Abstract
Background/Objectives: Identification of human remains is of utmost importance for criminal investigations and providing closure to the families. The reconstruction of a biological profile of the individual will narrow down the list of candidates for identification. From another perspective, facial approximations performed by a forensic artist can provide investigative leads, with the identity being confirmed by primary or secondary methods of identification. In recent years, DNA analysis has evolved, trying to create a portrait of the perpetrator/victim based on External Visible Characteristics (EVCs), the color of the eyes, hair, and skin and Biogeographical ancestry (BGA), called DNA phenotyping. Despite these advances, currently, there are no studies integrating the biological profile performed by forensic anthropologists, the facial approximation created by forensic artists and EVCs determined by DNA. The goal of this work was to integrate these three investigative leads to enhance the possibility of human identification. Methods: Five donated remains from Mercyhurst were studied through these approaches: reconstruction of biological profile, facial approximation and estimation of EVCs based on previous studies. Results: Our results indicated the feasibility of integrating this biological profile and EVCs data into the facial approximation developed by the forensic artist, aiming to an enhance portrait of the remains. In a second phase of this project, the accuracy of the integrated facial approximation will be assessed. Conclusions: This study pointed out the importance of an interdisciplinary approach towards the identification of human remains, as well as the combination of current methods with new technologies.
1. Introduction
When human remains are found, the first step in the identification process is to reconstruct the biological profile of the individual, which consists of a broad description of the deceased regarding population affinity, biological sex, age and stature. The reconstruction of the biological profile of the skeleton is one of the pillars of forensic anthropology and provides critical information in the identification of unknown human remains. Each component (population affinity, biological sex, age and stature) relies on established morphological and/or metric methods. From all the biological profile components, biological sex and age are the most relevant for narrowing down the list of candidates for the identity.
Estimation of population affinity remains one of the most challenging aspects of the biological profile. Hefner’s method for population affinity estimation [1] as well as Hefner and Ousley [2] rely on a combination of nonmetric cranial traits and discriminant function analysis. Hefner’s approach uses a probabilistic framework to classify individuals into populational groups based on cranial traits. Fordisc, a software program developed by Ousley and Jantz [3], is another widely used tool for population affinity estimation. Fordisc employs discriminant function analysis to classify individuals based on cranial measurements (although postcranial measurements can be used, they generally are not very informative). The software allows forensic anthropologists to compare an unknown individual’s measurements to these reference groups, providing a statistical probability of group membership. While Fordisc is a powerful tool, its accuracy depends on the availability of population-specific reference data and the appropriateness of the comparative samples.
Biological sex estimation is most accurately determined by the os coxae, namely the ventral arc, subpubic concavity and medial aspect of the ischiopubic ramus, as described by Phenice [4]. Klales et al. [5] reported a revised scoring system for these traits that improved reliability and reduced observer error. Cranial morphology also provides informative sex indicators, including the nuchal crest, mastoid process and supraorbital margin. Walker [6] developed a probabilistic approach to sex estimation from cranial features that has been tested and validated on several populations. MorphoPASSE [7], is a computer program for sex estimation relying of the scoring of pelvic and cranial traits for sex estimation, which is widely used nowadays.
Different methods are used to estimate age-at-death for juveniles and adults. Age estimation methods for juveniles rely on growth and developmental changes in bones and teeth. Whilst age estimation in adults relies on degenerative changes in the skeleton. Most widely used adult age estimation methods are the Suchey–Brooks method of pubic symphysis [8] and the Lovejoy et al. [9] system for the auricular surface. Several authors have explored the use of cranial sutures obliteration for age estimation [10,11]. However, all methods utilizing cranial sutures closure have shown a poor correlation with chronological age [12]. İşcan et al. [13,14] developed a phase-based system that evaluates changes in the costal cartilage surface at the sternal end of the 4th rib. This method has been validated in multiple populations and is particularly useful for its precision in middle-aged and older adults. Hartnett [15,16] introduced revised standards for estimating age from the pubic symphysis and sternal rib ends that provide increased accuracy and restrict interobserver error.
Overall, the biological profile of the skeleton remains a vital component in forensic anthropology. While traditional methods are a good basis, ongoing research and technological advancement continue to improve their precision and applicability. The integration of anthropological analysis with other methods of identification such as genetic analysis and forensic art techniques could help overcome the current limitations of each group of techniques individually.
Another traditional method often employed to assist with the identification of human remains is forensic art. Forensic art is a broad and diverse discipline offering a wide range of forensic techniques to assist with suspect and victim identification in a law enforcement context. Its primary focus is to generate investigative leads by triggering recognition through the creation of facial images which enable other forensic disciplines, such as DNA analysis, to make legally valid identifications [17].
Forensic facial approximation is a forensic art technique that assists identification efforts by simulating the in-life appearance of an unidentified decedent. This is a highly collaborative effort where the forensic artist works in tandem with other forensic professionals such as pathologists, forensic anthropologists and law enforcement investigators who provide information on biological sex, population affinity and cause and/or manner of death. It is based on a procedure of approximating the soft tissue contours of the face using statistical tissue depth data. The morphological information of the skull provides the underlying architecture of the development of the soft tissue features. These facial approximations can be developed in several ways including two-dimensional and three-dimensional formats.
While forensic facial approximation is not a conclusive means of identification, it serves as a means of expediting the investigative process while investigators await more conclusive determinations of identity. The forensic facial approximation process may serve as a means of generating leads when other forensic avenues of identification have been exhausted. Its function is to generate leads rather than serve as the primary means of identification.
As one of the other primary methods of identification, DNA analysis from human remains could be crucial in cases of missing persons and disaster victim identification [18]. As a resilient molecule, DNA degrades gradually in hard tissues, such as bones and teeth, with extraction of the DNA being possible under favorable conditions [19]. However, environmental factors could affect the remains, leading to problems of degradation, inhibition and contamination of DNA and hampering the possibility of obtaining a STR profile [20]. Additionally, as more time passes since the disaster or when the person went missing, it is possible that less ante-mortem samples from relatives or the person itself would be available [21]. In these cases, other means of identification are required. Single Nucleotide Polymorphisms (SNPs) have been emerged as potential solutions to these problems [22]. SNPs are nucleotide substitutions, insertions or deletions that are normally biallelic with low mutation rates and high heritability [23,24,25]. Moreover, the small size of their PCR amplicons makes them useful to analyze in cases of degraded or scarce DNA. Based on them, it has been possible to develop assays for the prediction of Externally Visible Characteristics (EVCs) and biogeographical ancestry (BGA) [26,27], referred to as forensic DNA phenotyping (FDP). As a result, FDP could be also useful in missing persons’ investigations and in the identification of human remains [28].
In this study, we chose to apply the HIrisPlex-S system for the prediction of eye, hair and skin color [26]. It consists of 41 DNA SNPs: 24 included in the original HIrisPlex assay and 17 SNPs investigated in another analysis to complement the skin color. It was carried out based on this original protocol, with SNaPshot™ (Single Based Extension (SBE) and Capillary Electrophoresis (CE)). The results of the analysis were introduced in the open-source software available at https://hirisplex.Erasmusmc.nl/ (accessed on 25 April 2025) to evaluate the prediction probabilities for three iris colors, four hair colors and five skin color categories [26].
These three techniques independently showed different successes in aiding with the identification of human remains, providing investigative leads. However, currently, there are no studies assessing the combination of these methods. Overall, the aim of this study was to integrate a forensic anthropology biological profile, facial approximation by a forensic artist and forensic DNA phenotyping to improve human identification.
2. Methods and Materials
2.1. Samples
Five skeletons from the Donated Collection of the Department of Applied Forensic Sciences at Mercyhurst University were included in this study.
2.2. Anthropological Analysis
Population affinity, biological sex and age-at-death estimations were conducted for the five individuals included in this project.
Non-metric population affinity was estimated using six macromorphoscopic traits described in Hefner [1] and the optimized summed scored attributes (OSSAs) method outlined in Hefner and Ousley [2]. Metric analysis for population affinity estimation was conducted using twenty-six standard cranial measurements recorded from an individual which were compared to individuals of known sex and ancestry through linear discriminant function analysis in FORDISC 3.1 [2,3] following the guidelines outlined in Ousley and Jantz [29].
Non-metric sex estimation was based on features of the os coxae and cranium, using ordinal scores of the 12 variables (2 unilateral and 2 binary skull traits, as well as 3 bilateral pelvis traits) and the random forest classification provided by MorphoPASSE [7].
For age estimation, the pubic symphysis, auricular surface and cranial sutures were analyzed using the computer-based age estimation program ADBOU, which utilizes transition analysis [30,31].
Stature (though not related to the facial approximation) was estimated using FORDISC 3.1 [2,3].
2.3. Bone Samples for DNA Analysis
Bone fragments of approximately 2 × 2 cm were collected from the femoral diaphysis for the five individuals using a Stryker 810 Autopsy Saw (Stryker, Portage, MI, USA).
2.4. Facial Approximation
The process of developing forensic facial approximations for the five unknown skeletal samples was initiated by three-dimensionally scanning the remains and generating a virtual 3-D model of each of the skulls. The scans contained both the mesh and texture data of the skulls facilitating the creation of virtual replicas of the skulls using a 3-D modeling application (Autodesk 3ds Max).
Multiple tissue depth data sets were then researched based on the sample profiles to ensure the most accurate soft tissue approximations. The Rhine and Moore tissue data sets for normal male and female, European-derived, samples were selected for this study. Appropriate tissue depth markers were created and placed at various craniometric landmarks on each of the skulls based on Karen Taylor’s two-dimensional facial reconstruction techniques [32].
The skulls were then aligned in the Frankfort Horizontal Plane to minimize perspective distortion. Frontal and lateral 2-D images were rendered and exported in a lossless format (.PNG). A scale was generated to facilitate accurate scaling of exported images in a digital imaging application (Adobe Photoshop).
The frontal and lateral images were aligned to correspond to one another to facilitate the development of two-dimensional frontal and lateral facial approximations in concurrent fashion. Empirical data from the skull was gathered to locate phenotypic traits on the splanchnocranium. The lateral projection of the nose was calculated using data based on the anterior nasal spine and Dr. Robert George’s technique for lateral projection [33].
2.5. DNA Isolation from Bone Samples
A diamond cutting disk was used to cut femur bones samples into smaller pieces. Then, the bone pieces were mechanically ground using an agate mortar and pestle and were divided into aliquots of approximately 200 mg each. DNA was isolated from the bone aliquots by using the DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) with some modifications to the manufacturer’s instructions. First, 180 μL of ATL buffer plus 20 μL of proteinase K were added to each sample and they were mixed thoroughly by vortex. The resulting solution was then incubated (overnight) under agitation at 56 °C until the tissue was completely lysated. The following day, 200 μL of AL buffer was added and mixed by vortexing. Then, 200 μL ethanol (100%) was added to the solution and mixed again by vortexing. The solution was transferred to a DNeasy Mini Spin column placed in a 2 mL collection tube and centrifuged at 6000× g for 1 min. The flow-through was discarded, and the column was placed in a new 2 mL collection tube. Next, 500 μL of AW1 Buffer was added, and the column was centrifuged at 6000× g for 1 min. The flow-through was discarded, and the column was placed in a new 2 mL collection tube. Later, 500 μL of AW2 buffer was added, and the column was centrifuged at 20,000× g for 3 min. The flow-through was discarded and the column was centrifuged one more time at 20,000× g for 1 min to eliminate the ethanol residues. The flow-through was discarded, and the column was placed in a new 1.5 mL tube. Then, 35 microliters of AE buffer were added directly onto the DNeasy column’s membrane and incubated for 1 min at room temperature. Finally, the column was centrifuged at 6000× g for 1 min to elute the DNA. The resulting DNA samples were stored at −20 °C.
As preventive measures to avoid DNA contamination of the samples, the whole molecular biology workflow, from DNA extraction through sequencing, was performed under sterile working conditions: working under a biological safety cabinet II UV-sterilized before and after being used, utilizing new sterile material such as pipette tips with filters, nitrile gloves discarded after one single use and utilizing molecular-grade sterile water.
2.6. DNA Quantification
The DNA samples were quantified by using the Qubit dsDNA HS Assay Kit (Invitrogen, Life Technologies, Carlsbad, CA, USA), according to the manufacturer’s protocol.
2.7. Hair and Eye Color Genotyping System Protocol
For the genotyping of hair and eye color, we used the HIrisPlex system. The HIrisPlex system is based on the evaluation of 23 SNPs and 1 insertion/deletion (INDEL) polymorphism from 11 genes [34]. All the marker details, primer sequences, and concentrations are provided in Table 1. PCR amplification of all SNPs was performed in a single multiplex PCR assay with a total volume of 12 μL containing PCR primers, concentrations described in Table 1, 1 μL genomic DNA isolated from bones (1 ng/μL), 1X PCR buffer (Applied Biosystems. Waltham, MA, USA), 2.7 mM MgCl2 (Applied Biosystems), 200 μM of each dNTP (Promega Corporation. Madison, WI, USA) and 0.5 U AmpliTaq Gold 360 DNA Polymerase (Applied Biosystems). The thermocycler PCR parameters were set as follows: 95 °C for 10 min; 33 cycles of 95 °C for 30 s, 56 °C for 30 s, 72 °C for 30 s and a final elongation phase of 72 °C for 7 min. The PCR products were purified using ExoSAP-IT Express (Applied Biosystems) and incubated at 37 °C for 4 min and 80 °C for 1 min. The multiplex SBE (single base extension) was carried out for all 24 products at the same time in a single multiplex reaction using 2 μL of the purified PCR product, 2 μL of the SBE primer mix (final concentrations of each SBE primer in Table 1) and 1 μL of the ABI Prism® SNaPshot Multiplex Kit (Applied Biosystems) with the following thermocycler parameters: 96 °C for 2 min; 25 cycles of 96 °C for 10 s, 50 °C for 5 s and 60 °C for 30 s. The resulting products were purified using 1 μL of shrimp alkaline phosphatase (SAP) (Applied Biosystems) and incubated 37 °C for 45 min and 75 °C for 15 min. Finally, the purified SBE products were analyzed on the SeqStudio Genetic Analyzer machine (Applied Biosystems) with Applied Biosystems POP-1 polymer on a 28 cm capillary length under an injection voltage of 1.2 kV for 7 s and with a running time of 330 s at 60 °C. Gene Mapper ID-X v1.6 software (Thermo Fisher Scientific, Waltham, MA, USA) was used for analysis of the results.

Table 1.
Information about the 24 DNA variants of the HIrisPlex assay, including PCR and single base extension (SBE) primer sequences and concentrations. Major and minor alleles correspond to the input information entered to the HIrisPlex prediction model.
2.8. Skin Color Genotyping System Protocol
For the genotyping assay of skin color, the HIrisPlex-S (HPS) system was used following Chaitanya et al.’s [26] protocol, a 17-plex system involving the evaluation of 36 SNPs from 16 genes for skin color. A detailed mention of all the markers, primer sequences, and concentrations is provided in Table 2. PCR amplification of all SNPs was performed in a single multiplex PCR assay with a total volume of 12 μL containing PCR primers, concentrations described in Table 2, 1 μL genomic DNA isolated from bones (1 ng/μL), 1X PCR buffer (Applied Biosystems. Waltham, MA, USA), 2.7 mM MgCl2 (Applied Biosystems), 200 μM of each dNTP (Promega Corporation, Madison, WI, USA) and 0.5 U AmpliTaq Gold 360 DNA Polymerase (Applied Biosystems). The thermocycler PCR parameters were set as follows: 95 °C for 10 min; 33 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s and a final elongation phase of 72 °C for 7 min. The PCR products were purified using ExoSAP-IT Express (Applied Biosystems) and incubated at 37 °C for 4 min and 80 °C for 1 min. The multiplex SBE (single base extension) was carried out for all 17 products at the same time in a single multiplex reaction using 2 μL of the purified PCR product, 2 μL of the SBE primer mix (Table 2) and 1 μL of the ABI Prism® SNaPshot Multiplex Kit (Applied Biosystems) with the following thermocycler parameters: 96 °C for 2 min; 25 cycles of 96 °C for 10 s, 50 °C for 5 s and 60 °C for 30 s. The resulting products were purified using 1 μL of shrimp alkaline phosphatase (SAP) (Applied Biosystems) and incubated 37 °C for 45 min and 75 °C for 15 min. Finally, the purified SBE products were analyzed on the SeqStudio Genetic Analyzer machine (Applied Biosystems) with Applied Biosystems POP-1 polymer on a 28 cm capillary length under an injection voltage of 1.2 kV for 7 s and with a running time of 330 s at 60 °C. Gene Mapper ID-X v1.6 software program (Thermo Fisher Scientific, Waltham, MA, USA) was used for analysis of the results.

Table 2.
Information about the 17 DNA variants HIrisPlex-S (HPS) DNA test with PCR and single base extension (SBE) primer sequences and concentration. Major and minor alleles correspond to the input information entered to the HPS prediction model.
2.9. Hair, Eye and Skin Color Prediction
The prediction of eye, hair and skin color from the donors was performed based on the identification of the specific SNPs described in previous sections and populated on the open source website reported by Chaitanya et al. [26], known as HIrisplex-S: HIrisPlex-S Eye, Hair and Skin Colour DNA Phenotyping Webtool: https://hirisplex.Erasmusmc.nl/. This tool provided the probabilities of the predictive phenotypes based on the different categories of eye color, hair color and shade and skin color.
3. Results
3.1. Biological Profile
The results obtained from the skeletal biological profile estimations are summarized in Table 3, comprising population affinity, biological sex and age-at-death for the five individuals (stature was not used for facial approximation). Known data from the individuals is also included.

Table 3.
Summary of the skeletal biological profile analysis for each individual. Population affinity was estimated using Fordisc, biological sex estimation was conducted using MorphoPASSE and age at death was estimated using ADBOU. Additionally, the known population affinity, sex and age of the individuals are presented.
Individual 1’s skeletal profile estimation corresponded to a white male between 35 and 75 years of age at the time of death and between 165 and 185 cm in stature, which was consistent with the known data of the individual (white male, 66 years of age, and 170 cm). Individual 2’s skeletal profile estimation corresponded to a white male over 50 years and between 165 and 185 cm in stature, which was consistent with the known data of the individual (white male, 88 years of age and 165 cm). Individual 3 corresponded to a white male between 30 and 75 years, and 162 to 185 cm in stature, which was consistent with the known data of the individual (white male, 42 years of age and 165 cm). Individual 4’s skeletal profile estimation corresponded to a white male between 30 and 65 years, which was consistent with the known data of the individual (white male, 66 years of age and 185 cm). Individual 5’s skeletal profile estimation corresponded to a white female between 45 and 85 years of age at the time of death, which was consistent with the known data of the individual (white female, 62 years of age and 162 cm) (Table 3).
3.2. Phenotyping Predictions
A summary of the results from the HIrisPlex-S online tool is depicted in Table 4, including the prediction p-values. Regarding the eye color, the prediction was performed by comparing the probabilities obtained by entering our genotyping results into the online tool and matching the resulting probabilities to the pictures in the figures provided by Walsh et al. (2011) [35]. Hair color prediction was performed according to the recommendations from Walsh et al. (2013) based on the hair color and shade probabilities as derived from the HIrisPlex-S online tool [34]. In brief, the process for predicting the hair color and shade with the best probability of matching the true hair color is as follows: (1) Calling the category of the leading color (black, brown, red, redhead and blonde) prediction based on the highest probability value; (2) calling the final color prediction based on the contribution and effect of black and blonde using the probability values for dark and light attributes. For a clearer reference on the color and tone of the hair, we refer to the images shown in the same article [34]. Finally, skin color was predicted as described in Chaitanya et al. (2018) [26]. In their guide for skin color prediction, the authors set different threshold values for the probabilities obtained by using the HIrisPlex-S DNA test system and classify skin color in five different categories: very pale, pale, intermediate, dark, and dark to black. The pictures of the exemplified performance of their predictive model were also used in order to clarify the skin color of our samples.

Table 4.
Phenotyping predictions according to the HIrisPlex-S online tool.
According to the genotyping analysis, Individual 1 would have blue eyes and light red hair with pale skin tone. Individual 2 would have brown eyes, dark red hair and intermediate to pale skin tone. Individual 3 would have brown eyes, intermediate red hair and intermediate skin tone. Individual 4 would have blue eyes, light red hair and pale skin. Individual 5 would have blue eyes, dark red hair and pale skin tone. These phenotyping predictions have been included in the facial approximation based on the pictures displayed in previous publications [26,36].
3.3. Facial Approximation Integration
Using the forensic anthropological profile, 3-D skull model and accepted forensic art techniques, 2-D frontal and lateral facial approximations were developed for each of the samples in a monochromatic grayscale. These grayscale versions served as templates for developing the final facial approximation likenesses in full color using the forensic DNA phenotyping predictions for hair, eye and skin color.
Digital imaging techniques were used to enhance the final facial approximations. Additionally, superimpositions of the skulls over the facial approximations were created to illustrate the correspondence between the contours of the face and the distal points of the tissue depth markers. Figure 1 presents the frontal view of the facial approximation products.
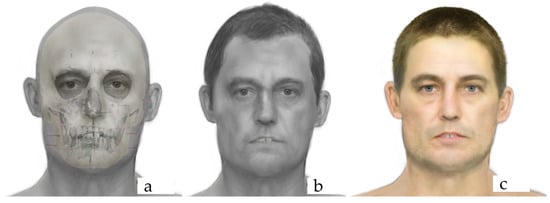
Figure 1.
Facial approximation products of Individual 4: overlay of the soft tissues over the skull (a), final facial approximation with the skeletal profile estimation information (b), and final facial approximation with the skeletal profile estimation and phenotyping information (c).
4. Discussion
This technical report presented, for the first time, the application of an interdisciplinary approach, integrating a forensic anthropology biological profile, facial approximation and forensic DNA phenotyping, with the aim of improving human identification.
The next step of this project will be the comparison of the facial approximations obtained (only with the anthropological information and with the anthropological and phenotyping information) with in-life photos of the sample decedents. Therefore, the facial approximations will be assessed in terms of overall likeness and accuracy of facial proportion.
Image comparison is a topic that has been adjudicated in legal contexts in a variety of cases. However, image comparison as it relates to forensic facial approximation is not typically subject to legal scrutiny. It is a forgone conclusion that forensic facial approximations capture an approximate likeness at best as the name would suggest. Therefore, evaluating the efficacy of facial approximation in even the broadest statistical terms can prove quite challenging. Facial approximations are subject to subtle interpretations of appearance and likeness and do not necessarily fit into the prescribed standards of one-to-one image comparison used in facial recognition algorithms and facial identification analyses.
Moreover, each technique by itself has its limitations. Forensic anthropology, in the first phase of the identification process, through the estimation of the biological profile (population affinity, biological sex, age at death and stature), as well as other unique characteristics (i.e., previous ante-mortem trauma), could help to reduce the number of missing persons candidates for the identity [37]. However, if there are no other ante-mortem data like dental records or DNA reference samples from the deceased or relatives, the identification is challenging. Facial approximation could help in these cases; although it is not a scientific method of identification, it can provide visibility, especially for cold cases, and it can assist in achieving a positive identification through scientific means.
Evaluating the efficacy of forensic facial approximations through feature interpretation is inherently grounded in a qualitative analysis of likeness best expressed in terms of language rather than numerical values. Consequently, the evaluation of a facial approximation remains a nuanced process that demands a holistic appreciation for the intricacies of facial feature interpretation and its qualitative dimensions [17]. Some “successful” forensic facial approximations have elements of “built-in ambiguity” [32]. This tendency is often preferred in situations where the specificity of an image may prove detrimental to the identification process where potential leads are ruled out. Assessing likeness through an image comparison then becomes a discussion of qualitative data versus quantitative data where the results are more aptly expressed through language and value judgments instead of numerical values [17]. As mentioned previously in this article, the evaluation of the facial approximations recognition of the method presented will be assessed in the second phase of this project.
This study is based the FDP on the HIrisPlex-S system [26,34,35,36,38,39], which is the most common one used in several studies [20,37,40,41,42] applying different methodologies with advantages and disadvantages. Many of these previous works have been focused on translating these HIrisPlex-S into Next Generation Sequencing (NGS) platforms [41,43,44,45,46]. Additionally, there are tools, like VISAGE Basic Tool (BT) from the VISAGE Consortium, which includes ancestry SNPs as well as the 41 SNPs from the HIrisPlex-S panel [47]. Moreover, there are commercial solutions like the MiSeq FGx™ Forensic Genomics System, from Qiagen, with a first panel including 27 autosomal, 7 X- and 24 Y-chromosomal STRs and 94 identity-SNPs; a second panel includes 56 ancestry SNPs and 22 phenotype-informative SNPs (for eye and hair color) [22]. Parabon Nanolabs also offers the Snapshot™ DNA Phenotyping Service, creating a complete profile, including genetic ancestry, eye, hair and skin color, freckling and face shape [22]. Overall, the aforementioned platforms point out one of the main limitations of FDP: the lack of standardization of methodologies. As described, current efforts are focused on translating these panels into NGS, as they allow higher throughput, multiplexing capacity and sequencing accuracy, as well as the possibility to automate and sequence different markers in the same run [48]. However, the overall cost is too high and may not be affordable for routine use by the forensic labs [20]. As a result, the gold-standard technique is still SNaPshot™ (SBE-CE assay) due to its robustness, simplicity and efficiency, but more precisely, because the instrument is already present in forensic laboratories. This is the reason why this study applied this classical methodology instead of NGS. The idea was to develop a protocol that is affordable and accessible for forensic laboratories, using the equipment they already have available. However, it is not exempt from drawbacks; SNaPshot™ has higher risk of contamination and error, and more importantly, is limited to analyzing three single traits with 30 to 40 markers (eye, hair and skin color) [43]. Despite this, previous studies and our present work demonstrated its applicability to deteriorated DNA [49,50]. Finally, it is worth noting that, overall, these previous works, both applying classical SNaPshot™ and NGS sequencing technologies, based their facial approximation only on forensic DNA phenotyping; the present study is the first one integrating these FDP traits into facial approximation and anthropological findings, enhancing human identification.
5. Conclusions
This is the first interdisciplinary study integrating anthropological biological profiling, facial approximation and forensic DNA phenotyping. The findings from this research indicate the possibility of performing forensic DNA phenotyping in a forensic lab, with the instrumentation used for STR profiling in degraded human remains, and work alongside the forensic artist and forensic anthropologist to enhance facial approximation and improve human identification. Although the integrated methodology is presented in this technical report, the second phase of this project will involve an assessment of the recognition of the facial approximations with and without the information obtained from the phenotyping analysis.
Further research is needed in this new multidisciplinary approach, including more individuals in the sample encompassing wider ranges of age and including different populations.
Author Contributions
Conceptualization, S.C.Z. and J.A.-G.; methodology, S.C.Z., J.A.-G., F.M.-P. and J.M.; validation, S.C.Z., J.A.-G., F.M.-P. and J.M.; formal analysis, S.C.Z., J.A.-G., F.M.-P. and J.M.; data curation, S.C.Z., J.A.-G., F.M.-P. and J.M.; writing—original draft preparation, S.C.Z., J.A.-G., F.M.-P. and J.M.; writing—review and editing, S.C.Z., J.A.-G., F.M.-P. and J.M.; funding acquisition, S.C.Z. and J.A.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Eppley Foundation for Research (Grant number 5266).
Institutional Review Board Statement
The New Jersey Institute of Technology Institutional Review Board (IRB) granted an exemption of these experiments under 45 CFR 46.104(d)(704), category 4 (protocol number: 2205021027).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available upon request to the authors.
Acknowledgments
We would like to acknowledge the students of the Department of Applied Forensic Sciences at Mercyhurst University who assisted in this project: Rhiannon Toy, Aurora Vana, Samanatha Myers and Dakota Bell. All our gratitude to the donated individuals included in this study; without their generous donation to science this (and many other) research project would not be possible.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethical Statement
This study was conducted in accordance with the ethical standards for research involving human remains.
References
- Hefner, J.T. Cranial nonmetric variation and estimating ancestry. J. Forensic Sci. 2009, 54, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Hefner, J.T.; Ousley, S.D. Statistical classification methods for estimating ancestry using morphoscopic traits. J. Forensic Sci. 2014, 59, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Ousley, S.D.; Jantz, R.L. Fordisc: A Computer Program for Forensic Anthropology; University of Tennessee: Knoxville, TN, USA, 1996. [Google Scholar]
- Phenice, T.W. A newly developed visual method of sexing the os pubis. Am. J. Phys. Anthropol. 1969, 30, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Klales, A.R.; Ousley, S.D.; Vollner, J.M. A revised method of sexing the human innominate using Phenice’s nonmetric traits and statistical methods. Am. J. Phys. Anthropol. 2012, 149, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.L. Sexing skulls using discriminant function analysis of visually assessed traits. Am. J. Phys. Anthropol. 2008, 136, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Klales, A.R.; Cole, S.J. MorphoPASSE: Morphological Pelvis and Skull Sex Estimation. In Sex Estimation of the Human Skeleton; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Brooks, S.; Suchey, J.M. Skeletal age determination based on the os pubis: A comparison of the Acsádi-Nemeskéri and Suchey-Brooks methods. Hum. Evol. 1990, 5, 227–238. [Google Scholar] [CrossRef]
- Lovejoy, C.O.; Meindl, R.S.; Pryzbeck, T.R.; Mensforth, R.P. Chronological metamorphosis of the auricular surface of the ilium: A new method for the determination of adult skeletal age at death. Am. J. Phys. Anthropol. 1985, 68, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Meindl, R.S.; Lovejoy, C.O. Ectocranial suture closure: A revised method for the determination of skeletal age at death based on the lateral-anterior sutures. Am. J. Phys. Anthropol. 1985, 68, 57. [Google Scholar] [CrossRef]
- Nawrocki, S.P. Regression formulae for the estimation of age from cranial suture closure. In Forensic Osteology: Advances in the Identification of Human Remains, 2nd ed.; Reichs, K.J., Ed.; CC Thomas: Springfield, IL, USA, 1998; pp. 276–292. [Google Scholar]
- Adserias-Garriga, J.; Wilson-Taylor, R. Chapter 5: Skeletal age estimation in adults. In Age Estimation; Adserias-Garriga, J., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 55–73. ISBN 9780128144916. [Google Scholar] [CrossRef]
- Iscan, M.Y.; Loth, S.R.; Wright, R.K. Age estimation from the rib by phase analysis: White males. J. Forensic Sci. 1984, 29, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- Iscan, M.Y.; Loth, S.R.; Wright, R.K. Age estimation from the rib by phase analysis: White females. J. Forensic Sci. 1985, 30, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, K.M. Analysis of age-at-death estimation using data from a new, modern autopsy sample—Part II: Sternal end of the fourth rib. J. Forensic Sci. 2010, 55, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, K.M. Analysis of age-at-death estimation using data from a new, modern autopsy sample—Part I: Pubic bone. J. Forensic Sci. 2010, 55, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.; Denny, T. Standardization Challenges in Forensic Art [Review of Standardization Challenges in Forensic Art]. ID News 2024, 54, 8–9. [Google Scholar]
- Hartman, D.; Drummer, O.; Eckhoff, C.; Scheffer, J.W.; Stringer, P. The contribution of DNA to the disaster victim identification (DVI) effort. Forensic. Sci. Int. 2011, 205, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Hofreiter, M.; Serre, D.; Poinar, H.N.; Kuch, M.; Paabo, S. Ancient DNA. Nat. Rev. Genet. 2001, 2, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, L.; Pajnic, I.Z.; Walsh, S.; Balazic, J.; Zupanc, T.; Kayser, M. Bringing colour back after 70 years: Predicting eye and hair colour from skeletal remains of World War II victims using the HIrisPlex system. Forensic Sci. Int. Genet. 2017, 26, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Ossowski, A.; Kus, M.; Brzezinski, P.; Pruffer, J.; Piatek, J.; Zielinska, G.; Bykowska, M.; Jalowinska, K.; Torgaszev, A.; Skoryukov, A.; et al. Example of human individual identification from World War II gravesite. Forensic. Sci. Int. 2013, 233, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Terrado-Ortuno, N.; May, P. Forensic DNA phenotyping: A review on SNP panels, genotyping techniques, and prediction models. Forensic. Sci. Res. 2025, 10, owae013. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sobrino, B.; Brion, M.; Carracedo, A. SNPs in forensic genetics: A review on SNP typing methodologies. Forensic. Sci. Int. 2005, 154, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, B.; Carracedo, A. SNP typing in forensic genetics: A review. Methods Mol. Biol. 2005, 297, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Budowle, B. SNP typing strategies. Forensic. Sci. Int. 2004, 146, S139–S142. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, L.; Breslin, K.; Zuniga, S.; Wirken, L.; Pospiech, E.; Kukla-Bartoszek, M.; Sijen, T.; Knijff, P.; Liu, F.; Branicki, W.; et al. The HIrisPlex-S system for eye, hair and skin colour prediction from DNA: Introduction and forensic developmental validation. Forensic. Sci. Int. Genet. 2018, 35, 123–135. [Google Scholar] [CrossRef] [PubMed]
- de la Puente, M.; Santos, C.; Fondevila, M.; Manzo, L.; Consortium, E.U.-N.; Carracedo, A.; Lareu, M.V.; Phillips, C. The Global AIMs Nano set: A 31-plex SNaPshot assay of ancestry-informative SNPs. Forensic Sci. Int. Genet. 2016, 22, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Watherston, J.; McNevin, D.; Gahan, M.E.; Bruce, D.; Ward, J. Current and emerging tools for the recovery of genetic information from post mortem samples: New directions for disaster victim identification. Forensic Sci. Int. Genet. 2018, 37, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Ousley, S.D.; Jantz, R.L. Fordisc 3 and Statistical Methods for Estimating Sex and Ancestry. In A Companion to Forensic Anthropology; Dirkmaat, D.C., Ed.; Blackwell Publishing: Hoboken, NJ, USA, 2012. [Google Scholar]
- Boldsen, J.L.; Milner, G.R.; Hylleberg, R. ADBOU Age Estimation Software. Version 2.0. 2002.
- Boldsen, J.L.; Milner, G.R.; Konigsberg, L.; Wood, J. Transition analysis: A new method for estimating age from skeleton. In Paleodemography: Age Distributions from Skeletal Samples; Hoppa, R., Vaupel, J.W., Eds.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Taylor, K. Forensic Art & Illustration; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Stephan, C.N.; Henneberg, M.; Sampson, W. Predicting nose projection and pronasale position in facial approximation: A test of published methods and proposal of new guidelines. Am. J. Phys. Anthropol. 2003, 122, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Liu, F.; Wollstein, A.; Kovatsi, L.; Ralf, A.; Kosiniak-Kamysz, A.; Branicki, W.; Kayser, M. The HIrisPlex system for simultaneous prediction of hair and eye colour from DNA. Forensic. Sci. Int. Genet. 2013, 7, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Liu, F.; Ballantyne, K.N.; van Oven, M.; Lao, O.; Kayser, M. IrisPlex: A sensitive DNA tool for accurate prediction of blue and brown eye colour in the absence of ancestry information. Forensic. Sci. Int. Genet. 2011, 5, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Chaitanya, L.; Clarisse, L.; Wirken, L.; Draus-Barini, J.; Kovatsi, L.; Maeda, H.; Ishikawa, T.; Sijen, T.; de Knijff, P.; et al. Developmental validation of the HIrisPlex system: DNA-based eye and hair colour prediction for forensic and anthropological usage. Forensic Sci. Int. Genet. 2014, 9, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Alfieri, L.; Mazdai, L.; Frisoni, P.; Gaudio, R.M.; Neri, M. Application of Forensic DNA Phenotyping for Prediction of Eye, Hair and Skin Colour in Highly Decomposed Bodies. Healthcare 2023, 11, 647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chaitanya, L.; Walsh, S.; Andersen, J.D.; Ansell, R.; Ballantyne, K.; Ballard, D.; Banemann, R.; Bauer, C.M.; Bento, A.M.; Brisighelli, F.; et al. Collaborative EDNAP exercise on the IrisPlex system for DNA-based prediction of human eye colour. Forensic Sci. Int. Genet. 2014, 11, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Wollstein, A.; Liu, F.; Chakravarthy, U.; Rahu, M.; Seland, J.H.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; Vingerling, J.R.; et al. DNA-based eye colour prediction across Europe with the IrisPlex system. Forensic Sci. Int. Genet. 2012, 6, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Palmal, S.; Adhikari, K.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; Silva de Cerqueira, C.C.; Bonfante, B.; Chacon-Duque, J.C.; Sohail, A.; Hurtado, M.; Villegas, V.; et al. Prediction of eye, hair and skin colour in Latin Americans. Forensic Sci. Int. Genet. 2021, 53, 102517. [Google Scholar] [CrossRef] [PubMed]
- Melchionda, F.; Silvestrini, B.; Robino, C.; Bini, C.; Fattorini, P.; Martinez-Labarga, C.; De Angelis, F.; Tagliabracci, A.; Turchi, C. Development and Validation of MPS-Based System for Human Appearance Prediction in Challenging Forensic Samples. Genes 2022, 13, 1688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zupanic Pajnic, I.; Zupanc, T.; Leskovar, T.; Cresnar, M.; Fattorini, P. Eye and Hair Color Prediction of Ancient and Second World War Skeletal Remains Using a Forensic PCR-MPS Approach. Genes 2022, 13, 1432. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breslin, K.; Wills, B.; Ralf, A.; Ventayol Garcia, M.; Kukla-Bartoszek, M.; Pospiech, E.; Freire-Aradas, A.; Xavier, C.; Ingold, S.; de La Puente, M.; et al. HIrisPlex-S system for eye, hair, and skin color prediction from DNA: Massively parallel sequencing solutions for two common forensically used platforms. Forensic Sci. Int. Genet. 2019, 43, 102152. [Google Scholar] [CrossRef] [PubMed]
- Diepenbroek, M.; Bayer, B.; Anslinger, K. Phenotype predictions of two-person mixture using single cell analysis. Forensic Sci. Int. Genet. 2023, 67, 102938. [Google Scholar] [CrossRef] [PubMed]
- Sapan, V.; Simsek, S.Z.; Filoglu, G.; Bulbul, O. Forensic DNA phenotyping using Oxford Nanopore Sequencing system. Electrophoresis 2025, 46, 198–211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ragazzo, M.; Puleri, G.; Errichiello, V.; Manzo, L.; Luzzi, L.; Potenza, S.; Strafella, C.; Peconi, C.; Nicastro, F.; Caputo, V.; et al. Evaluation of OpenArray as a Genotyping Method for Forensic DNA Phenotyping and Human Identification. Genes 2021, 12, 221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xavier, C.; de la Puente, M.; Mosquera-Miguel, A.; Freire-Aradas, A.; Kalamara, V.; Vidaki, A.; Gross, T.E.; Revoir, A.; Pospiech, E.; Kartasinska, E.; et al. Development and validation of the VISAGE AmpliSeq basic tool to predict appearance and ancestry from DNA. Forensic Sci. Int. Genet. 2020, 48, 102336. [Google Scholar] [CrossRef] [PubMed]
- Jager, A.C.; Alvarez, M.L.; Davis, C.P.; Guzman, E.; Han, Y.; Way, L.; Walichiewicz, P.; Silva, D.; Pham, N.; Caves, G.; et al. Developmental validation of the MiSeq FGx Forensic Genomics System for Targeted Next Generation Sequencing in Forensic DNA Casework and Database Laboratories. Forensic Sci. Int. Genet. 2017, 28, 52–70. [Google Scholar] [CrossRef] [PubMed]
- Draus-Barini, J.; Walsh, S.; Pospiech, E.; Kupiec, T.; Glab, H.; Branicki, W.; Kayser, M. Bona fide colour: DNA prediction of human eye and hair colour from ancient and contemporary skeletal remains. Investig. Genet. 2013, 4, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- King, T.E.; Fortes, G.G.; Balaresque, P.; Thomas, M.G.; Balding, D.; Maisano Delser, P.; Neumann, R.; Parson, W.; Knapp, M.; Walsh, S.; et al. Identification of the remains of King Richard III. Nat. Commun. 2014, 5, 5631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).