Abstract
Vascular diseases present a significant threat to human health worldwide. Atherosclerosis is the most prevalent vascular disease, accounting for the majority of morbidity and mortality globally. Vascular calcification is a dynamic pathological process underlying the development of atherosclerotic plaques and involves the phenotypic transformation of vascular smooth muscle cells (VSMCs) into osteogenic cells. Specifically, the phenotypic switch in VSMCs often involves modifications in gene expression due to epigenetic changes, including DNA methylation, histone modification, and non-coding RNAs. Understanding the role of these epigenetic changes in regulating the pathophysiology of vascular calcification, along with the proteins and pathways that mediate these changes, will aid in identifying new therapeutic candidates to enhance vascular health. This review discusses a comprehensive range of epigenetic modifications and their implications for vascular health and the development of vascular calcification.
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of morbidity and mortality worldwide, with an estimated 18 million deaths annually, 85% of which are due to myocardial infarction and stroke [1]. Accompanied by the increasing incidence of hypertension and type 2 diabetes in an aging population, the obesity epidemic has hindered our ability to address the worldwide burden of CVD despite advances in biomedical research [2]. Furthermore, the costs of CVD are projected to climb to approximately USD 1.1 trillion in the United States by 2050 [3]. Understanding the complex pathophysiology of CVD is essential to foster the development of novel therapies and reduce the burden of CVD.
Atherosclerosis is a disease characterized by the progressive buildup of plaque in the walls of arteries, ultimately leading to tissue ischemia [4,5]. Coronary artery disease (CAD), carotid artery disease, and peripheral artery disease are three common forms of atherosclerosis that impact blood supply to the heart, brain, and lower extremities, respectively. Vascular calcification occurs in atherosclerotic plaques due to deposition of calcium phosphate minerals in the extracellular matrix [6]. The crucial step leading to vascular calcification is the phenotypic alteration of VSMCs in the medial layer of blood vessels into osteogenic cells. VSMCs typically exist in a contractile, non-proliferative phenotype. However, in response to various stimuli, they undergo a phenotypic switch with increased proliferation and reduced contractility. Additionally, vascular aging, along with risk factors such as diabetes, hypertension, metabolic disorders, and inflammation, further contributes to this pathological transformation of VSMCs [7,8].
The molecular mechanisms involved in this phenotypic transition include changes in gene expression that lead to the upregulation of bone-related proteins, such as runt-related transcription factor 2 (RUNX2), bone morphogenetic proteins (BMPs), alkaline phosphatase (ALP), osteocalcin, osteopontin, etc., and downregulation of smooth muscle cell-specific marker proteins such as transgelin (SM22α), calponin, and alpha-smooth muscle actin (αSMA) [9,10,11,12]. Various signaling pathways, including Wnt and Notch, regulate VSMC gene expression by activating transcriptional factors or epigenetic modifications (Figure 1) [13,14].
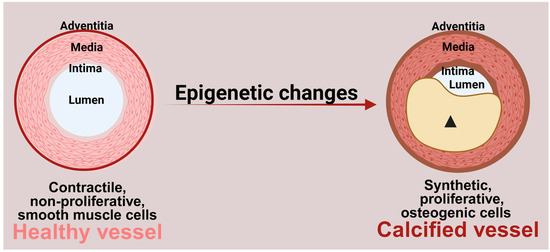
Figure 1.
Role of epigenetic changes in vascular calcification. Epigenetic changes contribute to the transition of contractile, non-proliferative smooth muscle cells to highly proliferative, osteogenic cells. These osteogenic cells secrete calcium phosphate minerals into the extracellular matrix, resulting in vascular calcification. (Arrowhead: calcified plaque). Figure made in Bio-Render.
Epigenetic modifications generally include DNA methylation and histone modifications (methylation, acetylation, lactylation, etc.) (Figure 2), which regulate gene expression at the transcriptional level [15,16]. Additionally, non-coding RNAs act as epigenetic regulators that modulate gene expression both post-transcriptionally and at the transcriptional level [17]. Numerous studies have highlighted the role of epigenetics in the phenotypic switch of VSMCs to osteogenic cells. Epigenetic regulation has garnered significant attention as a therapeutic target in vascular calcification. This review explores the mechanisms mediating the epigenetic regulation of vascular calcification, ultimately leading to the osteogenic trans-differentiation of VSMCs.
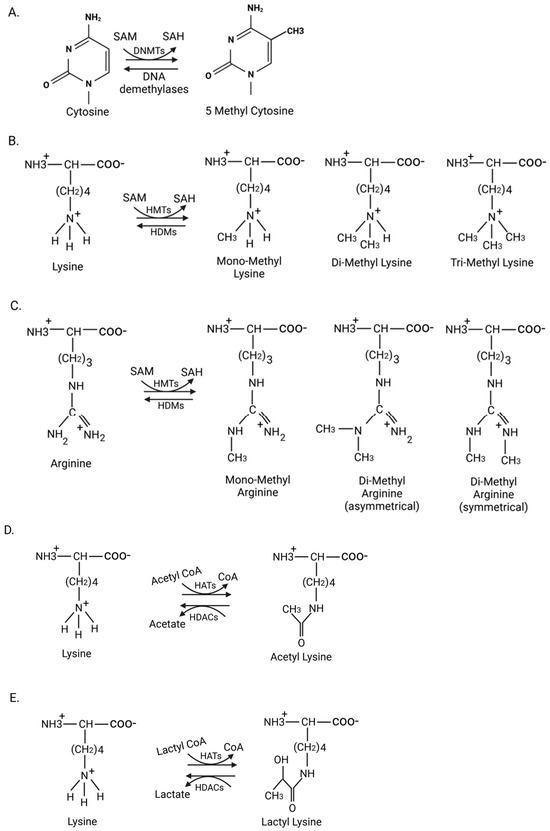
Figure 2.
Various epigenetic modifications and their mediators. (A) DNA methylation: DNA methyltransferases (DNMTs) mediate methylation of cytosine residues on CpG islands, giving rise to 5-methylcytosine, which can be reversed by DNA demethylases. (B,C) Histone methylation: Histone methyl transferases (HMTs) add methyl groups to the epsilon amino group of lysine or the guanidino group of arginine on histones, resulting in either mono-, di-, or trimethyl states of lysine, and mono- and dimethyl states of arginine. These histone methyl groups are removed by histone demethylases (HDMs). (D,E) Histone acetylation and lactylation: Histone acetyltransferases (HATs) transfer acetyl and lactyl groups to the epsilon amino group of lysine on histones, resulting in histone acetylation and lactylation, respectively. These groups are removed by histone deacetylases (HDACs). SAM: S-adenosyl methionine; SAH: S-adenosyl homocysteine. Figure made in Bio-Render.
2. DNA Methylation
DNA methylation involves the transfer of methyl groups to cytosine bases, resulting in the formation of 5-methylcytosine. This process is catalyzed by DNA methyltransferases (DNMTs) (Figure 2A). Based on their structure and function, DNMTs are classified as either maintenance enzymes, which include DNMT1, or de novo enzymes, which include DNMT3a and DNMT3b. DNMT1 copies the DNA methylation pattern from the parental strand to the newly synthesized strand during DNA replication, while DNMT3a and DNMT3b add methyl groups to unmethylated DNA [18,19].
DNA methylation has been extensively studied for its role in genome organization and the regulation of gene expression. A guanine residue follows cytosine residues that undergo methylation. These CpG dinucleotides are found in stretches of approximately 1000 base pair regions in the genome, known as CpG islands, which are often associated with gene promoter regions, and their methylation regulates gene expression, usually by inhibiting the binding of transcriptional factors [20,21]. Therefore, regulating the DNA methylation of CpG islands can influence the expression of genes that control processes such as cell differentiation, a crucial aspect of vascular calcification.
Vascular calcification is a clinical manifestation commonly linked with chronic kidney disease, which hinders renal filtration and accumulates various toxins and metabolites, including phosphate and indoxyl sulfate [22,23,24]. In vitro and ex vivo studies using human aortic VSMCs, and animal models have shown that high levels of phosphate and indoxyl sulfate induce calcification through alterations in DNA methylation [25,26]. High concentrations of phosphate also enhance the activity of DNMT1, leading to the upregulation of the osteogenic marker runt-related transcription factor 2 (RUNX2/Cbfa1) and the downregulation of the smooth muscle contractile protein SM22α, which results in a phenotypic switch of VSMCs and vascular calcification (Figure 3a). Conversely, inhibiting DNMT1 with procaine, a DNA demethylating agent that decreases 5-methylcytosine levels [27], prevents phosphate-induced calcification [25]. Similar results have been observed after treating cells and animal models with indoxyl sulfate. Indoxyl sulfate promotes vascular calcification by enhancing the expression of DNMT1, which leads to promoter methylation and decreases the expression of the anti-calcification protein Klotho. The pro-calcific effect of indoxyl sulfate is inhibited by treatment with DNMT1 inhibitor 5-aza-2′-deoxycytidine (5Aza-2dc) [28], that binds to DNA and inhibits its activity [26].
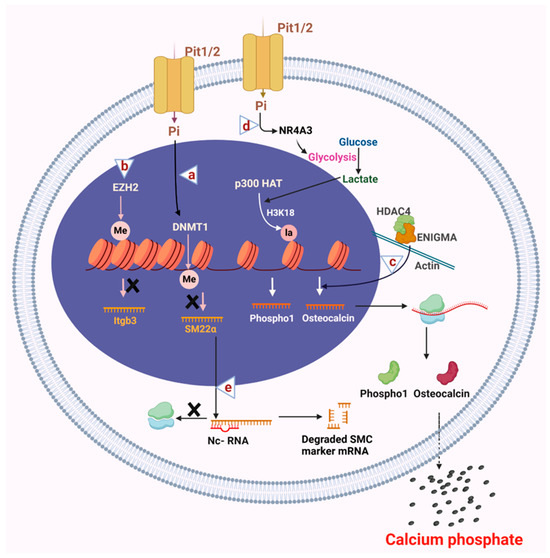
Figure 3.
Epigenetic alterations in the VSMC osteogenic switch. (a) High phosphate conditions activate DNMT1, which causes methylation of CpG islands of DNA, leading to transcriptional repression of smooth muscle cell contractile protein SM22α. (b) Histone methyl transferase EZH2 binds to the promoter region of Itgb3 and inhibits its expression, which promotes smooth muscle cell migration and calcification. (c) In the cytosol, histone deacetylase HDAC4 interacts with the cytosolic binding protein ENIGMA, an actin cytoskeleton binding protein. The HDAC4 and ENIGMA complex activates the expression of osteogenic protein osteocalcin through mechanosensing that results in calcification. (d) Increased expression of NR4A3 under high phosphate conditions causes an increase in glycolytic flux that leads to accumulation of lactate, which p300 HAT uses to mediate histone lactylation of the promoter regions of the Phospho1 gene, thereby causing an increase in its expression and calcification. (e) Non-coding RNAs play an essential role in osteogenic differentiation by inhibiting the expression of VSMC contractile proteins by binding and degrading their mRNAs. Altogether, epigenetic mechanisms lead to the osteogenic differentiation of VSMCs that secrete calcium phosphate minerals into the extracellular space, leading to vascular calcification. ×: denotes inhibition of expression. Figure made in Bio-Render.
Aging is a significant risk factor for atherosclerosis [29,30]. Vascular aging is associated with remodeling of the extracellular matrix (ECM), primarily involving the breakdown of elastin, deposition of collagen, and cross-linking, which increases vascular stiffness [31,32]. Matrix stiffness has been shown to regulate the phenotype of vascular smooth muscle cells by influencing DNA methylation. VSMCs cultured on stiffer substrates that mimic increased ECM stiffness exhibit a reduced expression of DNMT1 compared to those on softer substrates. This decrease in DNMT1 leads to a diminished expression of smooth muscle cell marker proteins, such as alpha-smooth muscle actin, calponin, and SM22α, along with an increased expression of osteogenic marker proteins such as BMP2 and RUNX2, contributing to a phenotypic shift toward osteogenic cells, followed by calcification [33].
ECM stiffness regulates DNMT1 expression through mechanotransduction. This process involves sensing both external and internal mechanical cues (mechanosensing) and responding to these cues by inducing changes in gene expression and phenotypic transition [34,35]. For instance, increased ECM stiffness activates mechanosensory discoidin domain receptor 1 (DDR1) on VSMC cells, which triggers ERK-mediated phosphorylation of p53 and subsequent p53-mediated inhibition of DNMT1 expression, resulting in the transition of VSMCs to an osteogenic phenotype [36].
In summary, cardiovascular risk factors like chronic kidney disease and vascular aging change the functionally differentiated VSMC phenotype into a synthetic osteogenic phenotype by downregulating smooth muscle contractile proteins and upregulating synthetic proteins. These changes are driven by alterations in DNA methylation status in the promoter regions of the respective genes, leading to phenotypic changes and vascular calcification that ultimately contribute to atherosclerotic cardiovascular disease.
3. Histone Modifications
Histones are basic proteins that are rich in lysine and arginine. DNA, in association with histones, is organized into nucleosomes, which are the basic units of chromatin [37]. Besides DNA methylation, the post-translational modification of histones also regulates gene expression by controlling chromatin accessibility to transcriptional machinery. Histone-modifying enzymes, such as histone methyltransferases/demethylases and histone acetyltransferases/deacetylases, manage histone interactions with DNA by adding and removing methyl and acetyl groups. This process changes chromatin organization into either euchromatin, which is accessible to the binding of transcription factors and leads to gene expression, or heterochromatin, which is inaccessible to transcription factors and inhibits gene expression [38,39,40,41]. These changes in chromatin structure ultimately alter the VSMC phenotype. Among histone modifications, histone methylation, acetylation, and lactylation play crucial roles in the VSMC osteogenic phenotypic switch.
3.1. Histone Methylation
Histones undergo post-translational modifications on their lysine and arginine residues through histone methyltransferases (Figure 2B,C) [42,43]. These methyltransferases contain a catalytic SET domain that has been identified as a conserved region in three proteins of Drosophila melanogaster: Suppressor of variegation 3-9 (Su(var)3-9), Enhancer of zeste (E(z)), and Trithorax (Trx) [44]. Histone demethylases have a jumonji C (Jmjc) domain as their catalytic domain [45,46]. Three distinct states of histone methylation exist: mono-, di-, and tri-methylation. Depending on the position and state of methylation, histone modifications can either activate (e.g., H3K4, H3K36, H3K79) or repress (e.g., H3K9, H3K27, H4K20) transcription [47]. Numerous studies have identified histone methylation as a crucial regulator of the VSMC phenotypic switch in vascular calcification.
Inflammation is linked to vascular calcification. Pro-inflammatory cytokines released by macrophages are crucial in vascular calcification, as they promote the expression of osteogenic markers and induce phenotypic changes in VSMCs [48,49].
A recent study demonstrated that the proinflammatory cytokine interleukin-6 promotes vascular calcification partly by regulating histone methylation. Interleukin-6 (IL-6)/soluble interleukin-6 receptor (sIL-6R) encourages vascular calcification through an increase in phosphorylated STAT3 (signal transducer and activator of transcription 3), which, in combination with JMJD2B (histone demethylase), suppresses the repressive H3K9Me3 mark near the RUNX2 promoter region, leading to an increased expression of RUNX2 and the transition of VSMCs to osteogenic cells [50].
The transition of VSMCs to an osteogenic phenotype is associated with increased expression of synthetic genes and decreased expression of contractile genes, followed by enhanced proliferation, migration, and calcification [51]. The histone methyltransferase Enhancer of zeste homolog 2 (EZH2) is a polycomb repressive complex 2 (PRC2) component that suppresses gene expression through repressive H3K27 dimethylation or trimethylation. Knockdown of EZH2 in mouse VSMCs resulted in reduced synthetic gene expression and decreased proliferation, migration, and calcification of VSMCs. It was also discovered that EZH2 interacts with the promoter of integrin Itgb3 and represses its expression, leading to VSMC phenotypic transition towards a synthetic state (Figure 3b) [52]. Increased dimethylation of arginine 17 on histone H3 in low-density lipoprotein receptor-related protein 6 (LRP6) knockout mice is linked to dysregulated osteopontin expression, a glycoprotein produced by various cell types that plays a crucial role in vascular calcification by promoting the proliferation and migration of VSMCs [53].
3.2. Histone Acetylation
Histone acetyltransferases (HATs) and histone deacetylases (HDACs) maintain the homeostatic balance of histone acetylation. These enzymes catalyze the addition and removal of acetyl groups from histones, changing the chromatin structure and the expression of target genes (Figure 2D) [54,55]. Modifying the activity of HATs and HDACs is essential in regulating the VSMC phenotype.
Based on their homology to yeast HDACs, human HDACs are grouped into four classes: class I (HDAC 1,2,3,8), class II (HDAC 4,5,6,7,9,10), class III, which consists of sirtuins, and class IV, which includes HDAC 11. Based on the domain composition, class II is subdivided into class IIa (HDAC 4,5,7,9) and class IIb (HDAC 6,10) [56]. HDAC classes I and II and sirtuins have been shown to regulate vascular calcification. This review discusses the role of class I and II HDACs in vascular calcification. We refer to reviews by Pan et al. [57] and Wang et al. [58] for the role of sirtuins in vascular calcification.
The class I HDACs are predominantly localized in the nucleus and contain a deacetylase domain for histone deacetylation. Among class I HDACs, HDAC1 and HDAC2 inhibit vascular calcification, at least in part, through the regulation of autophagy. Autophagy is protective in vascular calcification by inhibiting VSMC phenotypic transition [59,60]. In rat VSMCs, HDAC1 inhibits vascular calcification by reducing the expression of histone demethylase LSD1 via regulation of H3K9 acetylation of the LSD1 promoter region. Reduced LSD1 leads to increased autophagy and reduced vascular calcification [61]. HDAC2 overexpression attenuates the expression of osteopontin and osteocalcin. It inhibits the downregulation of αSMA and SM22α through increased autophagic flux in mouse models of CKD and human VSMCs treated with β-glycerophosphate [62].
Among class II HDACs, class IIa HDACs contain a deacetylase domain at their C-terminus and a MEF2 transcription factor binding domain at their N-terminus [63]. Class IIa HDACs shuttle between the nucleus and cytosol. Several important phosphorylation sites in class IIa HDACs regulate their nucleo-cytoplasmic localization. Once phosphorylated by specific kinases, the binding of 14-3-3 chaperones in the cytosol blocks their nuclear localization and allows cytoplasmic retention [64]. The cytosolic localization of HDAC4, mediated by a salt-inducible kinase (SIK), promotes vascular calcification by increasing osteocalcin expression [65]. Additionally, cytosolic HDAC4 engages with ENIGMA, a protein that binds to the actin cytoskeleton. The cytoskeleton connects to the nucleus via the linker of the nucleoskeleton and cytoskeleton complex (LINC), regulating gene expression through mechanosensing [66]. Speculation suggests the HDAC4-ENIGMA complex might influence VSMC gene expression via the LINC complex under osteogenic conditions (Figure 3c).
Our recent genome-wide meta-analysis study found that single nucleotide polymorphisms (SNPs) in the HDAC9 locus are associated with abdominal aortic calcification. Further, we showed that siRNA-mediated knockdown of HDAC9 in human VSMCs reduces RUNX2 expression and vascular calcification, while adenoviral expression of HDAC9 increased vascular calcification through increased expression of RUNX2 [67]. In addition, the loss of HDAC9 attenuated vascular calcification in the matrix Gla protein knockout (MGP KO) mouse model of vascular calcification, indicating that HDAC9 is a strong promoter of in vivo vascular calcification. Like HDAC4, HDAC9 shuttles between the nucleus and cytosol, and one study suggests that HDAC9 promotes vascular calcification by activating NF-κB [68]. However, the precise mechanisms by which HDAC9 regulates RUNX2 expression under osteogenic conditions remain incompletely elucidated.
3.3. Histone Lactylation
Besides its canonical role in adding acetyl groups, p300 acetyltransferase can also add lactyl groups in a process known as histone lactylation (Figure 2E). Lactate produced in glycolysis is a substrate to mediate histone lysine lactylation. This recently identified histone epigenetic modification plays a role in the metabolic regulation of gene expression [69].
Lactate is a byproduct of glycolysis, and VSMCs rely extensively on glycolysis, which results in lactate production [70]. Lactate promotes the VSMC synthetic phenotype with increased proliferation and migration [71]. Recent studies have emphasized the role of lactate in regulating VSMC calcification through histone lactylation. The transcription factor NR4A3 (nuclear receptor subfamily 4 group A member) regulates glucose metabolism. Under high phosphate stimulation, NR4A3 expression increases, promoting glycolysis and lactate production in VSMCs, which leads to enhanced p300 acetyltransferase-mediated lactylation of histone H3 (H3K18la) in the promoter region of Phospho1, a gene that facilitates VSMC calcification. This results in increased expression of Phospho1, which mediates the secretion of inorganic phosphate and is a potent inducer of vascular calcification (Figure 3d) [72]. Given that this is a relatively new field of study, more research is needed to understand the effects of histone lactylation globally on VSMC phenotype and function.
Overall, post-translational modification of histones, mediated by histone-modifying enzymes, plays a crucial role in regulating the VSMC phenotype. Cardiovascular risk factors such as inflammation and metabolic abnormalities promote vascular calcification through histone methylation and lactylation. On the one hand, some histone deacetylases, such as HDAC1 and HDAC2, inhibit vascular calcification by promoting autophagy, while others, such as HDAC4, promote it by regulating cytosolic non-histone proteins.
4. Non-Coding RNAs
Non-coding RNAs act as epigenetic regulators essential for post-transcriptional regulation (Figure 3e). They are classified into two main types based on size: long non-coding RNAs (lncRNAs) and small non-coding RNAs. Long non-coding RNAs are >200 nucleotides long. By contrast, small non-coding RNAs are <200 nucleotides long and include microRNAs (~20 nucleotides long), circular RNAs, small nuclear RNAs, and small nucleolar RNAs. Among small non-coding RNAs, microRNAs have been extensively studied for their role in vascular calcification [73,74,75]. The present review addresses the role of long non-coding RNAs and microRNAs in promoting vascular calcification.
4.1. Long Non-Coding RNAs
Long non-coding RNAs (lncRNAs) are defined as non-protein coding RNAs longer than 200 nucleotides. Their function depends on their binding target: lncRNAs can modulate the stability of DNA/RNA or can bind and change chromatin complex structures in the form of epigenetic regulation [76]. lncRNAs modulate the effects of many calcification factors, including the osteogenic effects of high-phosphate conditions [77,78,79], the inhibitory effect of antioxidants [80], and the calcifying effects of mineralocorticoid receptors [81]. The mechanisms by which lncRNAs modulate calcification are similarly diverse with wide-ranging effects on inflammation, autophagy, and cellular senescence, amongst other processes. Most pro-calcifying lncRNAs are found to act through miRNA sponging, in which the lncRNA binds a target miRNA to inactivate it. The lncRNA H19, which is imprinted maternally, sponges several microRNAs to increase inflammatory TLR3 signaling and Erk1/2 phosphorylation, and ultimately cause Capitalize RUNX2 upregulation and calcification [82,83,84]. Similarly, the lncRNA Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) promotes RUNX2-mediated calcification by sponging miR-30c, while LINC00458 (aka ES3) promotes calcification and senescence through direct sponging of miR-34c-5p and resulting increases in Bcl-2 modifying factor [85,86].
Other lncRNAs promote vascular calcification through their effects on cellular senescence. The pro-calcification lncRNAs small nucleolar RNA host gene 1 (SNHG1), ES3, and BMF antisense 1 (BMF-AS1) regulate microRNAs, transcription factors, and other proteins to promote dysregulated autophagy, thereby leading to senescence and calcification [86,87,88].
In addition to influencing master regulators of vascular calcification, lncRNAs can reduce calcification through various signaling pathways. Inhibition of vascular calcification via lncRNAs such as small nucleolar RNA host gene 29 (SNHG29) and long intergenic RNA-erythroid pro-survival (lincRNA-EPS) has been associated with increased Klotho signaling and decreased Smad3, Wnt, and β-catenin signaling [89,90]. lncRNA GAS5 may inhibit vascular calcification by promoting PTEN (phosphatase and tensin homolog) signaling and suppressing VSMC phenotypic switching, proliferation, and migration [91,92].
As assays evolve and annotations become increasingly available for genes and transcripts, searches for influential lncRNAs have increased in scope and produced large sets of lncRNAs associated with vascular calcification. Multiple studies have conducted transcriptome-wide searches for gene transcripts influenced by calcifying conditions [77,93,94]. These studies have generated regulatory networks to identify lncRNAs that influence important proteins in calcification pathways, such as the transcription factors FOXO1 and SNAI2 [95]. Thus, these assays provide additional lncRNAs that identify new molecular mechanisms of calcification and potential therapeutic targets for modifying signaling pathways, transcription factors, and other mechanisms important to vascular calcification.
In summary, lncRNAs are a class of epigenetic regulators known to target many well-known signaling pathways and cellular phenomena in the vasculature. Though it is a newer area of investigation, targeting lncRNAs may represent a powerful class of epigenetic therapies in vascular calcification.
4.2. Small Non-Coding RNAs
MicroRNAs have been extensively studied among small non-coding RNAs for their role in vascular calcification [96]. MicroRNAs are approximately 22 nts long and mostly bind to the 3′UTR of target mRNA, leading to their degradation and inhibition of gene expression. Several miRNAs regulate the VSMC osteogenic transition; some promote, while others inhibit. Some well-studied miRNAs promoting vascular calcification include miR-32, miR-34a, and miR-155. Among these, miR-32 has been shown to promote vascular calcification through various mechanisms, including inhibition of PTEN, which activates PI3K/AKT signaling, resulting in increased expression and phosphorylation of osteogenic mediator RUNX2 [97]. A recent study demonstrated that treating high-phosphate-induced VSMCs with the Bushen Huoxue Formula, a traditional Chinese medicine, reduced exosomal miR-32, enhanced PTEN expression, and reduced osteogenic mediator proteins BMP-2 and RUNX2 [98]. Additionally, miR-32 promotes vascular calcification by inhibiting autophagy [99] and by increasing the expression of proinflammatory mediator TNFα [100]. Vascular miR-34a expression increases with aging and is essential in age-mediated vascular senescence. Increased miR-34a levels were associated with increased secretion of pro-inflammatory cytokine IL-6 and increased arterial inflammation and vascular calcification [101]. Further, miR-34a reduces the expression of vascular calcification inhibitors Axl (AXL Receptor Tyrosine Kinase) and SIRT1 (Sirtuin 1) [102]. Indoxyl sulfate has been shown to induce miR-155, downregulating the anti-calcific protein matrix Gla protein (MGP), thereby increasing vascular calcification [103]. Further, depletion of miR-155 inhibits vascular calcification by inhibiting AKT phosphorylation and FOXO3a degradation [104]. Several other miRNAs exert an inhibitory effect on vascular calcification. For example, endothelial-derived miR-204-5p inhibits vascular calcification by reducing the expression of osteogenic mediators RUNX2 and BMP2 [105]. Another miRNA, mir-133a, inhibits osteogenic differentiation and VSMC calcification by downregulating the expression of RUNX2 [106].
Data supporting the vital role of microRNAs in vascular calcification continue to emerge. Each miRNA likely has more than one target, making the biology of miRNAs in vascular calcification multi-factorial. Moreover, the fact that miRNAs are expressed in a tissue-specific manner but are also secreted (i.e., in exosomes) confers the ability for miRNAs to exert effects in an autocrine, paracrine, and long-range endocrine fashion that adds to the complex downstream effects of miRNAs in vivo.
5. Targeting Epigenetic Regulators to Treat Vascular Calcification
Thus far, no therapies have been approved to treat vascular calcification. Preventative strategies include lifestyle modifications to prevent risk factors such as diabetes, hypertension, and chronic kidney disease. When prevention fails, interventionalists can offer methods to treat stenotic lesions of vascular calcification. For instance, rotational atherectomy has been used to treat calcified coronary and peripheral lesions through the high-speed rotation of a diamond-tipped burr that fractures and debulks calcium plaque. Intravascular lithotripsy is another technique that disrupts calcium plaques using ultrasonic shock waves [107,108]. While these methods help treat patients with established vascular calcium deposits, preventative treatments for calcification are urgently needed to prevent adverse cardiovascular outcomes.
Several intervention studies have investigated the potential efficacy of drugs to attenuate vascular calcification. A recent review summarized the results of several randomized clinical trials on calcification attenuation [109]. Aged garlic extract (AGE) has been shown to inhibit lipid accumulation in the thoracic aorta and prevent the phenotypic switch of VSMCs [110]. In multiple studies, AGE treatment significantly attenuated coronary artery calcification progression and subclinical atherosclerosis [111,112,113,114,115,116]. Statins, which are hydroxy methyl glutaryl-CoA (HMG-CoA) reductase inhibitors that inhibit cholesterol biosynthesis in the liver, do not attenuate calcification [117,118,119,120,121,122,123,124,125,126,127,128,129]. Vitamin K is a co-factor for the gamma-glutamyl carboxylase enzyme that catalyzes the carboxylation of glutamate residues in matrix Gla protein [130], whose carboxylation status is critical for its anti-calcific properties [131]. Vitamin K treatment may reduce the progression of coronary artery calcification [132,133], but larger-scale clinical trials are needed to define these potential benefits. Lifestyle changes, including strict dietary control and physical activity, did not attenuate coronary calcification [134,135,136,137]. In a trial using nifedipine, an anti-hypertensive agent, the authors found a significant attenuation in coronary calcium [138]; however, another study with the same drug showed no effect [139]. Omega-3 fatty acids [140,141], hypoglycemic agents to reduce blood sugar levels [142,143], hormone replacement therapies [144,145,146], and treatment with inhibitors of platelet aggregation, such as sarpogrelate [147] and cilostazol [148], have all been studied to assess the impact on coronary artery calcification and non-calcified atherosclerotic plaque with these trials having mixed results and for the most part not showing a significant reduction in calcified plaque.
Since transforming VSMCs to osteogenic cells is a crucial step in calcification, therapies that prevent this phenotype switch may help treat vascular calcification. As discussed above, in vitro and in vivo evidence from many studies suggests that epigenetic regulators of VSMC osteogenic phenotype switch may be an excellent therapeutic strategy for preventing (or slowing the progression of) vascular calcification. For example, inhibition of DNMTs attenuates osteogenic differentiation under high phosphate conditions that model chronic kidney disease. The FDA has approved the DNMT inhibitor 5-azacytidine to treat myelodysplastic syndromes, and it could be a potential therapeutic agent for inhibiting osteogenic differentiation [149]. In addition to DNMTs, HMTs promote osteogenic differentiation of VSMCs. Tazemetostat is another drug approved by the FDA for treating epithelioid sarcoma and is an inhibitor of histone methyltransferase EZH2 [150]. Histone acetylation is vital in VSMC osteogenic differentiation, and class IIa histone deacetylases promote osteogenic differentiation. Four HDAC inhibitors, vorinostat [151,152], romidepsin [153], panobinostat [154], and belinostat [155], have been approved by the FDA for treating hematologic cancers. Further study is needed to determine if these FDA-approved therapies can be used as an alternative indication of vascular calcification. However, the HDAC inhibitors are non-selective and inhibit more than one class of HDACs. Since class I HDACs protect against vascular calcification while class II HDACs promote it, non-specific HDAC inhibitors may not be ideal for treating it. Therefore, there is a need to develop more selective pharmacological agents to inhibit HDACs in calcification.
Many in vitro studies have shown that vascular calcification can be mitigated by correcting perturbations in lncRNA levels. The downregulation of H19 reduces calcification in vitro through either MAPK or NF-κB signaling reduction [83,84]. Similarly, inhibition of lncRNAs TUG1 [156] and MALAT1 [85] reduced calcification through suppression of RUNX2 expression and FAS-AS1 inhibition reduced calcification by suppressing inflammatory response to hyperphosphatemia [157]. Restoration of the anti-calcifying lncRNAs GAS5 [79] and ANCR [78] has been shown to mitigate vascular calcification by increasing signaling through the PTEN pathway or enhancing autophagy. ANCR is particularly interesting and has been tested as an agent for reducing calcification in vascular graft tissues. By supplying exosomes containing ANCR targeted at Gli+ (smooth muscle progenitor) cells, osteogenic differentiation was mitigated, and the development of calcification was suppressed [158]. Similarly, small non-coding RNAs, such as microRNAs, affect vascular calcification by inhibiting PTEN, autophagy, or attaining a senescence-associated secretory phenotype of smooth muscle cells that secrete proinflammatory cytokines that aid in VSMC calcification [97,99,101]. Alternatively, some miRNAs inhibit calcification by reducing the expression of osteogenic markers such as RUNX2 and BMPs [105,106]. Future therapies for non-coding RNAs may be promising, as precisely designed antisense oligonucleotides can specifically target non-coding RNAs. However, various issues must be addressed to put these therapies into practice, such as oligonucleotide stability, targeted delivery to tissue (vasculature) and the possibility of off-target effects [159].
In conclusion, many primary risk factors for cardiovascular diseases—including chronic kidney disease, inflammation, vascular stiffness, and metabolic abnormalities—contribute to the osteogenic phenotypic switch of vascular smooth muscle cells by altering the epigenetic landscape, resulting in vascular calcification. With no currently approved therapies, attenuating vascular calcification remains a challenge and an opportunity for future research endeavors.
Author Contributions
Conceptualization, L.P.K. and R.M; writing—original draft preparation, L.P.K., Y.Y.G., S.L. and M.C.; writing—review and editing, L.P.K., Y.Y.G., S.L. and R.M.; supervision, R.M.; funding acquisition, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
R. Malhotra (R01HL159514 and R01HL162928) and S. Lee (F32HL164025) are supported by the National Institutes of Health. Y. Y. Guo is supported by the Sarnoff Foundation.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AGE | Aged garlic extract |
| αSMA | Alpha-smooth muscle actin |
| ALP | Alkaline phosphatase |
| ANCR | Angelman syndrome chromosome region |
| 5aza-2dc | 5-aza-2′-deoxycytidine |
| BMPs | Bone morphogenic proteins |
| CVDs | Cardiovascular diseases |
| DDR1 | Discoidin domain receptor 1 |
| DNMTs | DNA methyltransferases |
| ECM | Extracellular matrix |
| ERK | Extracellular signal-regulated kinase |
| EZH2 | Enhancer of zeste homolog 2 |
| FOXO | Forkhead box O |
| GAS5 | Growth arrest-specific 5 |
| HATs | Histone acetyltransferases |
| HDACs | Histone deacetylases |
| HMTs | Histone methyl transferases |
| IL-6 | Interleukin-6 |
| LINC | Linker of the nucleoskeleton and cytoskeleton complex |
| lncRNAs | Long non-coding RNAs |
| lincRNA-EPS | Long intergenic RNA-erythroid pro-survival |
| LRP6 | Lipoprotein receptor-related protein 6 |
| MALAT1 | Metastasis associated lung adenocarcinoma transcript 1 |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa B |
| NR4A3 | Nuclear receptor subfamily 4 group A member |
| PRC2 | Polycomb repressive complex 2 |
| PTEN | Phosphate and tensin homolog |
| RUNX2 | Runt-related transcription factor 2 |
| sIL-6R | Soluble interleukin-6 receptor |
| SM22α | Smooth muscle protein 22-alpha |
| SNAI2 | Snail family transcriptional repressor 2 |
| SNHG1 | Small nucleolar RNA host gene 1 |
| STAT3 | Signal transducer and activator of transcription 3 |
| TLR3 | Toll like receptor 3 |
| TUG1 | Taurine upregulated 1 |
| VSMCs | Vascular smooth muscle cells |
| Wnt | Wingless integrated |
References
- Mocumbi, A.O. Cardiovascular Health Care in Low- and Middle-Income Countries. Circulation 2024, 149, 557–559. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Joynt Maddox, K.E.; Elkind, M.S.V.; Aparicio, H.J.; Commodore-Mensah, Y.; de Ferranti, S.D.; Dowd, W.N.; Hernandez, A.F.; Khavjou, O.; Michos, E.D.; Palaniappan, L.; et al. Forecasting the Burden of Cardiovascular Disease and Stroke in the United States Through 2050-Prevalence of Risk Factors and Disease: A Presidential Advisory From the American Heart Association. Circulation 2024, 150, e65–e88. [Google Scholar] [CrossRef]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef]
- Onnis, C.; Virmani, R.; Kawai, K.; Nardi, V.; Lerman, A.; Cademartiri, F.; Scicolone, R.; Boi, A.; Congiu, T.; Faa, G.; et al. Coronary Artery Calcification: Current Concepts and Clinical Implications. Circulation 2024, 149, 251–266. [Google Scholar] [CrossRef]
- Sutton, N.R.; Malhotra, R.; St Hilaire, C.; Aikawa, E.; Blumenthal, R.S.; Gackenbach, G.; Goyal, P.; Johnson, A.; Nigwekar, S.U.; Shanahan, C.M.; et al. Molecular Mechanisms of Vascular Health: Insights From Vascular Aging and Calcification. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 15–29. [Google Scholar] [CrossRef]
- Elmarasi, M.; Elmakaty, I.; Elsayed, B.; Elsayed, A.; Zein, J.A.; Boudaka, A.; Eid, A.H. Phenotypic switching of vascular smooth muscle cells in atherosclerosis, hypertension, and aortic dissection. J. Cell Physiol. 2024, 239, e31200. [Google Scholar] [CrossRef]
- Tyson, J.; Bundy, K.; Roach, C.; Douglas, H.; Ventura, V.; Segars, M.F.; Schwartz, O.; Simpson, C.L. Mechanisms of the Osteogenic Switch of Smooth Muscle Cells in Vascular Calcification: WNT Signaling, BMPs, Mechanotransduction, and EndMT. Bioengineering 2020, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Byon, C.H.; Yuan, K.; Chen, J.; Mao, X.; Heath, J.M.; Javed, A.; Zhang, K.; Anderson, P.G.; Chen, Y. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ. Res. 2012, 111, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Hruska, K.A.; Mathew, S.; Saab, G. Bone Morphogenetic Proteins in Vascular Calcification. Circ. Res. 2005, 97, 105–114. [Google Scholar] [CrossRef]
- Steitz, S.A.; Speer, M.Y.; Curinga, G.; Yang, H.Y.; Haynes, P.; Aebersold, R.; Schinke, T.; Karsenty, G.; Giachelli, C.M. Smooth muscle cell phenotypic transition associated with calcification: Upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001, 89, 1147–1154. [Google Scholar] [CrossRef]
- Bundy, K.; Boone, J.; Simpson, C.L. Wnt Signaling in Vascular Calcification. Front. Cardiovasc. Med. 2021, 8, 708470. [Google Scholar] [CrossRef]
- Shimizu, T.; Tanaka, T.; Iso, T.; Doi, H.; Sato, H.; Kawai-Kowase, K.; Arai, M.; Kurabayashi, M. Notch Signaling Induces Osteogenic Differentiation and Mineralization of Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1104–1111. [Google Scholar] [CrossRef]
- Portela, A.; Esteller, M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010, 28, 1057–1068. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Patil, V.S.; Zhou, R.; Rana, T.M. Gene regulation by non-coding RNAs. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 16–32. [Google Scholar] [CrossRef]
- Lyko, F. The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat. Rev. Genet. 2018, 19, 81–92. [Google Scholar] [CrossRef]
- Jin, B.; Robertson, K.D. DNA methyltransferases, DNA damage repair, and cancer. Adv. Exp. Med. Biol. 2013, 754, 3–29. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef]
- Hutcheson, J.D.; Goettsch, C. Cardiovascular Calcification Heterogeneity in Chronic Kidney Disease. Circ. Res. 2023, 132, 993–1012. [Google Scholar] [CrossRef]
- Palit, S.; Kendrick, J. Vascular calcification in chronic kidney disease: Role of disordered mineral metabolism. Curr. Pharm. Des. 2014, 20, 5829–5833. [Google Scholar] [CrossRef]
- Dube, P.; DeRiso, A.; Patel, M.; Battepati, D.; Khatib-Shahidi, B.; Sharma, H.; Gupta, R.; Malhotra, D.; Dworkin, L.; Haller, S.; et al. Vascular Calcification in Chronic Kidney Disease: Diversity in the Vessel Wall. Biomedicines 2021, 9, 404. [Google Scholar] [CrossRef]
- Montes de Oca, A.; Madueño, J.A.; Martinez-Moreno, J.M.; Guerrero, F.; Muñoz-Castañeda, J.; Rodriguez-Ortiz, M.E.; Mendoza, F.J.; Almaden, Y.; Lopez, I.; Rodriguez, M.; et al. High-phosphate-induced calcification is related to SM22α promoter methylation in vascular smooth muscle cells. J. Bone Miner. Res. 2010, 25, 1996–2005. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Zhang, H.; Liu, T.; Zhang, H.; Teng, J.; Ji, J.; Ding, X. Indoxyl Sulfate Enhance the Hypermethylation of Klotho and Promote the Process of Vascular Calcification in Chronic Kidney Disease. Int. J. Biol. Sci. 2016, 12, 1236–1246. [Google Scholar] [CrossRef]
- Scheinbart, L.S.; Johnson, M.A.; Gross, L.A.; Edelstein, S.R.; Richardson, B.C. Procainamide inhibits DNA methyltransferase in a human T cell line. J. Rheumatol. 1991, 18, 530–534. [Google Scholar]
- Creusot, F.; Acs, G.; Christman, J.K. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J. Biol. Chem. 1982, 257, 2041–2048. [Google Scholar] [CrossRef]
- Wang, J.C.; Bennett, M. Aging and Atherosclerosis. Circ. Res. 2012, 111, 245–259. [Google Scholar] [CrossRef]
- Head, T.; Daunert, S.; Goldschmidt-Clermont, P.J. The Aging Risk and Atherosclerosis: A Fresh Look at Arterial Homeostasis. Front. Genet. 2017, 8, 216. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Mecham, R.P. Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 2009, 89, 957–989. [Google Scholar] [CrossRef]
- Oh, Y.S.; Berkowitz, D.E.; Cohen, R.A.; Figueroa, C.A.; Harrison, D.G.; Humphrey, J.D.; Larson, D.F.; Leopold, J.A.; Mecham, R.P.; Ruiz-Opazo, N.; et al. A Special Report on the NHLBI Initiative to Study Cellular and Molecular Mechanisms of Arterial Stiffness and Its Association With Hypertension. Circ. Res. 2017, 121, 1216–1218. [Google Scholar] [CrossRef]
- Xie, S.A.; Zhang, T.; Wang, J.; Zhao, F.; Zhang, Y.P.; Yao, W.J.; Hur, S.S.; Yeh, Y.T.; Pang, W.; Zheng, L.S.; et al. Matrix stiffness determines the phenotype of vascular smooth muscle cell in vitro and in vivo: Role of DNA methyltransferase 1. Biomaterials 2018, 155, 203–216. [Google Scholar] [CrossRef]
- Di, X.; Gao, X.; Peng, L.; Ai, J.; Jin, X.; Qi, S.; Li, H.; Wang, K.; Luo, D. Cellular mechanotransduction in health and diseases: From molecular mechanism to therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 282. [Google Scholar] [CrossRef]
- Davis, M.J.; Earley, S.; Li, Y.S.; Chien, S. Vascular mechanotransduction. Physiol. Rev. 2023, 103, 1247–1421. [Google Scholar] [CrossRef]
- Wang, J.; Xie, S.A.; Li, N.; Zhang, T.; Yao, W.; Zhao, H.; Pang, W.; Han, L.; Liu, J.; Zhou, J. Matrix stiffness exacerbates the proinflammatory responses of vascular smooth muscle cell through the DDR1-DNMT1 mechanotransduction axis. Bioact. Mater. 2022, 17, 406–424. [Google Scholar] [CrossRef]
- Gross, D.S.; Chowdhary, S.; Anandhakumar, J.; Kainth, A.S. Chromatin. Curr. Biol. 2015, 25, R1158–R1163. [Google Scholar] [CrossRef][Green Version]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm (2020) 2023, 4, e292. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef]
- Morgan, M.A.J.; Shilatifard, A. Reevaluating the roles of histone-modifying enzymes and their associated chromatin modifications in transcriptional regulation. Nat. Genet. 2020, 52, 1271–1281. [Google Scholar] [CrossRef]
- Black, J.C.; Van Rechem, C.; Whetstine, J.R. Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol. Cell 2012, 48, 491–507. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Bedford, M.T. Histone arginine methylation. FEBS Lett. 2011, 585, 2024–2031. [Google Scholar] [CrossRef]
- Dillon, S.C.; Zhang, X.; Trievel, R.C.; Cheng, X. The SET-domain protein superfamily: Protein lysine methyltransferases. Genome Biol. 2005, 6, 227. [Google Scholar] [CrossRef]
- Tsukada, Y.-i.; Fang, J.; Erdjument-Bromage, H.; Warren, M.E.; Borchers, C.H.; Tempst, P.; Zhang, Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature 2006, 439, 811–816. [Google Scholar] [CrossRef]
- Klose, R.J.; Kallin, E.M.; Zhang, Y. JmjC-domain-containing proteins and histone demethylation. Nat. Rev. Genet. 2006, 7, 715–727. [Google Scholar] [CrossRef]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef]
- Shioi, A.; Katagi, M.; Okuno, Y.; Mori, K.; Jono, S.; Koyama, H.; Nishizawa, Y. Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: Roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circ. Res. 2002, 91, 9–16. [Google Scholar] [CrossRef]
- Tintut, Y.; Patel, J.; Parhami, F.; Demer, L.L. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation 2000, 102, 2636–2642. [Google Scholar] [CrossRef]
- Kurozumi, A.; Nakano, K.; Yamagata, K.; Okada, Y.; Nakayamada, S.; Tanaka, Y. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone 2019, 124, 53–61. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef]
- Xue, S.; Leng, S.; Zhang, F.; Dang, Z.; Su, G.; Yu, W. Enhancer of zeste homolog 2 facilitates phenotypic transition of vascular smooth muscle cells leading to aortic aneurysm/dissection. Exp. Ther. Med. 2024, 27, 145. [Google Scholar] [CrossRef]
- Cheng, S.L.; Ramachandran, B.; Behrmann, A.; Shao, J.S.; Mead, M.; Smith, C.; Krchma, K.; Bello Arredondo, Y.; Kovacs, A.; Kapoor, K.; et al. Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR-/- mice by restraining noncanonical Wnt signals. Circ. Res. 2015, 117, 142–156. [Google Scholar] [CrossRef]
- Lee, K.K.; Workman, J.L. Histone acetyltransferase complexes: One size doesn’t fit all. Nat. Rev. Mol. Cell Biol. 2007, 8, 284–295. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, J.-S. A short guide to histone deacetylases including recent progress on class II enzymes. Exp. Mol. Med. 2020, 52, 204–212. [Google Scholar] [CrossRef]
- Pan, X.; Pi, C.; Ruan, X.; Zheng, H.; Zhang, D.; Liu, X. Mammalian Sirtuins and Their Relevance in Vascular Calcification. Front. Pharmacol. 2022, 13, 907835. [Google Scholar] [CrossRef]
- Wang, S.; Hu, S. The Role of Sirtuins in Osteogenic Differentiation of Vascular Smooth Muscle Cells and Vascular Calcification. Front. Cardiovasc. Med. 2022, 9, 894692. [Google Scholar] [CrossRef]
- Phadwal, K.; Feng, D.; Zhu, D.; MacRae, V.E. Autophagy as a novel therapeutic target in vascular calcification. Pharmacol. Ther. 2020, 206, 107430. [Google Scholar] [CrossRef]
- Lino Cardenas, C.L.; Jiang, W.; Kajuluri, L.P.; Singh, K.; Ostrom, K.; Li, R.; Cherbonneau, F.; Boerboom, S.; Birchenough, C.; Roh, K.; et al. Treatment of calcific arterial disease via enhancement of autophagy using GSK343. iScience 2023, 26, 108360. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, H.; Liu, C.; Huang, L.; Lu, D.; Gao, C. HDAC1-mediated deacetylation of LSD1 regulates vascular calcification by promoting autophagy in chronic renal failure. J. Cell Mol. Med. 2020, 24, 8636–8649. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Liu, P.; Zhang, C.; Huang, Q.; Zhao, Z.; Wu, S.; Li, D.; Liu, H. HDAC2 counteracts vascular calcification by activating autophagy in chronic kidney disease. Faseb J. 2024, 38, e23470. [Google Scholar] [CrossRef]
- Asfaha, Y.; Schrenk, C.; Alves Avelar, L.A.; Hamacher, A.; Pflieger, M.; Kassack, M.U.; Kurz, T. Recent advances in class IIa histone deacetylases research. Bioorg Med. Chem. 2019, 27, 115087. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.G.; Miyazaki, M.; Hoshino, H.; Miwa, Y.; Horinouchi, S.; Yoshida, M. 14-3-3 regulates the nuclear import of class IIa histone deacetylases. Biochem. Biophys. Res. Commun. 2008, 377, 852–856. [Google Scholar] [CrossRef] [PubMed]
- Abend, A.; Shkedi, O.; Fertouk, M.; Caspi, L.H.; Kehat, I. Salt-inducible kinase induces cytoplasmic histone deacetylase 4 to promote vascular calcification. EMBO Rep. 2017, 18, 1166–1185. [Google Scholar] [CrossRef]
- Chen, Y.; Ju, L.; Rushdi, M.; Ge, C.; Zhu, C. Receptor-mediated cell mechanosensing. Mol. Biol. Cell 2017, 28, 3134–3155. [Google Scholar] [CrossRef]
- Malhotra, R.; Mauer, A.C.; Lino Cardenas, C.L.; Guo, X.; Yao, J.; Zhang, X.; Wunderer, F.; Smith, A.V.; Wong, Q.; Pechlivanis, S.; et al. HDAC9 is implicated in atherosclerotic aortic calcification and affects vascular smooth muscle cell phenotype. Nat. Genet. 2019, 51, 1580–1587. [Google Scholar] [CrossRef]
- Lan, Z.; Chen, A.; Li, L.; Ye, Y.; Liang, Q.; Dong, Q.; Wang, S.; Fu, M.; Li, Y.; Liu, X.; et al. Downregulation of HDAC9 by the ketone metabolite β-hydroxybutyrate suppresses vascular calcification. J. Pathol. 2022, 258, 213–226. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Cheng, A.; Xu, G.; He, F. Metabolism of vascular smooth muscle cells in vascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H613–H631. [Google Scholar] [CrossRef]
- Yang, L.; Gao, L.; Nickel, T.; Yang, J.; Zhou, J.; Gilbertsen, A.; Geng, Z.; Johnson, C.; Young, B.; Henke, C.; et al. Lactate Promotes Synthetic Phenotype in Vascular Smooth Muscle Cells. Circ. Res. 2017, 121, 1251–1262. [Google Scholar] [CrossRef]
- Ma, W.; Jia, K.; Cheng, H.; Xu, H.; Li, Z.; Zhang, H.; Xie, H.; Sun, H.; Yi, L.; Chen, Z.; et al. Orphan Nuclear Receptor NR4A3 Promotes Vascular Calcification via Histone Lactylation. Circ. Res. 2024, 134, 1427–1447. [Google Scholar] [CrossRef] [PubMed]
- Beermann, J.; Piccoli, M.T.; Viereck, J.; Thum, T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Wang, C.; Chen, H. MicroRNAs are critical in regulating smooth muscle cell mineralization and apoptosis during vascular calcification. J. Cell Mol. Med. 2020, 24, 13564–13572. [Google Scholar] [CrossRef]
- Jang, B.; Zhang, D.; Ma, Z.; Yang, X.; Liu, L.; Xing, H.; Feng, L.; Song, J.; Zhao, X.; Song, X.; et al. MicroRNAs in vascular smooth muscle cells: Mechanisms, therapeutic potential, and advances in delivery systems. Life Sci. 2025, 364, 123424. [Google Scholar] [CrossRef] [PubMed]
- Graf, J.; Kretz, M. From structure to function: Route to understanding lncRNA mechanism. Bioessays 2020, 42, e2000027. [Google Scholar] [CrossRef]
- Jeong, G.; Kwon, D.H.; Shin, S.; Choe, N.; Ryu, J.; Lim, Y.H.; Kim, J.; Park, W.J.; Kook, H.; Kim, Y.K. Long noncoding RNAs in vascular smooth muscle cells regulate vascular calcification. Sci. Rep. 2019, 9, 5848. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Meng, Q.; Li, D.; Hu, F.Z.; Zhu, Y.Q.; Huang, Y.Y.; Liu, Y.N.; Sun, L.; Liang, Q.H. The protective effects of long non-coding RNA-ANCR on arterial calcification. J. Bone Miner. Metab. 2020, 38, 421–431. [Google Scholar] [CrossRef]
- Chang, Z.; Yan, G.; Zheng, J.; Liu, Z. The lncRNA GAS5 Inhibits the Osteogenic Differentiation and Calcification of Human Vascular Smooth Muscle Cells. Calcif. Tissue Int. 2020, 107, 86–95. [Google Scholar] [CrossRef]
- Song, Z.; Wei, D.; Chen, Y.; Chen, L.; Bian, Y.; Shen, Y.; Chen, J.; Pan, Y. Association of astragaloside IV-inhibited autophagy and mineralization in vascular smooth muscle cells with lncRNA H19 and DUSP5-mediated ERK signaling. Toxicol. Appl. Pharmacol. 2019, 364, 45–54. [Google Scholar] [CrossRef]
- Li, X.Z.; Xiong, Z.C.; Zhang, S.L.; Hao, Q.Y.; Liu, Z.Y.; Zhang, H.F.; Wang, J.F.; Gao, J.W.; Liu, P.M. Upregulated LncRNA H19 Sponges MiR-106a-5p and Contributes to Aldosterone-Induced Vascular Calcification via Activating the Runx2-Dependent Pathway. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1684–1699. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, M.; Jin, J.; Bai, Y.; Zhang, D.; Zhang, S.; Xu, J. Hypomethylation of the LncRNA H19 promoter accelerates osteogenic differentiation of vascular smooth muscle cells by activating the Erk1/2 pathways. J. Int. Med. Res. 2024, 52, 3000605241234567. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qi, H.; Yao, L. A long non-coding RNA H19/microRNA-138/TLR3 network is involved in high phosphorus-mediated vascular calcification and chronic kidney disease. Cell Cycle 2022, 21, 1667–1683. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, X.C.; Chen, M.L.; Zhuang, Z.W.; Jiang, Y.; Wang, J.; Zhou, Y.J. LncRNA H19/Runx2 axis promotes VSMCs transition via MAPK pathway. Am. J. Transl. Res. 2020, 12, 1338–1347. [Google Scholar] [PubMed]
- Gong, Y.; Zhong, Q.; Xia, Y.; Wen, Y.; Gan, H. Long non-coding RNA MALAT1 sponges miR-30c to promote the calcification of human vascular smooth muscle cells by regulating Runx2. Ren. Fail. 2023, 45, 2204953. [Google Scholar] [CrossRef]
- Lin, X.; Zhan, J.K.; Zhong, J.Y.; Wang, Y.J.; Wang, Y.; Li, S.; He, J.Y.; Tan, P.; Chen, Y.Y.; Liu, X.B.; et al. lncRNA-ES3/miR-34c-5p/BMF axis is involved in regulating high-glucose-induced calcification/senescence of VSMCs. Aging 2019, 11, 523–535. [Google Scholar] [CrossRef]
- Li, S.; Ni, Y.; Li, C.; Xiang, Q.; Zhao, Y.; Xu, H.; Huang, W.; Wang, Y.; Wang, Y.; Zhan, J.; et al. Long noncoding RNA SNHG1 alleviates high glucose-induced vascular smooth muscle cells calcification/senescence by post-transcriptionally regulating Bhlhe40 and autophagy via Atg10. J. Physiol. Biochem. 2023, 79, 83–105. [Google Scholar] [CrossRef]
- Zhong, J.Y.; Cui, X.J.; Zhan, J.K.; Wang, Y.J.; Li, S.; Lin, X.; Xiang, Q.Y.; Ni, Y.Q.; Liu, L.; Liu, Y.S. LncRNA-ES3 inhibition by Bhlhe40 is involved in high glucose-induced calcification/senescence of vascular smooth muscle cells. Ann. N. Y. Acad. Sci. 2020, 1474, 61–72. [Google Scholar] [CrossRef]
- Huang, C.; Zhan, J.F.; Chen, Y.X.; Xu, C.Y.; Chen, Y. LncRNA-SNHG29 inhibits vascular smooth muscle cell calcification by downregulating miR-200b-3p to activate the α-Klotho/FGFR1/FGF23 axis. Cytokine 2020, 136, 155243. [Google Scholar] [CrossRef]
- Li, Y.; Xi, Z.; Yu, Z.; Yang, C.; Tan, C. LincRNA-EPS increases TGF-β expression to inhibit the Wnt/β-catenin pathway, VSMC osteoblastic differentiation and vascular calcification in diabetic mice. Exp. Ther. Med. 2022, 23, 425. [Google Scholar] [CrossRef]
- Horita, H.; Wysoczynski, C.L.; Walker, L.A.; Moulton, K.S.; Li, M.; Ostriker, A.; Tucker, R.; McKinsey, T.A.; Churchill, M.E.; Nemenoff, R.A.; et al. Nuclear PTEN functions as an essential regulator of SRF-dependent transcription to control smooth muscle differentiation. Nat. Commun. 2016, 7, 10830. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kontos, C.D. Inhibition of vascular smooth muscle cell proliferation, migration, and survival by the tumor suppressor protein PTEN. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Guo, Y.; Diao, Z.; Guo, W.; Liu, W. Genome-wide identification of lncRNAs and mRNAs differentially expressed in human vascular smooth muscle cells stimulated by high phosphorus. Ren. Fail. 2020, 42, 437–446. [Google Scholar] [CrossRef]
- Wicik, Z.; Jales Neto, L.H.; Guzman, L.E.F.; Pavão, R.; Takayama, L.; Caparbo, V.F.; Lopes, N.H.M.; Pereira, A.C.; Pereira, R.M.R. The crosstalk between bone metabolism, lncRNAs, microRNAs and mRNAs in coronary artery calcification. Genomics 2021, 113, 503–513. [Google Scholar] [CrossRef]
- Wei, X.; Su, Y.; Li, Q.; Zheng, Z.; Hou, P. Analysis of crucial genes, pathways and construction of the molecular regulatory networks in vascular smooth muscle cell calcification. Exp. Ther. Med. 2021, 21, 589. [Google Scholar] [CrossRef]
- Goettsch, C.; Hutcheson, J.D.; Aikawa, E. MicroRNA in cardiovascular calcification: Focus on targets and extracellular vesicle delivery mechanisms. Circ. Res. 2013, 112, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, X.; Shen, Y.; Chen, L.; Xu, C.; Zhao, H.; Wu, Y.; Zhang, Q.; Zhong, J.; Tang, Z.; et al. MicroRNA-32 promotes calcification in vascular smooth muscle cells: Implications as a novel marker for coronary artery calcification. PLoS ONE 2017, 12, e0174138. [Google Scholar] [CrossRef]
- Guo, X.; Liu, S.; Wu, X.; Yang, R.; Ren, Q.; Zhou, Y.; Shi, K.; Yuan, L.; Zhang, N.; Liu, S. Alleviating vascular calcification with Bushen Huoxue formula in rats with chronic kidney disease by inhibiting the PTEN/PI3K/AKT signaling pathway through exosomal microRNA-32. J. Pharm. Pharmacol. 2025, 77, 550–563. [Google Scholar] [CrossRef]
- Cao, J.; Chen, C.; Chen, Q.; Gao, Y.; Zhao, Z.; Yuan, Q.; Li, A.; Yang, S.; He, Y.; Zu, X.; et al. Extracellular vesicle miR-32 derived from macrophage promotes arterial calcification in mice with type 2 diabetes via inhibiting VSMC autophagy. J. Transl. Med. 2022, 20, 307. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.; Zhong, X.; Shen, Y.; Gao, Y.; Chen, Q.; Zu, X.; Liu, J. miR32-5p promoted vascular smooth muscle cell calcification by upregulating TNFα in the microenvironment. BMC Immunol. 2020, 21, 3. [Google Scholar] [CrossRef]
- Zuccolo, E.; Badi, I.; Scavello, F.; Gambuzza, I.; Mancinelli, L.; Macrì, F.; Tedesco, C.C.; Veglia, F.; Bonfigli, A.R.; Olivieri, F.; et al. The microRNA-34a-Induced Senescence-Associated Secretory Phenotype (SASP) Favors Vascular Smooth Muscle Cells Calcification. Int. J. Mol. Sci. 2020, 21, 4454. [Google Scholar] [CrossRef] [PubMed]
- Badi, I.; Mancinelli, L.; Polizzotto, A.; Ferri, D.; Zeni, F.; Burba, I.; Milano, G.; Brambilla, F.; Saccu, C.; Bianchi, M.E.; et al. miR-34a Promotes Vascular Smooth Muscle Cell Calcification by Downregulating SIRT1 (Sirtuin 1) and Axl (AXL Receptor Tyrosine Kinase). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, Z.; Wei, L.; Cheng, X.; Chen, L.; Gao, F.; Jiang, H. Indoxyl sulfate promotes osteogenic differentiation of vascular smooth muscle cells by miR-155-5p-dependent downregulation of matrix Gla protein via ROS/NF-κB signaling. Exp. Cell Res. 2020, 397, 112301. [Google Scholar] [CrossRef]
- Li, Y.; Sun, W.; Saaoud, F.; Wang, Y.; Wang, Q.; Hodge, J.; Hui, Y.; Yin, S.; Lessner, S.M.; Kong, X.; et al. MiR155 modulates vascular calcification by regulating Akt-FOXO3a signalling and apoptosis in vascular smooth muscle cells. J. Cell Mol. Med. 2021, 25, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Ning, H.; Wang, X.; Wang, Y.; Han, T.; Sun, C. Endothelial Autophagy Promotes Atheroprotective Communication Between Endothelial and Smooth Muscle Cells via Exosome-Mediated Delivery of miR-204-5p. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1813–1832. [Google Scholar] [CrossRef]
- Liao, X.B.; Zhang, Z.Y.; Yuan, K.; Liu, Y.; Feng, X.; Cui, R.R.; Hu, Y.R.; Yuan, Z.S.; Gu, L.; Li, S.J.; et al. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology 2013, 154, 3344–3352. [Google Scholar] [CrossRef]
- Kaul, A.; Dhalla, P.S.; Bapatla, A.; Khalid, R.; Garcia, J.; Armenta-Quiroga, A.S.; Khan, S. Current Treatment Modalities for Calcified Coronary Artery Disease: A Review Article Comparing Novel Intravascular Lithotripsy and Traditional Rotational Atherectomy. Cureus 2020, 12, e10922. [Google Scholar] [CrossRef]
- Brinton, T.J.; Ali, Z.A.; Hill, J.M.; Meredith, I.T.; Maehara, A.; Illindala, U.; Lansky, A.; Götberg, M.; Van Mieghem, N.M.; Whitbourn, R.; et al. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation 2019, 139, 834–836. [Google Scholar] [CrossRef]
- Murali, S.; Smith, E.R.; Tiong, M.K.; Tan, S.J.; Toussaint, N.D. Interventions to Attenuate Cardiovascular Calcification Progression: A Systematic Review of Randomized Clinical Trials. J. Am. Heart Assoc. 2023, 12, e031676. [Google Scholar] [CrossRef]
- Campbell, J.H.; Efendy, J.L.; Smith, N.J.; Campbell, G.R. Molecular basis by which garlic suppresses atherosclerosis. J. Nutr. 2001, 131, 1006s–1009s. [Google Scholar] [CrossRef]
- Budoff, M.J.; Ahmadi, N.; Gul, K.M.; Liu, S.T.; Flores, F.R.; Tiano, J.; Takasu, J.; Miller, E.; Tsimikas, S. Aged garlic extract supplemented with B vitamins, folic acid and L-arginine retards the progression of subclinical atherosclerosis: A randomized clinical trial. Prev. Med. 2009, 49, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Takasu, J.; Flores, F.R.; Niihara, Y.; Lu, B.; Lau, B.H.; Rosen, R.T.; Amagase, H. Inhibiting progression of coronary calcification using Aged Garlic Extract in patients receiving statin therapy: A preliminary study. Prev. Med. 2004, 39, 985–991. [Google Scholar] [CrossRef]
- Zeb, I.; Ahmadi, N.; Nasir, K.; Kadakia, J.; Larijani, V.N.; Flores, F.; Li, D.; Budoff, M.J. Aged garlic extract and coenzyme Q10 have favorable effect on inflammatory markers and coronary atherosclerosis progression: A randomized clinical trial. J. Cardiovasc. Dis. Res. 2012, 3, 185–190. [Google Scholar] [CrossRef]
- Wlosinska, M.; Nilsson, A.C.; Hlebowicz, J.; Hauggaard, A.; Kjellin, M.; Fakhro, M.; Lindstedt, S. The effect of aged garlic extract on the atherosclerotic process—A randomized double-blind placebo-controlled trial. BMC Complement. Med. Ther. 2020, 20, 132. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Nakanishi, R.; Li, D.; Alani, A.; Rezaeian, P.; Prabhu, S.; Abraham, J.; Fahmy, M.A.; Dailing, C.; Flores, F.; et al. Aged Garlic Extract Reduces Low Attenuation Plaque in Coronary Arteries of Patients with Metabolic Syndrome in a Prospective Randomized Double-Blind Study. J. Nutr. 2016, 146, 427s–432s. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, K.; Kinninger, A.; Cherukuri, L.; Birudaraju, D.; Nakanishi, R.; Almeida, S.; Jayawardena, E.; Shekar, C.; Flores, F.; Hamal, S.; et al. Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with diabetes: A randomized, double-blind, placebo-controlled study. Exp. Ther. Med. 2020, 19, 1457–1461. [Google Scholar] [CrossRef]
- Arad, Y.; Spadaro, L.A.; Roth, M.; Newstein, D.; Guerci, A.D. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: The St. Francis Heart Study randomized clinical trial. J. Am. Coll. Cardiol. 2005, 46, 166–172. [Google Scholar] [CrossRef]
- Cowell, S.J.; Newby, D.E.; Prescott, R.J.; Bloomfield, P.; Reid, J.; Northridge, D.B.; Boon, N.A. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N. Engl. J. Med. 2005, 352, 2389–2397. [Google Scholar] [CrossRef]
- Dichtl, W.; Alber, H.F.; Feuchtner, G.M.; Hintringer, F.; Reinthaler, M.; Bartel, T.; Süssenbacher, A.; Grander, W.; Ulmer, H.; Pachinger, O.; et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). Am. J. Cardiol. 2008, 102, 743–748. [Google Scholar] [CrossRef]
- Houslay, E.S.; Cowell, S.J.; Prescott, R.J.; Reid, J.; Burton, J.; Northridge, D.B.; Boon, N.A.; Newby, D.E. Progressive coronary calcification despite intensive lipid-lowering treatment: A randomised controlled trial. Heart 2006, 92, 1207–1212. [Google Scholar] [CrossRef]
- Miyoshi, T.; Kohno, K.; Asonuma, H.; Sakuragi, S.; Nakahama, M.; Kawai, Y.; Uesugi, T.; Oka, T.; Munemasa, M.; Takahashi, N.; et al. Effect of Intensive and Standard Pitavastatin Treatment With or Without Eicosapentaenoic Acid on Progression of Coronary Artery Calcification over 12 Months—Prospective Multicenter Study. Circ. J. 2018, 82, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Petri, M.A.; Kiani, A.N.; Post, W.; Christopher-Stine, L.; Magder, L.S. Lupus Atherosclerosis Prevention Study (LAPS). Ann. Rheum. Dis. 2011, 70, 760–765. [Google Scholar] [CrossRef]
- Longenecker, C.T.; Sattar, A.; Gilkeson, R.; McComsey, G.A. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. Aids 2016, 30, 2195–2203. [Google Scholar] [CrossRef]
- Raggi, P.; Callister, T.Q.; Davidson, M.; Welty, F.K.; Bachmann, G.A.; Laskey, R.; Pittman, D.; Kafonek, S.; Scott, R. Aggressive versus moderate lipid-lowering therapy in postmenopausal women with hypercholesterolemia: Rationale and design of the Beyond Endorsed Lipid Lowering with EBT Scanning (BELLES) trial. Am. Heart J. 2001, 141, 722–726. [Google Scholar] [CrossRef]
- Schmermund, A.; Achenbach, S.; Budde, T.; Buziashvili, Y.; Förster, A.; Friedrich, G.; Henein, M.; Kerkhoff, G.; Knollmann, F.; Kukharchuk, V.; et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: A multicenter, randomized, double-blind trial. Circulation 2006, 113, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Terry, J.G.; Carr, J.J.; Kouba, E.O.; Davis, D.H.; Menon, L.; Bender, K.; Chandler, E.T.; Morgan, T.; Crouse, J.R., 3rd. Effect of simvastatin (80 mg) on coronary and abdominal aortic arterial calcium (from the coronary artery calcification treatment with zocor [CATZ] study). Am. J. Cardiol. 2007, 99, 1714–1717. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kang, S.J.; Ahn, J.M.; Chang, M.; Yun, S.C.; Roh, J.H.; Lee, P.H.; Park, H.W.; Yoon, S.H.; Park, D.W.; et al. Effect of Statin Treatment on Modifying Plaque Composition: A Double-Blind, Randomized Study. J. Am. Coll. Cardiol. 2016, 67, 1772–1783. [Google Scholar] [CrossRef]
- Egede, R.; Jensen, L.O.; Hansen, H.S.; Hansen, K.N.; Junker, A.; Thayssen, P. Influence of high-dose lipid lowering treatment compared to low-dose lipid lowering treatment on plaque composition assessed by intravascular ultrasound virtual histology in patients with ST-segment elevation acute myocardial infarction: The VIRHISTAMI trial. EuroIntervention 2013, 8, 1182–1189. [Google Scholar] [CrossRef]
- Lo, J.; Lu, M.T.; Ihenachor, E.J.; Wei, J.; Looby, S.E.; Fitch, K.V.; Oh, J.; Zimmerman, C.O.; Hwang, J.; Abbara, S.; et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: A randomised, double-blind, placebo-controlled trial. Lancet HIV 2015, 2, e52–e63. [Google Scholar] [CrossRef]
- Ayombil, F.; Camire, R.M. Insights into vitamin K-dependent carboxylation: Home field advantage. Haematologica 2020, 105, 1996–1998. [Google Scholar] [CrossRef]
- Yao, Y.; Shahbazian, A.; Boström, K.I. Proline and γ-Carboxylated Glutamate Residues in Matrix Gla Protein Are Critical for Binding of Bone Morphogenetic Protein-4. Circ. Res. 2008, 102, 1065–1074. [Google Scholar] [CrossRef]
- Shea, M.K.; O’Donnell, C.J.; Hoffmann, U.; Dallal, G.E.; Dawson-Hughes, B.; Ordovas, J.M.; Price, P.A.; Williamson, M.K.; Booth, S.L. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am. J. Clin. Nutr. 2009, 89, 1799–1807. [Google Scholar] [CrossRef]
- Bellinge, J.W.; Francis, R.J.; Lee, S.C.; Bondonno, N.P.; Sim, M.; Lewis, J.R.; Watts, G.F.; Schultz, C.J. The effect of vitamin K1 on arterial calcification activity in subjects with diabetes mellitus: A post hoc analysis of a double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2022, 115, 45–52. [Google Scholar] [CrossRef]
- Henzel, J.; Kępka, C.; Kruk, M.; Makarewicz-Wujec, M.; Wardziak, Ł.; Trochimiuk, P.; Dzielińska, Z.; Demkow, M. High-Risk Coronary Plaque Regression After Intensive Lifestyle Intervention in Nonobstructive Coronary Disease: A Randomized Study. JACC Cardiovasc. Imaging 2021, 14, 1192–1202. [Google Scholar] [CrossRef]
- Fitch, K.; Abbara, S.; Lee, H.; Stavrou, E.; Sacks, R.; Michel, T.; Hemphill, L.; Torriani, M.; Grinspoon, S. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV-infected patients with the metabolic syndrome. Aids 2012, 26, 587–597. [Google Scholar] [CrossRef]
- Kuller, L.H.; Pettee Gabriel, K.K.; Kinzel, L.S.; Underwood, D.A.; Conroy, M.B.; Chang, Y.; Mackey, R.H.; Edmundowicz, D.; Tyrrell, K.S.; Buhari, A.M.; et al. The Women on the Move Through Activity and Nutrition (WOMAN) study: Final 48-month results. Obesity 2012, 20, 636–643. [Google Scholar] [CrossRef]
- Lehmann, N.; Paul, A.; Moebus, S.; Budde, T.; Dobos, G.J.; Michalsen, A. Effects of lifestyle modification on coronary artery calcium progression and prognostic factors in coronary patients--3-year results of the randomized SAFE-LIFE trial. Atherosclerosis 2011, 219, 630–636. [Google Scholar] [CrossRef]
- Motro, M.; Shemesh, J. Calcium channel blocker nifedipine slows down progression of coronary calcification in hypertensive patients compared with diuretics. Hypertension 2001, 37, 1410–1413. [Google Scholar] [CrossRef]
- Motro, M.; Kirwan, B.A.; de Brouwer, S.; Poole-Wilson, P.A.; Shemesh, J. Tracking coronary calcification and atherosclerotic lesions in patients with stable angina pectoris undergoing nifedipine therapy. Cardiology 2007, 107, 165–171. [Google Scholar] [CrossRef]
- Budoff, M.J.; Muhlestein, J.B.; Bhatt, D.L.; Le Pa, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Kinninger, A.; Lakshmanan, S.; Roy, S.K.; et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: A prospective, placebo-controlled randomized trial (EVAPORATE): Interim results. Cardiovasc. Res. 2021, 117, 1070–1077. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Elajami, T.K.; Ashfaque, H.; Saleh, M.; Bistrian, B.R.; Welty, F.K. Effect of Eicosapentaenoic and Docosahexaenoic Acids Added to Statin Therapy on Coronary Artery Plaque in Patients With Coronary Artery Disease: A Randomized Clinical Trial. J. Am. Heart Assoc. 2017, 6, 6981. [Google Scholar] [CrossRef]
- Davidson, M.H.; Beam, C.A.; Haffner, S.; Perez, A.; D’Agostino, R., Sr.; Mazzone, T. Pioglitazone versus glimepiride on coronary artery calcium progression in patients with type 2 diabetes mellitus: A secondary end point of the CHICAGO study. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1873–1876. [Google Scholar] [CrossRef]
- Nozue, T.; Fukui, K.; Koyama, Y.; Fujii, H.; Kunishima, T.; Hikita, H.; Hibi, K.; Miyazawa, A.; Michishita, I. Effects of sitagliptin on coronary atherosclerosis in patients with type 2 diabetes-A serial integrated backscatter-intravascular ultrasound study. Am. J. Cardiovasc. Dis. 2016, 6, 153–162. [Google Scholar]
- Basaria, S.; Harman, S.M.; Travison, T.G.; Hodis, H.; Tsitouras, P.; Budoff, M.; Pencina, K.M.; Vita, J.; Dzekov, C.; Mazer, N.A.; et al. Effects of Testosterone Administration for 3 Years on Subclinical Atherosclerosis Progression in Older Men With Low or Low-Normal Testosterone Levels: A Randomized Clinical Trial. Jama 2015, 314, 570–581. [Google Scholar] [CrossRef]
- Budoff, M.J.; Ellenberg, S.S.; Lewis, C.E.; Mohler, E.R., 3rd; Wenger, N.K.; Bhasin, S.; Barrett-Connor, E.; Swerdloff, R.S.; Stephens-Shields, A.; Cauley, J.A.; et al. Testosterone Treatment and Coronary Artery Plaque Volume in Older Men With Low Testosterone. Jama 2017, 317, 708–716. [Google Scholar] [CrossRef]
- Harman, S.M.; Black, D.M.; Naftolin, F.; Brinton, E.A.; Budoff, M.J.; Cedars, M.I.; Hopkins, P.N.; Lobo, R.A.; Manson, J.E.; Merriam, G.R.; et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: A randomized trial. Ann. Intern. Med. 2014, 161, 249–260. [Google Scholar] [CrossRef]
- Lee, D.H.; Chun, E.J.; Hur, J.H.; Min, S.H.; Lee, J.E.; Oh, T.J.; Kim, K.M.; Jang, H.C.; Han, S.J.; Kang, D.K.; et al. Effect of sarpogrelate, a selective 5-HT(2A) receptor antagonist, on characteristics of coronary artery disease in patients with type 2 diabetes. Atherosclerosis 2017, 257, 47–54. [Google Scholar] [CrossRef]
- Lee, D.H.; Chun, E.J.; Oh, T.J.; Kim, K.M.; Moon, J.H.; Choi, S.H.; Park, K.S.; Jang, H.C.; Lim, S. Effect of cilostazol, a phosphodiesterase-3 inhibitor, on coronary artery stenosis and plaque characteristics in patients with type 2 diabetes: ESCAPE study. Diabetes Obes. Metab. 2019, 21, 1409–1418. [Google Scholar] [CrossRef]
- Kaminskas, E.; Farrell, A.T.; Wang, Y.C.; Sridhara, R.; Pazdur, R. FDA drug approval summary: Azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist 2005, 10, 176–182. [Google Scholar] [CrossRef]
- Straining, R.; Eighmy, W. Tazemetostat: EZH2 Inhibitor. J. Adv. Pract. Oncol. 2022, 13, 158–163. [Google Scholar] [CrossRef]
- Mann, B.S.; Johnson, J.R.; Cohen, M.H.; Justice, R.; Pazdur, R. FDA approval summary: Vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 2007, 12, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.A. Discovery and development of SAHA as an anticancer agent. Oncogene 2007, 26, 1351–1356. [Google Scholar] [CrossRef]
- Grant, C.; Rahman, F.; Piekarz, R.; Peer, C.; Frye, R.; Robey, R.W.; Gardner, E.R.; Figg, W.D.; Bates, S.E. Romidepsin: A new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert. Rev. Anticancer. Ther. 2010, 10, 997–1008. [Google Scholar] [CrossRef]
- El Omari, N.; Bakrim, S.; Khalid, A.; Abdalla, A.N.; Almalki, W.H.; Lee, L.H.; Ardianto, C.; Ming, L.C.; Bouyahya, A. Molecular mechanisms underlying the clinical efficacy of panobinostat involve Stochasticity of epigenetic signaling, sensitization to anticancer drugs, and induction of cellular cell death related to cellular stresses. Biomed. Pharmacother. 2023, 164, 114886. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Thomas, C.M. Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma. J. Oncol. Pharm. Pract. 2017, 23, 143–147. [Google Scholar] [CrossRef]
- Yu, C.; Li, L.; Xie, F.; Guo, S.; Liu, F.; Dong, N.; Wang, Y. LncRNA TUG1 sponges miR-204-5p to promote osteoblast differentiation through upregulating Runx2 in aortic valve calcification. Cardiovasc. Res. 2018, 114, 168–179. [Google Scholar] [CrossRef]
- Wu, J.; Wan, M.; Jiang, Z.; Gong, W.; Zhou, X. lncRNA FAS-AS1 served as a diagnostic biomarker of end-stage renal disease and mediated vascular calcification via regulating oxidative stress and inflammation. Gene 2024, 896, 148035. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Xiao, H.; Zhou, X.; Li, Y.; Zhao, S.; Zhao, X.; Liu, Y.; Liu, M.; Xue, F.; Zhang, Q.; et al. Engineered exosomes reprogram Gli1(+) cells in vivo to prevent calcification of vascular grafts and autologous pathological vessels. Sci. Adv. 2023, 9, eadf7858. [Google Scholar] [CrossRef]
- Ratti, M.; Lampis, A.; Ghidini, M.; Salati, M.; Mirchev, M.B.; Valeri, N.; Hahne, J.C. MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside. Target. Oncol. 2020, 15, 261–278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).