiente, los Animales y Nosotros; Colección Indagaciones, Editorial Universidad Nacional de Cuyo: Mendoza, Argentina, 2023; p. 167. [Google Scholar]
- ECHA European Chemicals Agency. Guidance on Information Requirements and Chemical Safety Assessment: Chapter, R.7a: Endpoint Specific Guidance, Version 6; ECHA European Chemicals Agency: Helsinki, Finland, 2017. [Google Scholar]
- Luan, Y.; Honma, M. Genotoxicity testing and recent advances. Genome Instab. Dis. 2022, 3, 1–21. [Google Scholar] [CrossRef]
- Groves, C.P.; Napier, J.R. “Primate”. Encyclopedia Britannica, 2025. Available online: https://www.britannica.com/animal/primate-mammal (accessed on 25 January 2025).
- Estrada, A.; Garber, P.A.; Rylands, A.B.; Roos, C.; Fernandez-Duque, E.; Di Fiore, A.; Nekaris, K.A.-I.; Nijman, V.; Heymann, E.W.; Lambert, J.E.; et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 2017, 3, e1600946. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishna, S.; Sengupta, A. What does human-animal studies have to offer ethology? Acta Ethol. 2020, 23, 193–199. [Google Scholar] [CrossRef]
- García de la Chica, A.; Oklander, L.I.; Kowalewski, M.M.; Fernandez-Duque, E. Human and non-human primate coexistence in Argentina: Conflicts and solutions. Animals 2023, 13, 3331. [Google Scholar] [CrossRef] [PubMed]
- Wildlife Conservation Society. One World-One Health Manhattan Principles; Wildlife Conservation Society: Bronx, NY, USA, 2004; Available online: http://www.wcs.org/conservation-challenges/wildlife-health/wildlife-humans-and-livestock/one-world-one-health.aspx (accessed on 15 January 2025).
- Cibot, M.; McLennan, M.R.; Kváč, M.; Sak, B.; Asiimwe, C.; Petrželková, K. Sparse Evidence for Giardia intestinalis, Cryptosporidium spp. and Microsporidia Infections in Humans, Domesticated Animals and Wild Nonhuman Primates Sharing a Farm–Forest Mosaic Landscape in Western Uganda. Pathogens 2021, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.M.; Salzer, J.S.; Deutsch, J.C.; Raño, M.; Kuhlenschmidt, M.S.; Gillespie, T.R. Black and gold howler monkeys (Alouatta caraya) as sentinels of ecosystem health: Patterns of zoonotic protozoa infection relative to degree of human–primate contact. Am. J. Primatol. 2011, 73, 75–83. [Google Scholar] [CrossRef]
- Cantarelli, V.I.; Perez-Rueda, M.A.; Kowalewski, M.M.; Mastromonaco, G.F.; Ponzio, M.F. Validation of an enzyme immunoassay and comparison of fecal cortisol metabolite levels in black and gold howler monkeys (Alouatta caraya) inhabiting fragmented and continuous areas of the humid Chaco region, Argentina. Am. J. Primatol. 2017, 79, e22625. [Google Scholar] [CrossRef]
- de Azevedo Fernandes, N.C.; Guerra, J.M.; Díaz-Delgado, J.; Cunha, M.; del C Saad, L.; Iglezias, S.D.; Ressio, R.A.; dos Santos Cirqueira, C.; Kanamura, C.T.; Jesus, I.P.; et al. Differential yellow fever susceptibility in new world nonhuman primates, comparison with humans, and implications for surveillance. Emerg. Infect. Dis. 2021, 27, 47. [Google Scholar] [CrossRef]
- Devaux, C.A.; Mediannikov, O.; Medkour, H.; Raoult, D. Infectious disease risk across the growing human-non human primate interface: A review of the evidence. Front. Public Health 2019, 7, 305. [Google Scholar] [CrossRef]
- McLennan, M.R.; Spagnoletti, N.; Hockings, K.J. The implications of primate behavioral flexibility for sustainable human–primate coexistence in anthropogenic habitats. Int. J. Primatol. 2017, 38, 105–121. [Google Scholar] [CrossRef]
- Matzenbacher, C.A.; Da Silva, J.; Garcia, A.L.H.; Cappetta, M.; de Freitas, T.R. Anthropogenic effects on natural mammalian populations: Correlation between telomere length and coal exposure. Sci. Rep. 2019, 9, 6325. [Google Scholar] [CrossRef] [PubMed]
- Benvindo-Souza, M.; Hosokawa, A.V.; Dos Santos, C.G.A.; de Assis, R.A.; Pedroso, T.M.A.; Borges, R.E.; Pacheco, S.M.; de Souza Santos, L.R.; e Silva, D.d.M. Evaluation of genotoxicity in bat species found on agricultural landscapes of the Cerrado savanna, central Brazil. Environ. Pollut. 2022, 293, 118579. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.D.R.; Benvindo-Souza, M.; Assis, R.A.; Dos Santos, C.G.A.; de Lima Amorim, N.P.; Borges, R.E.; de Melo, C.; de Souza Santos, L.R. Micronucleus and different nuclear abnormalities in wild birds in the Cerrado, Brazil. Environ. Sci. Pollut. Res. 2022, 29, 14279–14287. [Google Scholar] [CrossRef]
- Chilton, J.A.; Laing, S.T.; Bradley, A. Animal Models in Toxicologic Research: Nonhuman Primate. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Academic Press: Cambridge, MA, USA, 2022; pp. 777–809. [Google Scholar]
- de Souza, A.B.; Rodrigues, R.P.; Pessoa, G.T.; da Silva, A.; Moura, L.S.; Sousa, F.C.; da Silva, E.G.; Diniz, A.N.; Barbosa, M.A.P.S.; Araújo, J.R.; et al. Standard electrocardiographic data from capuchin monkeys (Cebus apella, Linnaeus, 1758). J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 13–17. [Google Scholar]
- Rogers, J.; Raveendran, M.; Harris, R.A.; Mailund, T.; Leppälä, K.; Athanasiadis, G.; Schierup, M.H.; Cheng, J.; Munch, K.; Walker, J.A.; et al. The comparative genomics and complex population history of Papio baboons. Sci. Adv. 2019, 5, eaau6947. [Google Scholar] [CrossRef] [PubMed]
- do Monte Barretto, M.L.; de Albuquerque, P.P.F.; de Souza Costa, J.B.; Leal, S.G.; Paim, A.P.S.; da Fonseca Oliveira, A.A. Concentrations of iron and chromium in free-ranging common marmosets (Callithrix jacchus) from Pernambuco, Brazil. Environ. Monit. Assess. 2023, 195, 895. [Google Scholar] [CrossRef]
- Morris, S.M.; Dobrovolsky, V.N.; Shaddock, J.G.; Mittelstaedt, R.A.; Bishop, M.E.; Manjanatha, M.G.; Shelton, S.D.; Doerge, D.R.; Twaddle, N.C.; Chen, J.J.; et al. The genetic toxicology of methylphenidate hydrochloride in non-human primates. Mutat. Res. 2009, 673, 59–66. [Google Scholar] [CrossRef]
- Borrell, A.; Ponsa, M.; Egozcue, J.; Rubio, A.; Garcia, M. Chromosome abnormalities in peripheral blood lymphocytes from Cebus apella (Cebidae, Platyrrhini) after X-ray irradiation. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 401, 65–76. [Google Scholar] [CrossRef]
- Borrell, A.; Ponsa, M.; Egozcue, J.; Rubio, A.; Garcia, M. Chromosome abnormalities in peripheral blood lymphocytes from Macaca fascicularis and Erythrocebus patas (Cercopithecidae, Catarrhini) after X-ray irradiation. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 403, 185–198. [Google Scholar] [CrossRef]
- Hotchkiss, C.E.; Bishop, M.E.; Dertinger, S.D.; Slikker, W., Jr.; Moore, M.M.; MacGregor, J.T. Flow cytometric analysis of micronuclei in peripheral blood reticulocytes IV: An index of chromosomal damage in the rhesus monkey (Macaca mulatta). Toxicol. Sci. 2008, 102, 352–358. [Google Scholar] [CrossRef]
- Zúñiga-González, G.M.; Gómez-Meda, B.C.; Zamora-Perez, A.L.; Ramos-Ibarra, M.L.; Batista-González, C.M.; Lemus-Varela, M.L.; Rodríguez-Ávila, J.L.; Gallegos-Arreola, M.P. Micronucleated erythrocyte frequencies in old and new world primates: Measurement of micronucleated erythrocyte frequencies in peripheral blood of Callithrix jacchus as a model for evaluating genotoxicity in primates. Environ. Mol. Mutagen. 2005, 46, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Correa, R.M.D.S.; Mota, T.C.; Guimarães, A.C.; Bonfim, L.T.; Burbano, R.R.; Bahia, M.D.O. Cytotoxic and genotoxic effects of fluconazole on African green monkey kidney (Vero) cell line. BioMed Res. Int. 2018, 1, 6271547. [Google Scholar] [CrossRef]
- Matos, L.A.; Alcântara, D.F.A.; Sousa, J.M.C.; Ribeiro, H.F.; Leal, M.F.; Muniz, J.A.P.C.; Imbeloni, A.A.; Smith, M.A.C.; Burbano, R.R.; Bahia, M.O. Evaluation of the Biochemical, Hematological and Genotoxic Parameters of Cebus apella Treated with N-Methyl-Nitrosurea (NMU) Followed by Treatment with a Complex Homeopathic Compound (Canova). J. Biother. 2016, 2, e2. [Google Scholar]
- Nieves, M.; Puntieri, F.; Bailey, S.M.; Mudry, M.D.; Maranon, D.G. Exploring the Relationship between Spontaneous Sister Chromatid Exchange and Genome Instability in Two Cryptic Species of Non-Human Primates. Animals 2023, 13, 510. [Google Scholar] [CrossRef]
- Puntieri, F.; Andrioli, N.B.; Nieves, M. Association between genomic instability and evolutionary chromosomal rearrangements in Neotropical Primates. Genome Biol. Evol. 2018, 10, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Mudry, M.D.; Martinez, R.A.; Nieves, M.; Carballo, M.A. Biomarkers of genotoxicity and genomic instability in a non-human primate, Cebus libidinosus (Cebidae, Platyrrhini), exposed to nitroimidazole derivatives. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 721, 108–113. [Google Scholar] [CrossRef]
- Fundia, A.; Gorostiaga, M.; Mudry, M. Expression of common fragile sites in two Ceboidea species: Saimiri boliviensis and Alouatta caraya (Primates: Platyrrhini). Genet. Sel. Evol. 2000, 32, 87–97. [Google Scholar] [CrossRef]
- Gomes, L.M.; Moysés, D.A.; Nascimento, H.F.; Mota, T.C.; Bonfim, L.T.; Cardoso, P.C.; Burbano, R.M.R.; Bahia, M.O. Genotoxic and cytotoxic effects of the drug dipyrone sodium in African green monkey kidney (Vero) cell line exposed in vitro. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1529–1535. [Google Scholar] [CrossRef]
- Seo, J.E.; Davis, K.; Malhi, P.; He, X.; Bryant, M.; Talpos, J.; Burks, S.; Mei, N.; Guo, X. Genotoxicity evaluation using primary hepatocytes isolated from rhesus macaque (Macaca mulatta). Toxicology 2021, 462, 152936. [Google Scholar] [CrossRef]
- Redón, C.E.; Nakamura, A.J.; Gouliaeva, K.; Rahman, A.; Blakely, W.F.; Bonner, W.M. The use of gamma-H2AX as a biodosimeter for total-body radiation exposure in non-human primates. PLoS ONE 2010, 5, e15544. [Google Scholar] [CrossRef]
- Fundia, A.F.; Mudry, M. Inducción de sitios frágiles en Cebus apella. Bol. Primatológico Argent. 1987, 5, 7–12. [Google Scholar]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Reprinted by UFAW, 1992: 8 Hamilton Close, South Mimms, Potters Bar, Herts EN6 3QD England; Methuen: London, UK, 1959; p. 238. [Google Scholar]
- Páez, C. Caracterización de los Cambios Estructurales y Dinámicos del Genoma de Sapajus Cay Como Respuesta a la Exposición In Vitro a Bleomicina. Bachelor Thesis, Universidad Nacional de San Martin, Buenos Aires, Argentina, 2022; p. 29. [Google Scholar]
- Nieves, M.; Páez, R.C.; Ferreras, E.O.; Ortuño Orgaz, M.N.; Andrioli, N.B. Analysis of the in vitro response of the Sapajus cay (Primates: Platyrrhini) genome to exposure to the radiomimetic bleomycin. Comp. Biochem. Physiol. 2025. submitted. [Google Scholar]
- Nieves, M.; Mendez, G.; Ortiz, A.; Mühlmann, M.; Mudry, M.D. Karyological diagnosis of Cebus (Primates, Platyrrhini) in captivity: Detection of hybrids and management program applications. Anim. Reprod. Sci. 2008, 108, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Nieves, M.; Remis, M.I.; Sesarini, C.; Hassel, D.L.; Argüelles, C.F.; Mudry, M.D. Assessment of genetic variability in captive capuchin monkeys (Primates: Cebidae). Sci. Rep. 2021, 11, 7306. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, A.G.; Ayala, M.I.; Fernández, C.B.; Ferreras, E.O.; Manzur, T.; Nieves, M.; Bolzán, A.D. Efecto de los agroquímicos tiabendazol y zineb sobre los telómeros de Macaca fascicularis (Catarrhini, Cercophitecidae, Primates). JBAG 2023, MCTA 4, 198. [Google Scholar]
- Ferreras, E.O.; Ayala, M.I.; Fernández, C.B.; Cardozo, A.G.; Kubickova, S.; Vozdova, M.; Manzur, T.; Bolzán, A.D.; Andrioli, N.B.; Nieves, M. Estudio comparativo de inestabilidad genómica por efecto de la bleomicina en Macaca fascicularis y Sapajus cay (Primates). JBAG 2023, MCTA 3, 198. [Google Scholar]
- Castellano, R.C. Will the great primates be recognized as non-human persons in the 21st century? Stoa 2024, 15, 45–59. [Google Scholar] [CrossRef]
- Vermeire, T.; Epstein, M.; Hoet, P.; Krätke, R.; Testai, E.; Badin, R.A.; Flecknell, P.; Hudson-Shore, M.; Jones, D.; Langermans, J.A.; et al. Final Opinion on the Need for Non-Human Primates in Biomedical Research, Production and Testing of Products and Devices, Update 2017; Scientific Committee on Health, Environmental and Emerging Risks (SCHEER), European Commission: Brussels, Belgium, 2017; Available online: https://ec.europa.eu/health/sites/health/files/scientific_committees/scheer/docs/scheer_o_004.pdf (accessed on 10 March 2021).
- Lee, K.H.; Lee, D.W.; Kang, B.C. The ‘R’principles in laboratory animal experiments. Lab. Anim. Res. 2020, 36, 45. [Google Scholar] [CrossRef]
- Johansen, M.D.; Irving, A.; Montagutelli, X.; Tate, M.D.; Rudloff, I.; Nold, M.F.; Hansbro, N.G.; Kim, R.Y.; Donovan, C.; Liu, G. Animal and translational models of SARS-CoV-2 infection and COVID. Mucosal Immunol. 2020, 13, 877–891. [Google Scholar] [CrossRef]
- Gao, C.E.; Song, Q.; Zhang, M.; Li, J.; Miao, Y.; Li, Z.; Dong, J. Generation, ex vivo expansion and safety of engineered PD1-knockout primary T cells from cynomolgus macaques. Mol. Immunol. 2020, 124, 100–108. [Google Scholar] [CrossRef]
- Grow, D.A.; McCarrey, J.R.; Navara, C.S. Advantages of nonhuman primates as preclinical models for evaluating stem cell-based therapies for Parkinson’s disease. Stem Cell Res. 2016, 17, 352–366. [Google Scholar] [CrossRef]
- Mudry, M.D.; Nieves, M.; Steinberg, E.R. Cytogenetics of howler monkeys. In Howler Monkeys: Adaptive Radiation, Systematics, and Morphology; Springer: New York, NY, USA, 2015; pp. 85–105. [Google Scholar]
- Nieves, M.; Steinberg Eliana, R.; Fantini, L.; Hassel, D.L.; Bruno, G.A.; Mudry, M.D. Primates from the Inside: Genomes and Chromosomes; La Primatología en Argentina, I., Kowalewski, M., Oklander, L.I., Eds.; SAREM: Istanbul, Türkiye, 2017; pp. 69–85. ISBN 987-98497-1-X. [Google Scholar]
- Steinberg, E.R.; Bressa, M.J.; Mudry, M.D. Sex chromosome systems in Neotropical Primates: What have we learnt so far from cytogenetics and genomics? J. Evol. Biol. 2022, 35, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Hug, M.G. Evaluación Productiva de Sistemas de Cría Bovina en Función de la Tecnología Aplicada en la Región Centro-Sur de Corrientes. Magister Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2018. [Google Scholar]
- Greenwood, P.L. Review: An overview of beef production from pasture and feedlot globally, as demand for beef and the need for sustainable practices increase. Anim. Int. J. Anim. Biosci. 2021, 15, 100295. [Google Scholar] [CrossRef]
- Veneciano, J.H.; Frasinelli, C.A. Cría y Recría de Bovinos. Sitio Argentino de Producción Animal. 2014. Available online: https://www.produccion-animal.com.ar/informacion_tecnica/cria/177-TextoCriaRecria.pdf (accessed on 16 January 2025).
- Udroiu, I.; Sgura, A. Cytogenetic tests for animal production: State of the art and perspectives. Anim. Genet. 2017, 48, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Holečková, B.; Schwarzbacherová, V.; Galdíková, M.; Koleničová, S.; Halušková, J.; Staničová, J.; Verebová, V.; Jutková, A. Chromosomal Aberrations in Cattle. Genes 2021, 12, 1330. [Google Scholar] [CrossRef]
- Iannuzzi, A.; Parma, P.; Iannuzzi, L. Chromosome.Abnormalities and Fertility in Domestic Bovids: A Review. Animals 2021, 11, 802. [Google Scholar] [CrossRef]
- Lioi, M.B.; Santoro, A.; Barbieri, R.; Salzano, S.; Ursini, M.V. Ochratoxin A and zearalenone: A comparative study on genotoxic effects and cell death induced in bovine lymphocytes. Mutat. Res. 2004, 557, 19–27. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzi, L.; De Giovanni, A.; Malagutti, L.; Molteni, L.; Sciaraffia, F.; Tamburini, A.; Zannotti, M. Genotoxic activity of the Fumonisin B1 mycotoxin in cultures of bovine lymphocytes. Ital. J. Anim. Sci. 2005, 4, 395–402. [Google Scholar] [CrossRef]
- Ali, M.M.; Sahar, T.; Firyal, S.; Ijaz, M.; Majeed, K.A.; Awan, F.; Adil, M.; Akbar, H.; Imran Rashid, M.; Hakki Ciğerci, İ. Assessment of Cytotoxic, Genotoxic, and Oxidative Stress of Dibutyl Phthalate on Cultured Bovine Peripheral Lymphocytes. Oxidative Med. Cell. Longev. 2022, 2022, 9961513. [Google Scholar] [CrossRef]
- Šutiaková, I.; Kovalkovičová, N.; Šutiak, V. Micronucleus assay in bovine lymphocytes after exposure to bisphenol A in vitro. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 502–506. [Google Scholar] [CrossRef]
- Föllmann, W.; Birkner, S. The Use of Cultured Primary Bovine Colon Epithelial Cells as a Screening Model to Detect Genotoxic Effects of Heterocyclic Aromatic Amines in the Comet Assay. J. Toxicol. Environ. Health Part A Curr. Issues 2008, 71, 947–953. [Google Scholar] [CrossRef]
- Piešová, E.; Šiviková, K.; Dianovský, J.; Holečková, B. The induction of micronuclei into bovine lymphocyte cultures exposed to cyclohexanone. Folia Vet. 2003, 47, 161–163. [Google Scholar]
- Piešová, E.; Šiviková, K. The induction of micronuclei in bovine lymphocytes by exposure to benzene and S-9 mix. Ann. Agric. Environ. Med. 2003, 10, 261–263. [Google Scholar] [PubMed]
- Šiviková, K.; Holečková, B.; Dianovský, J. Chromosome damage induced by benzene after the use of conventional and FISH chromosome painting. Neoplasma 2005, 52, 1. [Google Scholar]
- Ferré, D.M.; Jotallán, P.J.; Lentini, V.R.; Ludueña, H.R.; Romano, R.R.; Gorla, N.B.M. Biomonitoring of the hematological, biochemical and genotoxic effects of the mixture cypermethrin plus chlorpyrifos applications in bovine. Sci. Total Environ. 2020, 726, 138058. [Google Scholar] [CrossRef]
- Stupáková, K.; Galdíková, M.; Schwarzbacherová, V.; Holečková, B. Analysis of chromosomal damage caused by acetamiprid. Folia Vet. 2019, 63, 25–29. [Google Scholar] [CrossRef]
- Ficová, I.; Galdíková, M. Testing the potential clastogenic/cytotoxic effects of pesticide Calypso 480 SC. Folia Vet. 2017, 61, 47–51. [Google Scholar] [CrossRef]
- Galdíková, M.; Šiviková, K.; Holečková, B.; Dianovský, J.; Mesarč, M. Use of FISH technique with whole chromosome painting probes for the detection of chromosomal aberrations induced by positive mutagens in bovine peripheral lymphocytes. Caryologia 2011, 64, 427–434. [Google Scholar] [CrossRef]
- Orosová, M.; Holečková, B.; Šiviková, K.; Dianovský, J. Effect of fungicide euparen multi (tolylfluanid) on the induction of chromosomal aberrations in cultivated bovine lymphocytes. Acta Biol. Hung. 2010, 61, 411–422. [Google Scholar] [CrossRef]
- Holečková, B.; Dianovský, J.; Šivíková, K. Effect of pesticides on cultivated lymphocytes of cattle. Ekológia Veterinárna Medicína 2008, 7, 146–151. [Google Scholar]
- Holečková, B. Evaluation of the in vitro effect of glyphosate-based herbicide on bovine lymphocytes using chromosome painting. Bull. Vet. Inst. Pulawy 2006, 50, 533–536. [Google Scholar]
- Holečková, B. The detection of chromosome aberrations by the fish method in bovine peripheral lymphocytes after in vitro glyphophosate-based herbicide exposure. Folia Vet. 2005, 49, 182–185. [Google Scholar]
- Holečková, B.; Dianovský, J.; Šiviková, K. Cytogenetic effect of pesticides on cultivated peripheral lymphocytes of cattle. Folia Vet. 2009, 53, 83–86. [Google Scholar]
- Holečková, B.; Šiviková, K.; Dianovský, J.; Galdíková, M. Effect of triazole pesticide formulation on bovine culture cells. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2013, 48, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Dianovský, J.; Šiviková, K.; Holečková, B. Apoptosis and cytogenetic effect induced by tolylfluanid fungicide. J. Agrobiol. 2008, 25, 85–87. [Google Scholar]
- Šiviková, K.; Dianovský, J. Cytogenetic effect of technical glyphosate on cultivated bovine peripheral lymphocytes. Int. J. Hyg. Environ. Health 2006, 209, 15–20. [Google Scholar] [CrossRef]
- Šiviková, K.; Holečková, B.; Schwarzbacherová, V.; Galdíková, M.; Dianovský, J. Potential chromosome damage, cell-cycle kinetics/and apoptosis induced by epoxiconazole in bovine peripheral lymphocytes in vitro. Chemosphere 2018, 193, 82–88. [Google Scholar] [CrossRef]
- Šiviková, K.; Dianovský, J.; Holečková, B.; Galdíková, M.; Kolesárová, V. Assessment of cytogenetic damage in bovine peripheral lymphocytes exposed to in vitro tebuconazole-based fungicide. Chemosphere 2013, 92, 555–562. [Google Scholar] [CrossRef]
- Galdíková, M.; Holečková, B.; Šiviková, K.; Schwarzbacherová, V.; Koleničová, S. Evaluating the genotoxic damage in bovine whole blood cells in vitro after exposure to thiacloprid. Toxicol. Vitr. 2019, 61, 104616. [Google Scholar] [CrossRef]
- Drážovská, M.; Šiviková, K.; Holečková, B.; Dianovský, J.; Galdíková, M.; Schwarzbacherová, V. Evaluation of potential genotoxic/cytotoxic effects induced by epoxiconazole and fenpropimorph-based fungicide in bovine lymphocytes in vitro. J. Environ. Sci. Health Part B 2016, 51, 769–776. [Google Scholar] [CrossRef]
- Galdíková, M.; Šiviková, K.; Holečková, B.; Dianovský, J.; Drážovská, M.; Schwarzbacherová, V. The effect of thiacloprid formulation on DNA/chromosome damage and changes in GST activity in bovine peripheral lymphocytes. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2015, 50, 698–707. [Google Scholar] [CrossRef]
- Siviková, K.; Dianovský, J.; Holečková, B. Induction of SCEs and DNA fragmentation in bovine peripheral lymphocytes by in vitro exposure to tolylfluanid-based fungicide. Genet. Mol. Biol. 2011, 34, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Šiviková, K.; Dianovský, J.; Holečková, B.; Galdíková, M.; Mesarč, M. Evaluation of cytogenetic effects on bovine peripheral lymphocytes after the treatment with tebuconazole. Egypt. Acad. J. Biolog. Sci. 2010, 2, 1–4. [Google Scholar]
- Ferré, D.M.; Ludueña, H.R.; Romano, R.R.; Gorla, N.B.M. Evaluation of the genotoxic potential of cypermethrin, chlorpyrifos and their subsequent mixture, on cultured bovine lymphocytes. Chemosphere 2020, 243, 125341. [Google Scholar] [CrossRef] [PubMed]
- Anchordoquy, J.M.; Anchordoquy, J.P.; Nikoloff, N.; Gambaro, R.; Padula, G.; Seoane, A.; Furnus, C. Doramectin induced cytotoxic and genotoxic effects on bovine peripheral lymphocytes and cumulus cells in vitro. J. Environ. Sci. Health Part B 2019, 54, 147–154. [Google Scholar] [CrossRef]
- Schwarzbacherová, V.; Wnuk, M.; Deregowska, A.; Holečková, B.; Lewinska, A. In vitro exposure to thiacloprid-based insecticide formulation promotes oxidative stress, apoptosis and genetic instability in bovine lymphocytes. Toxicol. Vitr. 2019, 61, 104654. [Google Scholar] [CrossRef]
- Anchordoquy, J.P.; Anchordoquy, J.M.; Nikoloff, N.; Gambaro, R.; Padula, G.; Furnus, C.; Seoane, A. Cytotoxic and genotoxic effects induced by enrofloxacin-based antibiotic formulation Floxagen® in two experimental models of bovine cells in vitro: Peripheral lymphocytes and cumulus cells. Environ. Sci. Pollut. Res. 2018, 26, 2998–3005. [Google Scholar] [CrossRef]
- Schwarzbacherová, V.; Šiviková, K.; Drážovská, M.; Dianovský, J. Evaluation of DNA damage and cytotoxicity induced by triazole fungicide in cultured bovine lymphocytes. Caryol. Int. J. Cytol. Cytosystematics Cytogenet. 2015, 68, 233–238. [Google Scholar] [CrossRef]
- Ramos-Ibarra, M.; Villa-Castellanos, J.; Barba-León, J.; Flores-Valdez, M.; Zavala-Aguirre, L.; Torres Bugarín, O. Estudio exploratorio de la genotoxicidad de vacunas recombinantes para tuberculosis bovina. Abanico Vet. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Schnabel, K.; Schmitz, R.; Frahm, J.; Meyer, U.; Breves, G.; Dänicke, S. Functionality and DNA-damage properties of blood cells in lactating cows exposed to glyphosate contaminated feed at different feed energy levels. Arch. Anim. Nutr. 2020, 74, 87–106. [Google Scholar] [CrossRef] [PubMed]
- PAHO-Pan American Health Organization; WHO-World Health Organization. Las Funciones Esenciales de la Salud Pública en las Américas una Renovación para el Siglo XXI. Marco Conceptual y Descripción; Organización Panamericana de la Salud: Washington, DC, USA, 2020; ISBN 978-92-75-32265-9. [Google Scholar]
- CCRVDF Comité del Codex sobre Residuos de Medicamentos Veterinarios en los Alimentos. Límites Máximos de Residuos (LMR) y Recomendaciones Sobre la Gestión de Riesgos (rgr) Para Residuos de Medicamentos Veterinarios en los Alimentos CXM 2–202. 2023. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXM%2B2%252FMRL2s.pdf (accessed on 16 January 2025).
- VICH, International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products. VICH GL 23: Studies to Evaluate the Safety of Residues of Veterinary Drugs in Human Food: Genotoxicity Testing. 2015. Available online: https://www.ema.europa.eu/en/vich-gl23-studies-evaluate-safety-residues-veterinary-drugs-human-food-genotoxicity-testing (accessed on 17 January 2025).
- EFSA European Food Safety Authority. Opinion of the Scientific Panel on Plant Protection Products and Their Residues to Evaluate the Suitability of Existing Methodologies and, if Appropriate, the Identification of New Approaches to Assess Cumulative and Synergistic Risks from Pesticides to Human Health with a View to Set MRLs for Those Pesticides in the Frame of Regulation (EC) 396. EFSA J. 2008, 704, 1–84. [Google Scholar]
- Moreno, L.; Lanusse, C. Chapter 23 Veterinary Drug Residues in Meat-Related Edible Tissues. PART III The Current Qualities of Consumer and Public Perceptions. In New Aspects of Meat Quality; Woodhead Publishing: Sawston, UK, 2017; pp. 581–603. [Google Scholar] [CrossRef]
- Cristaldi, M.; Ieradi, L.A.; Udroiu, I.; Zilli, R. Comparative evaluation of background micronucleus frequencies in domestic mammals. Mutat. Res. 2004, 559, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Glazko, T.T.; Astaf’eva, E.E.; Pheophilov, A.V.; Kushnir, A.V.; Stolpovskii, Y.A.; Glazko, V.I. Intrabreed genetic differentiation of local breeds of mongolian cattle and small stock under different ecogeographic breeding conditions. Russ. Agric. Sci. 2012, 38, 133–136. [Google Scholar] [CrossRef]
- Balode, Z. Assessment of radio-frequency electromagnetic radiation by the micronucleus test in Bovine peripheral erythrocytes. Sci. Total Environ. 1996, 180, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Sato, I.; Sasaki, J.; Satoh, H.; Natsuhori, M.; Murata, T.; Okada, K. Assessments of DNA Damage and Radiation Exposure Dose in Cattle Living in the Contaminated Area Caused by the Fukushima Nuclear Accident. Bull. Environ. Contam. Toxicol. 2020, 105, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Picco, S.J.; Fazzio, L.D.; Rosa, D.; Pintos, M.E.; Furnus, C.C.; Dulout, F.N.; Mattioli, G.A. Alteraciones oxidativas y daño en el ADN en bovinos con hipocuprosis. Analecta Vet. 2006, 25, 11–17. [Google Scholar]
- Bissett, W.; Smith, R.; Adams, L.G.; Field, R.; Moyer, W.; Phillips, T.; Scott, H.M.; Thompson, J.A. Geostatistical analysis of biomarkers of genotoxicity in cattle, Bos taurus and Bos taurus × Bos indicus, sentinels near industrial facilities. Ecotoxicology 2009, 18, 87–93. [Google Scholar] [CrossRef]
- Shekhar, S.; Sahoo, A.K.; Dalai, N.; Chaudhary, P.; Praveen, P.K.; Saikhom, R.; Rai, R. Chromosome analysis of arsenic affected cattle. Vet. World 2014, 7, 859–862. [Google Scholar] [CrossRef]
- Di Meo, G.P.; Perucatti, A.; Genualdo, V.; Caputi-Jambrenghi, A.; Rasero, R.; Nebbia, C.; Iannuzzi, L. Chromosome fragility in dairy cows exposed to dioxins and dioxin-like PCBs. Mutagenesis 2011, 26, 269–272. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, C.M.; Kim, S.R.; Kim, J.C.; Bae, C.S.; Oh, K.S.; Jo, S.K.; Kim, T.H.; Jong-Sik, J.; Kim, S.H. The micronucleus frequency in cytokinesis-blocked lymphocytes of cattle in the vicinity of a nuclear power plant. J. Vet. Sci. 2007, 8, 117–120. [Google Scholar] [CrossRef]
- Kadmiri, M.; Glouib, K.; Verschaeve, L.; Hilali, A. Cytogenetic monitoring of domestic mammals exposed to wastewaters from the localities of Dladla and Boukallou near Settat, Morocco. Environ. Int. 2006, 32, 690–696. [Google Scholar] [CrossRef]
- US Department of Agriculture. Países Líderes en Producción de Carne Vacuno a Nivel Mundial en 2023 y 2024. 2025. Available online: https://es.statista.com/estadisticas/635290/carne-de-vacuno-principales-paises-productores/ (accessed on 17 January 2025).
- Favetta, L.A.; Villagómez, D.A.F.; Iannuzzi, L.; Di Meo, G.; Webb, A.; Crain, S.; King, W.A. Disorders of Sexual Development and Abnormal Early Development in Domestic Food-Producing Mammals: The Role of Chromosome Abnormalities, Environment and Stress Factors. Sex Dev. 2011, 6, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Gonçalvez, C.V.; Arellano, F.A.; Pérez Carrera, A.L. Técnicas de estudio para la evaluación del daño al ADN y su aplicación en la producción animal. SNS Publicación Periódica Científico Tecnológica 2015, 7, 21–37. [Google Scholar]
- Iannuzzi, A.; Iannuzzi, L.; Parma, P. Molecular. Cytogenetics in Domestic Bovids: A Review. Animals 2023, 13, 944. [Google Scholar] [CrossRef] [PubMed]
- Starodub, L.; Mokhnachova, N.; Zhukorskyi, O. Cytogenetic and ISSR-Markers Polymorphism in the Population of Local Ukrainian Lebedyn Cows. OBM Genet. 2024, 8, 1–13. [Google Scholar] [CrossRef]
- Schmidt, P.L. Companion animals as sentinels for public health. Vet. Clin. N. Am. Small Anim. Pract. 2009, 39, 241–250. [Google Scholar] [CrossRef]
- Garden, O.A.; Volk, S.W.; Mason, N.J.; Perry, J.A. Companion animals in comparative oncology: One Medicine in action. Vet. J. 2018, 240, 6–13. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mora-Tiscareno, A.; Fordham, L.A.; Chung, C.J.; Garcia, R.; Osnaya, N.; Hernández, J.; Acuna, H.; Gambling, T.M.; Villarreal-Calderón, A.; et al. Canines as sentinel species for assessing chronic exposures to air pollutants: Part Respiratory pathology. Toxicol. Sci. 2001, 61, 342–355. [Google Scholar] [CrossRef]
- Aslan, B.; Viola, L.; Saini, S.S.; Stockman, J.; Ryan, E.P. Pets as Sentinels of Human Exposure to Pesticides and Co-exposure Concerns with Other Contaminants/Toxicants. In Pets as Sentinels, Forecasters and Promoters of Human Health; Pastorinho, M., Sousa, A., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Caliri, M.; Ferré, D.M.; Ledda, V.; Gorla, N.B.M. Chromosomal aberrations induced by Mitomycin C in canine lymphocytes. Vet. Arh. 2023, 93, 341–350. [Google Scholar] [CrossRef]
- Carracedo, R.; Caliri, M.N.; Ferré, D.M.; Pedrosa, A.; Lentini, V.; Gorla, N.B.M. Description of dog buccal epithelial cells to approximate their use as a biomarker of induced damage in the Buccal Micronucleus Cytome assay. Vet. Sci. 2025. submitted. [Google Scholar]
- McKeon, M.; Xu, Y.; Kirkland, D.; Schmuck, G.; Krebsfänger, N.; Avlasevich, S.L.; Dertinger, S.D. Cyclophosphamide and etoposide canine studies demonstrate the cross-species potential of the flow cytometric peripheral blood micronucleated reticulocyte endpoint. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 742, 79–83. [Google Scholar] [CrossRef]
- Harper, S.; Dertinger, S.; Bishop, M.; Lynch, A.; Lorenzo, M.; Saylor, M.; MacGregor, J. Flow Cytometric Analysis of Micronuclei in Peripheral Blood Reticulocytes III. An Efficient Method of Monitoring Chromosomal Damage in the Beagle Dog. Toxicol. Sci. 2007, 100, 406–414. [Google Scholar] [CrossRef]
- Zúñiga-González, G.; Torres-Bugarın, O.; Zamora-Perez, A.; Gómez-Meda, B.C.; Ibarra, M.R.; Martınez-González, S.; González-Rodríguez, A.; Luna-Aguirre, J.; Ramos-Mora, A.; Ontiveros-Lira, D.; et al. Differences in the number of micronucleated erythrocytes among young and adult animals including humans: Spontaneous micronuclei in 43 species. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2001, 494, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Dönmez-Altuntas, H.; Hamurcu, Z.; Liman, N.; Demirtas, H.; Imamoglu, N. Increased micronucleus frequency after oral administration of cadmium in dogs. Biol. Trace Elem. Res. 2006, 112, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Backer, L.C.; Grindem, C.B.; Corbett, W.T.; Cullins, L.; Hunter, J.L. Pet dogs as sentinels for environmental contamination. Sci. Total Environ. 2001, 274, 161–169. [Google Scholar] [CrossRef]
- Catena, C.; Conti, D.; Villani, P.; Nastasi, R.; Archilei, R.; Righi, E. Micronuclei and 3AB index in human and canine lymphocytes after in vitro X-irradiation. Mutat. Res. Environ. Mutagen. Relat. Subj. 1994, 312, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tidu, L.; Ciccarelli, S.; De Sanctis, S.; Lista, F.; Ferreri, R.; Regalbuto, E.; Grizzi, F.; Taverna, G.; Poli, A.; Bruzzone, M.; et al. Sentinel role of military dogs in detecting genotoxic agents in the environment during military operations: A pilot study. Toxicol. Mech. Methods 2025, 1–15. [Google Scholar] [CrossRef]
- da Costa, B.F.T.; Teixeira, A.; Prata, J.C.; Pérez-Mongiovi, D. Application of the Buccal Micronucleus Cytome Assay for Genotoxicity Detection in Dogs. Animals 2025, 15, 382. [Google Scholar] [CrossRef]
- Santovito, A.; Saracco, M.; Scarfo, M.; Nota, A.; Bertolino, S. Purebred dogs show higher levels of genomic damage compared to mixed breed dogs. Mamm. Genome 2024, 35, 90–98. [Google Scholar] [CrossRef]
- Santovito, A.; Buglisi, M.; Sciandra, C. Buccal micronucleus assay as a useful tool to evaluate the stress-associated genomic damage in shelter dogs and cats: New perspectives in animal welfare. J. Vet. Behav. 2022, 47, 22–28. [Google Scholar] [CrossRef]
- Lentini, V. Descripción Citológica del Epitelio Bucal Canino y Citotoxicidad de la Piperazina Mediante el Ensayo de Micronúcleos Citoma. Bachelor Thesis, Universidad Juan Agustín Maza, Mendoza, Argentina, 2015. [Google Scholar]
- Grzesiakowska-Dul, A.; Kasprowicz, M.J.; Otwinowska-Mindur, A.; Baran, P.; Kuchta-Gładysz, M. Cytokinesis-Blocking Micronucleus Assay for Assessing Nuclear Chromatin Integrity Abnormalities in Dog’s Somatic Cells After Exposure to HVAD-Produced Silver Nanoparticles. Int. J. Mol. Sci. 2024, 25, 12691. [Google Scholar] [CrossRef]
- Pérez, N.; Berrío, A.; Jaramillo, J.E.; Urrego, R.; Arias, M.P. Exposure to cigarette smoke causes DNA damage in oropharyngeal tissue in dogs. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014, 769, 13–19. [Google Scholar] [CrossRef]
- Kimura, K.C.; Fukumasu, H.; Chaible, L.M.; Lima, C.E.; Horst, M.A.; Matsuzaki, P.; Sanches, D.S.; Pires, C.G.; Silva, T.C.; Pereira, T.C.; et al. Evaluation of DNA damage by the alkaline comet assay of the olfactory and respiratory epithelia of dogs from the city of Sao Paulo, Brazil. Exp. Toxicol. Pathol. 2010, 62, 209–219. [Google Scholar] [CrossRef]
- Waters, D.J.; Shen, S.; Glickman, L.T.; Cooley, D.M.; Bostwick, D.G.; Qian, J.; Combs, G.F., Jr.; Morris, J.S. Prostate cancer risk and DNA damage: Translational significance of selenium supplementation in a canine model. Carcinogenesis 2005, 26, 1256–1262. [Google Scholar] [CrossRef]
- Peterson, H.M.; Manley, C.I.; Trepanier, L.A.; Pritchard, J.C. Genotoxicity from metronidazole detected in vitro, but not in vivo, in healthy dogs in a randomized clinical trial. Am. J. Vet. Res. 2023, 84. [Google Scholar] [CrossRef]
- Avila, A.; Prieto, L.; Luna-Acosta, A. Nine decades of data on environmental chemical pollutant exposure in dogs: A bibliometric analysis. Environ. Sci. Pollut. Res. 2023, 30, 45515–45527. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Ariu, F.; Bocca, B.; Solinas, G.; Leoni, G.G.; Podda, A.; Bogliolo, L. Heavy metal(loid) accumulation in the ovarian tissue of free-ranging queens and bitches inhabiting highly polluted urban environments. Animals 2023, 13, 650. [Google Scholar] [CrossRef] [PubMed]
- González-Gómez, X.; Cambeiro-Pérez, N.; Martínez-Carballo, E.; Simal-Gándara, J. Screening of organic pollutants in pet hair samples and the significance of environmental factors. Sci. Total Environ. 2018, 625, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Suárez, N.; Rial, C.; Boada, L.D.; Henríquez-Hernández, L.A.; Valerón, P.F.; Camacho, M.; Luzardo, O.P. Are pet dogs good sentinels of human exposure to environmental polycyclic aromatic hydrocarbons, organochlorine pesticides and polychlorinated biphenyls? J. Appl. Anim. Res. 2016, 44, 135–145. [Google Scholar] [CrossRef]
- Glickman, L.T.; Domanski, L.M.; Maguire, T.G.; Dubielzig, R.R.; Churg, A. Mesothelioma in pet dogs associated with exposure of their owners to asbestos. Environ. Res. 1983, 32, 305–313. [Google Scholar] [CrossRef]
- Hayes, H.M., Jr.; Hoover, R.; Tarone, R.E. Bladder cancer in pet dogs: A sentinel for environmental cancer? Am. J. Epidemiol. 1981, 144, 229–233. [Google Scholar] [CrossRef]
- Reif, J.S. Companion dogs as sentinels for the carcinogenic effects of pesticides. Pestic. People Nat. 1999, 1, 7–14. [Google Scholar]
- Sévère, S.; Marchand, P.; Guiffard, I.; Morio, F.; Venisseau, A.; Veyrand, B.; Le Bizec, B.; Antignac, J.P.; Abadie, J. Pollutants in pet dogs: A model for environmental links to breast cancer. Springerplus 2015, 4, 27. [Google Scholar] [CrossRef]
- Hayes, H.M.; Tarone, R.E.; Casey, H.W. A cohort study of the effects of Vietnam service on testicular pathology of U.S. military working dogs. Mil. Med. 1995, 160, 248–255. [Google Scholar] [CrossRef]
- Shearin, A.L.; Ostrander, E.A. Leading the way: Canine models of genomics and disease. Dis. Models Mech. 2010, 3, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.; Creevy, K.; Dan, G.; Promislow, D.E. The companion dog as a model for human aging and mortality. Anat. Soc. 2018, 17, 12737. [Google Scholar] [CrossRef]
- Wang, T.; Jianzhu, M.; Andrew, N.; Hogan, S.; Katherine, L.; Brian, T.; Jason, F.; Kreisberg, P.; Adams, P.T.; Carvunis, A.-R.; et al. Quantitative translation of dog-to-human aging by conserved remodeling of the DNA methylome. Cell Syst. 2020, 11, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Gorla, N.B.M. Los animales domésticos y silvestres como centinelas de salud Ambiental. Investig. Cienc. Univ. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981, 66, 1191–1308. [Google Scholar] [CrossRef] [PubMed]
- Thorgeirsson, U.P.; Dalgard, D.W.; Reeves, J.; Adamson, R.H. Tumor incidence in a chemical carcinogenesis study of nonhuman primates. Regul. Toxicol. Pharmacol. 1994, 19, 130–151. [Google Scholar] [CrossRef]
- Takayama, S.; Sieber, S.M.; Dalgard, D.W.; Thorgeirsson, U.P.; Adamson, R.H. Effects of long-term oral administration of DDT on nonhuman primates. J. Cancer Res. Clin. Oncol. 1999, 125, 219–225. [Google Scholar] [CrossRef]
- Hollstein, M.; McCann, J.; Angelosanto, F.A.; Nichols, W.W. Short-term tests for carcinogens and mutagens. Mutat. Res. Rev. Genet. Toxicol. 1979, 65, 133–226. [Google Scholar] [CrossRef]
- Dearfiel, K.L.; Gollapudi, B.B.; Bemis, J.C.; Benz, R.D.; Douglas, G.R.; Elespuru, R.K.; Johnson, G.E.; Kirkland, D.J.; LeBaron, M.J.; Li, A.P.; et al. Next generation testing strategy for assessment of genomic damage: A conceptual framework and considerations. Environ. Mol. Mutagen. 2017, 58, 264–283. [Google Scholar] [CrossRef] [PubMed]
- Stucki, A.O.; Barton-Maclaren, T.S.; Bhuller, Y.; Henriquez, J.E.; Henry, T.R.; Hirn, C.; Miller-Holt, J.; Nagy, E.G.; Perron, M.M.; Ratzlaff, D.E.; et al. Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of industrial chemicals and pesticides for effects on human health. Front. Toxicol. 2022, 4, 964553. [Google Scholar] [CrossRef] [PubMed]
- Salk, J.J.; Kennedy, S.R. Next-generation genotoxicology: Using modern sequencing technologies to assess somatic mutagenesis and cancer risk. Environ. Mol. Mutagen. 2020, 61, 135–151. [Google Scholar] [CrossRef] [PubMed]
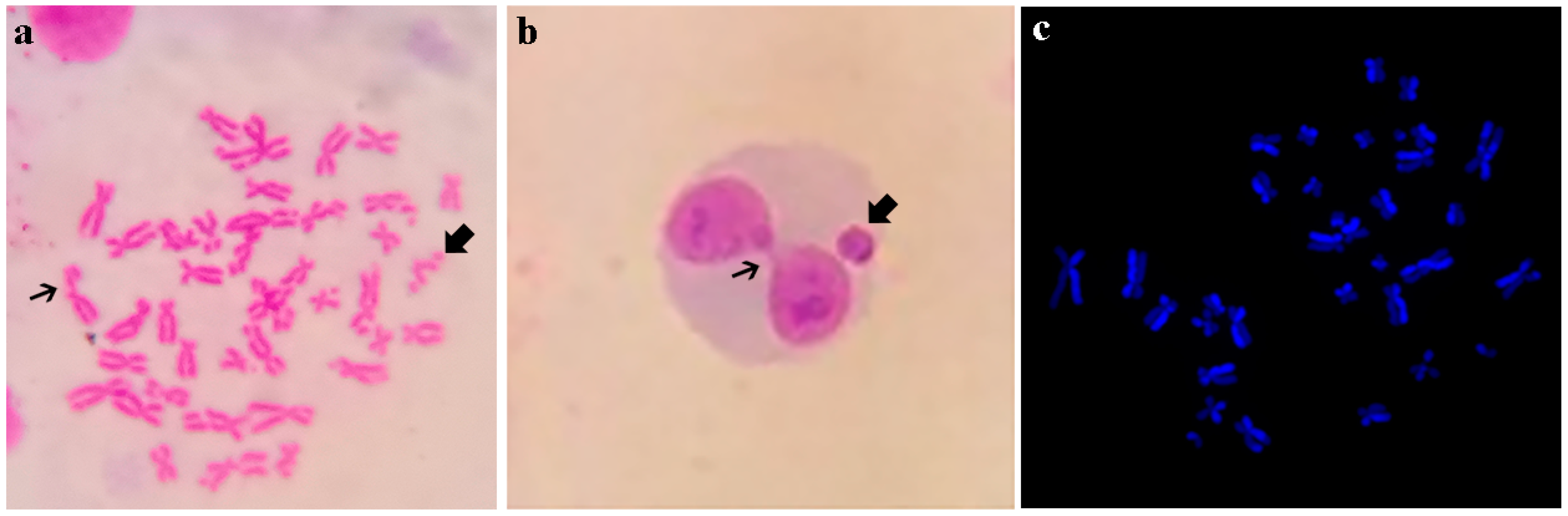
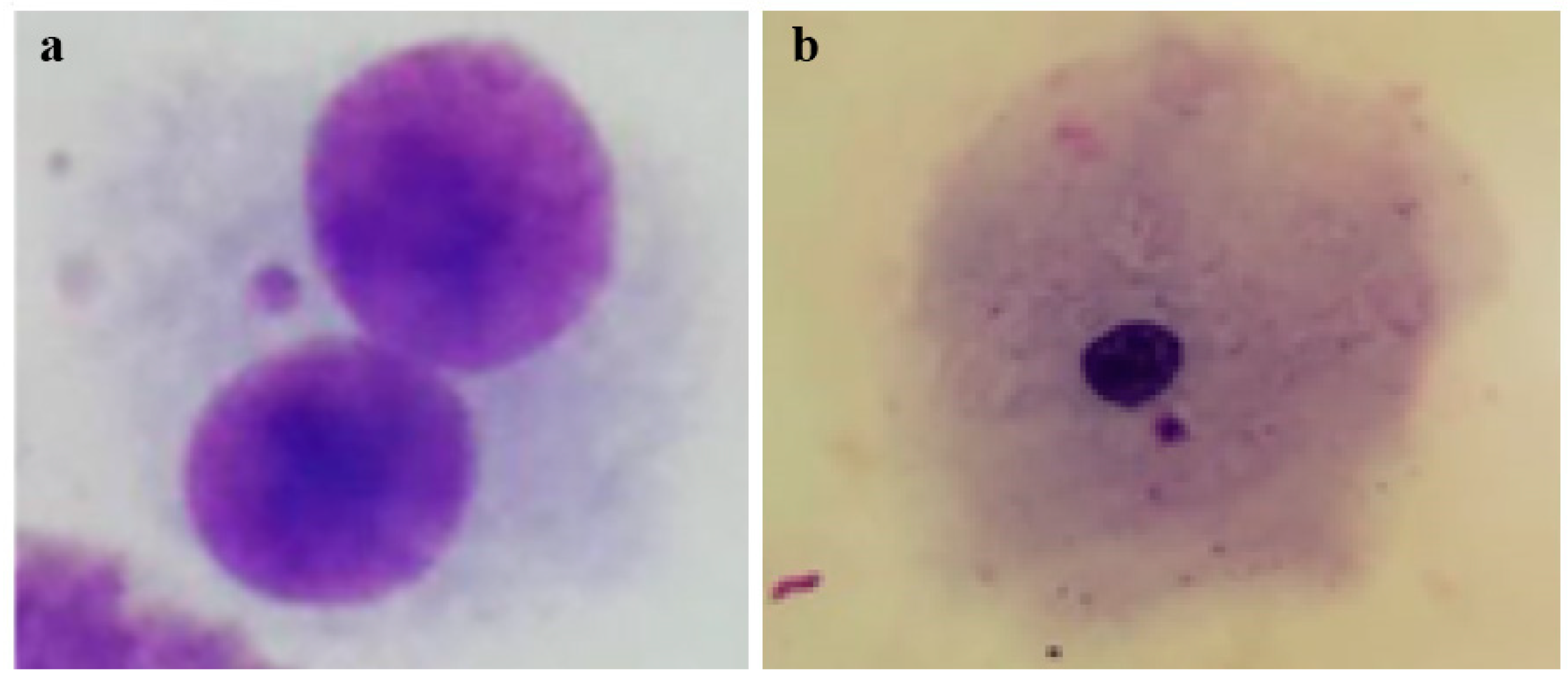
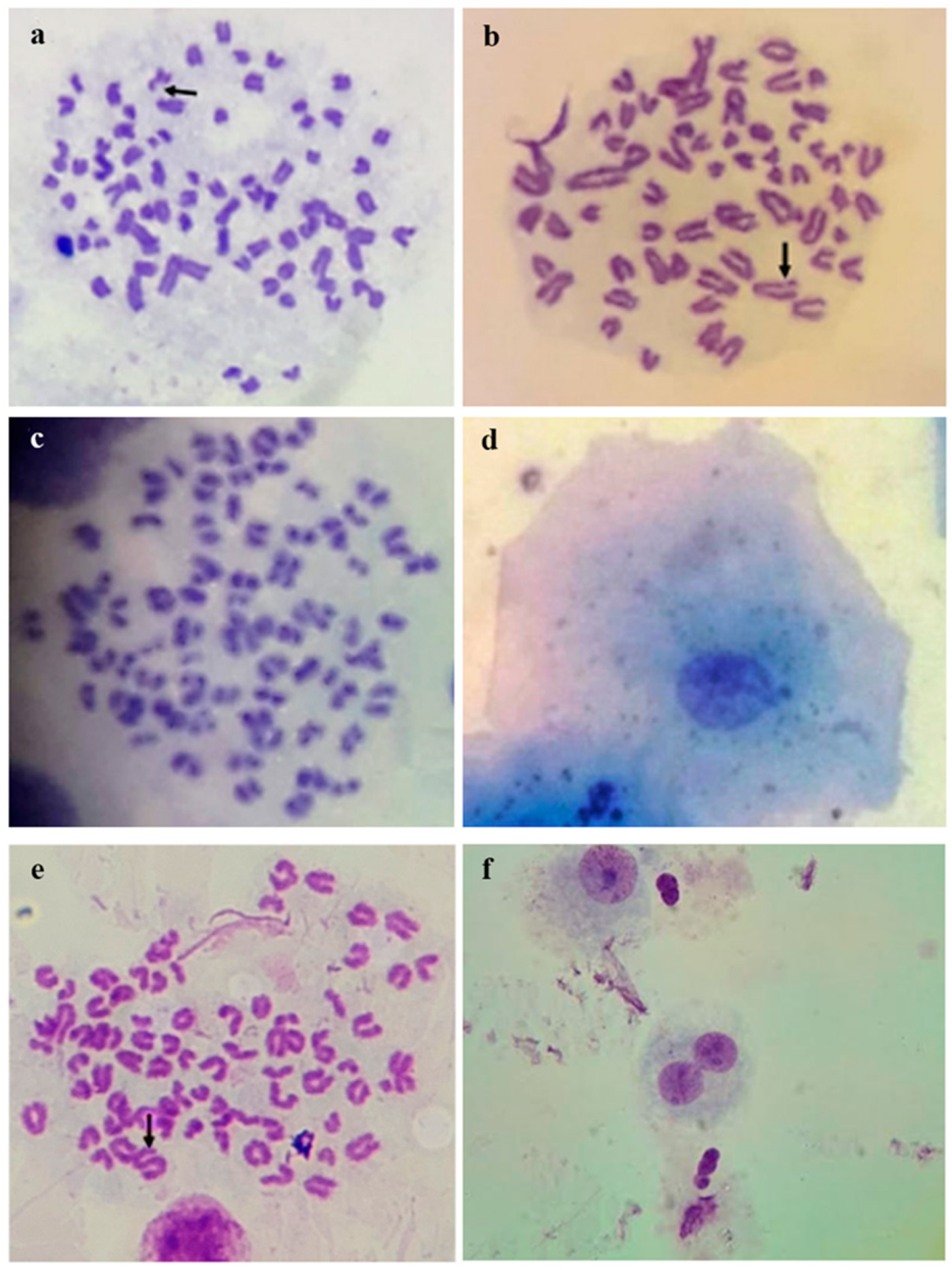
| OECD Genetic Toxicology Tests | Species | Xenobiotics/ Contaminants | Findings and Conclusions of the Genotoxicological Evaluation | Merits of the Paper | References |
|---|---|---|---|---|---|
| TG 473 | In vitro mammalian chromosomal aberration (CA) test | ||||
| and TG 474 | Macaca mulatta | Methyl-phenidate hydrochloride | No significant increases in CA frequencies in treated animals. Non-genotoxic or clastogenic effects on cells under the experimental conditions. | Drug commonly used to treat Attention-Deficit/Hyperactivity Disorder in pediatric populations. Drug not classified by IARC. Conflicting results have been reported in relation to Methyl-phenidate genotoxic activity. Peripheral blood lymphocytes cultures were used for testing | [58] |
| * Cebus apella, Macaca fascicularis, Erythrocebus patas | X-ray irradiation | CA increases in a dose-dependent way. Genotoxic effect on NHP’peripheral blood lymphocytes cultures | Ionizing radiation is “carcinogenic to humans” (Group 1 IARC). Species selected due to the chromosomal response is supposed to be similar than human ones. | [59,60] | |
| TG 474 | Mammalian erythrocyte micronucleus test | ||||
| M. mulatta | Cyclophosphamide (CP) | Single administration of CP induced a 10-fold increase in blood MN-Ret frequency. Clastogenetic effect on NHP’ peripheral blood lymphocytes culture and bone marrow cells. | Well-established clastogen. Drug “Carcinogenic to humans” (Group 1 IARC). To validate the use of blood lymphocytes instead of the invasive bone marrow sampling. | [61] | |
| Callitrhix jacchus | Methotrexate, CP, Cytosine-rabinoside (Ara-C), 5-fluorouracil | MN erythrocyte frequencies were increased by the Ara-C treatment. This NWM species could serve as a suitable model for testing the genotoxic effects of chemicals. | Four antineoplastic pediatric therapeutic drugs. Methotrexate and 5-fluorouracil are “not classifiable as to their carcinogenicity to humans” (Group 3 IARC). Cyclophosphamide is “carcinogenic to humans” (Group 1 IARC). Ara-C, is not classified by IARC. Blood smears were used for testing. | [62] | |
| TG 479 | In vitro sister chromatid exchange assay (SCE) in mammalian cells | ||||
| Ateles chamek, A. paniscus | None (healthy NHP) | Spontaneous G-SCEs and T-SCEs were quantified. | The study was designed to validate the use of SCE test for detection of DNA breakage and repair in Neotropical Primates species. Primary cell cultures of fibroblast from both species were used. | [63] | |
| Alouatta caraya, A. chamek, A. paniscus, ** Sapajus cay | None (healthy NHP) | Spontaneous G-SCEs were quantified. | Four different genome structures were analyzed and compared in the same study. Primary cell cultures of fibroblasts from each species were used. | [64] | |
| Homo sapiens, ** S. cay | Ornidazole (ONZ), Metronidazole (MTZ) | S. cay SCE values following treatments were significantly different from control. Genotoxic effect in the assessed conditions. | Two nitro-imidazole antibiotics. Main use: antimicrobial in human and veterinary medicine. MTZ is “possibly carcinogenic to humans” (Group 2B IARC). ONZ is not classified by IARC. Conflicting results have been reported in relation to the genotoxic activity of imidazole derivatives. Peripheral blood lymphocytes culture was used for testing. | [65] | |
| TG 487 and | In vitro mammalian cell micronucleus test and | ||||
| No TG | In vitro mammalian alkaline comet assay | ||||
| Cercophitecus aethiops | Fluconazole | MN frequency increased at 1306 µM of fluconazole, cytotoxic and genotoxic in the assessed conditions. | Triazole antifungal drug. Main use: obstetrics and gynecology for the treatment of vaginal candidiasis, patients with compromised immunity. Not classified by IARC. Scarce studies on the genotoxicity of fluconazole and the need of testing effects in different systems were the driving force of the study. VERO cell line was used for testing. | [66] | |
| * C. apella | N-Methyl-Nitrosourea (MNU) and Canova complex | MNU significantly increased MN frequency, but declined with Canova. Genotoxic effect of MNU can be controlled by Canova | Synthetic, mutagenic and carcinogenic drug used in research to induce tumors. Possibly carcinogenic to humans (Group 2B IARC). The potential antimutagenic action of Canova, a homeopathic complex of compound was evaluated. Peripheral blood lymphocytes culture was used for testing. | [67] | |
| No TG | In vitro mammalian alkaline comet assay | ||||
| C. aethiops | Dipyrone | Dipyrone causes DNA damage with a dose-response effect. Genotoxic and cytotoxic effects in the assessed conditions. | Common analgesic drug used in human medicine. Not classified by IARC. VERO cell line was used for testing. | [68] | |
| No TG | In vitro mammalian chromosomal aberration test with fragile sites induction | ||||
| A. caraya, Saimiri boliviensis | Fluorodeoxyuridine | Low coincidence between c-fra location, and breakpoints involved in rearrangements. | DNA synthesis inhibitor. Not classified by IARC. Common fragile sites are reliable markers of genetic instability, useful to evaluate the spontaneous change in chromosome structure. Peripheral blood lymphocytes cultures were used. | [69] | |
| No TG | Comet chip assay in Primary macaque hepatocytes (PMHs) | ||||
| M. mulatta | Enzymes, sugars, hormones, anti-inflammatories, corticosteroids, and others (22 compounds) | Primary macaque hepatocytes constitute a reliable surrogate of primary human’s hepatocytes for evaluating the genotoxic hazards of chemical substances. | In vivo and in vitro study/ Validation of a non-human primate putative surrogate for primary human hepatocytes in genotoxicity assessments for compounds known to have different genotoxic/carcinogenic effects. | [70] | |
| No TG | In vivo and ex vivo Peripheral blood cell with the γ-H2AX assay | ||||
| M. mulatta | 137Cs γ-rays | Quantitation of γ-H2AX foci is a good bio-dosimeter for analyzing body exposure to radiation. | Gamma-radiation is “Carcinogenic to humans” (Group 1 IARC). Study performed to validate the γ-H2AX biodosimetry for estimating severity and chronicity of therapeutic body radiation exposure effects. Peripheral blood lymphocytes and hair bulbs were tested. | [71] | |
| OECD Genetic Toxicology Tests | Xenobiotics | Findings and Conclusions of the Genotoxicological Evaluation | Merits of the Paper | References |
|---|---|---|---|---|
| TG 473 | In vitro mammalian chromosomal aberration (CA) test a | |||
| Acetamiprid-Mospilan 20SP® | Decrease of MI and a high percentage of ruptures was observed (25 and 50 μg/mL). Possible genotoxic action on chromosomes | Commercial formulation. Neonicotinoid pesticide (main use: insecticide). Not classified by IARC | [104] | |
| Thiacloprid-Calypso 480 SC® | Increase of Cas (240 and 480 ug/mL). Reduction in the MI. Genotoxic action on chromosomes | Commercial formulation. See Thiacloprid’s merits of the paper above | [105] | |
| Mitomycin C | Increase CAs and decrease of MI. Structural CAs (FISH-WCP). Genotoxic ability | Alkylating agent used in veterinary medicine. Other uses in genotoxicity studies. Possibly carcinogenic to humans (Group 2B IARC) | [106] | |
| Tolylfluanid-Euparen Multi® | Decrease of MI. Dose-dependent polyploidy and non-reciprocal translocations chromosomes 5 and 7 (FISH-WCP). Cytostatic and cytotoxic capacity | Commercial formulation. Sulfamides pesticide (fungicide) use in agriuclture. Not classified by IARC | [107] | |
| Tolylfluanid-Euparen Multi®-Bendiocarbamate | Decrease of MI (160 µg/mL bendiocarb). Dose-dependence polyploidy (FISH-WCP). Cytotoxic capacity | Commercial formulations (Tolylfluanid) for use in agriculture. See Tolylfluanid’s merits of the paper above. Bendiocarbamate is a insecticide used in agriculture and in indoor areas. Not classified by IARC | [108] | |
| Glyphosate–Monsanto Europe® | Increase of polyploidy (4n) (56 umol/L) (FISH-WCP). Potential genotoxic effect (aneugenic) | Commercial formulation. Phosphanate herbicide. Probably carcinogenic to humans (Group 2A IARC) | [109] | |
| Glyphosate–Monsanto Europe® | Increase non-significant of monosomy, trisomy, and polyploidy (4n) (FISH-WCP). Genotoxic ability | Commercial formulation. See Glyphosate’s merits of the paper above | [110] | |
| Tolylfluanid–Euparen Multi®, Bendiocarbamate | Inhibition of MI, polyploidy, chromosomes 1 and 5 translocations, (FISH-WCP). Tolylfluanid caused numerical CAs. Genotoxic capacity (tolyfluanid) | Commercial formulation. See merits of the paper of Tolylfluanid and Bendiocarbamate above | [111] | |
| and TG 479 | Prothioconazole + tebuconazole- Bayer® | MI Reduction (dose-dependent 7.5–30 μg/mL). Breakages and polyploidy (3 μg/mL) (FISH-WCP). Decreased the PI. No significant increase of SCE. Cytotoxic capacity on lymphocytes | Commercial formulation. Mixture. Both are triazoles pesticides (fungicides), main use in agriculture, other human and veterinary medicine. Not classified by IARC | [112] |
| Tolylfluanid–Euparen Multi® | No evidence on the clastogenicity. Increases of chromosomal damage (17.5 μg/mL). Cytostatic ability | Commercial formulation. See Tolylfluanid’s merits of the paper above | [113] | |
| Glyphosate–Monsanto Europe® | Decrease of MI (560 and 1120 mmol/L). Increase non-significant of chromatid breaks and gaps. Increase in SCE (24 h exposure). Further increase in S9lymphocytes (2 h at 140 mmol/L). Genotoxicity and cytostatic ability | Commercial formulation. See Glyphosate’s merits of the paper above | [114] | |
| and TG 479, TG 487 | Epoxiconazole | Increase of CAs (2.5 and 50 μg/mL). Decrease of MI (100 μg/mL) and PI (24 h at 100 μg/mL and 48 h lower exposures). Dose-dependent decrease of CBPI. Cytostatic and cytotoxic capacity on lymphocytes | Trizole pesticide (fungicide), main use in agriculture, other human and veterinary medicine. Not classified by IARC | [115] |
| Tebuconazole–Orius 25 EW® | Increase of CAs (6–30 µg/mL). Numerical CAs chromosomes 5 and 7 (FISH-WCP). Increase SCE (24 h at 15–60 µg/mL). Dose-dependent decrease of CBPI. Cytotoxic ability and possible genotoxic (clastogenic) effect. | Commercial formulation. See Tebuconazole’s merits of the paper above | [116] | |
| and TG 479, TG 487, SCGE in vitro assay (No TG) | Thiacloprid | Elevation of CAs (120 μg/mL). Elevations in SCEs (120–480 μg/mL). Decrease of CBPI 30–480 μg/mL. DNA damage (240–480 µg/mL). Cytostatic and cytotoxic ability in bovine blood cells | Neonicotinoid pesticide (main use: insecticide). Not classified by IARC | [117] |
| Epoxiconazole + fenpropimorph- Tango Super® | Decrease in MI and PI (3–15 µg/mL). Decrease in CBPI (1.5–15 µg/mL). Moderate elevation in DNA damage non significance. Cytostatic and cytotoxic capacity on lymphocytes | Commercial formulation Mixture. See Epoxiconazole ’s merits of the paper above. Fenpropimorph is a morpholine-derived pesticide (fungicide) use in agriculture. Not classified by IARC | [118] | |
| Thiacloprid | Increased breakage rate (120, 240, 480 µg/mL). Non-significant increase in CAs (FISH-WCP). Decrease in PI (48 h at 240 and 480 µg/mL). Decrease in the CBPI. Increases in lymphocyte DNA damage (120 and 480 μg/mL). Genotoxic and cytotoxic ability in lymphocytes | See Thiacloprid’s merits of the paper above | [119] | |
| TG 479 | In vitro Sister Chromatid Exchange Assay in Mammalian Cells (SCE) a | |||
| Tolylfluanid-Bayer® | Increased genetic damage (17.5 µg/mL). Decrease of PI (3.5–17.5 µg/mL). Genotoxic capacity | Commercial formulation. See Tolylfluanid’s merits of the paper above | [120] | |
| Tebuconazole-Orius® | Elevations in the mean of SCEs at 24 h exposure. Genotoxic and cytotoxic capacity | Commercial formulation. See Tebuconazole’s merits of the paper above | [121] | |
| TG 487 | In vitro mammalian cell micronucleus test (CBMN assay) a | |||
| Cypermethrin (Cyp), chlorpyrifos (Cpf) and mixture Cyp+Cpf | Cyp produced a decrease in CBPI and an increase in BNMN and BNBuds. Cyp and Cyp+Cpf increased BNMN. Genotoxic (Cyp and Cpf) and cytoxic (Cyp) capacity | Pesticides used in agriculture and like parasicticides in veterynary medicine. Cyp is a insecticide pyrethroid and Cpf is an organophosphate insecticide and acaricide. Both not classified by IARC. Cyp has high priority to be evaluated by IARC | [122] | |
| and SCGE in vitro assay (no TG) | Doramectin-Dectomax-sf® | Increased BNMN and NBuds in lymphocytes and cumulus cells, (40–60 ng/mL). Increased proportion of damaged lymphocyte nuclei. Genotoxic and cytotoxic capacity | Commercial formulation. Avermectin class used for the treatment of parasites in animals. Not classified by IARC. | [123] |
| Thiacloprid-Calypso® | Decrease of CBPI (30–240 μg/mL). Dose-dependent increase of BNMN (120 and 240 μg/mL). Induction of DNA- dsb in lymphocytes using neutral single-cell microgel. Potential genotoxic and cytotoxic capacity | Commercial formulation. See Thiacloprid’s merits of the paper above | [124] | |
| Enrofloxacin-Floxagen® | Increase of BNMN in lymphocytes independent of the concentration and increased of NBuds. Dose-dependent increase of ID in lymphocytes (50, 100 and 150 μg/mL). Genotoxic and cytotoxic capacity | Commercial formulation. Quinolinemonocarboxylic acid (Quinolone) used like veterinary antibacterial agent. Not classified by IARC. | [125] | |
| Tebuconazole+ prothioconazole-Prosaro 250EC® | Dose-dependent decrease in the CBPI at 48 h. Dose-dependent increase in DNA damage. Genotoxic and cytotoxic capacity | Commercial formulation. Mixture. See merits of the paper of Tebuconazole and Prothioconazole above | [126] | |
| TG 474 | Mammalian in vivo Erythrocyte Micronucleus Test | |||
| BCG vaccine recombinant Mycobacterium bovis | 3 vaccines with different M. bovis proteins cause MNE, although these decrease over time. Genotoxic and possible cytotoxic capacity (myelosuppression) | Not classified by IARC. | [127] | |
| TG 489 | In Vivo Mammalian Alkaline Comet Assay (SCGE assay) a | |||
| and CBMN ex vivo assay (No TG) | Glyphosate-Roundup® | Glyphosate feed did not induce DNA damage, did not induce genotoxic effects. No evidence of genotoxic capacity | Commercial formulation. See Glyphosate’s merits of the paper above | [128] |
| No TG | Ex vivo mammalian cell micronucleus test (CBMN assay) a | |||
| Cypermethrin+ chlorpyrifos-Ecto 2A Plus® | Positive correlation between BNMN and BNBud post dermal exposure to a therapeutic dose. No evidence of genotoxic capacity | Commercial formulation. Mixture. See merits of the paper of Cypermethrin and Chlorpyrifos above | [103] | |
| OECD Genetic Toxicology Tests | Xenobiotic/ Contaminant | Findings and Conclusions of the Genotoxicological Evaluation | Merits of the Paper | References |
|---|---|---|---|---|
| TG 473 | In vitro mammalian chromosomal aberration (CA) test | |||
| Mytomicin C (MMC) | CA were significantly higher due to the effect of Mitomycin C (p = 0.0247). | MMC is one of the drugs recommended by the OECD for the CA assay. Given the scarce use of dog lymphocytes in this assay, it is the first step to be performed. | [153] | |
| TG 474 | Mammalian erythrocyte micronucleus (MN) test | |||
| Cyclophosphamide (CP) and etoposide (ETP) | Dose-related MN responses were evident for both agents. | In vivo study/5 days CP and 2 days ETP treatments. Support for utility of flow cytometry-based blood and bone marrow MN-retyculocytes (RET) measurements in dogs. Both are chemotherapeutics and “Carcinogenic to humans” (Group 1 IARC). | [155] | |
| CP | The kinetics of appearance of MN in blood maximum frequency occurred ~48 h after dosing. | In vivo study/One therapeutic dose of CP, The three-color flow cytometric method uses anti-CD71 labeling to identify reticulocytes. | [156] | |
| None (Healthy dogs) | Quantification of spontaneous values | In vivo study/Blood smears, acridine orange 100x objective- This species could be used as monitors for genotoxic events. | [157] | |
| TG 487 | In vitro mammalian cell micronucleus test (CBMN in lymphocyte cultures) | |||
| Cadmium (Cd) | Cd might be directly and/or indirectly genotoxic after a monthly oral administration in dogs. | Occupational exposure to Cd is associated with cancers of the lung. Ex vivo study/orall adminstration. Priority to be classified by IARC. | [158] | |
| Superfund sites | Pet dogs living near the Superfund sites had a higher micronucleus frequency than control animals | In situ exposure/ex vivo test Area contaminated with high levels of organochlorine pesticides, DDE, DDT, lindane; volatile organic chemicals. | [159] | |
| X-irradiation and 3-aminobenzamide (3 AB) | Canine lymphocytes have been found to be about three times more radiosensitive than human lymphocyte | Ionizing radiation is “carcinogenic to humans” (Group 1 IARC). In vitro, 3 AB inhibits poly(ADP-ribose) polymerase activity and increase thegenotoxic effect of X-rays | [160] | |
| Military working dogs | Increase in chromosomal damage. | In situ exposure/ex vivo test Animals assessed before and 6 months after deployment | [161] | |
| No TG | In vivo mammalian cell micronucleus cytome test (BMNCyt) | |||
| None (Healthy dogs) | Dogs presented genetic damagemarkers similar to those in humans but at a different frequency. | Support the potential development of BMCyt in buccal epithelial cells in dogs as biomarkers of disease | [162] | |
| None (Healthy dogs) | A significant increase of micronuclei, nuclear buds and total nuclear aberrations frequencies in purebred dogs compared to mixed-bred dogs | The observed increased genomic damage amongst purebred dogs may not be due to a different genomicinstability typical of a particular breed, but to inbreeding itself. | [163] | |
| Stress | Evidence of a possible correlation between physiological stress conditions and higher levels of genomic damage in sheltered dogs | Priority to be classified by IARC (job stress) | [164] | |
| Piperazine | BMCyt/in administered puppies, karyolitic cells were observed to be twice as frequent following treatment (p < 0.05) | The animals were studied in the first usual deworming scheme for newborn. | [165] | |
| No TG | Mammalian Alkaline Comet Assay (Single Cell Gel Electrophoresis, SCGE assay) | |||
| Silver nanoparticles | increase in micronuclei, nuclear buds and nucleoplasmic bridges | In vitro peripheral blood. Priority to be classified by IARC | [166] | |
| Household cigarette smoke | Statistically significant differences were found between exposedand non-exposed to cigarette smoke in comet assays carried out on biopsy samples not in swab samples. | In situ exposure/ex vivo test Priority to be classified by IARC (second nand smoke). More than 40 mutagenic and carcinogenic agents present in cigarette smoke. | [167] | |
| Air particulate matter index (PM10) | Alkaline comet length from olfactory or respiratory epithelia of dogs were 67.86 and 72.46, fespectively. Comet length increases with dog age | In situ exposure/in vivo study in olfactory and respiratory epithelia. Priority to be classified by IARC. Air pollution, as measured by PM10, can be responsible for this DNA damage. | [168] | |
| Nutritionally levels of selenium | U-shaped dose-response between selenium and DNA damage within the prostate | In vivo/Study lasting 7 months. To determine the optimal intake of selenium for prostate cancer prevention. Priority to be classified by IARC | [169] | |
| No TG | In vitro and in vivo Peripheral Blood Mononuclear Cell with the γ-H2AX assay | |||
| Metro- nidazole (MTZ) | MTZ 36 μg/m serum without henotoxic effect. In vitro, MTZ led to a significant increase in DNA damage at 100 μg/mL | In vitro and in vivo studies comparison. In vivo MTZ dosages for 1 week. MTZ is “possibly carcinogenic to humans”. H2AX histone phosphorylation in the cellular response against DNA double-strand breaks. | [170] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorla, N.B.M.; Nieves, M.; Ferré, D.M. Genotoxicity in Unconventional Mammalian Models of Wild, Urban, and Agricultural Ecosystems: A Systematic Review Under the One Health Approach. Genes 2025, 16, 525. https://doi.org/10.3390/genes16050525
Gorla NBM, Nieves M, Ferré DM. Genotoxicity in Unconventional Mammalian Models of Wild, Urban, and Agricultural Ecosystems: A Systematic Review Under the One Health Approach. Genes. 2025; 16(5):525. https://doi.org/10.3390/genes16050525
Chicago/Turabian StyleGorla, Nora Bibiana M., Mariela Nieves, and Daniela Marisol Ferré. 2025. "Genotoxicity in Unconventional Mammalian Models of Wild, Urban, and Agricultural Ecosystems: A Systematic Review Under the One Health Approach" Genes 16, no. 5: 525. https://doi.org/10.3390/genes16050525
APA StyleGorla, N. B. M., Nieves, M., & Ferré, D. M. (2025). Genotoxicity in Unconventional Mammalian Models of Wild, Urban, and Agricultural Ecosystems: A Systematic Review Under the One Health Approach. Genes, 16(5), 525. https://doi.org/10.3390/genes16050525