Effects of Melatonin on H2O2-Induced Oxidative Damage of the Granulosa Cells in Hen Ovarian Follicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Cell Culture
2.3. Oxidative Damage Model Cell Treatment
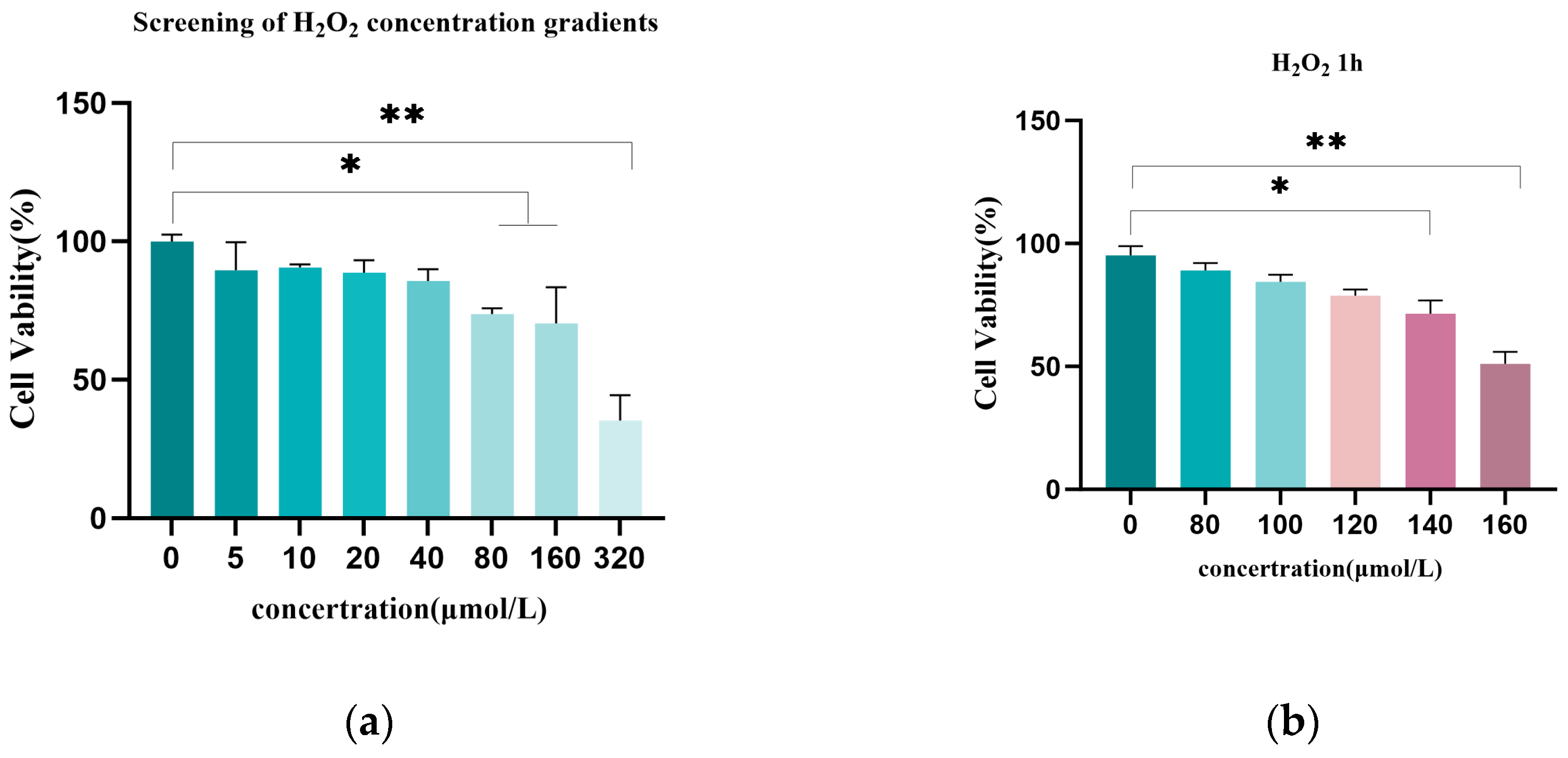
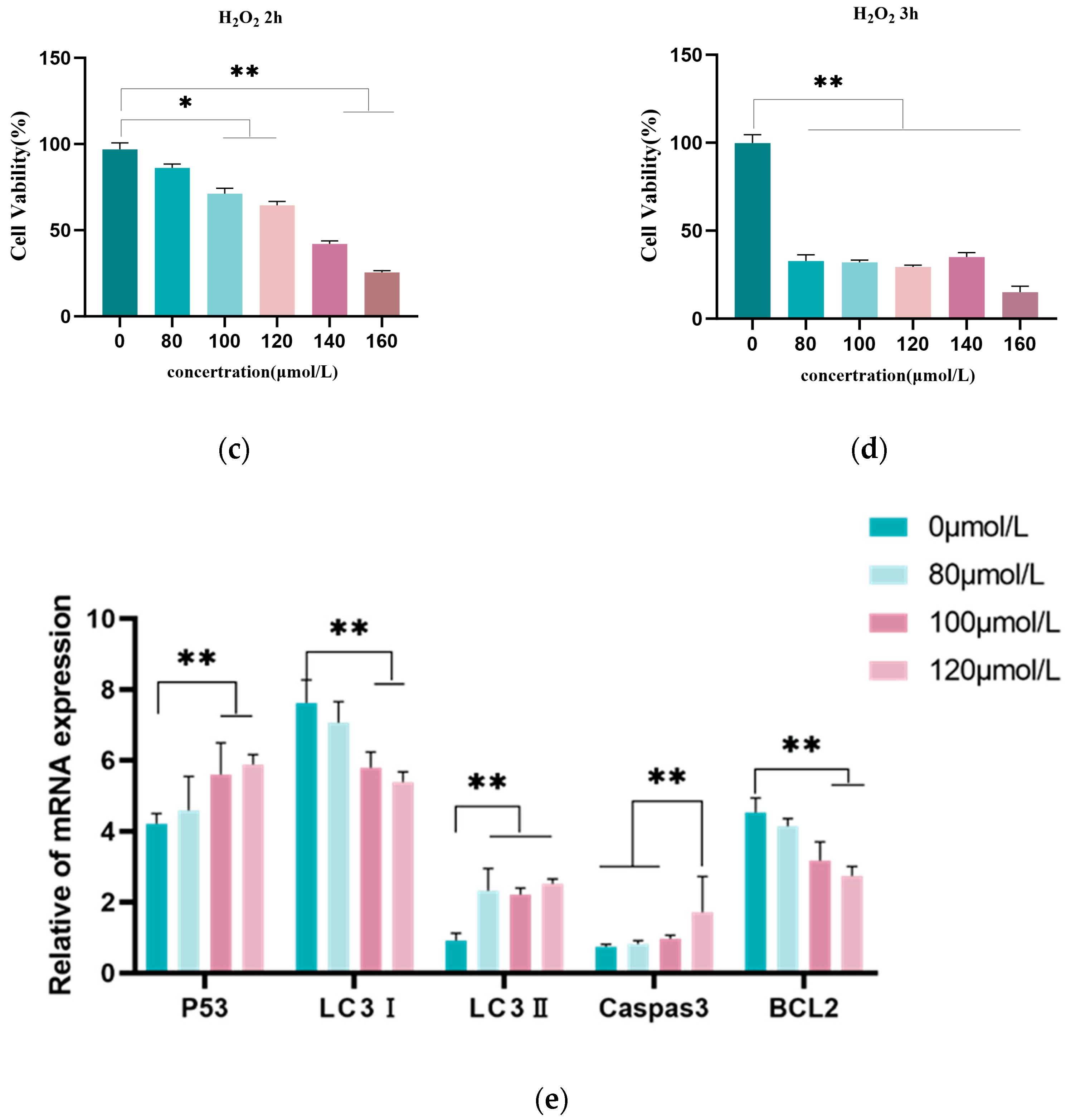
2.4. Cell Viability Assay
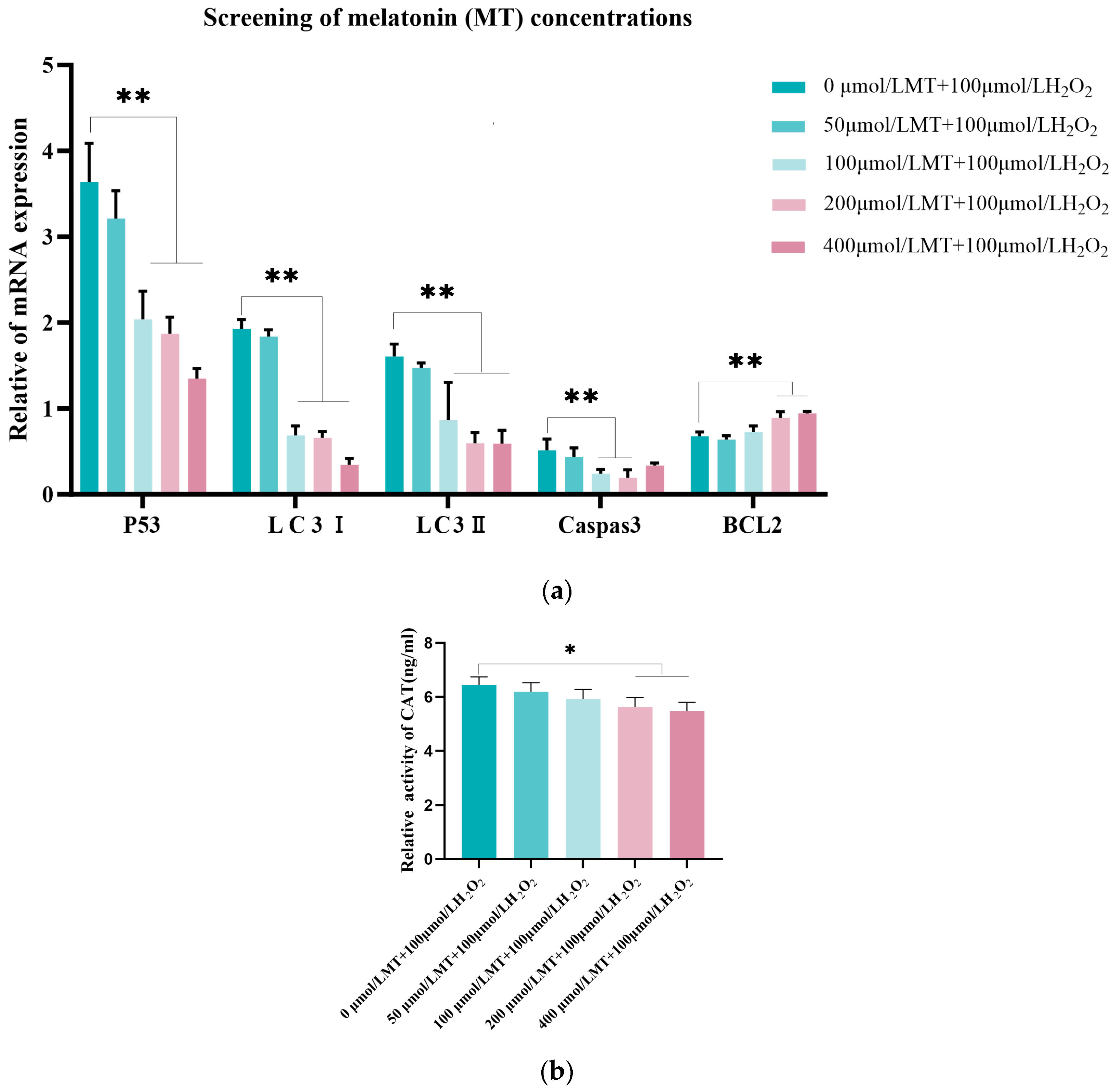
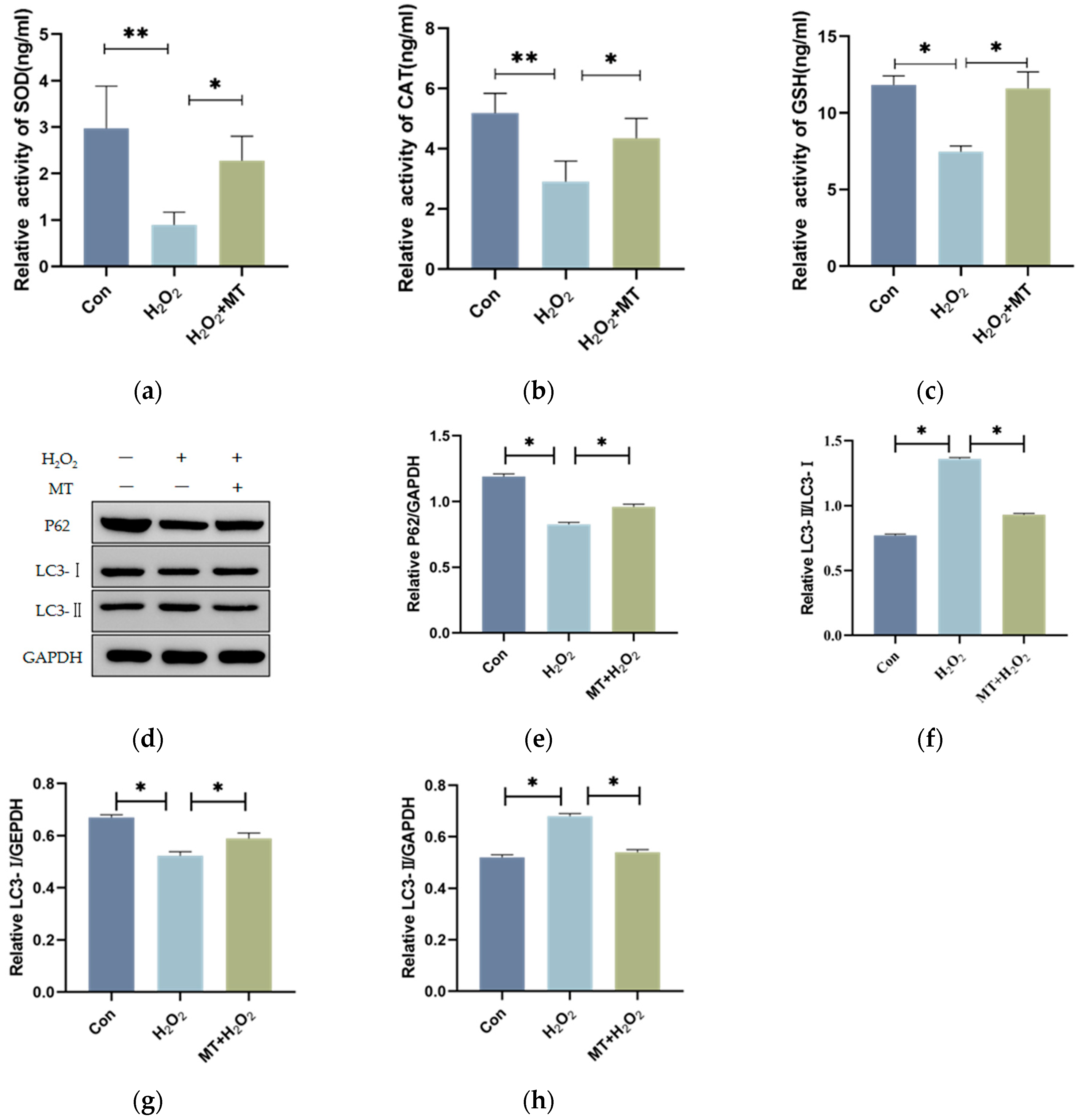
2.5. ELISA Assay
2.6. RNA Isolation and Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
2.7. Western Blotting
2.8. Autophagy Detection
2.9. Mitochondrial Membrane Potential Detection
2.10. Data Analysis
3. Results
3.1. H2O2-Induced Granulosa Cell Oxidative Damage Model
3.2. Screening of the Optimal Melatonin Concentration for Relieving Oxidative Damage in Model Cells
3.3. Melatonin Alleviates the Initiation of Autophagy in Granulosa Cells
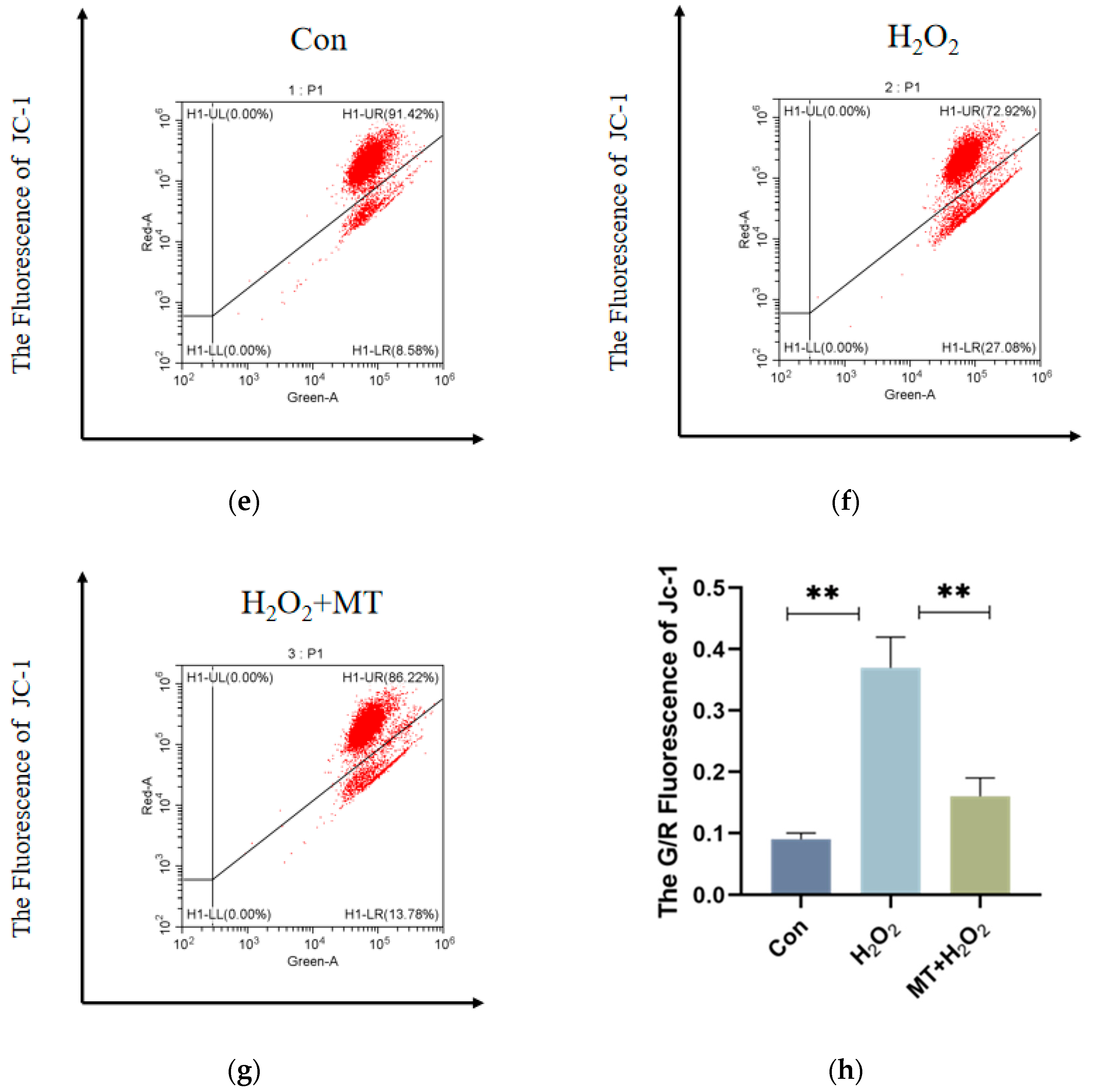
3.4. Melatonin Alleviates Mitochondrial Autophagy-Induced Oxidative Damage in Granulosa Cells (GCs)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| H2O2 | hydrogen peroxide |
| LC3-I | Microtubule-associated Protein 1A/1B-light Chain 3-I |
| LC3-II | Microtubule-associated Protein 1A/1B-light Chain 3-II |
| CAT | catalase |
| GSH | glutathione |
| SOD | superoxide dismutase |
| MT | melatonin |
| ROS | reactive oxygen species |
| P62 | Sequestosome 1 |
| GCs | granulosa cells |
| Ct | cycle threshold |
References
- Huang, Y.M.; Shi, Z.D.; Liu, Z.; Liu, Y.; Li, X.W. Endocrine Regulations of Reproductive Seasonality, Follicular Development and Incubation in Magang Geese. Anim. Reprod. Sci. 2008, 104, 344–358. [Google Scholar] [CrossRef]
- Rimon-Dahari, N.; Yerushalmi-Heinemann, L.; Alyagor, L.; Dekel, N. Ovarian Folliculogenesis. In Molecular Mechanisms of Cell Differentiation in Gonad Development; Piprek, R.P., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 167–190. ISBN 978-3-319-31973-5. [Google Scholar]
- Johnson, A.L.; Bridgham, J.T.; Witty, J.P.; Tilly, J.L. Expression of Bcl-2 and Nr-13 in Hen Ovarian Follicles during Development1. Biol. Reprod. 1997, 57, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, Y.; Guan, Z.; Cao, Y.; Li, L.; Liu, H.; Sun, S.-C. Protective Mechanism of FSH against Oxidative Damage in Mouse Ovarian Granulosa Cells by Repressing Autophagy. Autophagy 2017, 13, 1364–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jia, Y.; Meng, S.; Luo, Y.; Yang, Q.; Pan, Z. Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review. IJMS 2023, 24, 9205. [Google Scholar] [CrossRef]
- Goud, A.P.; Goud, P.T.; Diamond, M.P.; Gonik, B.; Abu-Soud, H.M. Reactive Oxygen Species and Oocyte Aging: Role of Superoxide, Hydrogen Peroxide, and Hypochlorous Acid. Free Radic. Biol. Med. 2008, 44, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Qiu, Y.; Wang, Q.; Gao, M.; Cao, Z.; Luan, X. ADPN Regulates Oxidative Stress-Induced Follicular Atresia in Geese by Modulating Granulosa Cell Apoptosis and Autophagy. Int. J. Mol. Sci. 2024, 25, 5400. [Google Scholar] [CrossRef]
- Zhu, M.; Miao, S.; Zhou, W.; Elnesr, S.S.; Dong, X.; Zou, X. MAPK, AKT/FoxO3a and mTOR Pathways Are Involved in Cadmium Regulating the Cell Cycle, Proliferation and Apoptosis of Chicken Follicular Granulosa Cells. Ecotoxicol. Environ. Saf. 2021, 214, 112091. [Google Scholar] [CrossRef]
- Zhang, G.-M.; An, S.-Y.; El-Samahy, M.A.; Zhang, Y.-L.; Wan, Y.-J.; Wang, Z.-Y.; Xiao, S.-H.; Meng, F.-X.; Wang, F.; Lei, Z.-H. Suppression of miR-1197-3p Attenuates H2O2-Induced Apoptosis of Goat Luteinized Granulosa Cells via Targeting PPARGC1A. Theriogenology 2019, 132, 72–82. [Google Scholar] [CrossRef]
- Luo, J.; Shi, R. Acrolein Induces Oxidative Stress in Brain Mitochondria. Neurochem. Int. 2005, 46, 243–252. [Google Scholar] [CrossRef]
- Yu, J.; Lou, Y.; He, K.; Yang, S.; Yu, W.; Han, L.; Zhao, A. Goose Broodiness Is Involved in Granulosa Cell Autophagy and Homeostatic Imbalance of Follicular Hormones. Poult. Sci. 2016, 95, 1156–1164. [Google Scholar] [CrossRef]
- Oroojan, A.A.; Chenani, N.; An’aam, M. Antioxidant Effects of Eugenol on Oxidative Stress Induced by Hydrogen Peroxide in Islets of Langerhans Isolated from Male Mouse. Int. J. Hepatol. 2020, 2020, 5890378. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One Molecule, Many Derivatives: A Never-ending Interaction of Melatonin with Reactive Oxygen and Nitrogen Species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Talpur, H.S.; Chandio, I.B.; Brohi, R.D.; Worku, T.; Rehman, Z.; Bhattarai, D.; Ullah, F.; JiaJia, L.; Yang, L. Research Progress on the Role of Melatonin and Its Receptors in Animal Reproduction: A Comprehensive Review. Reprod. Domest. Anim. 2018, 53, 831–849. [Google Scholar] [CrossRef]
- Xu, G.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. Melatonin Inhibits Apoptosis and Oxidative Stress of Mouse Leydig Cells via a SIRT1-Dependent Mechanism. Molecules 2019, 24, 3084. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, Z.; Di, S.; Yang, Y.; Yang, J.; Xu, L.; Reiter, R.J.; Qiao, S.; Yuan, J. AMPK/PGC1α Activation by Melatonin Attenuates Acute Doxorubicin Cardiotoxicity via Alleviating Mitochondrial Oxidative Damage and Apoptosis. Free Radic. Biol. Med. 2018, 129, 59–72. [Google Scholar] [CrossRef]
- Leon, J.; Acuña-Castroviejo, D.; Sainz, R.M.; Mayo, J.C.; Tan, D.-X.; Reiter, R.J. Melatonin and Mitochondrial Function. Life Sci. 2004, 75, 765–790. [Google Scholar] [CrossRef]
- Xu, G.; Dong, Y.; Wang, Z.; Ding, H.; Wang, J.; Zhao, J.; Liu, H.; Lv, W. Melatonin Attenuates Oxidative Stress-Induced Apoptosis of Bovine Ovarian Granulosa Cells by Promoting Mitophagy via SIRT1/FoxO1 Signaling Pathway. IJMS 2023, 24, 12854. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. J. Physiol. 2020, 598, 3793–3801. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative Stress in Oocyte Aging and Female Reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Li, J.; Pan, L.; Pan, W.; Li, N.; Tang, B. Recent Progress of Oxidative Stress Associated Biomarker Detection. Chem. Commun. 2023, 59, 7361–7374. [Google Scholar] [CrossRef]
- Kaczara, P.; Sarna, T.; Burke, J.M. Dynamics of H2O2 Availability to ARPE-19 Cultures in Models of Oxidative Stress. Free Radic. Biol. Med. 2010, 48, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Zhang, X.; Ge, S.-Q.; Zhang, E.-H.; Zhang, B. Expression of SIRT1 in the Ovaries of Rats with Polycystic Ovary Syndrome before and after Therapeutic Intervention with Exenatide. Int. J. Clin. Exp. Pathol. 2015, 8, 8276–8283. [Google Scholar]
- Devine, P.J.; Perreault, S.D.; Luderer, U. Roles of Reactive Oxygen Species and Antioxidants in Ovarian Toxicity. Biol. Reprod. 2012, 86, 27. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal 2017, 27, 989–1010. [Google Scholar] [CrossRef]
- Bai, K.; Hao, E.; Huang, C.; Yue, Q.; Wang, D.-H.; Shi, L.; Chen, Y.; Chen, H.; Huang, R. Melatonin Alleviates Ovarian Function Damage and Oxidative Stress Induced by Dexamethasone in the Laying Hens through FOXO1 Signaling Pathway. Poult. Sci. 2023, 102, 102745. [Google Scholar] [CrossRef]
- Inoue, N.; Matsuda, F.; Goto, Y.; Manabe, N. Role of Cell-Death Ligand-Receptor System of Granulosa Cells in Selective Follicular Atresia in Porcine Ovary. J. Reprod. Dev. 2011, 57, 169–175. [Google Scholar] [CrossRef]
- Martin, D.N.; Baehrecke, E.H. Caspases Function in Autophagic Programmed Cell Death in Drosophila. Development 2004, 131, 275–284. [Google Scholar] [CrossRef]
- Boya, P.; González-Polo, R.A.; Casares, N.; Perfettini, J.L.; Dessen, P.; Larochette, N.; Métivier, D.; Meley, D.; Souquere, S.; Yoshimori, T.; et al. Inhibition of Macroautophagy Triggers Apoptosis. Mol. Cell. Biol. 2005, 25, 1025–1040. [Google Scholar] [CrossRef]
- Escobar, M.L.; Echeverría, O.M.; Ortíz, R.; Vázquez-Nin, G.H. Combined Apoptosis and Autophagy, the Process That Eliminates the Oocytes of Atretic Follicles in Immature Rats. Apoptosis 2008, 13, 1253–1266. [Google Scholar] [CrossRef]
- Zhang, J. Autophagy and Mitophagy in Cellular Damage Control. Redox Biol. 2013, 1, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Padman, B.S.; Nguyen, T.N.; Uoselis, L.; Skulsuppaisarn, M.; Nguyen, L.K.; Lazarou, M. LC3/GABARAPs Drive Ubiquitin-Independent Recruitment of Optineurin and NDP52 to Amplify Mitophagy. Nat. Commun. 2019, 10, 408. [Google Scholar] [CrossRef]
- Deng, D.; Yan, J.; Wu, Y.; Wu, K.; Li, W. Morroniside Suppresses Hydrogen Peroxide-Stimulated Autophagy and Apoptosis in Rat Ovarian Granulosa Cells through the PI3K/AKT/mTOR Pathway. Hum. Exp. Toxicol. 2021, 40, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Ling, Z.; Luan, D.; Kang, J.; Dong, X.; Quan, F. HD-sEVs in Bovine Follicular Fluid Regulate Granulosa Cell Apoptosis and Estradiol Secretion through the Autophagy Pathway. Theriogenology 2023, 212, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Olson, S.D.; Whitney, M.J.; Prockop, D.J. Mitochondrial Transfer between Cells Can Rescue Aerobic Respiration. Proc. Natl. Acad. Sci. USA 2006, 103, 1283–1288. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and Reactive Oxygen Species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Jiang, Y.; Shen, M.; Chen, Y.; Wei, Y.; Tao, J.; Liu, H. Melatonin Represses Mitophagy to Protect Mouse Granulosa Cells from Oxidative Damage. Biomolecules 2021, 11, 968. [Google Scholar] [CrossRef]
- Bai, J.; Wang, X.; Chen, Y.; Yuan, Q.; Yang, Z.; Mi, Y.; Zhang, C. Nobiletin Ameliorates Aging of Chicken Ovarian Prehierarchical Follicles by Suppressing Oxidative Stress and Promoting Autophagy. Cells 2024, 13, 415. [Google Scholar] [CrossRef]
- Liu, X.; Lin, X.; Zhang, S.; Guo, C.; Li, J.; Mi, Y.; Zhang, C. Lycopene Ameliorates Oxidative Stress in the Aging Chicken Ovary via Activation of Nrf2/HO-1 Pathway. Aging 2018, 10, 2016–2036. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240. [Google Scholar] [CrossRef]
- Wang, J.; Jia, R.; Gong, H.; Celi, P.; Zhuo, Y.; Ding, X.; Bai, S.; Zeng, Q.; Yin, H.; Xu, S.; et al. The Effect of Oxidative Stress on the Chicken Ovary: Involvement of Microbiota and Melatonin Interventions. Antioxid. 2021, 10, 1422. [Google Scholar] [CrossRef]
- Yang, F.; Pei, R.; Zhang, Z.; Liao, J.; Yu, W.; Qiao, N.; Han, Q.; Li, Y.; Hu, L.; Guo, J.; et al. Copper Induces Oxidative Stress and Apoptosis through Mitochondria-Mediated Pathway in Chicken Hepatocytes. Toxicol. Vitr. 2019, 54, 310–316. [Google Scholar] [CrossRef]
- Song, C.; Peng, W.; Yin, S.; Zhao, J.; Fu, B.; Zhang, J.; Mao, T.; Wu, H.; Zhang, Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 2016, 6, 35165. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward | Reverse |
|---|---|---|
| BCL2 | GGACGCTTGGCTATCCCTAC | CTATGATGCGATGGCACGAC |
| Caspase3 | GCTGAAGGCTCCTGGTTTAT | TTCTGCCACTCTGCGATTTA |
| LC3-I | TTACACCCATATCAGATTCTTG | ATTCCAACCTGTCCCTCA |
| LC3-II | AGTGAAGTGTAGCAGGATGA | AAGCCTTGTGAACGAGAT |
| P53 | GCAAGATCGAGGAGGAGAACT | ATCTCATTGTCGGGGTTCAG |
| β-actin | GCCAACAGAGAGAAGATGACACAG | CATCACCAGAGTCCATCACAATACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ou, Y.; Cao, S.; Sun, X.; Qin, N.; Liswaniso, S.; Xu, R. Effects of Melatonin on H2O2-Induced Oxidative Damage of the Granulosa Cells in Hen Ovarian Follicles. Genes 2025, 16, 362. https://doi.org/10.3390/genes16040362
Wang S, Ou Y, Cao S, Sun X, Qin N, Liswaniso S, Xu R. Effects of Melatonin on H2O2-Induced Oxidative Damage of the Granulosa Cells in Hen Ovarian Follicles. Genes. 2025; 16(4):362. https://doi.org/10.3390/genes16040362
Chicago/Turabian StyleWang, Sheng, Yu Ou, Shengxiao Cao, Xue Sun, Ning Qin, Simushi Liswaniso, and Rifu Xu. 2025. "Effects of Melatonin on H2O2-Induced Oxidative Damage of the Granulosa Cells in Hen Ovarian Follicles" Genes 16, no. 4: 362. https://doi.org/10.3390/genes16040362
APA StyleWang, S., Ou, Y., Cao, S., Sun, X., Qin, N., Liswaniso, S., & Xu, R. (2025). Effects of Melatonin on H2O2-Induced Oxidative Damage of the Granulosa Cells in Hen Ovarian Follicles. Genes, 16(4), 362. https://doi.org/10.3390/genes16040362





