Role of MicroRNAs in Acute Myeloid Leukemia
Abstract
1. Introduction
2. Types of Non-Coding RNAs
3. MicroRNA Biogenesis
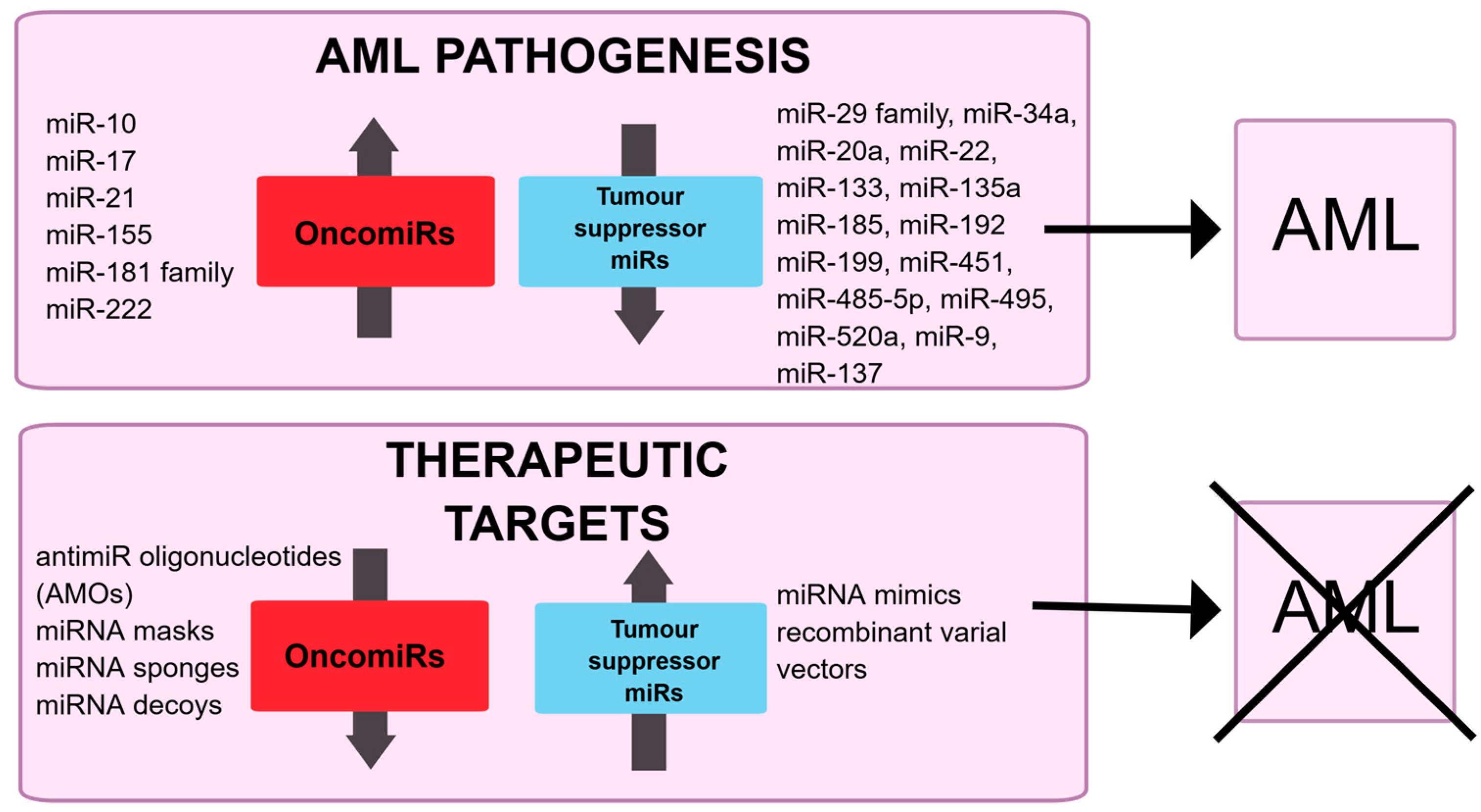
4. microRNA in AML
| ↓-miRNA | Target | Effect on AML/Leukemic Cells When miRNA Is Overexpressed | Method (Study Group/Control Group or Cell Lines) | Reference |
|---|---|---|---|---|
| miR-9 | CXCR4 | Reduction of proliferation and mobility of AML cells. Increased apoptosis rate of AML cells. | RT-qPCR (36 AML BM/10 BM; NB4, HL-60, Kasumi-1, SKNO-1, KG-1a/normal CD34+ cells), miRNA mimics (Kasumi-1, SKNO-1) | [116] |
| miR-20a-5p | PPP6C | Inhibition of cell proliferation, induction of cell cycle arrest and apoptosis. Decrease in tumor size in vivo. | RT-qPCR (61 AML BM/61 BM; Kasumi-1, THP-1, U937, HL-60/HS-5), miRNA mimics (THP-1, U937) | [117] |
| miR-22 | CRTC1 FLT3 | Inhibition of cell colony forming, viability, and growth. Inhibition of leukemogenesis in mice in vivo. Promotion of cell apoptosis. | RT-qPCR (42 AML MNC/5 MNC), miRNA mimics (MONOMAC-6, THP-1, KOCL-48) | [181] |
| EVI1 | Relieved blockage in the differentiation of bone marrow blasts. Inhibition of cell growth. | RT-qPCR (79 AML PB MNC/114 PB MNC; 41 AML BM MNC/8 BM MNC; 50 AML BM CD34+/10 BM CD34+), miRNA mimics (HL-60, THP1) | [182] | |
| miR-29a | MYC | Inhibition of cell tumorigenic ability in mice in vivo. | RT-qPCR (62 AML BM MNC/20 BM MNC; KG-1/BM MNC), miRNA mimics (KG-1) | [98] |
| miR-29b-3p | HuR | Inhibition of cell proliferation, colony-forming capability, and cell migration. Promotion of cell cycle arrest at G0/G1 phase and apoptosis. | RT-qPCR (K562, NB4, U937, K562/G01, Kasumi-1, HL60/PB MNC), miRNA mimics (K562, U937) | [99] |
| miR-30e-5p | CYB561 | Impaired cell self-renewal. Inhibited onset of KMT2A::MLLT3-driven leukemia in mice in vivo. | RT-qPCR (29 AML BM MNC/6 BM MNC), miRNA mimics (KMT2A::MLLT3 AML cells) | [151] |
| miR-34a | PD-L1 | Reduction in cell surface PD-L1 expression. Reduction in INF-γ-induced PD-L1 surface expression, apoptosis of PD-L1/CD 8+ T cells and IL-10 production upon IFN-γ | RT-qPCR (13 AML BM/5 BM), miRNA mimics (HL-60, Kasumi-1) | [103] |
| DHAC2 | Suppressed proliferation. Induced LSC death. Prolonged survival in AML mice in vivo. | RT-qPCR (30 AML BM/10 BM), miRNA mimics (KG-1a) | [104] | |
| miR-92a | MTHFD2 | Inhibition of cell proliferation and promotion of apoptosis. | RT-qPCR (HL-60, THP-1/HS-5), miRNA mimics (HL-60, THP-1) | [185] |
| miR-103a-2-5p | LILRB3 | Inhibition of cell proliferation, migration, and clonality. Promotion of apoptosis and cell cycle arrest. | RT-qPCR (30 AML BM MNC/BM MNC), miRNA mimics (THP-1, OCI-AML2, OCI-AML3, MV4-11) | [120] |
| miR-133 | ZC3H15 BCLAF1 | Inhibition of AML cell proliferation. Acceleration of AML cell apoptosis. | miRNA sequencing (102 AML samples/CD34+) *, miRNA mimics (NB4) | [123] |
| miR-135a | HOXA10 | Inhibition of AML cell proliferation and cell cycle. Acceleration of AML cell apoptosis. | RT-qPCR (29 AML PB/11 PB; HL-60, AML193, AML2, AML5/HS-5), miRNA mimics (HL-60, AML5) | [124] |
| miR-133a miR-135a | CDX2 | Inhibition of AML cell proliferation. | RT-qPCR (59 AML BM/9 BM), miRNA mimics (HEK-293, NB4, HL-60) | [125] |
| miR-137 | C-kit | Inhibition of proliferation and promotion differentiation of AML cells. | RT-qPCR (49 AML BM/57 BM), miRNA mimics (Kasumi-1, K562) | [127] |
| TRIM25 | Inhibited AML cells proliferation, migration and invasion. | RT-qPCR (45 AML BM/45 BM; HEL, Kasumi-1, HL-60, MEG01/HS-5), miRNA mimics (Ksaumi-1, HL-60) | [128] | |
| miR-142-3p | HMGB1 | Improvement of drug sensitivity in AML cells. | RT-qPCR (23 AML PB MNC/15 PB MNC), miRNA mimics (HL-60/ATRA, HL-60/ADR) | [110] |
| miR-142-5p | PFKP | Inhibition of AML cell proliferation, viability, cloning, and cycle. Induction of AML cell apoptosis. | RT-qPCR (THP-1, HL-60, TF-1, NB4, U937/HS-5), miRNA mimics (THP-1, U937) | [111] |
| miR-148 | DNMT1 | Inhibition of proliferation and induction of apoptosis of AML cells. | RT-qPCR (80 AML BM MNC/20 BM MNC; U937, THP-1, Kasumi-1/20 BM MNC), miRNA mimics (U937, Kasumi-1) | [130] |
| miR-185-5p | GPX1 | Inhibition of viability, proliferation, invasion, and promotion of apoptosis. | RT-qPCR (37 AML BM/37 BM; MOLM-14, HL-60, KG-1/HS-5), miRNA mimics (KG-1, HL-60) | [131] |
| miR-192-5p | ZBTB20 | Inhibition of cell viability. Induction of cell apoptosis and cell cycle arrest. | RT-qPCR (52 AML BM/34 BM THP-1, HL-60, NB4/HS-5), miRNA mimics (THP-1, HL-60) | [134] |
| CCNT2 | Induction of cell cycle arrest, apoptosis, and cell differentiation. | RT-qPCR (10 AML BM/10 BM; NB4, HL-60/10 BM), miRNA mimics (NB4, HL-60) | [135] | |
| miR-199a-5p | DRAM1 | Reduction of chemoresistance upon ADM treatment. Inhibition of autophagy. | RT-qPCR (32 AML relapsed/refractor BM/11 complete remission BM), miRNA mimics (K562/ADM, K562) | [190] |
| miR-211-5p | JAK2 | Reduction of AML cell proliferation, viability, and inflammation. Induction of AML cell apoptosis. | RT-qPCR (50 AML PB/50 PB), miRNA mimics (CPT treated LSCs) | [152] |
| miR-222-3p | IRF2 | Increased Th1/Th2 ratio. Induction of cell apoptosis. | RT-qPCR (20 BM of AML patients and healthy donors), miRNA mimics (HL-60) | [194] |
| miR-361-3p | KMT2A | Reduction of cell proliferation, migration, and invasion. | RT-qPCR (30 AML PB/30 PB; HL-60, KG-1a, KO52, THP-1/HS-5), miRNA mimics (HL-60, H562) | [198] |
| miR-409-3p | RAB10 | Inhibition of cell proliferation and induced apoptosis. | RT-qPCR (THP-1, NB4/HS-5), miRNA mimics (THP-1) | [153] |
| miR-451 | YWHAZ | Suppression of AML cell proliferation. Increased AML cell apoptosis. | RT-qPCR (69 AML PB MNC/80 PB MNC; 56 AML BM MNC/9 BM MNC; 32 AML BM CD34+/9 BM CD34+), miRNA mimics (NB4, HL-60) | [140] |
| miR-454-3p | ZEB2 | Inhibited viability and induced cell cycle arrest. Induction of apoptosis and autophagy. | RT-qPCR (NB4, THP-1, KG-1a, U937/PB MNC), miRNA mimics (THP-1) | [154] |
| miR-455-3p | UBN2 | Inhibition of cell proliferation and viability. Induction of cell apoptosis, autophagy, and cell cycle arrest. | RT-qPCR (16 AML PB/16 PB; HL-60, Kasumi-1, KG1, THP-1, MV4-11/PB MNC), miRNA mimics (HL-60) | [142] |
| miR-485-5p | SALL4 | Inhibition of cell proliferation and induction of cell apoptosis. | RT-qPCR (35 AML BM/35 BM; AML2, AML193, Kasumi-1, HL-60, AML5, U937/HS-5), miRNA mimics (AML5, U937) | [144] |
| miR-495-3p miR-543 | PDK1 | Inhibition of cell proliferation, cell glycolysis, viability under matrine. Induction of cell apoptosis and cell cycle arrest under matrine. | RT-qPCR (31 AML BM/31 BM; HL-60, Kasumi-1/HS-5), miRNA mimics (HL-60, Kasumi-1) | [147] |
| miR-520a-3p | MUC1 | Inhibition of cell proliferation and induction of apoptosis. | RT-qPCR (25 AML PB/25 PB), miRNA mimics (THP-1) | [150] |
| miR-654-3p | CCND1 | Inhibition of cell proliferation, induction of apoptosis and cell cycle arrest. | RT-qPCR (51 AML PB/51 PB; HL-60, Kasumi-1/HS-5), miRNA mimics (HL-60, Kasumi-1) | [155] |
| miR-1294 | ARHGEF10L | Inhibition of AML cell proliferation and invasion. Induction of AML cell apoptosis. | RT-qPCR (16 plasma/16 plasma; HL-60/HS-5), miRNA mimics (HL-60) | [156] |
| ↑-miRNA | Target | Effect on AML Cells While miRNA Is Downregulated | Method (Study Group/Control Group or Cell Lines) | Reference |
|---|---|---|---|---|
| miR-10b | HOXD10 | ND | RT-qPCR (108 AML serum samples/25 serum samples) | [158] |
| miR-17-5p | JAK1 | Inhibition of cell proliferation, migration, invasion, and promotion of apoptosis when lncRNA SUCLG2-AS1 overexpressed. | RT-qPCR (THP1, HL60/HS-5), miRNA mimics (THP-1, HL-60) | [165] |
| BECN1 | Inhibition of AML cell proliferation after treatment of vitamin D. | RT-qPCR (144 AML PB/45 PB), miRNA mimics (HL-60) | [166] | |
| miR-21 | KLF5 | Reduction of AML cells proliferation. | RT-qPCR (SKM-1, HL-60/HS-5), miRNA mimics (SKM-1, HL-60) | [161] |
| miR-92a-3p | PTEN | ND | RT-qPCR (115 AML BM MNC/48 BM MNC) | [186] |
| miR-93 | DAB2 | Inhibition of AML cells proliferation. Promotion of cell cycle arrest and apoptosis of AML cells. | RT-qPCR (28 AML BM MNC/30 BM MNC), miRNA mimics (THP-1, HL-60, HS-5) | [167] |
| miR-106-5p | RAB10 | ND | RT-qPCR (85 AML BM MNC/15 BM MNC) | [168] |
| miR-146a | CNTFR | Reduction of leukemic cells proliferation and migration. Increasing leukemic cells apoptosis. | RT-qPCR (11 AML BM/10 BM), miRNA mimics (HL-60) | [170] |
| miR-155 | SHIP1 | Inhibition of AML cell proliferation and promotion of apoptosis. | RT-qPCR (30 AML BM or PB MNC/10 BM MNC), miRNA mimics (U937, THP-1) | [173] |
| miR-181 family | PRKCD CTDSPL CAMKK1 | Modulation of granulocytic and macrophage-like differentiation. | RT-qPCR (95 AML PB MNC/75 PB MNC; 36 AML BM CD34+/9 BM CD34+), miRNA mimics (HL-60) | [179] |
| miR-221 miR-222 | YOD1 | Increasing p53 protein level and reducing its ubiquitination. | RT-qPCR (18 AML PB/20 PB and 6 BM), miRNA mimics (U2OS, HCT116) | [195] |
| miR-222-3p | AXIN2 | Decreased cell viability and induced apoptosis. | RT-qPCR (KG1a, NB4, U937, THP1/PB MNC), miRNA mimics (NB4, U937) | [196] |
| miR-361-3p | BTG2 | Inhibition of cell proliferation and induction of apoptosis. | RT-qPCR (34 AML PB/5 PB; HL-60/5 PB), miRNA mimics (HL-60) | [199] |
| miR-1306-5p | PHF6 | Inhibition of AML cell proliferation and induction of apoptosis rate. | RT-qPCR (48 AML BM/30 BM; HL-60, Kgla, K562, THP-1/HS-5), miRNA mimics (HL-60, K562) | [180] |
miRNA Associated with Genetic Abnormalities
| Genetic Abnormality | miRNA | Reference |
|---|---|---|
| t(8;21)(q22;q22.1)/ RUNX1::RUNX1T1 | let-7b | [200] |
| miR-223 | [201] | |
| miR-9-1 | [202] | |
| miR-126 | [203] | |
| miR-130a | [204] | |
| miR-383 | [205] | |
| t(15;17)(q24.1;q21.2)/ PML::RARA | miR-15b | [206] |
| miR-125b | [207] | |
| miR-382-5p | [208] | |
| inv(16)(p13.1q22) or t(16;16)(p13.1;q22)/ CBFB::MYH11 | miR-126 | [169] |
| t(9;11)(p21.3;q23.3)/ MLLT3::KMT2A | miR-30e | [151] |
| NPM1 | miR-10a | [209] |
| miR-21 | [163] | |
| miR-215-5p | [210] | |
| TP53 | miR-34a | [211] |
| miR-100 | ||
| RUNX1 | miR-363-3p | [213] |
| miR-146a | [214] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ranganathan, K.; Sivasankar, V. MicroRNAs—Biology and clinical applications. J. Oral Maxillofac. Pathol. 2014, 18, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, G.A.D. MicroRNAs: Circulating biomarkers for the early detection of imperceptible cancers via biosensor and machine-learning advances. Oncogene 2024, 43, 2135–2142. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell. Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Supplitt, S.; Karpinski, P.; Sasiadek, M.; Laczmanska, I. Current Achievements and Applications of Transcriptomics in Personalized Cancer Medicine. Int. J. Mol. Sci. 2021, 22, 1422. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Bhaskaran, M.; Mohan, M. MicroRNAs: History, biogenesis, and their evolving role in animal development and disease. Vet. Pathol. 2014, 51, 759–774. [Google Scholar] [CrossRef] [PubMed]
- Otmani, K.; Lewalle, P. Tumor Suppressor miRNA in Cancer Cells and the Tumor Microenvironment: Mechanism of Deregulation and Clinical Implications. Front. Oncol. 2021, 11, 708765. [Google Scholar] [CrossRef]
- Poller, W.; Sahoo, S.; Hajjar, R.; Landmesser, U.; Krichevsky, A.M. Exploration of the Noncoding Genome for Human-Specific Therapeutic Targets-Recent Insights at Molecular and Cellular Level. Cells 2023, 12, 2660. [Google Scholar] [CrossRef]
- Walter, N.G. Are non-protein coding RNAs junk or treasure?: An attempt to explain and reconcile opposing viewpoints of whether the human genome is mostly transcribed into non-functional or functional RNAs. Bioessays 2024, 46, e2300201. [Google Scholar] [CrossRef]
- Kelly, R.C.; Morgan, R.A.; Brown, M.; Overton, I.; Hardiman, G. The Non-coding Genome and Network Biology. In Systems Biology II Springer Medizin; Springer Nature Switzerland: Cham, Switzerland, 2024; Volume 15, pp. 163–181. [Google Scholar] [CrossRef]
- Nobusada, T.; Yip, C.W.; Agrawal, S.; Severin, J.; Abugessaisa, I.; Hasegawa, A.; Hon, C.C.; Ide, S.; Koido, M.; Kondo, A.; et al. Update of the FANTOM web resource: Enhancement for studying noncoding genomes. Nucleic Acids Res. 2025, 53, D419–D424. [Google Scholar] [CrossRef]
- Loganathan, T.; Doss, G.P.C. Non-coding RNAs in human health and disease: Potential function as biomarkers and therapeutic targets. Funct. Integr. Genom. 2023, 23, 33. [Google Scholar] [CrossRef] [PubMed]
- George, T.P.; Subramanian, S.; Supriya, M.H. A brief review of noncoding RNA. Egypt. J. Med. Hum. Genet. 2024, 25, 98. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Zhou, S.; Mao, J.; Zhan, Z.; Duan, S. miRNA interplay: Mechanisms and therapeutic interventions in cancer. MedComm—Oncol. 2024, 3, e93. [Google Scholar] [CrossRef]
- Poliseno, L.; Lanza, M.; Pandolfi, P.P. Coding, or non-coding, that is the question. Cell Res. 2024, 9, 609–629. [Google Scholar] [CrossRef]
- Li, S.; Hu, W.; Qian, L.; Sun, D. Insights into non-coding RNAS: Biogenesis, function and their potential regulatory roles in acute kidney disease and chronic kidney disease. Mol. Cell. Biochem. 2025, 480, 1287–1304. [Google Scholar] [CrossRef]
- GENCODE. Available online: https://www.gencodegenes.org/human/stats.html (accessed on 27 March 2025).
- Malgundkar, S.H.; Tamimi, Y. The pivotal role of long non-coding RNAs as potential biomarkers and modulators of chemoresistance in ovarian cancer (OC). Hum. Genet. 2024, 143, 107–124. [Google Scholar] [CrossRef]
- Kasprzyk, M.E.; Kazimierska, M.; Podralska, M. Navigating Non-Coding RNA from Biogenesis to Therapeutic Application; Academic Press: Cambridge, MA, USA, 2023; Chapter 3; pp. 89–138. [Google Scholar]
- Su, Y.; Wu, J.; Chen, W.; Shan, J.; Chen, D.; Zhu, G.; Ge, S.; Liu, Y. Spliceosomal snRNAs, the Essential Players in pre-mRNA Processing in Eukaryotic Nucleus: From Biogenesis to Functions and Spatiotemporal Characteristics. Adv. Biol. 2024, 8, 2400006. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Fang, Y.; Shen, X.; Liu, H.; Yu, L.; Zeng, S.; Cai, S.; Zhou, J.; Li, Z. Design and analysis of self-priming extension DNA hairpin probe for miRNA detection based on a unified dynamic programming framework. Anal. Chim. Acta 2024, 1303, 342530. [Google Scholar] [CrossRef]
- Venneri, M.; Passantino, A. MiRNA: What clinicians need to know. Eur. J. Intern. Med. 2023, 113, 6–9. [Google Scholar] [CrossRef]
- Maji, R.K.; Leisegang, M.S.; Boon, R.A.; Schulz, M.H. Revealing microRNA regulation in single cells. Trends Genet. 2025, S0168-9525(24)00317-2. [Google Scholar] [CrossRef]
- Chauhan, W.; Sudharshan, S.J.; Kafle, S.; Zennadi, R. SnoRNAs: Exploring Their Implication in Human Diseases. Int. J. Mol. Sci. 2024, 25, 7202. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.P.; Zhang, W.C.; Deng, J.R.; Qi, Z.H.; Lin, Z.W.; Wang, Z.D. Advances in the mechanism of small nucleolar RNA and its role in DNA damage response. Mil. Med. Res. 2024, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Giles, R.N.; Koutmou, K.S. Anticodon stem-loop tRNA modifications influence codon decoding and frame maintenance during translation. Semin. Cell Dev. Biol. 2024, 154, 105–113. [Google Scholar] [CrossRef]
- Wang, L.; Lin, S. Emerging functions of tRNA modifications in mRNA translation and diseases. J. Genet. Genom. 2023, 50, 223–232. [Google Scholar] [CrossRef] [PubMed]
- News Medical Life Sciences. Available online: https://www.news-medical.net/life-sciences/-Types-of-RNA-mRNA-rRNA-and-tRNA.aspx#:~:text=In%20bacteria%2C%20the%20small%20and,1800%20and%205000%20nucleotides%2C%20respectively (accessed on 27 March 2025).
- Rauscher, R.; Polacek, N. Ribosomal RNA expansion segments and their role in ribosome biology. Biochem. Soc. Trans. 2024, 52, 1317–1325. [Google Scholar] [CrossRef]
- Li, R.; Zhu, M.; Hu, X.; Chen, J.; Yu, F.; Barth, S.; Sun, L.; He, H. Overcoming endosomal/lysosomal barriers: Advanced strategies for cytosolic siRNA delivery. Chin. Chem. Lett. 2024, 110736. [Google Scholar] [CrossRef]
- Saleem, A.; Khan, M.U.; Zahid, T.; Khurram, I.; Ghani, M.U.; Ullah, I.; Munir, R.; Calina, D.; Sharifi-Rad, J. Biological role and regulation of circular RNA as an emerging biomarker and potential therapeutic target for cancer. Mol. Biol. Rep. 2024, 51, 296. [Google Scholar] [CrossRef]
- Drula, R.; Braicu, C.; Neagoe, I.B. Current advances in circular RNA detection and investigation methods: Are we running in circles? Wiley Interdiscip. Rev.-RNA 2024, 15, e1850. [Google Scholar] [CrossRef]
- Werry, N.; Russell, S.J.; Sivakumar, R.; Miller, S.; Hickey, K.; Larmer, S.; Lohuis, M.; Librach, C.; LaMarre, J. piRNA expression patterns in high vs. low fertility bovine sperm. Syst. Biol. Reprod. Med. 2024, 70, 183–194. [Google Scholar] [CrossRef]
- Claro-Linares, F.; Rojas-Ríos, P. PIWI proteins and piRNAs: Key regulators of stem cell biology. Front. Cell Dev. Biol. 2025, 13, 1540313. [Google Scholar] [CrossRef]
- Jove. Available online: https://app.jove.com/science-education/v/11630/concepts/pirna-piwi-interacting-rnas (accessed on 27 March 2025).
- Márton, É.; Varga, A.; Domoszlai, D.; Buglyó, G.; Balázs, A.; Penyige, A.; Balogh, I.; Nagy, B.; Szilágyi, M. Non-Coding RNAs in Cancer: Structure, Function, and Clinical Application. Cancers 2025, 17, 579. [Google Scholar] [CrossRef] [PubMed]
- Colino-Sanguino, Y.; Clark, S.J.; Valdes-Mora, F. The H2A.Z-nucleosome code in mammals: Emerging functions. Trends Genet. 2022, 38, 273–289. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Xiong, Z.; Ge, Y.; Xu, M.-Y.; Zhang, J.; Peng, Y.; Zhang, Q.; Sun, J.; Xi, Z.; et al. Enhancer transcription profiling reveals an enhancer RNA-driven ferroptosis and new therapeutic opportunities in prostate cancer. Signal Transduct. Target. Ther. 2025, 10, 87. [Google Scholar] [CrossRef]
- Rodriguez, A.; Griffiths-Jones, S.; Ashurst, J.L.; Bradley, A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004, 14, 1902–19010. [Google Scholar] [CrossRef]
- Yin, Z.; Shen, H.; Gu, C.M.; Zhang, M.Q.; Liu, Z.; Huang, J.; Zhu, Y.; Zhong, Q.; Huang, Y.; Wu, F.; et al. MiRNA-142-3P and FUS can be Sponged by Long Noncoding RNA DUBR to Promote Cell Proliferation in Acute Myeloid Leukemia. Front. Mol. Biosci. 2021, 8, 754936. [Google Scholar] [CrossRef]
- Orang, A.V.; Safaralizadeh, R.; Kazemzadeh-Bavili, M. Mechanisms of miRNA-Mediated Gene Regulation from Common Downregulation to mRNA-Specific Upregulation. Int. J. Genom. 2014, 2014, 970607. [Google Scholar] [CrossRef]
- Machowska, M.; Galka-Marciniak, P.; Kozlowski, P. Consequences of genetic variants in miRNA genes. Comput. Struct. Biotechnol. J. 2022, 20, 6443–6457. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Shyr, Y.; Cai, J.; Liu, Q. Interplay between miRNAs and host genes and their role in cancer. Brief. Funct. Genom. 2018, 18, 255–266. [Google Scholar] [CrossRef]
- Vilimova, M.; Pfeffer, S. Post-transcriptional regulation of polycistronic microRNAs. Wiley Interdiscip. Rev. 2023, 14, e1749. [Google Scholar] [CrossRef]
- Le, T.A.H.; Lao, T.D. Circulating microRNAs as the Potential Diagnostic and Prognostic Biomarkers for Nasopharyngeal Carcinoma. Genes 2022, 13, 1160. [Google Scholar] [CrossRef]
- Santovito, D.; Weber, C. Non-canonical features of microRNAs: Paradigms emerging from cardiovascular disease. Nat. Rev. Cardiol. 2022, 19, 620–638. [Google Scholar] [CrossRef]
- Macfarlane, L.A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef]
- Shademan, B.; Karamad, V.; Nourazarian, A.; Masjedi, S.; Isazadeh, A.; Sogutlu, F.; Avci, C.B. MicroRNAs as Targets for Cancer Diagnosis: Interests and Limitations. Adv. Pharm. Bull. 2023, 13, 435–445. [Google Scholar] [CrossRef]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA 2004, 10, 1957–1966. [Google Scholar] [CrossRef]
- Lee, D.; Shin, C. Emerging roles of DROSHA beyond primary microRNA processing. RNA Biol. 2018, 15, 186–193. [Google Scholar] [CrossRef]
- Han, J.; Pedersen, J.S.; Kwon, S.C.; Belair, C.D.; Kim, Y.K.; Yeom, K.H.; Yang, W.Y.; Haussler, D.; Blelloch, R.; Kim, V.N. Posttranscriptional crossregulation between Drosha and DGCR8. Cell 2009, 136, 75–84. [Google Scholar] [CrossRef]
- Kwon, S.C.; Nguyen, T.A.; Choi, Y.G.; Jo, M.H.; Hohng, S.; Kim, V.N.; Woo, J.S. Structure of Human DROSHA. Cell 2016, 164, 81–90. [Google Scholar] [CrossRef]
- Bofill-De Ros, X.; Vang Ørom, U.A. Recent progress in miRNA biogenesis and decay. RNA Biol. 2024, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stavast, C.J.; Erkeland, S.J. The Non-Canonical Aspects of MicroRNAs: Many Roads to Gene Regulation. Cells 2019, 8, 1465. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.T.; Chang, Y.M.; Chern, Y. The Impact of Dysregulated microRNA Biogenesis Machinery and microRNA Sorting on Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 3443. [Google Scholar] [CrossRef]
- Michlewski, G.; Cáceres, J.F. Post-transcriptional control of miRNA biogenesis. RNA 2019, 25, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K. When Argonaute takes out the ribonuclease sword. J. Biol. Chem. 2024, 300, 105499. [Google Scholar] [CrossRef]
- Grenda, A.; Budzyński, M.; Filip, A.A. Biogenesis of microRNAs and their role in the development and course of selected hematologic disorders. Postępy Hig. Med. Doświadczalnej Online 2013, 8, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef]
- Jungers, C.F.; Djuranovic, S. Modulation of miRISC-Mediated Gene Silencing in Eukaryotes. Front. Mol. Biosci. 2022, 9, 832916. [Google Scholar] [CrossRef] [PubMed]
- Kehl, T.; Backes, C.; Kern, F.; Fehlmann, T.; Ludwig, N.; Meese, E.; Lenhof, H.P.; Keller, A. About miRNAs, miRNA seeds, target genes and target pathways. Oncotarget 2017, 8, 107167–107175. [Google Scholar] [CrossRef]
- Li, L.; Sheng, P.; Li, T.; Fields, C.J.; Hiers, N.M.; Wang, Y.; Li, J.; Guardia, C.M.; Licht, J.D.; Xie, M. Widespread microRNA degradation elements in target mRNAs can assist the encoded proteins. Genes Dev. 2021, 35, 1595–1609. [Google Scholar] [CrossRef]
- Hackl, L.M.; Fenn, A.; Louadi, Z.; Baumbach, J.; Kacprowski, T.; List, M.; Tsoy, O. Alternative splicing impacts microRNA regulation within coding regions. NAR Genom. Bioinform. 2023, 5, lqad081. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The intricate balance between microRNA-induced mRNA decay and translational repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef]
- Kuzuoğlu-Öztürk, D.; Bhandari, D.; Huntzinger, E.; Fauser, M.; Helms, S.; Izaurralde, E. miRISC and the CCR4-NOT complex silence mRNA targets independently of 43S ribosomal scanning. EMBO J. 2016, 35, 1186–1203. [Google Scholar] [CrossRef]
- Amorim, I.S.; Lach, G.; Gkogkas, C.G. The Role of the Eukaryotic Translation Initiation Factor 4E (eIF4E) in Neuropsychiatric Disorders. Front. Genet. 2018, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Eulalio, A.; Huntzinger, E.; Nishihara, T.; Rehwinkel, J.; Fauser, M.; Izaurralde, E. Deadenylation is a widespread effect of miRNA regulation. RNA 2009, 15, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Grove, C.S.; Vassiliou, G.S. Acute myeloid leukemia: A paradigm for the clonal evolution of cancer? Dis. Models Mech. 2014, 7, 941–951. [Google Scholar] [CrossRef]
- American Cancer Society. Available online: https://www.cancer.org/cancer/types/acute-myeloid-leukemia/causes-risks-prevention/what-causes.html (accessed on 11 September 2024).
- American Cancer Society. Available online: https://www.cancer.org/cancer/types/acute-myeloid-leukemia/treating.html (accessed on 11 September 2024).
- Jimenez-Chillon, C.; Dillon, R.; Russell, N. Optimal Post-Remission Consolidation Therapy in Patients with AML. Acta Haematol. Pol. 2024, 147, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Abuelgasim, K.A.; Albuhayri, B.; Munshi, R.; Mugairi, A.A.; Alahmari, B.; Gmati, G.; Salama, H.; Alzahrani, M.; Alhejazi, A.; Alaskar, A.; et al. Impact of age and induction therapy on outcome of 180 adult patients with acute myeloid leukemia; retrospective analysis and literature review. Leuk. Res. Rep. 2020, 9, 100206. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 11 September 2024).
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 28, 15004. [Google Scholar] [CrossRef]
- Wallace, J.A.; O’Connell, R.M. MicroRNAs and acute myeloid leukemia: Therapeutic implications and emerging concepts. Blood 2017, 14, 1290–1301. [Google Scholar] [CrossRef]
- Fletcher, D.; Brown, E.; Javadala, J.; Uysal-Onganer, P.; Guinn, B.A. microRNA expression in acute myeloid leukemia: New targets for therapy? eJHaem 2022, 3, 596–608. [Google Scholar] [CrossRef]
- Kim, T.; Croce, C.M. MicroRNA: Trends in clinical trials of cancer diagnosis and therapy strategies. Exp. Mol. Med. 2023, 55, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Target Scan Human. Available online: https://www.targetscan.org/vert_80/ (accessed on 28 March 2025).
- miRDB. Available online: https://mirdb.org/ (accessed on 28 March 2025).
- ENCORI. Available online: https://rnasysu.com/encori/ (accessed on 28 March 2025).
- Ramsingh, G.; Jacoby, M.A.; Shao, J.; De Jesus Pizzaro, R.E.; Shen, D.; Trissal, M.; Getz, A.H.; Ley, T.J.; Walter, M.J.; Link, D.C. Acquired copy number alterations of miRNA genes in acute myeloid leukemia are uncommon. Blood 2013, 122, e44–e51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Starczynowski, D.T.; Morin, R.; McPherson, A.; Lam, J.; Chari, R.; Wegrzyn, J.; Kuchenbauer, F.; Hirst, M.; Tohyama, K.; Humphries, R.K.; et al. Genome-wide identification of human microRNAs located in leukemia-associated genomic alterations. Blood 2011, 117, 595–607. [Google Scholar] [CrossRef]
- Eyholzer, M.; Schmid, S.; Wilkens, L.; Mueller, B.U.; Pabst, T. The tumor-suppressive miR-29a/b1 cluster is regulated by CEBPA and blocked in human AML. Br. J. Cancer 2010, 103, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Liu, R.; Kasinski, A.L.; Shen, H.; Slack, F.J.; Tang, D.G. MicroRNA-34a: Potent Tumor Suppressor, Cancer Stem Cell Inhibitor, and Potential Anticancer Therapeutic. Front. Cell Dev. Biol. 2021, 9, 640587. [Google Scholar] [CrossRef]
- Bousquet, M.; Quelen, C.; Rosati, R.; Mansat-De Mas, V.; La Starza, R.; Bastard, C.; Lippert, E.; Talmant, P.; Lafage-Pochitaloff, M.; Leroux, D.; et al. Myeloid cell differentiation arrest by miR-125b-1 in myelodysplastic syndrome and acute myeloid leukemia with the t(2;11)(p21;q23) translocation. J. Exp. Med. 2008, 205, 2499–2506. [Google Scholar] [CrossRef]
- Mi, S.; Li, Z.; Chen, P.; He, C.; Cao, D.; Elkahloun, A.; Lu, J.; Pelloso, L.A.; Wunderlich, M.; Huang, H.; et al. Aberrant overexpression and function of the miR-17-92 cluster in MLL-rearranged acute leukemia. Proc. Natl. Acad. Sci. USA 2010, 107, 3710–3715. [Google Scholar] [CrossRef]
- Chen, P.; Price, C.; Li, Z.; Li, Y.; Cao, D.; Wiley, A.; He, C.; Gurbuxani, S.; Kunjamma, R.B.; Huang, H.; et al. miR-9 is an essential oncogenic microRNA specifically overexpressed in mixed lineage leukemia-rearranged leukemia. Proc. Natl. Acad. Sci. USA 2013, 110, 11511–11516. [Google Scholar] [CrossRef]
- Senyuk, V.; Zhang, Y.; Liu, Y.; Ming, M.; Premanand, K.; Zhou, L.; Chen, P.; Chen, J.; Rowley, J.D.; Nucifora, G.; et al. Critical role of miR-9 in myelopoiesis and EVI1-induced leukemogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 5594–5599. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Sun, M.; Mi, S.; Zhang, H.; Luo, R.T.; Chen, P.; Wang, Y.; Yan, M.; Qian, Z.; et al. Distinct microRNA expression profiles in acute myeloid leukemia with common translocations. Proc. Natl. Acad. Sci. USA 2008, 105, 15535–15540. [Google Scholar] [CrossRef]
- Gao, X.N.; Lin, J.; Li, Y.H.; Gao, L.; Wang, X.R.; Wang, W.; Kang, H.Y.; Yan, G.T.; Wang, L.L.; Yu, L. MicroRNA-193a represses c-kit expression and functions as a methylation-silenced tumor suppressor in acute myeloid leukemia. Oncogene 2011, 30, 3416–3428. [Google Scholar] [CrossRef]
- Pulikkan, J.A.; Dengler, V.; Peramangalam, P.S.; Peer Zada, A.A.; Müller-Tidow, C.; Bohlander, S.K.; Tenen, D.G.; Behre, G. Cell-cycle regulator E2F1 and microRNA-223 comprise an autoregulatory negative feedback loop in acute myeloid leukemia. Blood 2010, 115, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Vineetha, R.C.; Raj, J.A.G.; Devipriya, P.; Mahitha, M.S.; Hariharan, S. MicroRNA-based therapies: Revolutionizing the treatment of acute myeloid leukemia. Int. J. Lab. Hematol. 2024, 46, 33–41. [Google Scholar] [CrossRef]
- Ghazaryan, A.; Wallace, J.A.; Tang, W.W.; Barba, C.; Lee, S.-H.; Bauer, K.M.; Nelson, M.C.; Kim, C.N.; Stubben, C.; Voth, W.P.; et al. miRNA-1 promotes acute myeloid leukemia cell pathogenesis through metabolic regulation. Front. Genet. 2023, 14, 1192799. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A. A novel therapeutic strategy: The significance of exosomal miRNAs in acute myeloid leukemia. Med. Oncol. 2024, 41, 62. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G. miRNAs: The Road from Bench to Bedside. Genes 2023, 14, 314. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Li, M.; Wang, W.; Liu, Y.; Wang, S. Silencing long non-coding RNA XIST suppresses drug resistance in acute myeloid leukemia through down-regulation of MYC by elevating microRNA-29a expression. Mol. Med. 2020, 26, 114. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Wu, W.; Chen, Q.-Q.; Liu, S.-H.; Zheng, Z.-Y.; Cui, Z.-L.; Xu, J.-P.; Xue, Y.; Lin, D.-H. miR-29b-3p suppresses the malignant biological behaviors of AML cells via inhibiting NF-κB and JAK/STAT signaling pathways by targeting HuR. BMC Cancer 2022, 22, 909. [Google Scholar] [CrossRef]
- Randazzo, V.; Salemi, D.; Agueli, C.; Cannella, S.; Marfia, A.; Bica, M.G.; Randazzo, G.; Russo Lacerna, C.; Di Raimondo, F.; Fabbiano, F.; et al. Upregulation of Mir-29 in Normal Karyotype Aml Showing Dnmt3a Mutation. J. Hematol. Transfus. 2016, 4, 1048. [Google Scholar]
- Ngankeu, A.; Ranganathan, P.; Havelange, V.; Nicolet, D.; Volinia, S.; Powell, B.L.; Kolitz, J.E.; Uy, G.L.; Stone, R.M.; Kornblau, S.M.; et al. Discovery and functional implications of a miR-29b-1/miR-29a cluster polymorphism in acute myeloid leukemia. Oncotarget 2017, 9, 4354–4365. [Google Scholar] [CrossRef]
- Abdellateif, M.S.; Hassan, N.M.; Kamel, M.M.; El-Meligui, Y.M. Bone marrow microRNA-34a is a good indicator for response to treatment in acute myeloid leukemia. Oncol. Res. 2024, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Dong, K.; Lin, F.; Long, M.; Ouyang, Y.; Wei, J.; Chen, X.; Weng, Y.; He, T.; et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell. Signal. 2015, 27, 443–452. [Google Scholar] [CrossRef]
- Hu, Y.; Ma, X.; Wu, Z.; Nong, Q.; Liu, F.; Wang, Y.; Dong, M. MicroRNA-34a-mediated death of acute myeloid leukemia stem cells through apoptosis induction and exosome shedding inhibition via histone deacetylase 2 targeting. IUBMB Life 2020, 72, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zou, Y.; Lin, L.; Ma, X.; Chen, H. Identification of serum miR-34a as a potential biomarker in acute myeloid leukemia. Cancer Biomark. 2018, 22, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, Y.; Liao, C.; Ma, X.; Wang, M.; Li, Q.; Wang, D.; Li, Y.; Zhang, X.; Li, L.; et al. Engineered mesenchymal stem cell exosomes loaded with miR-34c-5p selectively promote eradication of acute myeloid leukemia stem cells. Cancer Lett. 2023, 575, 216407. [Google Scholar] [CrossRef]
- Peng, D.; Wang, H.; Li, L.; Ma, X.; Chen, Y.; Zhou, H.; Luo, Y.; Xiao, Y.; Liu, L. miR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding. Leukemia 2018, 32, 1180–1188. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Zhou, J.-D.; Wang, Y.-X.; Deng, Z.-Q.; Yang, J.; Yao, D.-M.; Qian, Z.; Yang, L.; Lin, J.; Qian, J. Low miR-34c expression is associated with poor outcome in de novo acute myeloid leukemia. Int. J. Lab. Hematol. 2017, 39, 42–50. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.-K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.-L.; Kim, T.-Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumors. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Xu, X. Upregulation of miR-142-3p Improves Drug Sensitivity of Acute Myelogenous Leukemia through Reducing P-Glycoprotein and Repressing Autophagy by Targeting HMGB1. Transl. Oncol. 2017, 10, 410–418. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, T.; Wang, Y.; Li, J.; Guo, L. Effect of lncRNA XIST on acute myeloid leukemia cells via miR-142-5p-PFKP axis. Hematology 2024, 29, 2306444. [Google Scholar] [CrossRef]
- Yuan, D.M.; Ma, J.; Fang, W.B. Identification of non-coding RNA regulatory networks in pediatric acute myeloid leukemia reveals circ-0004136 could promote cell proliferation by sponging miR-142. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9251–9258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhao, D.; Chen, F.; Frankhouser, D.; Wang, H.; Pathak, K.V.; Dong, L.; Torres, A.; Garcia-Mansfield, K.; Zhang, Y.; et al. Acquired miR-142 deficit in leukemic stem cells suffices to drive chronic myeloid leukemia into blast crisis. Nat. Commun. 2023, 14, 5325. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, P.; Qiao, H.; Sun, K.; Lu, X.; Bao, F.; Yu, R.; Lian, C.; Li, Y.; Chen, W.; et al. miR-9 Enhances the Chemosensitivity of AML Cells to Daunorubicin by Targeting the EIF5A2/MCL-1 Axis. Int. J. Biol. Sci. 2019, 15, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, X.; Xia, J.; Sun, J.; Huang, H.; Liu, Y. MicroRNA-9 restrains the sharp increase and boost apoptosis of human acute myeloid leukemia cells by adjusting the Hippo/YAP signaling pathway. Bioengineered 2021, 12, 2906–2914. [Google Scholar] [CrossRef]
- Zhu, B.; Xi, X.; Liu, Q.; Cheng, Y.; Yang, H. MiR-9 functions as a tumor suppressor in acute myeloid leukemia by targeting CX chemokine receptor 4. Am. J. Transl. Res. 2019, 11, 3384–3397. [Google Scholar] [PubMed]
- Bao, F.; Zhang, L.; Pei, X.; Lian, C.; Liu, Y.; Tan, H.; Lei, P. MiR-20a-5p functions as a potent tumor suppressor by targeting PPP6C in acute myeloid leukemia. PLoS ONE 2021, 16, e0256995. [Google Scholar] [CrossRef]
- Ping, L.; Jian-Jun, C.; Chu-Shu, L.; Guang-Hua, L.; Ming, Z. Silencing of circ_0009910 inhibits acute myeloid leukemia cell growth through increasing miR-20a-5p. Blood Cells Mol. Dis. 2019, 75, 41–47. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Wang, W.-T.; Huang, W.; Fang, K.; Sun, Y.-M.; Liu, S.-R.; Luo, X.-Q.; Chen, Y.-Q. The lncRNA HOTAIRM1 regulates the degradation of PML-RARA oncoprotein and myeloid cell differentiation by enhancing the autophagy pathway. Cell Death Differ. 2017, 24, 212–224. [Google Scholar] [CrossRef]
- Cen, Q.; Chen, J.; Guo, J.; Chen, M.; Wang, H.; Wu, S.; Zhang, H.; Xie, X.; Li, Y. CLPs-miR-103a-2-5p inhibits proliferation and promotes cell apoptosis in AML cells by targeting LILRB3 and Nrf2/HO-1 axis, regulating CD8+ T cell response. J. Transl. Med. 2024, 22, 278. [Google Scholar] [CrossRef]
- Zheng, Z.-Z.; Ma, Y.-P.; Wu, R.-H.; Rong, G.; Li, C.; Li, G.-X.; Ren, F.-G.; Xu, L.-J. Serum miR-133 as a novel biomarker for predicting treatment response and survival in acute myeloid leukemia. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 777–783. [Google Scholar] [CrossRef]
- Yamamoto, H.; Lu, J.; Oba, S.; Kawamata, T.; Yoshimi, A.; Kurosaki, N.; Yokoyama, K.; Matsushita, H.; Kurokawa, M.; Tojo, A.; et al. miR-133 regulates Evi1 expression in AML cells as a potential therapeutic target. Sci. Rep. 2016, 6, 19204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yue, C.; Liu, Q.; Che, X. Exploration of differentially expressed mRNAs and miRNAs for pediatric acute myeloid leukemia. Front. Genet. 2022, 13, 865111. [Google Scholar] [CrossRef]
- Xu, H.; Wen, Q. Downregulation of miR-135a predicts poor prognosis in acute myeloid leukemia and regulates leukemia progression via modulating HOXA10 expression. Mol. Med. Rep. 2018, 18, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Fan, Z.; Liang, C.; Peng, C.J.; Li, Y.; Wang, L.N.; Luo, J.S.; Zhang, X.L.; Liu, Y.; Zhang, L.D. miR-133a and miR-135a Regulate All-Trans Retinoic Acid-Mediated Differentiation in Pediatric Acute Myeloid Leukemia by Inhibiting CDX2 Translation and Serve as Prognostic Biomarkers. Technol. Cancer Res. Treat. 2024, 23, 15330338241248576. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, K.; Yu, J.; Tao, W.; Wei, Y. MiR-133 promotes the multidrug resistance of acute myeloid leukemia cells (HL-60/ADR) to daunorubicin. Cytotechnology 2024, 76, 833–846. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, X.; Chu, G.; Lai, G.; Zhang, B.; Wang, L.; Zhao, Y. miR-137 downregulates c-kit expression in acute myeloid leukemia. Leuk. Res. 2017, 57, 72–77. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.S.; Yang, Y.; Li, Y.; Lv, J.L.; Cheng, Y. TRIM25 contributes to the malignancy of acute myeloid leukemia and is negatively regulated by microRNA-137. Open Med. 2020, 16, 95–103. [Google Scholar] [CrossRef]
- Wang, X.-X.; Zhang, R.; Li, Y. Expression of the miR-148/152 Family in Acute Myeloid Leukemia and its Clinical Significance. Med. Sci. Monit. 2017, 23, 4768–4778. [Google Scholar] [CrossRef]
- Wang, X.-X.; Zhang, H.; Li, Y. Preliminary study on the role of miR-148a and DNMT1 in the pathogenesis of acute myeloid leukemia. Mol. Med. Rep. 2019, 19, 2943–2952. [Google Scholar] [CrossRef]
- Pang, B.; Mao, H.; Wang, J.; Yang, W. MiR-185-5p suppresses acute myeloid leukemia by inhibiting GPX1. Microvasc. Res. 2022, 140, 104296. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Zhang, J.; Zheng, N. Long Non-Coding RNA Taurine Upregulated Gene 1 Targets miR-185 to Regulate Cell Proliferation and Glycolysis in Acute Myeloid Leukemia Cells in vitro. OncoTargets Ther. 2020, 13, 7887–7896. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Deng, J.; Chen, C.; Ma, X.; Yu, L.; Chen, L. Circ_0001602 aggravates the progression of acute myeloid leukemia by regulating the miR-192-5p/ZBTB20 axis. Hematology 2023, 28, 2240133. [Google Scholar] [CrossRef]
- Tian, C.; Zhang, L.; Li, X.; Zhang, Y.; Li, J.; Chen, L. Low miR-192 expression predicts poor prognosis in pediatric acute myeloid leukemia. Cancer Biomark. 2018, 22, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Li, R.C.; Lu, J.; Meng, F.K.; Feng, Y.K.; Fang, M.H. MicroRNA-192 regulates cell proliferation and cell cycle transition in acute myeloid leukemia via interaction with CCNT2. Int. J. Hematol. 2017, 106, 258–265. [Google Scholar] [CrossRef]
- Chen, D.P.; Chang, S.W.; Wen, Y.H.; Wang, W.T. Association between diminished miRNA expression and the disease status of AML patients: Comparing to healthy control. Biomed. J. 2023, 46, 100518. [Google Scholar] [CrossRef] [PubMed]
- Krakowsky, R.H.E.; Wurm, A.A.; Gerloff, D.; Katzerke, C.; Bräuer-Hartmann, D.; Hartmann, J.U.; Wilke, F.; Thiede, C.; Müller-Tidow, C.; Niederwieser, D.; et al. miR-451a abrogates treatment resistance in FLT3-ITD-positive acute myeloid leukemia. Blood Cancer J. 2018, 8, 36. [Google Scholar] [CrossRef]
- Li, L.; Mussack, V.; Görgens, A.; Pepeldjiyska, E.; Hartz, A.S.; Aslan, H.; Rackl, E.; Rank, A.; Schmohl, J.; El Andaloussi, S.; et al. The potential role of serum extracellular vesicle derived small RNAs in AML research as non-invasive biomarker. Nanoscale Adv. 2023, 5, 1691–1705. [Google Scholar] [CrossRef]
- Song, L.; Lin, H.-S.; Gong, J.-N.; Han, H.; Wang, X.-S.; Su, R.; Chen, M.-T.; Shen, C.; Ma, Y.-N.; Yu, J.; et al. microRNA-451-modulated hnRNP A1 takes a part in granulocytic differentiation regulation and acute myeloid leukemia. Oncotarget 2017, 8, 55453–55466. [Google Scholar] [CrossRef]
- Su, R.; Gong, J.-N.; Chen, M.-T.; Song, L.; Shen, C.; Zhang, X.-H.; Yin, X.-L.; Ning, H.-M.; Liu, B.; Wang, F.; et al. c-Myc suppresses miR-451⊣YWTAZ/AKT axis via recruiting HDAC3 in acute myeloid leukemia. Oncotarget 2016, 7, 77430–77443. [Google Scholar] [CrossRef][Green Version]
- Wu, K.; Li, Y.; Nie, B.; Guo, C.; Ma, X.; Li, L.; Cheng, S.; Li, Y.; Luo, S.; Zeng, Y.; et al. MEF2A is a transcription factor for circPVT1 and contributes to the malignancy of acute myeloid leukemia. Int. J. Oncol. 2024, 65, 111. [Google Scholar] [CrossRef]
- Xie, Y.; Tan, L.; Wu, K.; Li, D.; Li, C. MiR-455-3p mediates PPARα through UBN2 to promote apoptosis and autophagy in acute myeloid leukemia cells. Exp. Hematol. 2023, 128, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, Q.; Zhu, K.; Zhu, J.; Li, J.; Yuan, Y.; Zhang, P.; Zhou, L.; Liu, L. LncRNA LINC00265/miR-485-5p/IRF2-mediated autophagy suppresses apoptosis in acute myeloid leukemia cells. Am. J. Transl. Res. 2020, 12, 2451–2462. [Google Scholar]
- Wang, W.-L.; Wang, H.R.; Ji, W.G.; Guo, S.L.; Li, H.X.; Xu, X.Y. MiRNA-485-5p suppresses the proliferation of acute myeloid leukemia via targeting SALL4. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4842–4849. [Google Scholar] [CrossRef]
- Huang, L.; Dai, J. Expression and Clinical Significance of miRNA-495 in the Peripheral Blood of Acute Myeloid Leukemia Patients. Proc. Anticancer Res. 2022, 6, 5. [Google Scholar] [CrossRef]
- Lei, Y.; Li, X.; Zhu, L. Matrine regulates miR-495-3p/miR-543/PDK1 axis to repress the progression of acute myeloid leukemia via the Wnt/βcatenin pathway. Chem. Biol. Drug Des. 2024, 103, e14441. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Song, L.; Pan, H.; Jiang, J.; Sun, L. Long noncoding RNA MIAT promotes the progression of acute myeloid leukemia by negatively regulating miR-495. Leuk. Res. 2019, 87, 106265. [Google Scholar] [CrossRef]
- Zhang, W.; Wan, B.; Liu, B.; Wu, S.; Zhao, L. Clinical significance of miR-372 and miR-495 in acute myeloid leukemia. Oncol. Lett. 2020, 20, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Qin, X.H.; Xie, X.L.; Liao, C.X.; Liu, D.T.; Li, G.W. Overexpression miR-520a-3p inhibits acute myeloid leukemia progression via targeting MUC1. Transl. Oncol. 2022, 22, 101432. [Google Scholar] [CrossRef]
- Xiao, J.; Wan, F.; Tian, L.; Li, Y. Tumor suppressor miR-520a inhibits cell growth by negatively regulating PI3K/AKT signaling pathway in acute myeloid leukemia. Adv. Clin. Exp. Med. 2024, 33, 729–738. [Google Scholar] [CrossRef]
- Ge, Y.; Hong, M.; Zhang, Y.; Wang, J.; Li, L.; Zhu, H.; Sheng, Y.; Wu, W.-S.; Zhang, Z. miR-30e-5p regulates leukemia stem cell self-renewal through the Cyb561/ROS signaling pathway. Haematologica 2024, 109, 411–421. [Google Scholar] [CrossRef]
- Ye, Q.; Ren, L.; Jiang, Z.M.; Li, X.Y.; Wei, G.Y.; Ren, Y.F.; Ren, L.H. Cryptanshinone extract of Salvia miltiorrhiza stimulates pediatric acute myeloid leukemia stem cell apoptosis and the anti-inflammatory mechanism via accelerating microRNA-211-5p to supress Janus kinase 2/signal transducer and activator of transcription 3 signaling pathway activation. J. Physiol. Pharmacol. 2023, 74, 691–700. [Google Scholar] [CrossRef]
- Xie, W.; Wang, Z.; Guo, X.; Guan, H. MiR-409-3p regulates the proliferation and apoptosis of THP-1 through targeting Rab10. Leuk. Res. 2023, 132, 107350. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, L.; Dan, W.; Chu, X.; Luo, X.; Liu, C.; Wan, P.; Lu, Y.; Liu, Z.; Zhang, Z.; et al. MiR-454-3p promotes apoptosis and autophagy of AML cells by targeting ZEB2 and regulating AKT/mTOR pathway. Hematology 2023, 28, 2223874. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, C.; Zhou, W. CircPLXNB2 regulates acute myeloid leukemia progression through miR-654-3p/CCND1 axis. Hematology 2023, 28, 2220522. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.S.; Yang, Y.; Fu, L.L. CircFN1 promotes acute myeloid leukemia cell proliferation and invasion but refrains apoptosis via miR-1294/ARHGEF10L axis. Kaohsiung J. Med. Sci. 2024, 40, 221–230. [Google Scholar] [CrossRef]
- Bi, L.; Sun, L.; Jin, Z.; Zhang, S.; Shen, Z. MicroRNA-10a/b are regulators of myeloid differentiation and acute myeloid leukemia. Oncol. Lett. 2018, 15, 5611–5619. [Google Scholar] [CrossRef]
- Wang, C.J.; Zou, H.; Feng, G.F. MiR-10b regulates the proliferation and apoptosis of pediatric acute myeloid leukemia through targeting HOXD10. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 7371–7378. [Google Scholar] [CrossRef]
- Zhi, Y.; Xie, X.; Wang, R.; Wang, B.; Gu, W.; Ling, Y.; Dong, W.; Zhi, F.; Liu, Y. Serum level of miR-10-5p as a prognostic biomarker for acute myeloid leukemia. Int. J. Hematol. 2015, 102, 296–303. [Google Scholar] [CrossRef]
- Yuan, Z.; Wang, W. LncRNA SNHG4 regulates miR-10a/PTEN to inhibit the proliferation of acute myeloid leukemia cells. Hematology 2020, 25, 160–164. [Google Scholar] [CrossRef]
- Li, C.; Yan, H.; Yin, J.; Ma, J.; Liao, A.; Yang, S.; Wang, L.; Huang, Y.; Lin, C.; Dong, Z.; et al. MicroRNA-21 promotes proliferation in acute myeloid leukemia by targeting Krüppel-like factor 5. Oncol. Lett. 2019, 18, 3367–3372. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Ma, H.; Liu, Y.; Cheng, S.; Wang, H.; Sun, J. Upregulation of serum exosomal miR-21 was associated with poor prognosis of acute myeloid leukemia patients. Food Sci. Technol. 2022, 42, e51621. [Google Scholar] [CrossRef]
- Riccioni, R.; Lulli, V.; Castelli, G.; Biffoni, M.; Tiberio, R.; Pelosi, E.; Lo-Coco, F.; Testa, U. miR-21 is overexpressed in NPM1-mutant acute myeloid leukemias. Leuk. Res. 2015, 39, 221–228. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, Y.; Shang, L.; Chen, H.; Yue, Y.; Dong, W.; Guo, Y.; Yang, H.; Yang, X.; Liu, Y.; et al. Overexpression of miR-17 predicts adverse prognosis and disease recurrence for acute myeloid leukemia. Int. J. Clin. Oncol. 2022, 27, 1222–1232. [Google Scholar] [CrossRef]
- Liu, M.; Yu, B.; Tian, Y.; Li, F. Regulatory function and mechanism research for m6A modification WTAP via SUCLG2-AS1- miR-17-5p-JAK1 axis in AML. BMC Cancer 2024, 24, 98. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Chen, K.; Wang, J.; Dong, Q.; Xie, J.; Yuan, Y. Vitamin D promotes autophagy in AML cells by inhibiting miR-17-5p-induced Beclin-1 overexpression. Mol. Cell. Biochem. 2021, 76, 3951–3962. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, R.; Wang, X.; Khadka, B.; Fang, Z.; Yu, M.; Zhang, L.; Wu, J.; Liu, J. MicroRNA-93 knockdown inhibits acute myeloid leukemia cell growth via inactivating the PI3K/AKT pathway by upregulating DAB2. Int. J. Oncol. 2021, 59, 81. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, A.; Guan, H. microRNA-106b-5p and Rab10: Potential Markers of Acute Myeloid Leukemia. Cancer Biother Radiopharm 2024, 39, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nguyen, L.X.T.; Chen, Y.C.; Wu, D.; Cook, G.J.; Hoang, D.H.; Brewer, C.J.; He, X.; Dong, H.; Li, S.; et al. Targeting miR-126 in inv(16) acute myeloid leukemia inhibits leukemia development and leukemia stem cell maintenance. Nat. Commun. 2021, 12, 6154. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Lei, D. microRNA-146a Promotes Growth of Acute Leukemia Cells by Downregulating Ciliary Neurotrophic Factor Receptor and Activating JAK2/STAT3 Signaling. Yonsei Med. J. 2019, 60, 924–934. [Google Scholar] [CrossRef]
- Li, X.; Xu, L.; Sheng, X.; Cai, J.; Liu, J.; Yin, T.; Xiao, F.; Chen, F.; Zhong, H. Upregulated microRNA-146a expression induced by granulocyte colony-stimulating factor enhanced low-dosage chemotherapy response in aged acute myeloid leukemia patients. Exp. Hematol. 2018, 68, 66–79. [Google Scholar] [CrossRef]
- Garavand, J.; Mohammadi, M.H.; Jalali, M.T.; Saki, N. Correlation of miR-155-5p, KRAS, and CREB Expression in Patients with Acute Myeloid Leukemia. Clin. Lab. 2024, 70, 89–98. [Google Scholar] [CrossRef]
- Xue, H.; Hua, L.M.; Guo, M.; Luo, J.M. SHIP1 is targeted by miR-155 in acute myeloid leukemia. Oncol. Rep. 2014, 32, 2253–2259. [Google Scholar] [CrossRef]
- Palma, C.A.; Al Sheikha, D.; Lim, T.K.; Bryant, A.; Vu, T.T.; Jayaswal, V.; Ma, D.D. MicroRNA-155 as an inducer of apoptosis and cell differentiation in Acute Myeloid Leukemia. Mol. Cancer 2014, 13, 79. [Google Scholar] [CrossRef]
- Elgohary, T.; Abu-Taleb, F.; Ghonaim, R. The Impact of miRNA-155 Expression on Treatment Outcome in Adult Acute Myeloid Leukemia Patients. J. Cancer Ther. 2019, 10, 203–214. [Google Scholar] [CrossRef]
- Hatem, A.; Gab~Allah, A.; Ghonaim, R.; Haggag, R. Prognostic Impact of microRNAs (miR-155, miR-10a, let7a) on the Outcome of Adult Patients with Acute Myeloid Leukemia. Zagazig Univ. Med. J. 2021, 27, 810–825. [Google Scholar] [CrossRef]
- El-Hassib, D.M.A.; Zidan, M.A.-A.; Marei, Y.M.; El Gheit, N.E.S.N.A.; Alnoury, H.A. Study of Micro RNA 181 a3p As a Biomarker for Diagnosis of Acute Myeloid Leukemia. Egypt. J. Hosp. Med. 2023, 91, 4780–4785. [Google Scholar] [CrossRef]
- Butrym, A.; Rybka, J.; Baczyńska, D.; Poręba, R.; Mazur, G.; Kuliczkowski, K. Expression of microRNA-181 determines response to treatment with azacitidine and predicts survival in elderly patients with acute myeloid leukemia. Oncol. Lett. 2016, 12, 2296–2300. [Google Scholar] [CrossRef]
- Su, R.; Lin, H.S.; Zhang, X.H.; Yin, X.L.; Ning, H.M.; Liu, B.; Zhai, P.F.; Gong, J.N.; Shen, C.; Song, L.; et al. MiR-181 family: Regulators of myeloid differentiation and acute myeloid leukemia as well as potential therapeutic targets. Oncogene 2015, 34, 3226–3239. [Google Scholar] [CrossRef]
- Gao, X.; Fan, S.; Zhang, X. MiR-1306-5p promotes cell proliferation and inhibits cell apoptosis in acute myeloid leukemia by downregulating PHF6 expression. Leuk. Res. 2022, 120, 106906. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, C.; Arnovitz, S.; Bugno, J.; Yu, M.; Zuo, Z.; Chen, P.; Huang, H.; Ulrich, B.; Gurbuxani, S.; et al. miR-22 has a potent anti-tumor role with therapeutic potential in acute myeloid leukemia. Nat. Commun. 2016, 7, 11452. [Google Scholar] [CrossRef]
- Shen, C.; Chen, M.T.; Zhang, X.H.; Yin, X.L.; Ning, H.M.; Su, R.; Lin, H.S.; Song, L.; Wang, F.; Ma, Y.N.; et al. The PU.1-Modulated MicroRNA-22 Is a Regulator of Monocyte/Macrophage Differentiation and Acute Myeloid Leukemia. PLoS Genet. 2016, 12, e1006259. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Zheng, G.; Cheng, S.; Xie, W.; Liu, X.; Tao, Y.; Xie, B. Serum miR-22 is a novel prognostic marker for acute myeloid leukemia. J. Clin. Lab. Anal. 2020, 34, e23370. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Sun, P.; Duan, M.; Lin, L.; Pan, Y.; Wu, C.; Fu, X.; Wang, H.; Guo, L.; Jin, T.; et al. microRNA-22 can regulate expression of the long non-coding RNA MEG3 in acute myeloid leukemia. Oncotarget 2017, 8, 65211–65217. [Google Scholar] [CrossRef]
- Gu, Y.; Si, J.; Xiao, X.; Tian, Y.; Yang, S. miR-92a Inhibits Proliferation and Induces Apoptosis by Regulating Methylenetetrahydrofolate Dehydrogenase 2 (MTHFD2) Expression in Acute Myeloid Leukemia. Oncol. Res. 2017, 25, 1069–1079. [Google Scholar] [CrossRef]
- Su, X.Y.; Zhao, Q.; Ke, J.M.; Wu, D.H.; Zhu, X.; Lin, J.; Deng, Z.Q. Circ_0002232 Acts as a Potential Biomarker for AML and Reveals a Potential ceRNA Network of Circ_0002232/miR-92a-3p/PTEN. Cancer Manag. Res. 2020, 12, 11871–11881. [Google Scholar] [CrossRef]
- Saadi, M.I.; Arandi, N.; Yaghobi, R.; Azarpira, N.; Geramizadeh, B.; Ramzi, M. Up-Regulation of the MiR-92a and miR-181a in Patients with Acute Myeloid Leukemia and their Inhibition with Locked Nucleic acid (LNA)-antimiRNA.; Introducing c-Kit as a New Target Gene. Int. J. Hematol. Oncol. 2018, 28, 238–247. [Google Scholar] [CrossRef]
- Rashed, R.A.; Hassan, N.M.; Hussein, M.M. MicroRNA-92a as a marker of treatment response and survival in adult acute myeloid leukemia patients. Leuk. Lymphoma 2020, 61, 2475–2481. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, Y. MicroRNA-199a deficiency relates to higher bone marrow blasts, poor risk stratification and worse prognostication in pediatric acute myeloid leukemia patients. Pediatr. Hematol. Oncol. 2022, 39, 500–507. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, G.; Wu, B.; Yang, W.; Liu, Z. miR-199a-5p Represses Protective Autophagy and Overcomes Chemoresistance by Directly Targeting DRAM1 in Acute Myeloid Leukemia. J. Oncol. 2019, 2019, 5613417. [Google Scholar] [CrossRef]
- Ellson, I.; Martorell-Marugán, J.; Carmona-Sáez, P.; Ramos-Mejia, V. MiRNA expression as outcome predictor in pediatric AML: Systematic evaluation of a new model. npj Genom. Med. 2024, 9, 40. [Google Scholar] [CrossRef]
- Favreau, A.J.; McGlauflin, R.E.; Duarte, C.W.; Sathyanarayana, P. miR-199b, a novel tumor suppressor miRNA in acute myeloid leukemia with prognostic implications. Exp. Hematol. Oncol. 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Alemdehy, M.F.; Haanstra, J.R.; de Looper, H.W.; van Strien, P.M.; Verhagen-Oldenampsen, J.; Caljouw, Y.; Sanders, M.A.; Hoogenboezem, R.; de Ru, A.H.; Janssen, G.M.; et al. ICL-induced miR139-3p and miR199a-3p have opposite roles in hematopoietic cell expansion and leukemic transformation. Blood 2015, 125, 3937–3948. [Google Scholar] [CrossRef]
- Yuan, Y.; Tan, S.; Wang, H.; Zhu, J.; Li, J.; Zhang, P.; Wang, M.; Zhang, F. Mesenchymal Stem Cell-Derived Exosomal miRNA-222-3p Increases Th1/Th2 Ratio and Promotes Apoptosis of Acute Myeloid Leukemia Cells. Anal. Cell. Pathol. 2023, 2023, 4024887. [Google Scholar] [CrossRef]
- Pei, H.Z.; Peng, Z.; Zhuang, X.; Wang, X.; Lu, B.; Guo, Y.; Zhao, Y.; Zhang, D.; Xiao, Y.; Gao, T.; et al. miR-221/222 induce instability of p53 By downregulating deubiquitinase YOD1 in acute myeloid leukemia. Cell Death Discov. 2023, 9, 249. [Google Scholar] [CrossRef]
- Liu, Z.; Zhong, L.; Dan, W.; Chu, X.; Liu, C.; Luo, X.; Zhang, Z.; Lu, Y.; Wan, P.; Wang, X.; et al. miRNA-222-3p enhances the proliferation and suppresses the apoptosis of acute myeloid leukemia cells by targeting Axin2 and modulating the Wnt/β-catenin pathway. Biochem. Biophys. Res. Commun. 2022, 620, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Pavlovic, D.; Tosic, N.; Zukic, B.; Pravdic, Z.; Vukovic, N.S.; Pavlovic, S.; Gasic, V. Expression Profiles of Long Non-Coding RNA GAS5 and MicroRNA-222 in Younger AML Patients. Diagnostics 2021, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Jiang, J.; He, G.; Zhou, H.; Ji, C. KMT2A is targeted by miR-361-3p and modulates leukemia cell’s abilities to proliferate, migrate and invade. Hematology 2023, 28, 2225341. [Google Scholar] [CrossRef]
- Liu, S.; Xu, H.; Li, Z. Linoleic acid derivatives target miR-361-3p/BTG2 to confer anticancer effects in acute myeloid leukemia. J. Biochem. Mol. Toxicol. 2023, 37, e23481. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.T.; Davis, A.G.; Zhou, J.H.; Ball, E.D.; Zhang, D.E. MicroRNA let-7b downregulates AML1-ETO oncogene expression in t(8;21) AML by targeting its 3′UTR. Exp. Hematol. Oncol. 2021, 10, 8. [Google Scholar] [CrossRef]
- Fazi, F.; Racanicchi, S.; Zardo, G.; Starnes, L.M.; Mancini, M.; Travaglini, L.; Diverio, D.; Ammatuna, E.; Cimino, G.; Lo-Coco, F.; et al. Epigenetic silencing of the myelopoiesis regulator microRNA-223 by the AML1/ETO oncoprotein. Cancer Cell 2007, 12, 457–466. [Google Scholar] [CrossRef]
- Fu, L.; Shi, J.; Liu, A.; Zhou, L.; Jiang, M.; Fu, H.; Xu, K.; Li, D.; Deng, A.; Zhang, Q.; et al. A minicircuitry of microRNA-9-1 and RUNX1-RUNX1T1 contributes to leukemogenesis in t(8;21) acute myeloid leukemia. Int. J. Cancer 2017, 140, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, P.; Su, R.; Li, Y.; Hu, C.; Wang, Y.; Arnovitz, S.; He, M.; Gurbuxani, S.; Zuo, Z.; et al. Overexpression and knockout of miR-126 both promote leukemogenesis. Blood 2015, 126, 2005–2015. [Google Scholar] [CrossRef]
- Krivdova, G.; Voisin, V.; Schoof, E.M.; Marhon, S.A.; Murison, A.; McLeod, J.L.; Gabra, M.M.; Zeng, A.G.X.; Aigner, S.; Yee, B.A.; et al. Identification of the global miR-130a targetome reveals a role for TBL1XR1 in hematopoietic stem cell self-renewal and t(8;21) AML. Cell Rep. 2022, 38, 110481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ning, Q.; Shi, J.; Chen, Y.; Jiang, M.; Gao, L.; Huang, W.; Jing, Y.; Huang, S.; Liu, A.; et al. A novel epigenetic AML1-ETO/THAP10/miR-383 mini-circuitry contributes to t(8;21) leukaemogenesis. EMBO Mol. Med. 2017, 9, 933–949. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhong, L.; Liu, D.; Yao, J.; Liu, J.; Zhong, P.; Yao, S.; Zhao, Y.; Li, L.; Chen, M.; et al. MiR-15b regulates cell differentiation and survival by targeting CCNE1 in APL cell lines. Cell. Signal. 2019, 60, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Qin, R.; Chen, J.J.; Pan, W.; Lu, X.C. MicroRNA-125b Accelerates and Promotes PML-RARa-driven Murine Acute Promyelocytic Leukemia. Biomed. Environ. Sci. 2022, 35, 485–493. [Google Scholar] [CrossRef]
- Liu, D.; Zhong, L.; Yuan, Z.; Yao, J.; Zhong, P.; Liu, J.; Yao, S.; Zhao, Y.; Liu, L.; Chen, M.; et al. miR-382-5p modulates the ATRA-induced differentiation of acute promyelocytic leukemia by targeting tumor suppressor PTEN. Cell. Signal. 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Ovcharenko, D.; Stölzel, F.; Poitz, D.; Fierro, F.; Schaich, M.; Neubauer, A.; Kelnar, K.; Davison, T.; Müller-Tidow, C.; Thiede, C.; et al. miR-10a overexpression is associated with NPM1 mutations and MDM4 downregulation in intermediate-risk acute myeloid leukemia. Exp. Hematol. 2011, 39, 1030–1042. [Google Scholar] [CrossRef]
- Gadewal, N.; Kumar, R.; Aher, S.; Gardane, A.; Gaur, T.; Varma, A.K.; Khattry, N.; Hasan, S.K. miRNA-mRNA Profiling Reveals Prognostic Impact of SMC1A Expression in Acute Myeloid Leukemia. Oncol. Res. 2020, 28, 321–330. [Google Scholar] [CrossRef]
- Rücker, F.G.; Russ, A.C.; Cocciardi, S.; Kett, H.; Schlenk, R.F.; Botzenhardt, U.; Langer, C.; Krauter, J.; Fröhling, S.; Schlegelberger, B.; et al. Altered miRNA and gene expression in acute myeloid leukemia with complex karyotype identify networks of prognostic relevance. Leukemia 2013, 27, 353–361. [Google Scholar] [CrossRef]
- Shahzad, M.; Amin, M.K.; Daver, N.G.; Shah, M.V.; Hiwase, D.; Arber, D.A.; Kharfan-Dabaja, M.A.; Badar, T. What have we learned about TP53-mutated acute myeloid leukemia? Blood Cancer J. 2024, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, S.; Lu, J.; Yuan, D.; He, L.; Qin, P.; Tan, H.; Xu, L. MicroRNA-363-3p promote the development of acute myeloid leukemia with RUNX1 mutation by targeting SPRYD4 and FNDC3B. Medicine 2021, 100, e25807. [Google Scholar] [CrossRef] [PubMed]
- Barreyro, L.; Sampson, A.M.; Hueneman, K.; Choi, K.; Christie, S.; Ramesh, V.; Wyder, M.; Wang, D.; Pujato, M.; Greis, K.D.; et al. Dysregulated innate immune signaling cooperates with RUNX1 mutations to transform an MDS-like disease to AML. iScience 2024, 27, 109809. [Google Scholar] [CrossRef] [PubMed]

| Abbreviation/ Numbers of Nucleotides | Full Name | Main Function | Type | Number of Genes by GENCODE * | Reference |
|---|---|---|---|---|---|
| lncRNA/>200 | long non-coding RNA | epigenetic, transcriptional, post-transcriptional regulation | regulatory | 35,934 | [18] |
| snRNA/75–300 | small nuclear RNA | spliceosome formation, which catalyzes the splicing of pre-mRNA | housekeeping | 1901 | [19,20] |
| miRNA/19–25 | microRNA | gene expression regulation at the post-transcriptional level through the destabilization of mRNA or inhibition of translation | regulatory | ~2500 ** | [21,22,23] |
| snoRNA/60–300 | small nucleolar RNA | regulation of spliceosomal and ribosomal functions, maintenance of the structure of rRNA | housekeeping | 942 | [24,25] |
| tRNA/70–80 | transfer RNA | protein synthesis by codon–anticodon interactions during translation | housekeeping | 416 ** | [26,27] |
| rRNA/up to~5000 | ribosomal RNA | ribosome subunits formation, which take part in translation, indicating the precise positioning of ribosomal proteins within the ribosome | housekeeping | 47 | [28,29] |
| siRNA/21–23 | small interfering RNA | suppression of genes expression by RNA interference (RNAi) | regulatory | N/A | [30] |
| circRNA/N/A | circular RNA | regulation of miRNA through the sponge effect | regulatory | ~11,000 ** | [31,32] |
| piRNA/24–32 | piwi-interacting RNA | gene suppression by interactions with PIWI proteins | regulatory | ~20,000 ** | [33,34,35] |
| paRNA/200–500 | promoter-associated RNA | scaffolding for proteins regulating gene expression e.g., during chromatin remodelling or transcription | regulatory | N/A | [36] |
| eRNA/50–2000 | enhancer RNA | regulation of gene expression by modulating chromatin | regulatory | N/A | [37,38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśnik, A.; Jarych, D.; Krawiec, K.; Strzałka, P.; Potocka, N.; Czemerska, M.; Sałagacka-Kubiak, A.; Pluta, A.; Wierzbowska, A.; Zawlik, I. Role of MicroRNAs in Acute Myeloid Leukemia. Genes 2025, 16, 446. https://doi.org/10.3390/genes16040446
Wiśnik A, Jarych D, Krawiec K, Strzałka P, Potocka N, Czemerska M, Sałagacka-Kubiak A, Pluta A, Wierzbowska A, Zawlik I. Role of MicroRNAs in Acute Myeloid Leukemia. Genes. 2025; 16(4):446. https://doi.org/10.3390/genes16040446
Chicago/Turabian StyleWiśnik, Aneta, Dariusz Jarych, Kinga Krawiec, Piotr Strzałka, Natalia Potocka, Magdalena Czemerska, Aleksandra Sałagacka-Kubiak, Agnieszka Pluta, Agnieszka Wierzbowska, and Izabela Zawlik. 2025. "Role of MicroRNAs in Acute Myeloid Leukemia" Genes 16, no. 4: 446. https://doi.org/10.3390/genes16040446
APA StyleWiśnik, A., Jarych, D., Krawiec, K., Strzałka, P., Potocka, N., Czemerska, M., Sałagacka-Kubiak, A., Pluta, A., Wierzbowska, A., & Zawlik, I. (2025). Role of MicroRNAs in Acute Myeloid Leukemia. Genes, 16(4), 446. https://doi.org/10.3390/genes16040446






