MicroRNAs miR-148a-3p, miR-425-3p, and miR-20a-5p in Patients with IgA Nephropathy
Abstract
1. Introduction
2. Materials and Methods
2.1. miRNA Isolation
2.2. Real-Time Quantitative Reverse Transcription PCR (RQ-PCR)
2.3. Statistical Analysis
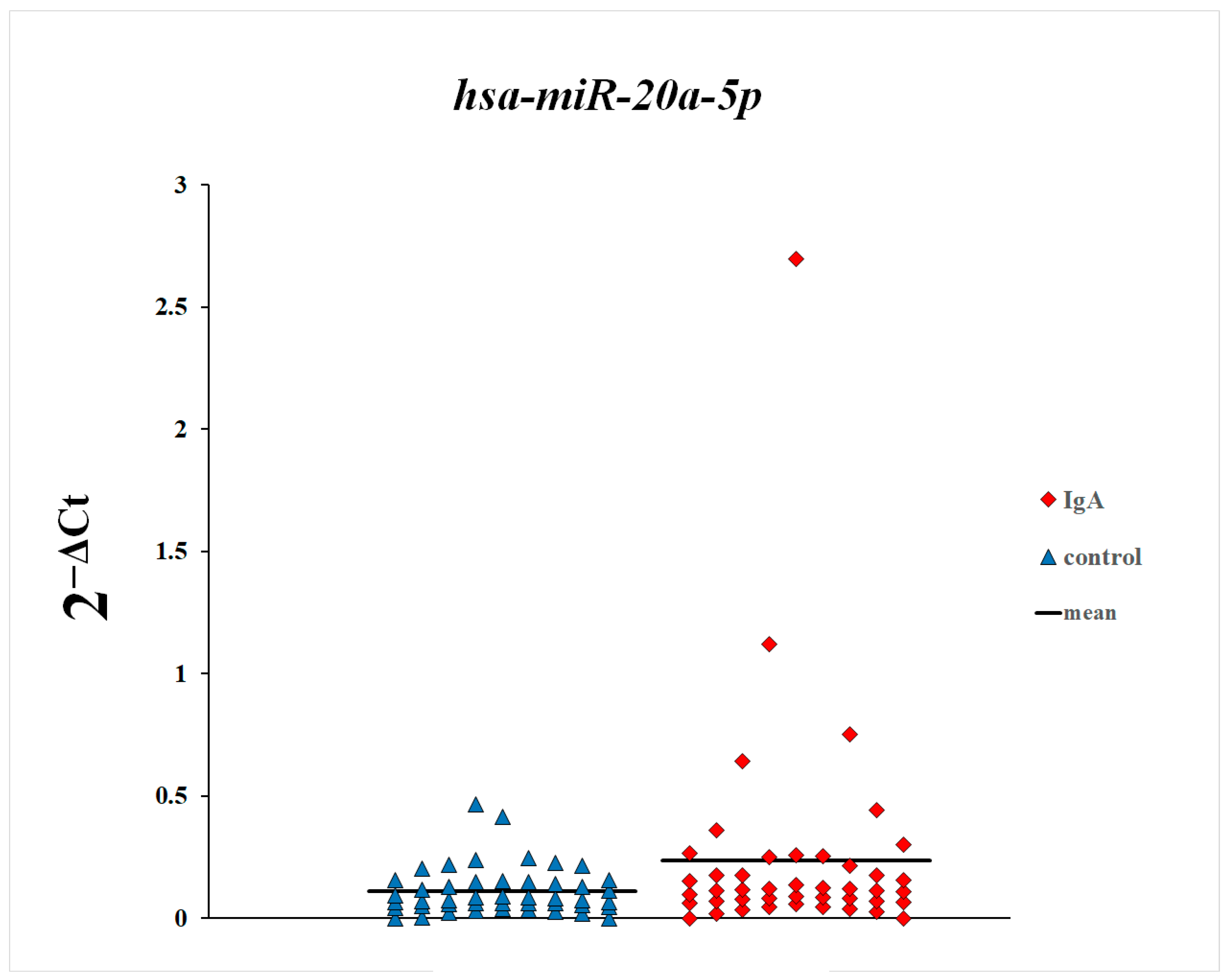
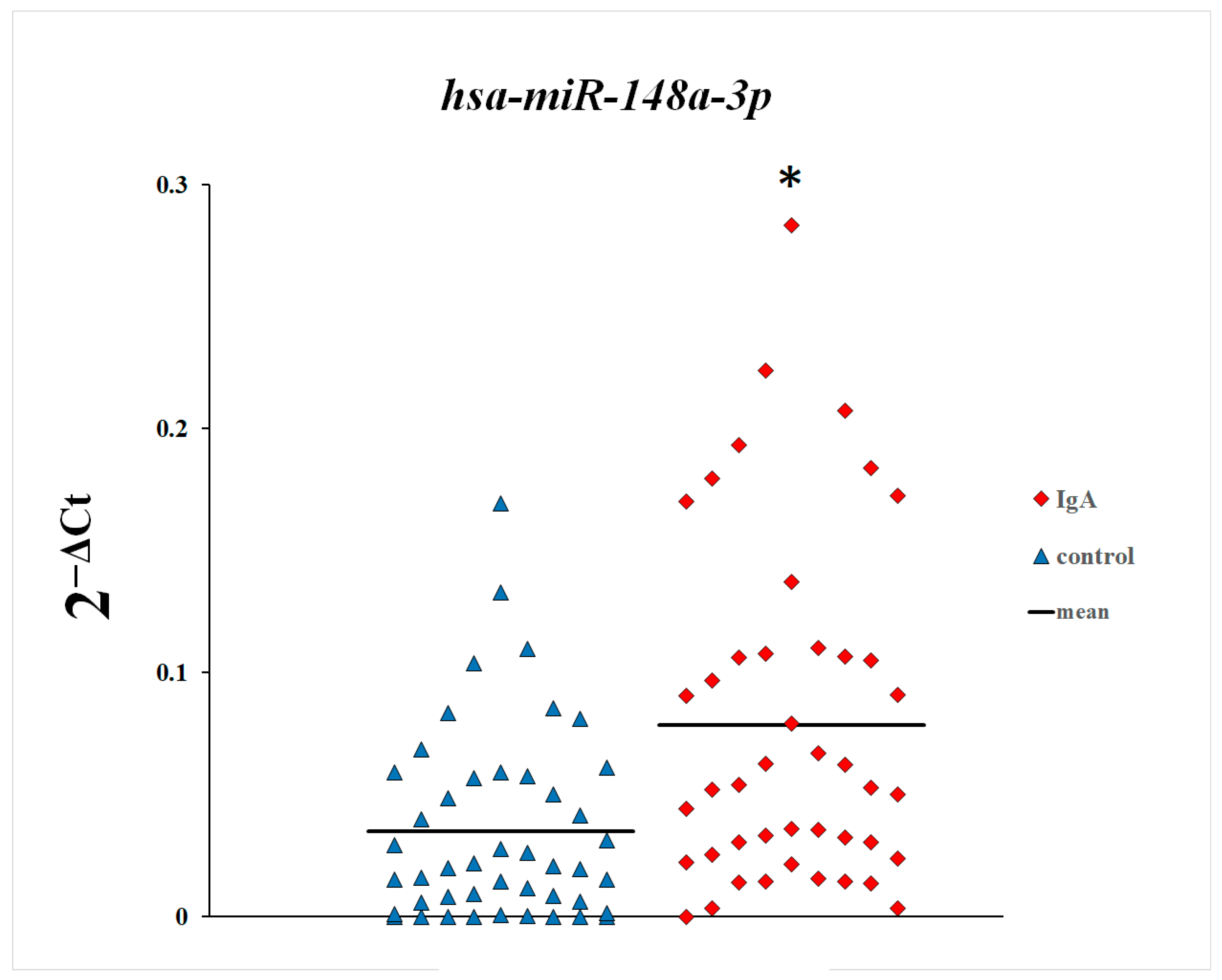
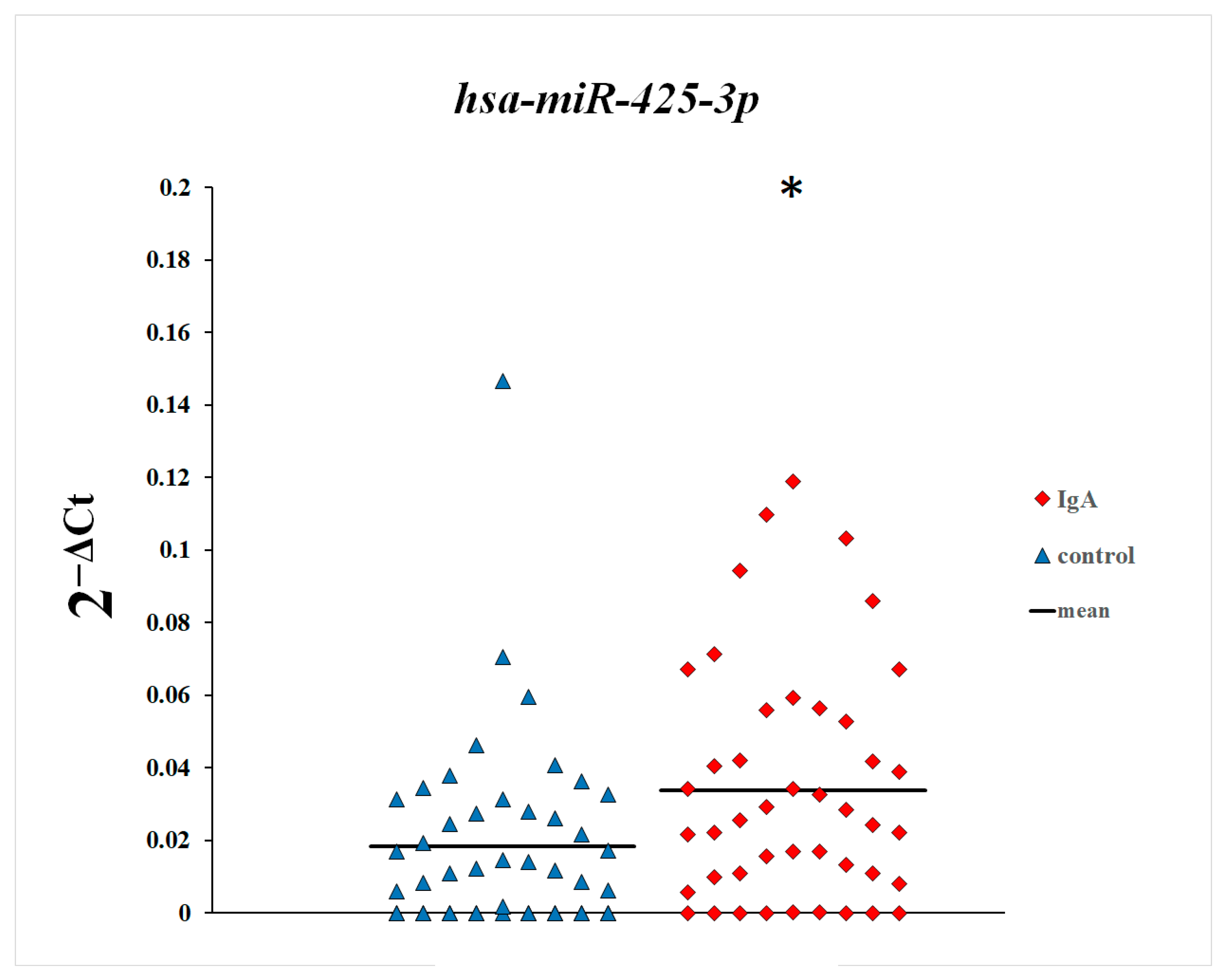
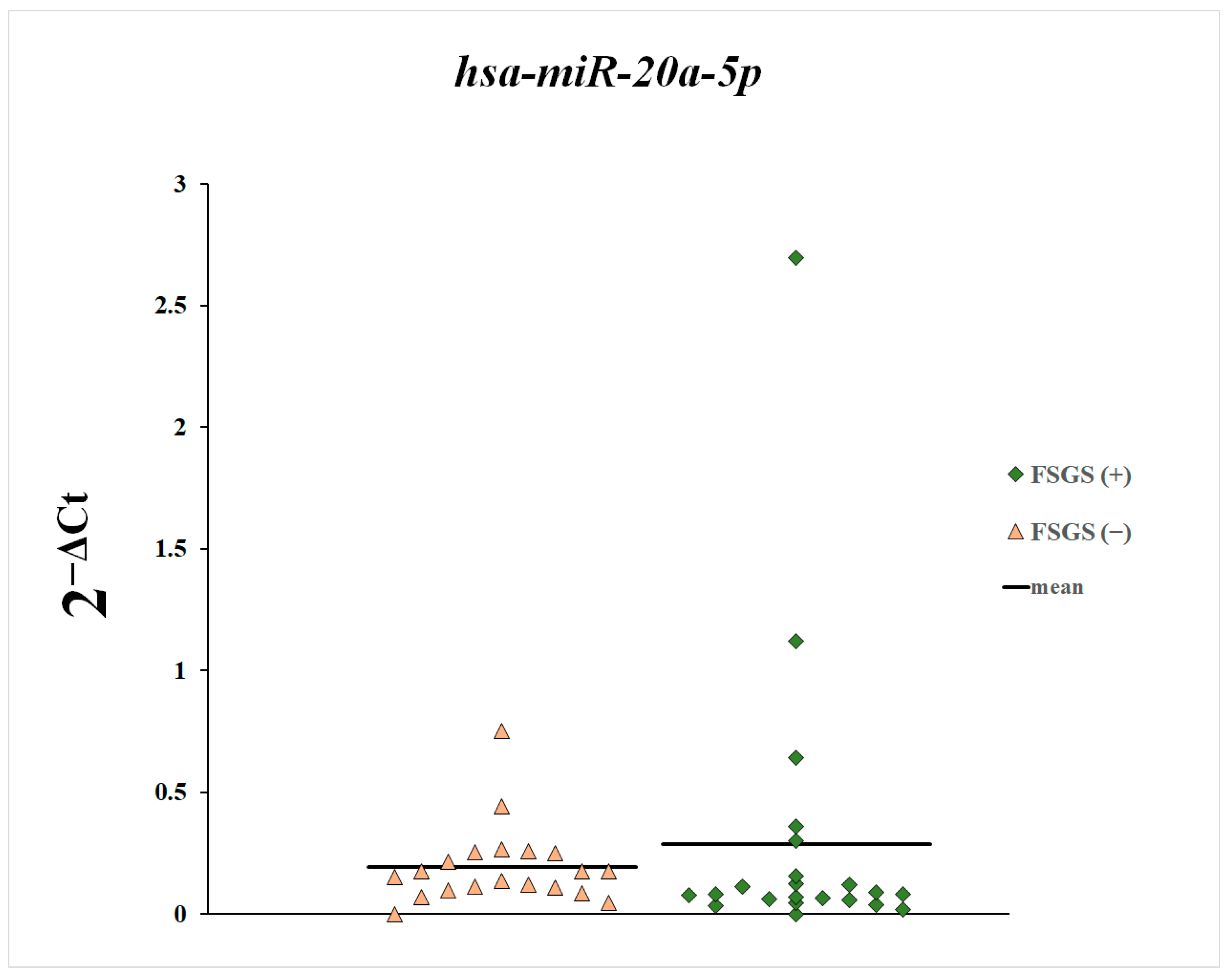
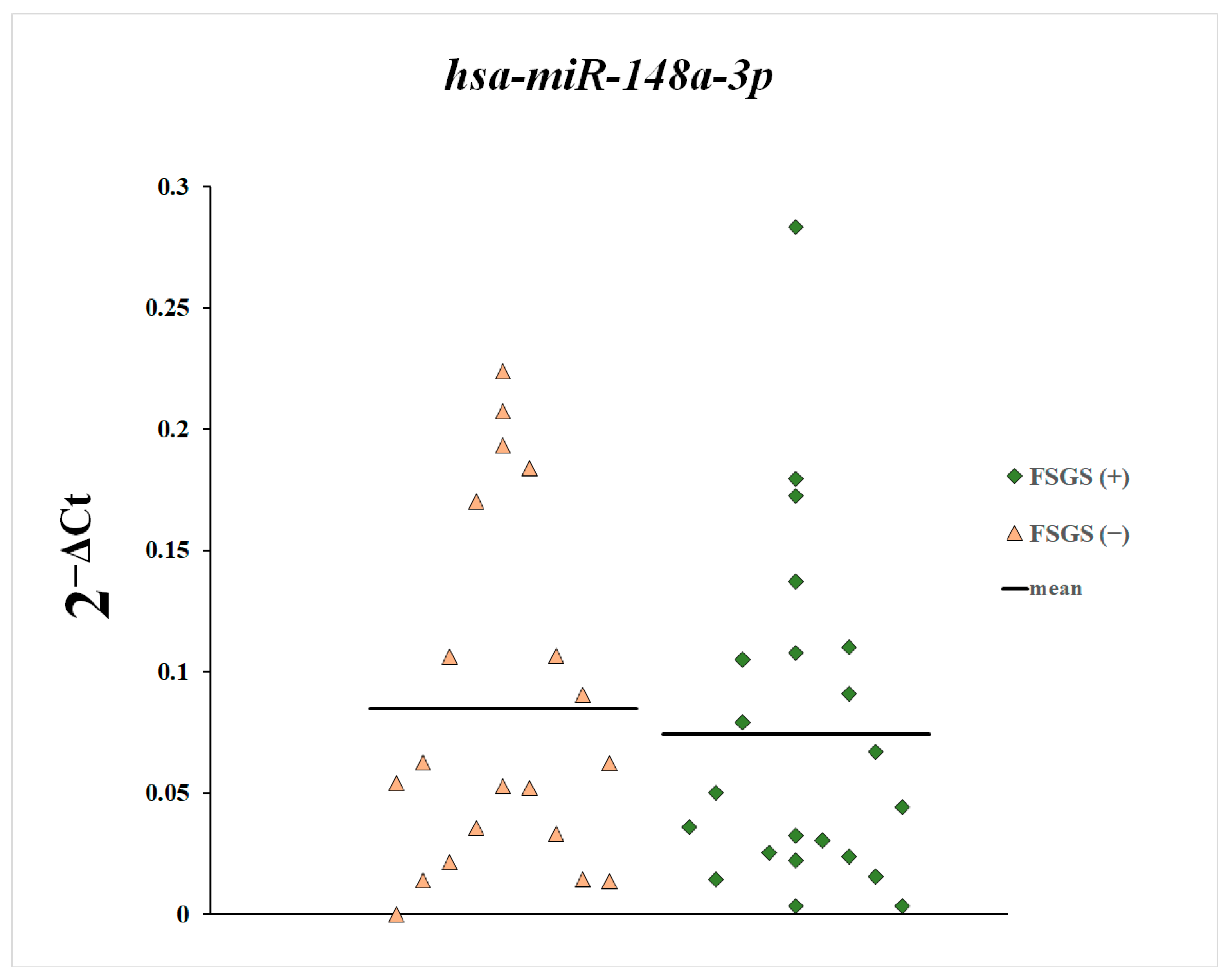
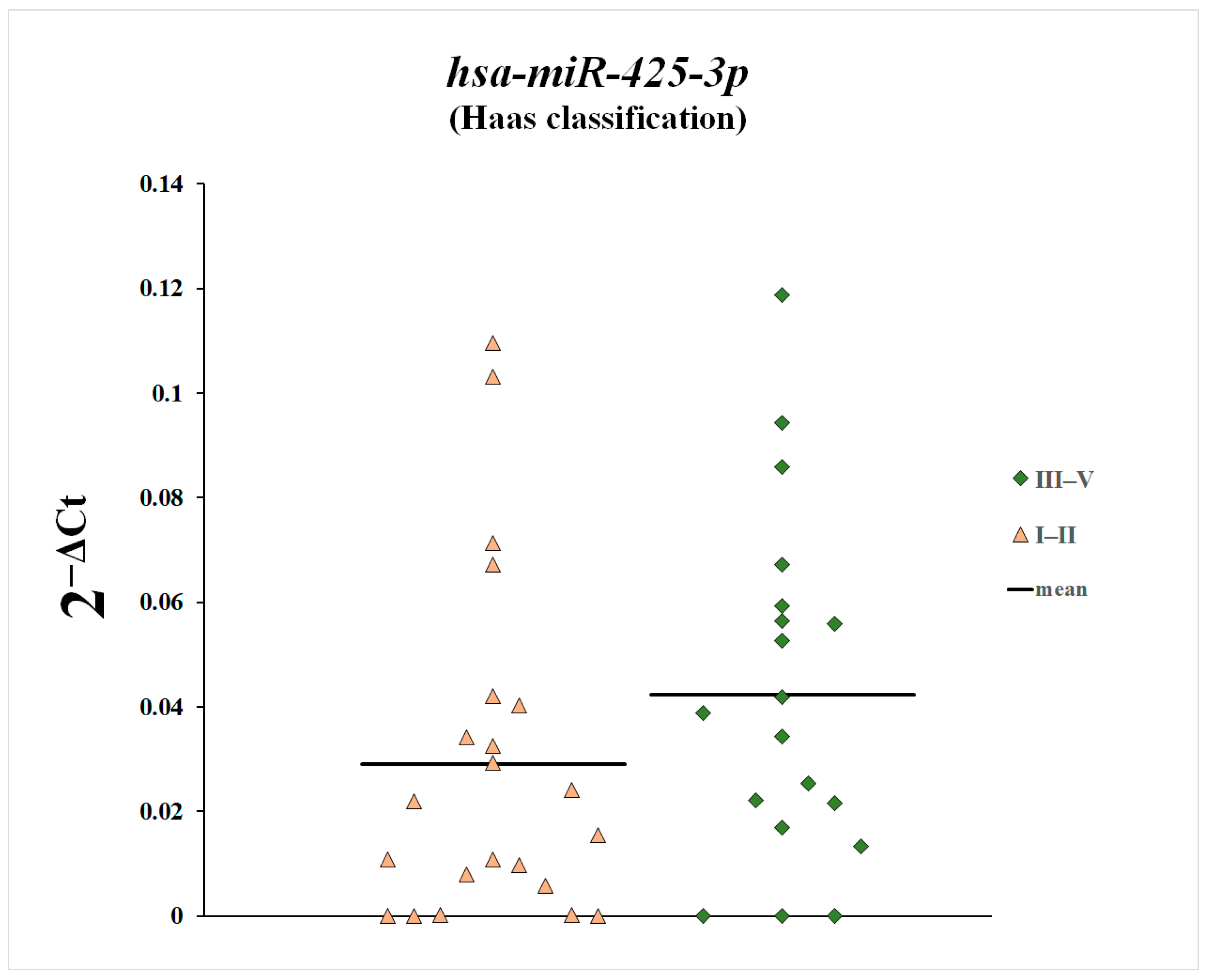
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Floege, J.; Wied, S.; Rauen, T. Assessing Prognosis in IgA Nephropathy. Kidney Int. 2022, 102, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Perše, M.; Večerić-Haler, Ž. The Role of IgA in the Pathogenesis of IgA Nephropathy. Int. J. Mol. Sci. 2019, 20, 6199. [Google Scholar] [CrossRef]
- Rajasekaran, A.; Julian, B.A.; Rizk, D.V. IgA Nephropathy: An Interesting Autoimmune Kidney Disease. Am. J. Med. Sci. 2021, 361, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Zachova, K.; Jemelkova, J.; Kosztyu, P.; Ohyama, Y.; Takahashi, K.; Zadrazil, J.; Orsag, J.; Matousovic, K.; Galuszkova, D.; Petejova, N.; et al. Galactose-Deficient IgA1 B Cells in the Circulation of IgA Nephropathy Patients Carry Preferentially Lambda Light Chains and Mucosal Homing Receptors. J. Am. Soc. Nephrol. 2022, 33, 908–917. [Google Scholar] [CrossRef]
- Rollino, C.; Vischini, G.; Coppo, R. IgA Nephropathy and Infections. J. Nephrol. 2016, 29, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Selvaskandan, H.; Jhaveri, K.D.; Rizk, D.V. Primary IgA Nephropathy: New Insights and Emerging Therapies. Adv. Kidney Dis. Health 2024, 31, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H. Biomarkers for IgA Nephropathy on the Basis of Multi-Hit Pathogenesis. Clin. Exp. Nephrol. 2019, 23, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Gesualdo, L.; Di Leo, V.; Coppo, R. The Mucosal Immune System and IgA Nephropathy. Semin. Immunopathol. 2021, 43, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.; Guo, C.; Rao, X.; Li, S. IgA Nephropathy to Proliferative Glomerulonephritis with Monoclonal IgAκ Deposits: A Pattern Switch. J. Nephrol. 2023, 36, 2375–2380. [Google Scholar] [CrossRef]
- Medjeral-Thomas, N.R.; Cook, H.T.; Pickering, M.C. Complement Activation in IgA Nephropathy. Semin. Immunopathol. 2021, 43, 679–690. [Google Scholar] [CrossRef]
- Hwang, V.J.; Ulu, A.; Van Hoorebeke, J.; Weiss, R.H. Biomarkers in IGA Nephropathy. Biomark. Med. 2014, 8, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Jiang, M.; Nie, X. Exploring the Pathogenesis and Treatment of IgA Nephropathy Based on Epigenetics. Epigenomics 2023, 15, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Zhai, Y.; An, H.; Gao, J.; Chen, Y.; Zhang, W.; Zhao, Z. MicroRNAs in IgA Nephropathy. Ren. Fail. 2021, 43, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Magistroni, R.; D’Agati, V.D.; Appel, G.B.; Kiryluk, K. New Developments in the Genetics, Pathogenesis, and Therapy of IgA Nephropathy. Kidney Int. 2015, 88, 974–989. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Kim, J.S.; Jung, S.W.; Kim, Y.G.; Hwang, H.S.; Moon, J.-Y.; Lee, S.-H.; Seo, J.-W.; Seok, J.; Tae, D.; et al. Clinical Significance of Urinary Exosomal microRNAs in Patients with IgA Nephropathy. Sci. Rep. 2023, 13, 17201. [Google Scholar] [CrossRef]
- Tripathy, A.; Yedla, P.; Vishnubhotla, R.; Sekaran, A.; Keithi Reddy, S. MicroRNAs as a Therapeutic Target in IgA Nephropathy in Indian Population. Biomed. Rep. 2023, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- Kademani, S.P.; Nelaturi, P.; Sathyasagar, K.; Ravikumar, S. Noncoding RNAs Associated with IgA Nephropathy. J. Nephrol. 2022, 36, 911–923. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Wang, W.; Zhu, M.; Qi, L.; Wang, T.; Cheng, W.; Zhu, J.; Shan, X.; Huang, Z.; et al. Plasma microRNA Signature of Patients with IgA Nephropathy. Gene 2018, 649, 80–86. [Google Scholar] [CrossRef]
- Anastasio, C.; Donisi, I.; Colloca, A.; D’Onofrio, N.; Balestrieri, M.L. MiR-148a-3p/SIRT7 Axis Relieves Inflammatory-Induced Endothelial Dysfunction. Int. J. Mol. Sci. 2024, 25, 5087. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, X.-T.; Miao, Y.-P.; Bai, X.-L.; Jin, F. MiR-148a-3p Attenuates Apoptosis and Inflammation by Targeting CNTN4 in Atherosclerosis. Ann. Transl. Med. 2022, 10, 1201. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Song, Z.; Li, Y.; Huang, J.; Zhao, F.; Luo, Y.; Wang, J.; Deng, F.; Shadekejiang, H.; Zhang, M.; et al. MiR-20a-5p Alleviates Kidney Ischemia/Reperfusion Injury by Targeting ACSL4-Dependent Ferroptosis. Am. J. Transplant. 2023, 23, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Qie, J.; Fu, Q.; Chen, J.; Jin, Y.; Ding, Z. miR-20a-5p/TGFBR2 Axis Affects Pro-Inflammatory Macrophages and Aggravates Liver Fibrosis. Front. Oncol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ma, M.; Liu, F.; Yin, X.; Shi, H.; Li, Q.; Yang, K.; Yu, M. miR-148a-3p Mitigation of Coronary Artery Disease through PCSK9/NF-κB Inhibition of Vascular Endothelial Cell Injury. J. Biochem. Mol. Toxicol. 2024, 38, e70011. [Google Scholar] [CrossRef]
- Haas, M. Histologic Subclassification of IgA Nephropathy: A Clinicopathologic Study of 244 Cases. Am. J. Kidney Dis. 1997, 29, 829–842. [Google Scholar] [CrossRef]
- Zalewski, K.; Misiek, M.; Kowalik, A.; Bakuła-Zalewska, E.; Kopczyński, J.; Zielińska, A.; Bidziński, M.; Radziszewski, J.; Góźdź, S.; Kowalewska, M. Normalizers for microRNA Quantification in Plasma of Patients with Vulvar Intraepithelial Neoplasia Lesions and Vulvar Carcinoma. Tumour Biol. 2017, 39, 101042831771714. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Dong, J.; Wang, L.-E.; Ma, H.; Liu, J.; Zhao, Y.; Tang, J.; Chen, X.; Dai, J.; Wei, Q.; et al. Serum microRNA Profiling and Breast Cancer Risk: The Use of miR-484/191 as Endogenous Controls. Carcinogenesis 2012, 33, 828–834. [Google Scholar] [CrossRef]
- Rice, J.; Roberts, H.; Rai, S.N.; Galandiuk, S. Housekeeping Genes for Studies of Plasma microRNA: A Need for More Precise Standardization. Surgery 2015, 158, 1345–1351. [Google Scholar] [CrossRef]
- Petersen, P.H.D.; Lopacinska-Jørgensen, J.; Høgdall, C.K.; Høgdall, E.V. Identification of Stably Expressed microRNAs in Plasma from High-Grade Serous Ovarian Carcinoma and Benign Tumor Patients. Mol. Biol. Rep. 2023, 50, 10235–10247. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Reddy, P.H. Are Circulating microRNAs Peripheral Biomarkers for Alzheimer’s Disease? Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wang, H.; Zhang, X.; Yang, Y.; Wang, L.; Du, L.; Li, W.; Li, J.; Qu, A.; Liu, Y.; et al. Identification and Validation of Reference Genes for qPCR Detection of Serum microRNAs in Colorectal Adenocarcinoma Patients. PLoS ONE 2013, 8, e83025. [Google Scholar] [CrossRef]
- Siedlecki-Wullich, D.; Català-Solsona, J.; Fábregas, C.; Hernández, I.; Clarimon, J.; Lleó, A.; Boada, M.; Saura, C.A.; Rodríguez-Álvarez, J.; Miñano-Molina, A.J. Altered microRNAs Related to Synaptic Function as Potential Plasma Biomarkers for Alzheimer’s Disease. Alzheimers Res. Ther. 2019, 11, 46. [Google Scholar] [CrossRef]
- Belmonte, T.; Perez-Pons, M.; Benítez, I.D.; Molinero, M.; García-Hidalgo, M.C.; Rodríguez-Muñoz, C.; Gort-Paniello, C.; Moncusí-Moix, A.; Madè, A.; Devaux, Y.; et al. Addressing the Unsolved Challenges in microRNA-Based Biomarker Development: Suitable Endogenous Reference microRNAs for SARS-CoV-2 Infection Severity. Int. J. Biol. Macromol. 2024, 269, 131926. [Google Scholar] [CrossRef]
- Pawluczyk, I.; Nicholson, M.; Barbour, S.; Er, L.; Selvaskandan, H.; Bhachu, J.S.; Barratt, J. A Pilot Study to Predict Risk of IgA Nephropathy Progression Based on miR-204 Expression. Kidney Int. Rep. 2021, 6, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Pawluczyk, I.Z.A.; Didangelos, A.; Barbour, S.J.; Er, L.; Becker, J.U.; Martin, R.; Taylor, S.; Bhachu, J.S.; Lyons, E.G.; Jenkins, R.H.; et al. Differential Expression of microRNA miR-150-5p in IgA Nephropathy as a Potential Mediator and Marker of Disease Progression. Kidney Int. 2021, 99, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- A Technical Guide to Identifying miRNA Normalizers Using TaqMan Advanced miRNA Assays. Available online: https://assets.thermofisher.com/TFS-Assets/GSD/Reference-Materials/identifying-mirna-normalizers-white-paper.pdf (accessed on 19 November 2024).
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Lai, J.Y.; Luo, J.; O’Connor, C.; Jing, X.; Nair, V.; Ju, W.; Randolph, A.; Ben-Dov, I.Z.; Matar, R.N.; Briskin, D.; et al. MicroRNA-21 in Glomerular Injury. J. Am. Soc. Nephrol. 2015, 26, 805–816. [Google Scholar] [CrossRef]
- He, K.; Chen, Z.; Zhao, J.; He, Y.; Deng, R.; Fan, X.; Wang, J.; Zhou, X. The Role of microRNA-155 in Glomerular Endothelial Cell Injury Induced by High Glucose. Mol. Biol. Rep. 2022, 49, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Bharati, J.; Kumar, M.; Kumar, N.; Malhotra, A.; Singhal, P.C. MicroRNA193a: An Emerging Mediator of Glomerular Diseases. Biomolecules 2023, 13, 1743. [Google Scholar] [CrossRef]
- Martino, E.; Balestrieri, A.; Aragona, F.; Bifulco, G.; Mele, L.; Campanile, G.; Balestrieri, M.L.; D’Onofrio, N. MiR-148a-3p Promotes Colorectal Cancer Cell Ferroptosis by Targeting SLC7A11. Cancers 2023, 15, 4342. [Google Scholar] [CrossRef]
- Xiong, J.; Ni, J.; Chen, C.; Wang, K. miR-148a-3p Regulates Alcoholic Liver Fibrosis through Targeting ERBB3. Int. J. Mol. Med. 2020, 46, 1003–1012. [Google Scholar] [CrossRef]
- Gao, J.; Qin, L.; Guo, Q.; Zhao, D.; Ma, G.; Zhou, K.; Wang, S.; Hao, H. Diagnostic Value and Clinical Significance of lncRNA NEAT1 Combined with miR-425-3p in Children with Viral Myocarditis. Turk. J. Pediatr. 2024, 66, 439–447. [Google Scholar] [CrossRef]
- Gasparello, J.; Papi, C.; Zurlo, M.; Gambari, L.; Manicardi, A.; Rozzi, A.; Ferrarini, M.; Corradini, R.; Gambari, R.; Finotti, A. MicroRNAs miR-584-5p and miR-425-3p Are Up-Regulated in Plasma of Colorectal Cancer (CRC) Patients: Targeting with Inhibitor Peptide Nucleic Acids Is Associated with Induction of Apoptosis in Colon Cancer Cell Lines. Cancers 2022, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Huang, X.; Zhang, B.; Pei, F.; Zhao, Z.; Cen, X. miR -20a-5p Regulated SMAD6 to Inhibit Chondrogenesis of hDPSCs. Oral Dis. 2023, 29, 3433–3446. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tang, G.; Liu, W.; Zhou, Y.; Fan, C.; Zhang, W. MiR-20a-5p Facilitates Cartilage Repair in Osteoarthritis via Suppressing Mitogen-Activated Protein Kinase Kinase Kinase 2. Bioengineered 2022, 13, 13801–13814. [Google Scholar] [CrossRef]
- Qingjuan, L.; Xiaojuan, F.; Wei, Z.; Chao, W.; Pengpeng, K.; Hongbo, L.; Sanbing, Z.; Jun, H.; Min, Y.; Shuxia, L. miR-148a-3p Overexpression Contributes to Glomerular Cell Proliferation by Targeting PTEN in Lupus Nephritis. Am. J. Physiol.-Cell Physiol. 2016, 310, C470–C478. [Google Scholar] [CrossRef]
- Li, X.; Dong, Z.-Q.; Chang, H.; Zhou, H.-B.; Wang, J.; Yang, Z.-J.; Qiu, M.; Bai, W.-F.; Shi, S.-L. Screening and Identification of Key microRNAs and Regulatory Pathways Associated with the Renal Fibrosis Process. Mol. Omics 2022, 18, 520–533. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, W.; Ge, H.; Li, K. Aberrant Expression of miR-148a-3p in Alzheimer’s Disease and Its Protective Role against Amyloid-β Induced Neurotoxicity. Neurosci. Lett. 2021, 756, 135953. [Google Scholar] [CrossRef]
- Li, J.; Tu, J.; Gao, H.; Tang, L. MicroRNA-425-3p Inhibits Myocardial Inflammation and Cardiomyocyte Apoptosis in Mice with Viral Myocarditis through Targeting TGF-β1. Immune Inflamm. Dis. 2021, 9, 288–298. [Google Scholar] [CrossRef]
- Bharti, N.; Agrawal, V.; Kamthan, S.; Prasad, N.; Agarwal, V. Blood TGF-Β1 and miRNA-21-5p Levels Predict Renal Fibrosis and Outcome in IgA Nephropathy. Int. Urol. Nephrol. 2023, 55, 1557–1564. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Y.; Xu, G. The Role of Mononuclear Phagocyte System in IgA Nephropathy: Pathogenesis and Prognosis. Front. Immunol. 2023, 14, 1192941. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wu, X.; Xiang, S.; Qiao, M.; Cen, X.; Pan, X.; Huang, X.; Zhao, Z. Regulatory Mechanism of miR-20a-5p Expression in Cancer. Cell Death Discov. 2022, 8, 262. [Google Scholar] [CrossRef]
- Gong, Y.; Xu, W.; Chen, Y.; Liu, Y.; Yang, Y.; Wang, B.; Lu, Z.; Lin, H.; Zhou, X.; Zhou, X. miR-20a-5p Regulates Pulmonary Surfactant Gene Expression in Alveolar Type II Cells. J. Cell. Mol. Med. 2019, 23, 7664–7672. [Google Scholar] [CrossRef] [PubMed]
- Koide, T.; Mandai, S.; Kitaoka, R.; Matsuki, H.; Chiga, M.; Yamamoto, K.; Yoshioka, K.; Yagi, Y.; Suzuki, S.; Fujiki, T.; et al. Circulating Extracellular Vesicle-Propagated microRNA Signature as a Vascular Calcification Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 415–431. [Google Scholar] [CrossRef]
- Wang, I.-K.; Sun, K.-T.; Tsai, T.-H.; Chen, C.-W.; Chang, S.-S.; Yu, T.-M.; Yen, T.-H.; Lin, F.-Y.; Huang, C.-C.; Li, C.-Y. MiR-20a-5p Mediates Hypoxia-Induced Autophagy by Targeting ATG16L1 in Ischemic Kidney Injury. Life Sci. 2015, 136, 133–141. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Hsu, C.-T.; Tsai, S.-F.; Chen, C.-H. Association between Circulating MicroRNAs (miR-21-5p, miR-20a-5p, miR-29b-3p, miR-126-3p and miR-101-3p) and Chronic Allograft Dysfunction in Renal Transplant Recipients. Int. J. Mol. Sci. 2022, 23, 12253. [Google Scholar] [CrossRef] [PubMed]
- Muendlein, A.; Geiger, K.; Leiherer, A.; Saely, C.H.; Fraunberger, P.; Drexel, H. Evaluation of the Associations between Circulating microRNAs and Kidney Function in Coronary Angiography Patients. Am. J. Physiol.-Ren. Physiol. 2020, 318, F315–F321. [Google Scholar] [CrossRef] [PubMed]



| Assay Name | Assay ID |
|---|---|
| ath-miR159a | 478411_mir |
| hsa-miR-191-5p | 477952_mir |
| hsa-miR-20a-5p | 478586_mir |
| hsa-miR-148a-3p | 477814_mir |
| hsa-miR-425-3p | 478093_mir |
| Parameters Correlated with Plasma Expression of miR-148a-3p | Rs | p |
|---|---|---|
| Age [years] | 0.21 | 0.16 |
| WBC [G/L] | 0.01 | 0.98 |
| RBC [T/L] | 0.04 | 0.85 |
| HGB [mmol/L] | −0.01 | 0.97 |
| HCT [L/L] | −0.01 | 0.96 |
| PLT [G/L] | −0.02 | 0.93 |
| Creatinine level [mg/dL] | 0.20 | 0.20 |
| Glomerular filtration rate [mL/min] | −0.30 | 0.04 |
| Daily urinary protein loss [g/24 h] | −0.03 | 0.88 |
| Parameters Correlated with Plasma Expression of miR-425-3p | Rs | p |
|---|---|---|
| Age [years] | 0.23 | 0.13 |
| WBC [G/L] | 0.03 | 0.88 |
| RBC [T/L] | −0.40 | 0.07 |
| HGB [mmol/L] | −0.37 | 0.08 |
| HCT [L/L] | −0.40 | 0.06 |
| PLT [G/L] | 0.08 | 0.74 |
| Creatinine level [mg/dL] | 0.25 | 0.10 |
| Glomerular filtration rate [mL/min] | −0.36 | 0.01 |
| Daily urinary protein loss [g/24 h] | 0.06 | 0.74 |
| Parameters Correlated with Plasma Expression of miR-20a-5p | Rs | p |
|---|---|---|
| Age [years] | 0.23 | 0.13 |
| WBC [G/L] | −0.29 | 0.19 |
| RBC [T/L] | −0.24 | 0.29 |
| HGB [mmol/L] | −0.18 | 0.41 |
| HCT [L/L] | −0.29 | 0.18 |
| PLT [G/L] | −0.15 | 0.50 |
| Creatinine level [mg/dL] | 0.01 | 0.95 |
| Glomerular filtration rate [mL/min] | −0.14 | 0.37 |
| Daily urinary protein loss [g/24 h] | −0.09 | 0.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybyciński, J.; Czerewaty, M.; Kwiatkowska, E.; Dziedziejko, V.; Safranow, K.; Domański, L.; Pawlik, A. MicroRNAs miR-148a-3p, miR-425-3p, and miR-20a-5p in Patients with IgA Nephropathy. Genes 2025, 16, 125. https://doi.org/10.3390/genes16020125
Przybyciński J, Czerewaty M, Kwiatkowska E, Dziedziejko V, Safranow K, Domański L, Pawlik A. MicroRNAs miR-148a-3p, miR-425-3p, and miR-20a-5p in Patients with IgA Nephropathy. Genes. 2025; 16(2):125. https://doi.org/10.3390/genes16020125
Chicago/Turabian StylePrzybyciński, Jarosław, Michał Czerewaty, Ewa Kwiatkowska, Violetta Dziedziejko, Krzysztof Safranow, Leszek Domański, and Andrzej Pawlik. 2025. "MicroRNAs miR-148a-3p, miR-425-3p, and miR-20a-5p in Patients with IgA Nephropathy" Genes 16, no. 2: 125. https://doi.org/10.3390/genes16020125
APA StylePrzybyciński, J., Czerewaty, M., Kwiatkowska, E., Dziedziejko, V., Safranow, K., Domański, L., & Pawlik, A. (2025). MicroRNAs miR-148a-3p, miR-425-3p, and miR-20a-5p in Patients with IgA Nephropathy. Genes, 16(2), 125. https://doi.org/10.3390/genes16020125






