Evaluation of DNA in Human Teeth—Ante-Mortem and Post-Mortem Factors Affecting Degradation and Preservation: A Literature Review
Abstract
1. Introduction
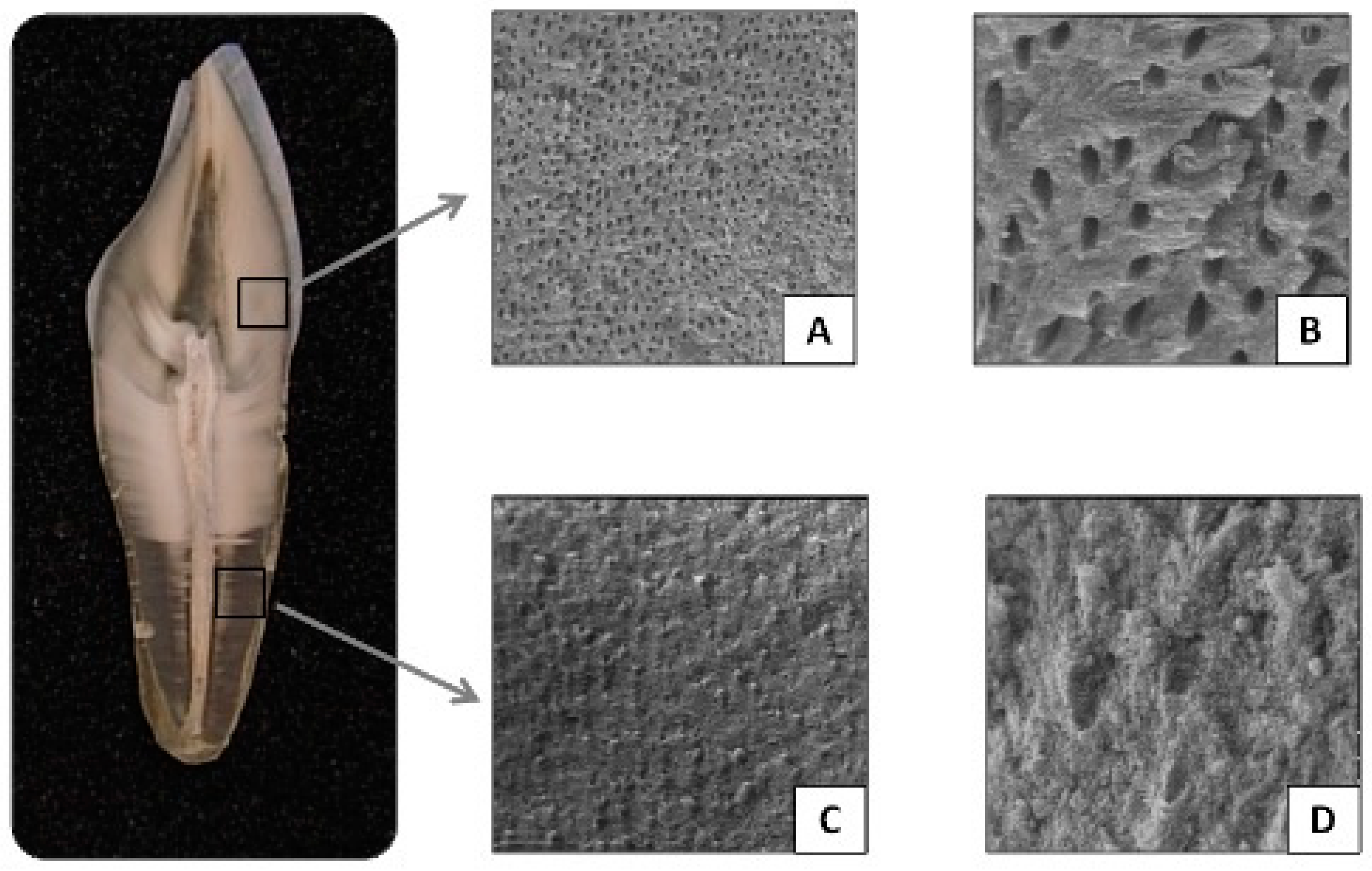
- The crown is exposed to the oral environment and composed of enamel, dentin, and pulp.
- The root is located within the bone tissue (alveolus) and composed of cementum, dentin, and pulp.
Tooth Characteristics and Affecting Factors
2. Methodology
2.1. Search Engines and Databases
2.2. Search Methodology
3. Results
3.1. Main Ante-Mortem Factors
- a. Type of Tooth: Humans have different types of teeth—molars, premolars, canines, and incisors—each varying in size and morphology according to their function. During odontogenesis, the pulp tissue develops in shape and size, correlating with the number of roots and the crown morphology of each tooth. The literature suggests that a larger pulp volume provides a better DNA source [3,6,12,13]. Additionally, multi-rooted teeth, such as molars and some premolars, increase the success rate of DNA extraction. This is due to the presence of more cementum and multiple pulp canals, which contribute a greater number of cells and, consequently, more genetic material. Some authors [12,14,15,16] have even reported a higher proportion of DNA in the root of up to 10 times more than in the crown.
- b. Biological Age of the Individual: Since the pulp–dentin complex and cementum are the primary sources of DNA in teeth, another factor that could influence DNA extraction is the individual’s age at the time of death. This is due to morphological and histological changes that occur in these tissues, as well as in enamel, over time. For example, enamel becomes thinner, and secondary dentin deposition alters the overall structure of the tooth [23].
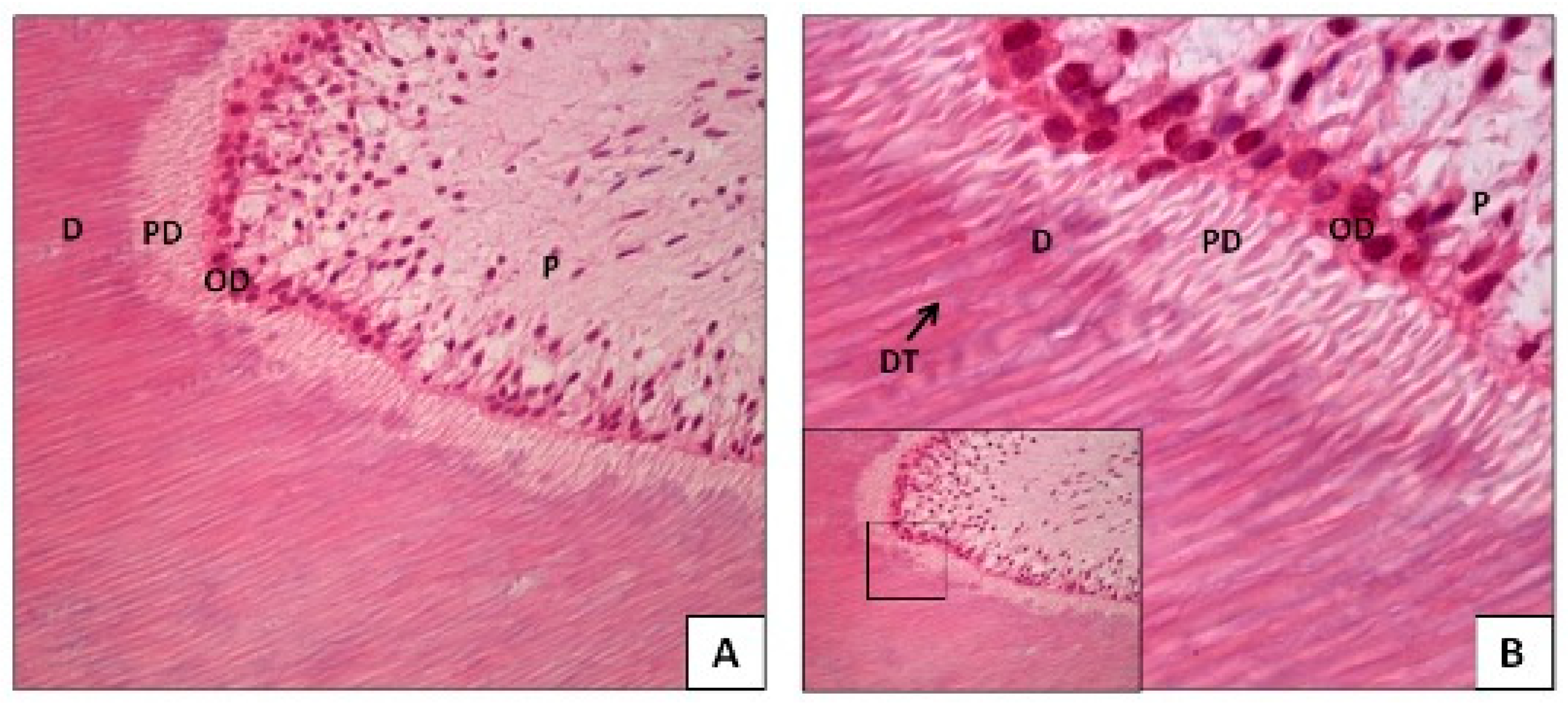
- c. Pathological Changes in Teeth: The previously mentioned changes that occur with age are physiological; however, pathological changes can also affect dental tissues. Severe pathological stimuli, such as dental caries, microbial infections, mechanical trauma (e.g., bruxism), or chemical erosion, can destroy the layer of odontoblasts in the pulp, leading to the rapid deposition of tertiary or reparative dentin. This type of dentin is tubular and reduces the volume of pulp tissue [21,27,29,31].
- d. Composition and Mineralization of the Teeth: The tissues with a high cellular content in the teeth (pulp–dentin complex and cement) are protected by mineralized tissues, such as bone and enamel, which are the tissues with the highest mineral content in humans. This characteristic makes these cells less susceptible to external post-mortem degradation factors. The mineralized collagen in bone tissue, enamel, dentin, and cement tends to be much more stable against these factors than the non-mineralized collagen in soft tissues, as this type of collagen in bone tissue and teeth forms calcium bonds with the apatite contained in them, causing the DNA macromolecule to become encapsulated within these [24,39,40,41].
3.2. Main Post-Mortem Factors
- a. Environmental Factors: In soft tissues, the post-mortem degradation process begins with the development of cellular autolysis, alongside the putrefaction of the tissues, where bacterial and fungal invasion occurs, leading to chemical degradation through the hydrolysis and oxidation of organic tissues, including the complete degradation of genetic material. This degradation process progresses over time and results in the breakdown of cellular organelles and DNA. Although the pulp–dentin complex and cement are well-protected against various degradation conditions, whether environmental or natural, they remain susceptible to these events [45]. It should be considered that degradation is affected by the nature and duration of this process and depends on the environmental conditions in which it occurs, such as exposure to moisture, temperature, ultraviolet light, substrate pH, and bacterial or fungal contamination. Depending on these conditions, the degradation process can be faster, slower, or even stop, meaning that the quantity and quality of the DNA obtained will also depend on these factors, which makes it difficult to predict [13,18,43].
- Sandy soils: Characterized by a light texture and excellent drainage, sandy soils exhibit variable pH, ranging from slightly acidic to neutral. Their high permeability reduces microbial activity, which can favor DNA preservation;
- Clayey soils: These soils possess a compact structure and high water retention capacity, often maintaining a neutral to slightly acidic pH. Their low oxygen permeability restricts microbial activity, thereby enhancing DNA conservation;
- Silty soils: With an intermediate texture and good moisture retention, silty soils typically have a neutral pH, providing favorable conditions for DNA stability, as a neutral to slightly alkaline pH is optimal for genetic material preservation;
- Calcareous soils: Rich in calcium carbonate, these soils maintain an alkaline pH, which inhibits microbial and enzymatic activity, promoting long-term DNA preservation;
- Acidic soils: Soils with a pH below 7 can accelerate the decomposition of soft tissues due to increased microbial and enzymatic activity, leading to rapid DNA degradation.
- b. Post-mortem Interval: Finally, the post-mortem time is another important factor in the DNA degradation process in dental tissues, and several studies consider the degradation process without taking this variable into account. Mansour H. et al., 2019 [12] specifies that post-mortem time is the most significant factor in DNA degradation, indicating that the most critical period for DNA preservation occurs in the first period after death, a finding that aligns with the results of other authors. The concentration and quality of DNA significantly decrease over time, with a notable difference observed in the median DNA concentrations [12,54,71]. These studies emphasize the importance of considering post-mortem time when analyzing DNA degradation in dental tissues, as DNA concentration decreases significantly in the first months after an individual’s death.
4. Future Perspectives
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Budowle, B.; Bieber, F.R.; Eisenberg, A.J. Forensic aspects of mass disasters: Strategic considerations for DNA-based human identification. Leg. Med. 2005, 7, 230–243. [Google Scholar] [CrossRef]
- Wei, Y.F.; Lin, C.Y.; Yu, Y.J.; Linacre, A.; Lee, J.C. DNA identification from dental pulp and cementum. Forensic Sci. Int. Genet. 2023, 67, 102945. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A. Enamel: Composition, Formation, and Structure in Ten Cate’s Oral Histology, Development, Structure and Function, 10th ed.; Elsevier—Health Sciences Division: Amsterdam, The Netherlands, 2024; pp. 176–177. [Google Scholar]
- Hughes-Stamm, S.; Warnke, F.; Van Daal, A. An alternate method for extracting DNA from environmentally challenged teeth for improved DNA analysis. Leg. Med. 2016, 18, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Izawa, H.; Tsutsumi, H.; Maruyama, S.; Komuro, T. DNA analysis of root canal-filled teeth. Leg. Med. 2017, 27, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.; Austin, J.J. Teeth as a source of DNA for forensic identification of human remains: A review. Sci. Justice 2013, 53, 433–441. [Google Scholar] [CrossRef]
- Shah, P.; Velani, P.R.; Lakade, L.; Dukle, S. Teeth in forensics: A review. Indian J. Dent. Res. 2019, 30, 291–299. [Google Scholar] [CrossRef]
- Sweet, D.J.; Sweet, C.H. DNA analysis of dental pulp to link incinerated remains of homicide victim to crime scene. J. Forensic Sci. 1995, 40, 310–314. [Google Scholar] [CrossRef]
- Mörnstad, H.; Pfeiffer, H.; Yoon, C.; Teivens, A. Demonstration and semi-quantification of mtDNA from human dentine and its relation to age. Int. J. Leg. Med. 1999, 112, 98–100. [Google Scholar] [CrossRef]
- Shiroma, C.Y.; Fielding, C.G.; Lewis, J.A., Jr.; Gleisner, M.R.; Dunn, K.N. A minimally destructive technique for sampling dentin powder for mitochondrial DNA testing. J. Forensic Sci. 2004, 49, 791–795. [Google Scholar] [CrossRef]
- Vavpotic, M.; Turk, T.; Martincic, D.S.; Balazic, J. Characteristics of the number of odontoblasts in human dental pulp post-mortem. Forensic Sci. Int. 2009, 193, 122–126. [Google Scholar] [CrossRef]
- Mansour, H.; Krebs, O.; Pinnschmidt, H.O.; Griem, N.; Hammann-Ehrt, I.; Püschel, K. Factors affecting dental DNA in various real post-mortem conditions. Int. J. Leg. Med. 2019, 133, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Martinez, L.J.; Martinez, E.; Martin de las Heras, S. Study of short- and long-term storage of teeth and its influence on DNA. J. Forensic Sci. 2009, 54, 1411–1413. [Google Scholar] [CrossRef]
- Gaytmenn, R.; Sweet, D. Quantification of forensic DNA from various regions of human teeth. J. Forensic Sci. 2003, 48, 622–625. [Google Scholar] [CrossRef]
- Dobberstein, R.C.; Huppertz, J.; von Wurmb-Schwark, N.; Ritz-Timme, S. Degradation of biomolecules in artificially and naturally aged teeth: Implications for age estimation based on aspartic acid racemization and DNA analysis. Forensic Sci. Int. 2008, 179, 181–191. [Google Scholar] [CrossRef]
- Higgins, D.; Kaidonis, J.; Townsend, G.; Hughes, T.; Austin, J.J. Targeted sampling of cementum for recovery of nuclear DNA from human teeth and the impact of common decontamination measures. Investig. Genet. 2013, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- INTERPOL. Disaster Victim Identification Guide. 2023. Available online: https://www.interpol.int/How-we-work/Forensics/Disaster-Victim-Identification-DVI (accessed on 24 November 2024).
- Heathfield, L.J.; Haikney, T.E.; Mole, C.G.; Finaughty, C.; Zachou, A.M.; Gibbon, V.E. Forensic human identification: Investigation into tooth morphotype and DNA extraction methods from teeth. Sci. Justice 2021, 61, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Carracedo, A.; Mayr, W.R.; Morling, N.; Parsons, T.J.; Sajantila, A.; Scheithauer, R.; Schmitter, H.; Schneider, P. International Society for Forensic Genetics. DNA Commission of the International Society for Forensic Genetics (ISFG): Recommendations regarding the role of forensic genetics for disaster victim identification (DVI). Forensic Sci. Int. Genet. 2007, 1, 3–12. [Google Scholar] [CrossRef]
- Yıldız, M.; Alp, H.H.; Gül, P.; Bakan, N.; Özcan, M. Lipid peroxidation and DNA oxidation caused by dental filling materials. J. Dent. Sci. 2017, 12, 233–240. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr.; Loghin, S.; Lin, L.M. Pulp and apical tissue response to deep caries in immature teeth: A histologic and histobacteriologic study. J. Dent. 2017, 56, 19–32. [Google Scholar] [CrossRef]
- Corte-Real, A.; Anjos, M.J.; Vieira, D.M.; Gamero, J.J. The tooth for molecular analysis and identification: A forensic approach. J. Forensic Odontostomatol. 2012, 30, 22–28. [Google Scholar]
- Carvalho, T.S.; Lussi, A. Age-related morphological, histological and functional changes in teeth. J. Oral Rehabil. 2017, 44, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, P.B.; Margaryan, A.; Schroeder, H.; Orlando, L.; Willerslev, E.; Allentoft, M.E. Improving access to endogenous DNA in ancient bones and teeth. Sci. Rep. 2015, 5, 11184. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, A.T.; Condon, N.; Cox, T.R.; Sexton, C.; Cooper, C.; Meyers, I.A.; Thomson, D.; Ford, P.J.; Roy, S.; Symons, A.L. Dynamic dentin: A quantitative microscopic assessment of age and spatial changes to matrix architecture, peritubular dentin, and collagens types I and III. J. Struct. Biol. 2022, 214, 107899. [Google Scholar] [CrossRef]
- Ryou, H.; Romberg, E.; Pashley, D.H.; Tay, F.R.; Arola, D. Importance of age on the dynamic mechanical behavior of intertubular and peritubular dentin. J. Mech. Behav. Biomed. Mater. 2015, 42, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.S.; Colon, P.; Ganss, C.; Huysmans, M.C.; Lussi, A.; Schlueter, N.; Schmalz, G.; Shellis, P.R.; Tveit, A.B.; Wiegand, A. Consensus Report of the European Federation of Conservative Dentistry: Erosive tooth wear—Diagnosis and management. Swiss Dent. J. 2016, 126, 342–346. [Google Scholar] [CrossRef]
- Hodjat, M.; Khan, F.; Saadat, K.A.S.M. Epigenetic alterations in aging tooth and the reprogramming potential. Ageing Res. Rev. 2020, 63, 101140. [Google Scholar] [CrossRef]
- Ge, Z.P.; Yang, P.; Li, G.; Zhang, J.-Z.; Ma, X.-C. Age estimation based on pulp cavity/chamber volume of 13 types of teeth from cone beam computed tomography images. Int. J. Leg. Med. 2016, 130, 1159–1167. [Google Scholar] [CrossRef]
- Porto, L.V.; Celestino da Silva Neto, J.; AnjosPontual, A.D.; Catunda, R.Q. Evaluation of volumetric changes of teeth in a Brazilian population by using cone beam computed tomography. J. Forensic Leg. Med. 2015, 36, 4–9. [Google Scholar] [CrossRef]
- Gomez de Ferraris, M.E. Periodonto de Inserción: Cemento, Ligamento Periodontal y Hueso Alveolar in Histología, Embriología e Ingeniería Tisular Bucodental, 4th ed.; Editorial Médica Panamericana: Madrid, Spain, 2019; pp. 272–275. [Google Scholar]
- Solheim, T. Dental cementum apposition as an indicator of age. Scand. J. Dent. Res. 1990, 98, 510–519. [Google Scholar] [CrossRef]
- Mansour, H.; Krebs, O.; Sperhake, J.P.; Augustin, C.; Koehne, T.; Amling, M.; Püschel, K. Cementum as a source of DNA in challenging forensic cases. J. Forensic Leg. Med. 2018, 54, 76–81. [Google Scholar] [CrossRef]
- Forshaw, R. Dental calculus—Oral health, forensic studies and archaeology: A review. Br. Dent. J. 2022, 233, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Warinner, C.; Speller, C.; Collins, M.J. A new era in palaeomicrobiology: Prospects for ancient dental calculus as a long-term record of the human oral microbiome. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20130376. [Google Scholar] [CrossRef]
- Weyrich, L.S.; Dobney, K.; Cooper, A. Ancient DNA analysis of dental calculus. J. Hum. Evol. 2015, 79, 119–124. [Google Scholar] [CrossRef]
- Cappellini, E.; Prohaska, A.; Racimo, F.; Welker, F.; Pedersen, M.W.; Allentoft, M.E.; Damgaard, P.d.B.; Gutenbrunner, P.; Dunne, J.; Hammann, S.; et al. Ancient biomolecules and evolutionary inference. Annu. Rev. Biochem. 2018, 87, 1029–1060. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.E.; Sabin, S.; Ziesemer, K.; Vågene, Å.J.; Schroeder, H.; Ozga, A.T.; Sankaranarayanan, K.; Hofman, C.A.; Yates, J.A.F.; Salazar-García, D.C.; et al. Differential preservation of endogenous human and microbial DNA in dental calculus and dentin. Sci. Rep. 2018, 8, 9822. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.J.; Riley, M.S.; Child, A.M.; Turner-Walker, G. A Basic Mathematical Simulation of the Chemical Degradation of Ancient Collagen. J. Archaeol. Sci. 1995, 22, 175–183. [Google Scholar] [CrossRef]
- Latham, K.E.; Miller, J.J. DNA recovery and analysis from skeletal material in modern forensic contexts. Forensic Sci. Res. 2018, 4, 51–59. [Google Scholar] [CrossRef]
- Kendall, C.; Høier Eriksen, A.M.; Kontopoulos, I.; Collins, M.J.; Turner-Walker, G. Diagenesis of archaeological bone and tooth. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 491, 21–37. [Google Scholar] [CrossRef]
- Korlević, P.; Gerber, T.; Gansauge, M.T.; Hajdinjak, M.; Nagel, S.; Aximu-Petri, A.; Meyer, M. Reducing microbial and human contamination in DNA extractions from ancient bones and teeth. Biotechniques 2015, 59, 87–93. [Google Scholar] [CrossRef]
- Raffone, C.; Baeta, M.; Lambacher, N.; Granizo-Rodríguez, E.; Etxeberria, F.; de Pancorbo, M.M. Intrinsic and extrinsic factors that may influence DNA preservation in skeletal remains: A review. Forensic Sci. Int. 2021, 325, 110859. [Google Scholar] [CrossRef]
- Okazaki, M.; Yoshida, Y.; Yamaguchi, S.; Kaneno, M.; Elliott, J.C. Affinity binding phenomena of DNA onto apatite crystals. Biomaterials 2001, 22, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, I.; Rodrigues, A.; Santos, R.; Augusto, D.; Focardi, M.; Aquino, J.; Fonseca, I.; Pereira, C.P. Post-mortem Interval estimate based on dental pulp: A histomorphology approach. J. Forensic Odontostomatol. 2024, 42, 60–75. [Google Scholar] [PubMed]
- Smenderovac, E.; Rheault, K.; Moisan, M.A.; Emilson, C.; Brazeau, É.; Morency, M.J.; Gagné, P.; Maire, V.; Emilson, E.; Venier, L.; et al. Desiccation as a suitable alternative to cold-storage of phyllosphere samples for DNA-based microbial community analyses. Sci. Rep. 2025, 15, 4243. [Google Scholar] [CrossRef]
- Shahzad, M.; De Maeyer, H.; Salih, G.A.; Nilsson, M.; Haratourian, A.; Shafique, M.; Shahid, A.A.; Allen, M. Evaluation of Storage Conditions and the Effect on DNA from Forensic Evidence Objects Retrieved from Lake Water. Genes 2024, 15, 279. [Google Scholar] [CrossRef] [PubMed]
- Burrows, A.M.; Kasu, M.; D’Amato, M.E. Preservation of DNA integrity in biological material. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 1, 416–418. [Google Scholar] [CrossRef]
- Burger, J.; Hummel, S.; Hermann, B.; Henke, W. DNA preservation: A microsatellite-DNA study on ancient skeletal remains. Electrophoresis 1999, 20, 1722–1728. [Google Scholar] [CrossRef]
- Alaeddini, R.; Walsh, S.J.; Abbas, A. Forensic implications of genetic analyses from degraded DNA—A review. Forensic Sci. Int. Genet. 2010, 4, 148–157. [Google Scholar] [CrossRef]
- Lozano-Peral, D.; Rubio, L.; Santos, I.; Gaitán, M.J.; Viguera, E.; Martín-de-Las-Heras, S. DNA degradation in human teeth exposed to thermal stress. Sci. Rep. 2021, 11, 12118. [Google Scholar] [CrossRef]
- Garriga, J.A.; Ubelaker, D.H.; Czapico, S. Evaluation of macroscopic changes and the efficiency of DNA profiling from burnt teeth. Sci. Justice 2016, 56, 437–442. [Google Scholar] [CrossRef]
- Terada, A.S.; Silva, L.A.; Galo, R.; Azevedo Ad Gerlach, R.F.; Silva, R.H. The use of a DNA stabilizer in human dental tissues stored under different temperature conditions and time intervals. J. Appl. Oral Sci. 2014, 22, 331–335. [Google Scholar] [CrossRef]
- Tozzo, P.; Scrivano, S.; Sanavio, M.; Caenazzo, L. The Role of DNA Degradation in the Estimation of Post-Mortem Interval: A Systematic Review of the Current Literature. Int. J. Mol. Sci. 2020, 21, 3540. [Google Scholar] [CrossRef]
- Asari, M.; Matsuura, H.; Isozaki, S.; Hoshina, C.; Okuda, K.; Tanaka, H.; Horioka, K.; Shiono, H.; Shimizu, K. Assessment of DNA degradation of buccal cells under humid conditions and DNA repair by DOP-PCR using locked nucleic acids. Leg. Med. 2018, 35, 29–33. [Google Scholar]
- Alvarez García, A.; Muñoz, I.; Pestoni, C.; Lareu, M.V.; Rodríguez-Calvo, M.S.; Carracedo, A. Effect of environmental factors on PCR-DNA analysis from dental pulp. Int. J. Leg. Med. 1996, 109, 125–129. [Google Scholar]
- Murakami, H.; Yamamoto, Y.; Yoshitome, K.; Ono, T.; Okamoto, O.; Shigeta, Y.; Doi, Y.; Miyaishi, S.; Ishizu, H. Forensic study of sex determination using PCR on teeth samples. Acta Med. Okayama 2000, 54, 21–32. [Google Scholar]
- Musse, J.; Nardis, A.; Anzai, E.; Hirata, M.; Cicarelli, R.; Oliveira, R. Freshwater and salt-water influence in human identification by analysis of DNA: An epidemiologic and laboratory study. Braz. J. Oral Sci. 2018, 8, 2. [Google Scholar]
- Corte-Real, A.; Andrade, L.; Anjos, M.J.; Carvalho, M.; Vide, M.C.; Corte-Real, F.; Vieira, D. The DNA extraction from the pulp dentine complex of both with and without carious. Int. Congr. Ser. 2006, 1288, 710–712. [Google Scholar]
- Chowdhury, R.M.; Singhvi, A.; Bagul, N.; Bhatia, S.; Singh, G.; Goswami, S. Sex determination by amplification of amelogenin gene from dental pulp tissue by polymerase Chain Reaction. Indian J. Dent. Res. 2018, 29, 470–476. [Google Scholar] [PubMed]
- Sun, Y.; Wang, Y.; Ji, C.; Ma, J.; He, B. The impact of hydroxyapatite crystal structures and protein interactions on bone’s mechanical properties. Sci. Rep. 2024, 14, 9786. [Google Scholar] [CrossRef]
- Gilbert, M.T.; Rudbeck, L.; Willerslev, E.; Hansen, A.J.; Smith, C.; Penkman, K.E.H.; Prangenberg, K.; Nielsen-Marsh, C.M.; Jans, M.E.; Arthur, P.; et al. Biochemical and physical correlates of DNA contamination in archaeological human bones and teeth excavated at Matera, Italy. J. Archaeol. Sci. 2005, 32, 785–793. [Google Scholar]
- Itoh, D.; Yoshimoto, N.; Yamamoto, S. Retention Mechanism of Proteins in Hydroxyapatite Chromatography—Multimodal Interaction Based Protein Separations: A Model Study. Curr. Protein Pept. Sci. 2019, 20, 75–81. [Google Scholar]
- Booncharoen, P.; Khacha-ananda, S.; Kanchai, C.; Ruengdit, S. Factors influencing DNA extraction from human skeletal remains: Bone characteristic and total demineralization process. Egypt. J. Forensic Sci. 2021, 11, 1. [Google Scholar] [CrossRef]
- Jiménez-Arce, G.; Morera-Brenes, B. Revisión sobre la extracción de ADN a partir de huesos humanos. Med. Leg. Costa Rica 1999, 16, 11–14. [Google Scholar]
- Khan, M.N.; Farooqui, M.A.; Khan, S. Role of soil factors in the degree of DNA degradation. J. Pak. Med. Stud. 2022, 13, 164–169. [Google Scholar]
- Schultz, J.J.; Martin, D.L. The importance of soil in human taphonomy and remains management. Forensic Sci. 2021, 2, 47. [Google Scholar]
- Christofidis, G.; Effects of Soil on Bone Preservation. AZoLifeSciences 2007. Available online: https://www.azolifesciences.com/article/Effects-of-Soil-on-the-Preservation-of-Bones.aspx (accessed on 13 March 2025).
- Barbaro, A.; Cormaci, P.; La Marca, A. DNA extraction from soil by EZ1 advanced XL (Qiagen). Forensic Sci. Int. 2019, 299, 161–167. [Google Scholar] [CrossRef]
- Ambers, A.; Turnbough, M.; Benjamin, R.; King, J.; Budowle, B. Assessment of the role of DNA repair in damaged forensic samples. Int. J. Leg. Med. 2014, 128, 913–921. [Google Scholar] [CrossRef]
- Bianchi, I.; Grassi, S.; Castiglione, F.; Bartoli, C.; De Saint Pierre, B.; Focardi, M.; Oliva, A.; Pinchi, V. Dental DNA as an Indicator of Post-Mortem Interval (PMI): A Pilot Research. Int. J. Mol. Sci. 2022, 23, 12896. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, A.M.; Huerta, P.A.; Coliboro-Dannich, V.; Castro, A.F.; Barbaro, A. Evaluation of DNA in Human Teeth—Ante-Mortem and Post-Mortem Factors Affecting Degradation and Preservation: A Literature Review. Genes 2025, 16, 364. https://doi.org/10.3390/genes16040364
Salazar AM, Huerta PA, Coliboro-Dannich V, Castro AF, Barbaro A. Evaluation of DNA in Human Teeth—Ante-Mortem and Post-Mortem Factors Affecting Degradation and Preservation: A Literature Review. Genes. 2025; 16(4):364. https://doi.org/10.3390/genes16040364
Chicago/Turabian StyleSalazar, Ana María, Patricia Alejandra Huerta, Viviana Coliboro-Dannich, Ariel F. Castro, and Anna Barbaro. 2025. "Evaluation of DNA in Human Teeth—Ante-Mortem and Post-Mortem Factors Affecting Degradation and Preservation: A Literature Review" Genes 16, no. 4: 364. https://doi.org/10.3390/genes16040364
APA StyleSalazar, A. M., Huerta, P. A., Coliboro-Dannich, V., Castro, A. F., & Barbaro, A. (2025). Evaluation of DNA in Human Teeth—Ante-Mortem and Post-Mortem Factors Affecting Degradation and Preservation: A Literature Review. Genes, 16(4), 364. https://doi.org/10.3390/genes16040364




