Overexpression of FLZ12 Suppresses Root Hair Development and Enhances Iron-Deficiency Tolerance in Arabidopsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Plasmid Constructions and Plant Transformation
2.3. Root Hair Measurement
2.4. GUS Histochemical Analysis
2.5. Subcellular Localization Analysis
2.6. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
2.7. Statistical Analysis
3. Results
3.1. Expression of FLZ12 Is Induced by Phosphate Deficiency, Drought, and Salinity
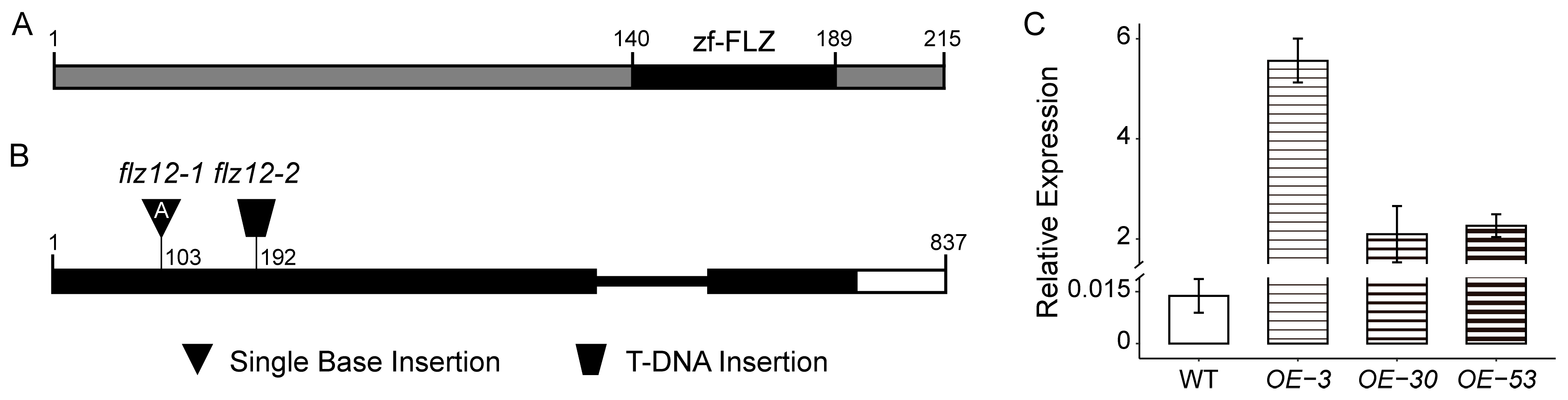
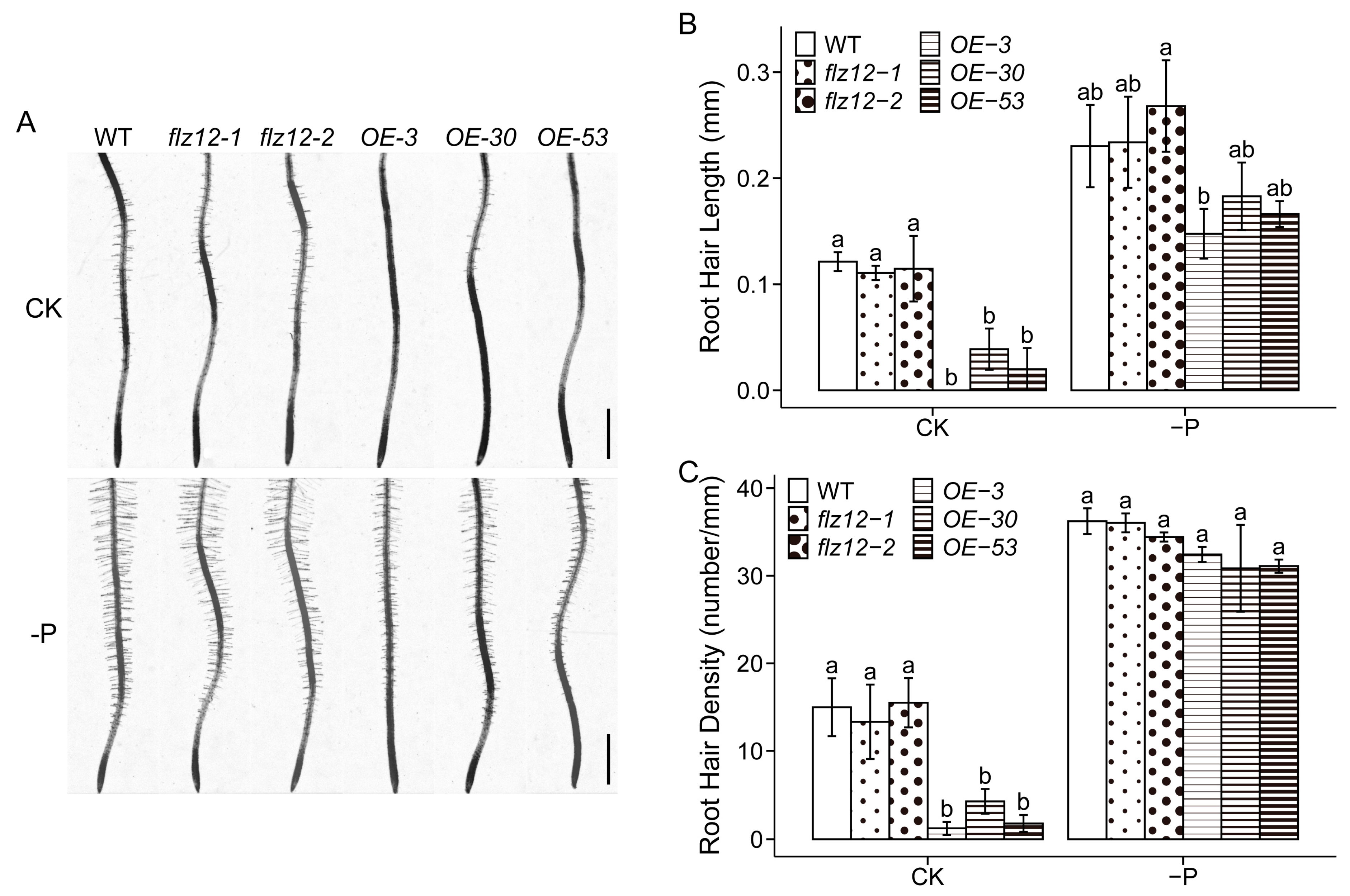
3.2. Overexpression of FLZ12 Inhibites Root Hair Development
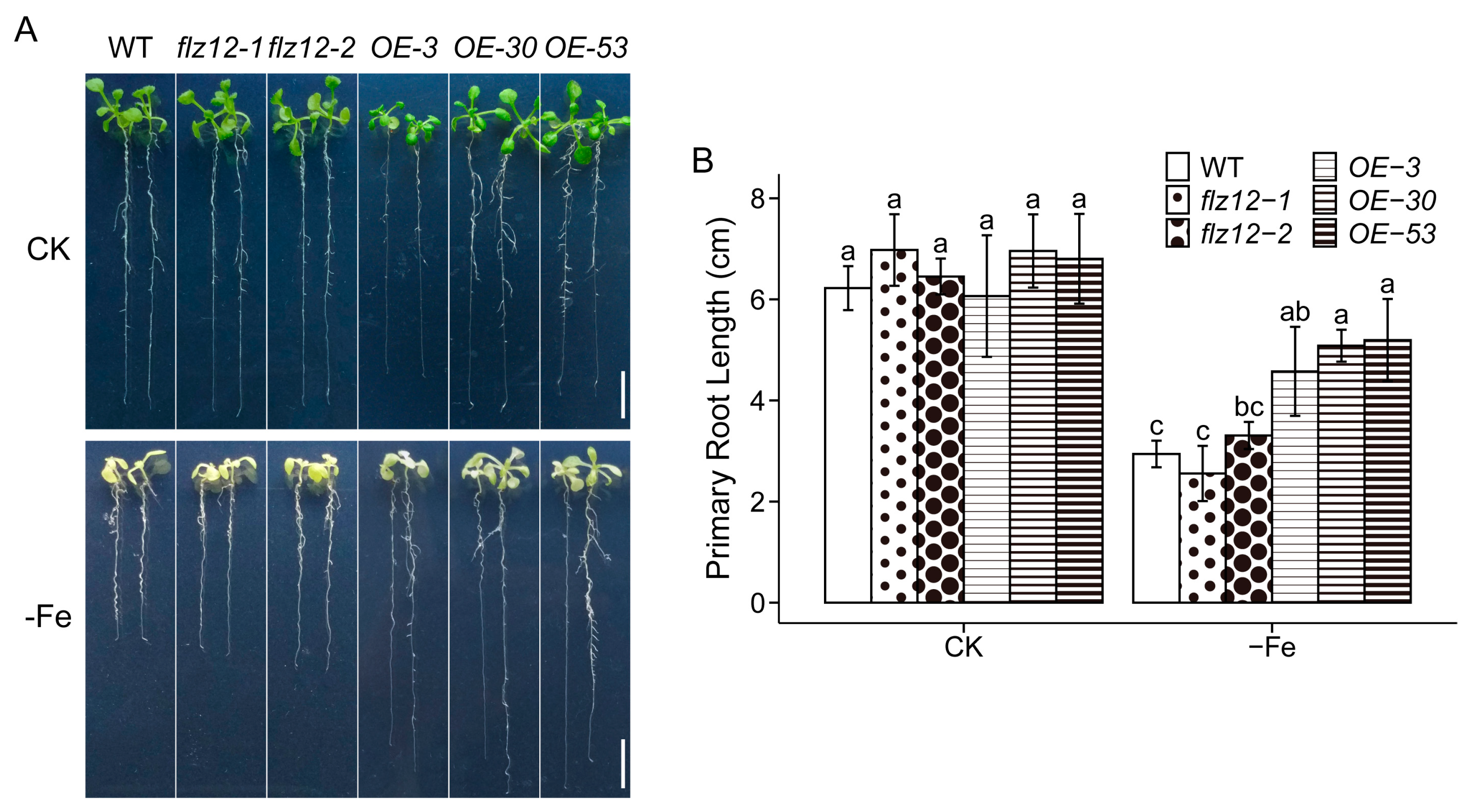
3.3. Overexpression of FLZ12 Enhances Seedling Tolerance to Iron Deficiency
3.4. FLZ12 Displays Little Duplicate Effect with FLZ13
3.5. FLZ12 Mainly Expresses in the Vasculature and the Protein Localizes in the Nucleus of the Cell
4. Discussion
4.1. Uncertain Disturbances in Seedling Growth Caused by Overexpression of FLZ12
4.2. Negative Role of FLZ12 in Root Hair Development and Its Link with SnRK1 and PLDζ2 (PHOSPHOLIPASE Dζ2)
4.3. FLZ12 Is a Promising Candidate in Improving Plant Iron-Deficiency Resilience via a FIT-IRT1-Independent Pathway
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI5 | ABSCISIC ACID-INSENSITIVE 5 |
| ANOVA | Analysis of variance |
| bHLH | BASIC HELIX-LOOP-HELIX |
| CRISPR/Cas9 | Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 |
| CYBDOM | CYTOCHROME B561 AND DOMON DOMAIN |
| DAG | Days after the start of germination |
| DAPI | 4′,6-Diamidino-2-phenylindole |
| Fe | Iron |
| -Fe | Iron deficiency |
| FER | FERRETIN |
| FIT | FE-DEFICIENCY INDUCED TRANSCRIPTION FACTOR 1 |
| FLC | FLOWERING LOCUS C |
| FLZ | FCS-LIKE ZINC FINGER |
| FRO2 | FERRIC REDUCTION OXIDASE 2 |
| IRM1 | INCREASED RESISTANCE TO MYZUS PERSICAE 1 |
| IRT1 | IRON-REGULATED TRANSPORTER 1 |
| KIN10 | SNF1-RELATED PROTEIN KINASE 1.1 |
| MARD1 | MEDIATOR OF ABA-REGULATED DORMANCY 1 |
| OE | Overexpression |
| P | Phosphorus |
| -P | Phosphate deficiency |
| Pi | Phosphate |
| PLDζ2 | PHOSPHOLIPASE Dζ2 |
| qRT-PCR | Quantitative real-time PCR |
| SnRK1 | Sucrose non-fermenting-1-related protein kinase |
| TUA3 | TUBULIN α-3 |
| YSL2 | YELLOW STRIPE LIKE 2 |
References
- Crombez, H.; Motte, H.; Beeckman, T. Tackling plant phosphate starvation by the roots. Dev. Cell 2019, 48, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Z.; Liu, D. Comparative functional analyses of PHR1, PHL1, and PHL4 transcription factors in regulating Arabidopsis responses to phosphate starvation. Front. Plant Sci. 2024, 15, 1379562. [Google Scholar]
- Tottey, S.; Block, M.A.; Allen, M.; Westergren, T.; Albrieux, C.; Scheller, H.V.; Merchant, S.; Jensen, P.E. Arabidopsis CHL27, located in both envelope and thylakoid membranes, is required for the synthesis of protochlorophyllide. Proc. Natl. Acad. Sci. USA 2003, 100, 16119–16124. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.V. Handbook of Plant Nutrition, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Satbhai, S.B.; Setzer, C.; Freynschlag, F.; Slovak, R.; Kerdaffrec, E.; Busch, W. Natural allelic variation of FRO2 modulates Arabidopsis root growth under iron deficiency. Nat. Commun. 2017, 8, 15603. [Google Scholar] [CrossRef]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron nutrition, biomass production, and plant product quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Ravet, K.; Touraine, B.; Boucherez, J.; Briat, J.-F.; Gaymard, F.; Cellier, F. Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J. 2009, 57, 400–412. [Google Scholar] [CrossRef]
- Lucena, C.; Porras, R.; Romera, F.J.; Alcántara, E.; García, M.J.; Pérez-Vicente, R. Similarities and differences in the acquisition of Fe and P by dicot plants. Agronomy 2018, 8, 148. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Zheng, Z.; Dong, J.; Song, L.; Sui, L.; Nussaume, L.; Desnos, T.; Liu, D. Genetic dissection of Fe-dependent signaling in root developmental responses to phosphate deficiency. Plant Physiol. 2019, 179, 300–316. [Google Scholar] [CrossRef]
- Venuti, S.; Zanin, L.; Marroni, F.; Franco, A.; Morgante, M.; Pinton, R.; Tomasi, N. Physiological and transcriptomic data highlight common features between iron and phosphorus acquisition mechanisms in white lupin roots. Plant Sci. 2019, 285, 110–121. [Google Scholar] [CrossRef]
- Nussaume, L.; Desnos, T. “Je t’aime moi non plus”: A love-hate relationship between iron and phosphate. Mol. Plant 2022, 15, 1–2. [Google Scholar] [CrossRef]
- Guo, M.; Ruan, W.; Zhang, Y.; Zhang, Y.; Wang, X.; Guo, Z.; Wang, L.; Zhou, T.; Paz-Ares, J.; Yi, K. A reciprocal inhibitory module for Pi and iron signaling. Mol. Plant 2022, 15, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.T.; Lahner, B.; Yakubova, E.; Salt, D.E.; Raghothama, K.G. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiol. 2008, 147, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Sanagala, R.; Sinilal, B.; Yadav, S.; Sarkar, A.K.; Dantu, P.K.; Jain, A. Iron availability affects phosphate deficiency-mediated responses, and evidence of cross-talk with auxin and zinc in Arabidopsis. Plant Cell Physiol. 2015, 56, 1107–1123. [Google Scholar] [CrossRef] [PubMed]
- Clúa, J.; Montpetit, J.; Jimenez-Sandoval, P.; Naumann, C.; Santiago, J.; Poirier, Y. A CYBDOM protein impacts iron homeostasis and primary root growth under phosphate deficiency in Arabidopsis. Nat. Commun. 2024, 15, 423. [Google Scholar] [CrossRef]
- Mueller, M. Arabidopsis Root Hair Development in Adaptation to Iron and Phosphate Supply. Ph.D. Dissertation, Humboldt University of Berlin, Berlin, Germany, 2007. [Google Scholar]
- Yang, X.; Liu, C.; Liang, C.; Wang, T.; Tian, J. The phosphorus-iron nexus: Decoding the nutrients interaction in soil and plant. Int. J. Mol. Sci. 2024, 25, 6992. [Google Scholar] [CrossRef]
- Choi, H.-S.; Cho, H.-T. Root hairs enhance Arabidopsis seedling survival upon soil disruption. Sci. Rep. 2019, 9, 11181. [Google Scholar] [CrossRef]
- Shibata, M.; Sugimoto, K. A gene regulatory network for root hair development. J. Plant Res. 2019, 132, 301–309. [Google Scholar] [CrossRef]
- Bates, T.R.; Lynch, J.P. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. Am. J. Bot. 2000, 87, 964–970. [Google Scholar]
- Cui, S.; Suzaki, T.; Tominaga-Wada, R.; Yoshida, S. Regulation and functional diversification of root hairs. Semin. Cell Dev. Biol. 2018, 83, 115–122. [Google Scholar] [CrossRef]
- Yen, M.-R.; Suen, D.-F.; Hsu, F.-M.; Tsai, Y.-H.; Fu, H.; Schmidt, W.; Chen, P.-Y. Deubiquitinating enzyme OTU5 contributes to DNA methylation patterns and is critical for phosphate nutrition signals. Plant Physiol. 2017, 175, 1826–1838. [Google Scholar] [CrossRef]
- Woo, J.; MacPherson, C.R.; Liu, J.; Wang, H.; Kiba, T.; Hannah, M.A.; Wang, X.-J.; Bajic, V.B.; Chua, N.-H. The response and recovery of the Arabidopsis thaliana transcriptome to phosphate starvation. BMC Plant Biol. 2012, 12, 62. [Google Scholar] [CrossRef]
- Raya-González, J.; Ojeda-Rivera, J.O.; Mora-Macias, J.; Oropeza-Aburto, A.; Ruiz-Herrera, L.F.; López-Bucio, J.; Herrera-Estrella, L. MEDIATOR16 orchestrates local and systemic responses to phosphate scarcity in Arabidopsis roots. New Phytol. 2021, 229, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Linn, J.; Ren, M.; Berkowitz, O.; Ding, W.; van der Merwe, M.J.; Whelan, J.; Jost, R. Root cell-specific regulators of phosphate-dependent growth. Plant Physiol. 2017, 174, 1969–1989. [Google Scholar] [CrossRef] [PubMed]
- Jamsheer K, M.; Laxmi, A. DUF581 is plant specific FCS-Like Zinc finger involved in protein-protein interaction. PLoS ONE 2014, 9, e99074. [Google Scholar] [CrossRef]
- Jamsheer K, M.; Mannully, C.T.; Gopan, N.; Laxmi, A. Comprehensive evolutionary and expression analysis of FCS-like zinc finger gene family yields insights into their origin, expansion and divergence. PLoS ONE 2015, 10, e0134328. [Google Scholar] [CrossRef]
- Nietzsche, M.; Schießl, I.; Börnke, F. The complex becomes more complex: Protein-protein interactions of SnRK1 with DUF581 family proteins provide a framework for cell- and stimulus type-specific SnRK1 signaling in plants. Front. Plant Sci. 2014, 5, 54. [Google Scholar] [CrossRef]
- Jamsheer K, M.; Laxmi, A. Expression of Arabidopsis FCS-Like Zinc finger genes is differentially regulated by sugars, cellular energy level, and abiotic stress. Front. Plant Sci. 2015, 6, 746. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Visser, R.G.F.; Broekgaarden, C.; Vosman, B. Overexpression of IRM1 enhances resistance to aphids in Arabidopsis thaliana. PLoS ONE 2013, 8, e70914. [Google Scholar] [CrossRef]
- He, Y.; Gan, S. A novel zinc-finger protein with a proline-rich domain mediates ABA-regulated seed dormancy in Arabidopsis. Plant Mol. Biol. 2004, 54, 1–9. [Google Scholar] [CrossRef]
- Jamsheer K, M.; Sharma, M.; Singh, D.; Mannully, C.T.; Jindal, S.; Shukla, B.N.; Laxmi, A. FCS-like zinc finger 6 and 10 repress SnRK1 signalling in Arabidopsis. Plant J. 2018, 94, 232–245. [Google Scholar] [CrossRef]
- Li, X.; Lai, M.; Li, K.; Yang, L.; Liao, J.; Gao, Y.; Wang, Y.; Gao, C.; Shen, W.; Luo, M.; et al. FLZ13 interacts with FLC and ABI5 to negatively regulate flowering time in Arabidopsis. New Phytol. 2024, 241, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, X.; Chen, S.; Liu, C.; Yang, L.; Li, K.; Liao, J.; Zheng, X.; Li, H.; Li, Y.; et al. ABI5–FLZ13 module transcriptionally represses growth-related genes to delay seed germination in response to ABA. Plant Commun. 2023, 4, 100636. [Google Scholar] [CrossRef] [PubMed]
- Jamsheer K, M.; Singh, D.; Sharma, M.; Sharma, M.; Jindal, S.; Mannully, C.T.; Shukla, B.N.; Laxmi, A. The FCS-LIKE ZINC FINGER 6 and 10 are involved in regulating osmotic stress responses in Arabidopsis. Plant Signal. Behav. 2019, 14, 1592535. [Google Scholar] [CrossRef]
- Wurzinger, B.; Nukarinen, E.; Nägele, T.; Weckwerth, W.; Teige, M. The SnRK1 kinase as central mediator of energy signaling between different organelles. Plant Physiol. 2018, 176, 1085–1094. [Google Scholar] [CrossRef]
- Nietzsche, M.; Landgraf, R.; Tohge, T.; Börnke, F. A protein–protein interaction network linking the energy-sensor kinase SnRK1 to multiple signaling pathways in Arabidopsis thaliana. Curr. Plant Biol. 2016, 5, 36–44. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Xing, H.-L.; Dong, L.; Zhang, H.-Y.; Han, C.-Y.; Wang, X.-C.; Chen, Q.-J. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 2015, 16, 144. [Google Scholar] [CrossRef]
- Strieder, M.L.; Pinto, K.G.; Bertoldi, C.; Schneider, A.d.B.; Delatorre, C.A. Response of Arabidopsis thaliana root growth to phosphorus and its relation to media chemical composition. Biol. Plant 2017, 61, 587–594. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium -mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Contreras, E.; Martinez, M. The RIN4-like/NOI proteins NOI10 and NOI11 modulate the response to biotic stresses mediated by RIN4 in Arabidopsis. Plant Cell Rep. 2024, 43, 70. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Zhang, C.-G.; Zhao, J.-F.; Chen, Y. GUS staining of guard cells to identify localised guard cell gene expression. Bio-Protocol 2017, 7, e2446. [Google Scholar] [CrossRef]
- Santi, S.; Schmidt, W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 2009, 183, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Krasnoperova, E.E.; Isayenkov, S.V.; Yemets, A.I.; Blume, Y.B. Influence of protein kinase KIN10 gene expression on root phenotype of Arabidopsis thaliana root system under condition of energy stress. Cytol. Genet. 2016, 50, 215–220. [Google Scholar] [CrossRef]
- Obayashi, T.; Hibara, H.; Kagaya, Y.; Aoki, Y.; Kinoshita, K. ATTED-II v11: A plant gene coexpression database using a sample balancing technique by subagging of principal components. Plant Cell Physiol. 2022, 63, 869–881. [Google Scholar] [CrossRef]
- Su, Y.; Li, M.; Guo, L.; Wang, X. Different effects of phospholipase Dζ2 and non-specific phospholipase C4 on lipid remodeling and root hair growth in Arabidopsis response to phosphate deficiency. Plant J. 2018, 94, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-L.; Yao, H.-Y.; Jia, L.-H.; Tan, J.-F.; Xu, Z.-H.; Zheng, W.-M.; Xue, H.-W. Phospholipase D-derived phosphatidic acid promotes root hair development under phosphorus deficiency by suppressing vacuolar degradation of PIN-FORMED2. New Phytol. 2020, 226, 142–155. [Google Scholar] [CrossRef]
- Lucena, C.; Porras, R.; García, M.J.; Alcántara, E.; Pérez-Vicente, R.; Zamarreño, Á.M.; Bacaicoa, E.; García-Mina, J.M.; Smith, A.P.; Romera, F.J. Ethylene and phloem signals are involved in the regulation of responses to Fe and P deficiencies in roots of strategy I plants. Front. Plant Sci. 2019, 10, 1237. [Google Scholar] [CrossRef]
- Peixoto, B.; Moraes, T.A.; Mengin, V.; Margalha, L.; Vicente, R.; Feil, R.; Höhne, M.; Sousa, A.G.G.; Lilue, J.; Stitt, M.; et al. Impact of the SnRK1 protein kinase on sucrose homeostasis and the transcriptome during the diel cycle. Plant Physiol. 2021, 187, 1357–1373. [Google Scholar] [CrossRef]
- Atwood, S.E.; O’Rourke, J.A.; Peiffer, G.A.; Yin, T.; Majumder, M.; Zhang, C.; Cianzio, S.R.; Hill, J.H.; Cook, D.; Whitham, S.A.; et al. Replication protein A subunit 3 and the iron efficiency response in soybean. Plant Cell Environ. 2014, 37, 213–234. [Google Scholar] [CrossRef]
- Peixoto, B.; Baena-González, E. Management of plant central metabolism by SnRK1 protein kinases. J. Exp. Bot. 2022, 73, 7068–7082. [Google Scholar] [CrossRef]
- Jamsheer, K.M.; Shukla, B.N.; Jindal, S.; Gopan, N.; Mannully, C.T.; Laxmi, A. The FCS-like zinc finger scaffold of the kinase SnRK1 is formed by the coordinated actions of the FLZ domain and intrinsically disordered regions. J. Biol. Chem. 2018, 293, 13134–13150. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Zhang, X.; Gao, J. Overexpression of FLZ12 Suppresses Root Hair Development and Enhances Iron-Deficiency Tolerance in Arabidopsis. Genes 2025, 16, 438. https://doi.org/10.3390/genes16040438
Yan M, Zhang X, Gao J. Overexpression of FLZ12 Suppresses Root Hair Development and Enhances Iron-Deficiency Tolerance in Arabidopsis. Genes. 2025; 16(4):438. https://doi.org/10.3390/genes16040438
Chicago/Turabian StyleYan, Mingke, Xin Zhang, and Jinghui Gao. 2025. "Overexpression of FLZ12 Suppresses Root Hair Development and Enhances Iron-Deficiency Tolerance in Arabidopsis" Genes 16, no. 4: 438. https://doi.org/10.3390/genes16040438
APA StyleYan, M., Zhang, X., & Gao, J. (2025). Overexpression of FLZ12 Suppresses Root Hair Development and Enhances Iron-Deficiency Tolerance in Arabidopsis. Genes, 16(4), 438. https://doi.org/10.3390/genes16040438




