Abstract
Background/Objectives: Limosilactobacillus fermentum KUB-D18, a heterofermentative lactic acid bacterium with promising probiotic properties, is known for promoting gut health and nutrient absorption. Originally isolated from chicken intestines, this strain demonstrates versatile metabolic capabilities in diverse gastrointestinal environments. However, the metabolic functions and sugar transport-related genes remain largely unexplored. This study thus aimed to dissect metabolic functions and sugar transports of L. fermentum KUB-D18. Methods: Next-generation and third-generation sequencing techniques using integrative genomic platform towards transportome analysis were performed. Results: The complete genome, sized at 2.12 Mbps with a GC content of 51.36%, revealed 2079 protein-encoding genes, of which 1876 protein functions were annotated and identified in top categories involved in amino acids, nucleotide, energy, and carbohydrate transports and metabolisms. Comparative genes analysis identified 50 core and 12 strain-specific genes linked to probiotic properties, e.g., acid resistances and bile tolerances, antioxidant functions, or anti-inflammatory properties. Further, sugar transportome analysis uncovered 57 transporter genes, demonstrating diverse carbon utilization and phosphotransferase (PTS) systems, corroborated by API 50 CHL test results for carbohydrate metabolism profile. Conclusions: These findings enhance the comprehensive metabolic understanding of L. fermentum KUB-D18, supporting its industrial potential and applications in engineered probiotics.
1. Introduction
Limosilactobacillus fermentum KUB-D18 is a member of the Lactobacillaceae family, which is well known for producing lactic acid [1]. As a probiotic strain isolated from chicken intestines, L. fermentum KUB-D18 exhibits several probiotic properties [2]. These characteristics highlight strong potential roles in food fermentation and health promotion, such as modulating the gut microbiome, improving metabolic health, and enhancing immune function [3].
To gain a deeper understanding of L. fermentum KUB-D18, obtaining a complete genome sequence is one of the most effective tools for systematic level. Recently, various sequencing and annotation techniques further expand the possibilities for exploring the whole-genome sequence of L. fermentum. In the previous research reported by Phujumpa et al. [2], the short reads of L. fermentum KUB-D18 were obtained through Illumina NovaSeq 6000 platform sequencing and assembled into the first draft genome size of approximately 2.02 Mbps under 398 scaffolds with GC content of 51.7% and 2158 protein-encoding genes, which were then functionally annotated. The short-read genome of L. fermentum KUB-D18 reveals genes for folate biosynthesis, L-ascorbic acid metabolism, and bile salt hydrolase (BSH) activity. BSH enhances gastrointestinal survival by deconjugating bile acids [4]. It also lowers cholesterol by altering bile acid solubility, promoting excretion, and triggering cholesterol use for bile acid synthesis [4]. With the complete genome still unavailable, key gaps remain in understanding the metabolic functions linked to probiotic properties, including stress adaptation, antioxidant defense, as well as antimicrobial and anti-inflammatory activities [2].
Beyond metabolic gaps, critical information on sugar transport in L. fermentum KUB-D18 remains largely unknown. As sugars are essential nutrients for probiotic growth, a comprehensive analysis of its transportome is necessary [5]. The sugar transportome facilitates the uptake of all sugar molecules, such as glucose from the external environment into the cell, directly influencing the metabolic activity, growth, and overall functionality of probiotics [6]. By exploring all sugar transport-related genes of L. fermentum KUB-D18 and linking them to functional properties, e.g., lactic acid production, antioxidant activities, as well as other industrial perspectives, are basically required.
Recent advancements in sequencing technologies and bioinformatics tools have enabled integrating both short-read sequences data provided by Next-generation sequencing (NGS) technologies, e.g., Illumina, and long-read sequences data provided by third-generation sequencing (TGS) technologies, e.g., PacBio or Oxford Nanopore, to produce high-quality genomic assembly with resulting gaps reduction [7]. Bioinformatics tools, e.g., MaSuRCA, hybridSPAdes, and Unicycler, exemplified this advancement in more complete and accurate genome assemblies, thereby facilitating downstream genomic analysis [8,9]. This approach not only improves the resolution of complex genomic regions but also facilitates the identification of protein-encoding genes related to metabolic functions and sugar transports in probiotic bacteria [9,10,11].
This study, therefore, aimed to dissect metabolic functions and sugar transports using integrative genomic platform towards transportome analysis of probiotic L. fermentum KUB-D18. In brief, the integration of NGS by Illumina and TGS data by Oxford Nanopore was initially used for genome assembly of L. fermentum KUB-D18. Once a completed genome became available, the functional annotation and analysis was performed for investigating metabolic gene functions and sugar transports of L. fermentum KUB-D18 using protein and pathway databases. Finally, to evaluate potential probiotic properties within the context of carbon utilization, API 50 CHL test results for carbohydrate metabolism profile were performed.
Through this study, we gained a more comprehensive understanding of the metabolic basis of L. fermentum KUB-D18. The precise mapping of the metabolic and sugar transport functions also offers new possibilities for optimizing the strain’s growth conditions and enhancing its probiotic properties, thereby increasing potentials for industrial applications.
2. Materials and Methods
2.1. Culture Condition and Carbohydrate Metabolism Test of L. fermentum KUB-D18
L. fermentum strain KUB-D18, originally isolated from chicken intestine samples, was obtained from the stock culture of the Specialized Research Unit of Probiotics and Prebiotics for Health, Department of Biotechnology, Kasetsart University [12]. This strain was preserved at −20 °C in MRS medium (Difco Laboratories, Detroit, MI, USA) supplemented with 25% (v/v) glycerol. For experimental use, the culture was cultivated by two successive propagations in MRS medium with an initial pH of 6.5 ± 0.2 at 37 °C for 15 h each time. The carbohydrate metabolism test of this strain was evaluated by using the API 50 CHL kit according to the manufacturer’s protocol (Biomérieux, Marcy l’Etoile, France). In brief, the isolates strain was adjusted to the concentration of 2 Mc Farland standard in 2 mL API suspension and inoculated to API 50 CHL medium. The API 50 CHL medium was homogenized and aliquoted to API microtube strip. Prior to incubation, a layer of mineral oil was added, and the strip was incubated at 37 °C. The result was evaluated after 24 h of incubation and compared to the API database.
2.2. Genomic DNA Extraction Towards Sequencing of L. fermentum KUB-D18
L. fermentum KUB-D18 was cultured for 18 h on MRS agar plates supplemented with 0.05% L-cysteine. Genomic DNA was extracted by using the MagAttract HMW DNA Kit (Qiagen, Hilden, Germany) with slight modifications to the manufacturer’s protocol.
In brief, colonies were scraped from the agar surface and suspended in buffer P1 until the optical density at 600 nm (OD600) reached 0.5. To lyse the bacterial cells, 40 µL of lysozyme (200 mg/mL, Thermo Scientific, Waltham, MA, USA) was added to the suspension and gently mixed by tapping. The mixture was incubated at 37 °C for 1 h in a thermoshaker MATRIX Orbital (IKA, Staufen, Germany) with shaking at 600 rpm. Subsequently, 40 µL of proteinase K was added, mixed gently, and incubated at 56 °C with shaking at 600 rpm for 15 min.
To eliminate RNA contaminants, 4 µL of RNase A (100 mg/mL) was added, and the mixture was incubated statically at 37 °C for 10 min. The remaining steps followed the manufacturer’s standard protocol, with an additional DNA purification step using magnetic beads. The lysate was combined with magnetic bead suspension and a binding buffer, thoroughly mixed, and incubated to achieve purified DNA. The quality of genomic DNA was analyzed by NanoDrop Spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and gel electrophoresis.
For genome sequencing, Oxford Nanopore Technology was performed to generate the integrative genome sequence of L. fermentum KUB-D18. For long-read Nanopore sequencing, the quality and adapter trimming of raw sequenced reads obtained was possessed by Nanoplot v1.38.0 [13] with a default parameter of a minimum length of 1000 base pairs (bps) and quality (Q) score of 10.
2.3. Integrative Genome Assembly and Functional Annotation of L. fermentum KUB-D18
Integrative genome assembly was based on the short-read Illumina NovaSeq 6000 platform [2] and long-read Oxford Nanopore Technology. It was conducted using Unicycler v0.4.8 [14] and generating high-quality assembled sequences in FASTA format. The genome features of these sequences were assessed using the quality evaluation tool, i.e., QUAST v5.0.2 [15]. To verify the completeness and contamination of the assembled genome, CheckM v1.2.1 [16] was employed. Additionally, plasmid typing was performed using MOB-suite v3.1.5 [17] (Figure 1). All the sequences have been deposited in the Sequence Read Archive (www.ncbi.nlm.nih.gov/sra, accessed on 19 November 2024) under the accession no. PRJNA1187331 (SRA number: SRR31373359).
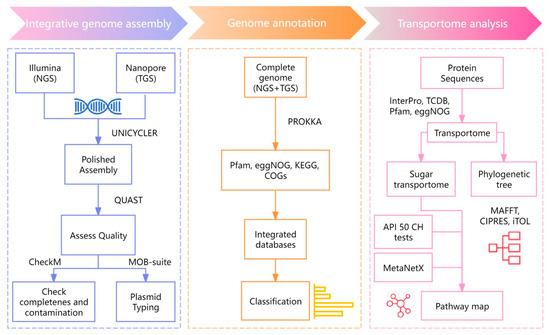
Figure 1.
The workflow of integrative genome assembly and functional annotation of L. fermentum KUB-D18.
The genome of L. fermentum KUB-D18 was annotated by prokaryotic genome annotation (Prokka v1.14.6) using the default parameters and minimizing contig size to 1000 bps [18]. Functional annotation of the genes was performed using integrated protein and pathway databases, e.g., Pfam [19], KEGG [20], COGs, and eggNOG [21,22]. To further annotate core and strain-specific genes, comparative genes analysis was performed across related L. fermentum strains, e.g., 3872, CECT5716, and F-6 [23,24,25]. Those strains were firstly selected for comparison based on their well-documented probiotic characteristics and the availability of comparative genomic data for a functional analysis [26]. In addition, a list of targeted genes was also explored across 75 strains of L. fermentum genomes from IMG and NCBI databases.
2.4. Transportome Towards Metabolic Pathway Mapping of L. fermentum KUB-D18
Sequences were aligned against the Transporter Classification Database (TCDB) under thresholds of sequence identity of 40% and E-value of 1 × 10 −5 [27]. Additionally, the Pfam, InterPro [28], and eggNOG databases were employed to screen for genes associated with transport functions. Then, Pfam, eggNOG, InterPro, and TCDB identifiers related to sugar transporters [29] were performed. Using the MAFFT v. 7.307 tool, multiple sequence alignment was performed for sugar transporters identification [30]. The phylogenetic tree was constructed using maximum likelihood analysis using RAxML–HPC BlackBox [31] through the CIPRES Science Gateway. The visualized phylogenetic tree was then illustrated using the online tool iTOL version 6 (https://itol.embl.de/, accessed on 26 December 2024) [32]. MetaNetX [33] was used to perform metabolic pathway mapping with functional annotation to further identify metabolic genes, enzymes and biochemical reactions to further propose metabolic functions illustrating diverse carbon utilization through various sugar transporters in L. fermentum KUB-D18.
3. Results and Discussion
3.1. Integrative Genomic Data Using NGS and TGS Towards Globally Annotated Results of L. fermentum KUB-D18
In this study, we conducted integrative genomics by assembling NGS (Illumina) [2] and TGS (Oxford Nanopore) data. As a result, the complete genome of L. fermentum KUB-D18 was successfully assembled into a single chromosomal contig, compared to the 398 scaffolds from the previous genome [2]. This greatly improved assembly continuity, minimized gaps and redundancies, and enhanced functional annotation (Table 1). As a result, the complete genome contains approximately 2.12 Mbps with GC content of 51.36%. Exploring annotation, it results in 2079 protein-encoding genes and 75 RNA-encoding genes.

Table 1.
Comparison of genomic characteristics of L. fermentum KUB-D18 in different studies.
In the context of enhancing functional annotation across updated multiple biological databases, the assigned protein functions were accordingly identified by COGs (1848 genes), KEGG (1222 genes), Pfam (1787 genes), and eggNOG (1849 genes) (Supplementary Tables S1–S4). After removing duplicated genes across integrated databases, the results were 1876 annotated genes (Table 1). This enables a comprehensive analysis of gene functions in the genome of the L. fermentum KUB-D18 and further links it to its probiotic functions.
Of the 1876 annotated genes, 1572 were classified into four main functional categories. The majority (705 genes, 44.85%) were involved in metabolism, followed by genetic information processing (596 genes, 37.91%), environmental information processing (163 genes, 10.37%), and cellular processes (108 genes, 6.87%) (Figure 2A). To further analyze the metabolic genes, we then categorized them into metabolic functional categories. The most abundant was involved in amino acid transport and metabolism (182 genes), followed by nucleotide transport and metabolism (112 genes), energy production and conversion (95 genes), carbohydrate transport and metabolism (94 genes), coenzyme transport and metabolism (78 genes), inorganic ion transport and metabolism (73 genes), lipid transport and metabolism (60 genes), and secondary metabolite biosynthesis, transport, and catabolism (11 genes) (Figure 2B).
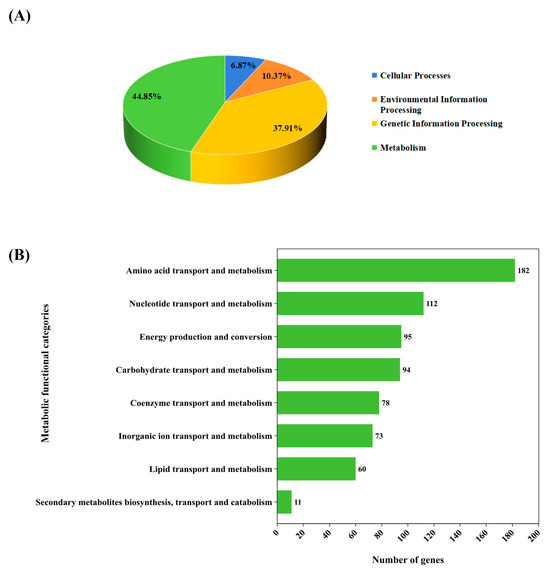
Figure 2.
Classification of gene functions of L. fermentum KUB-D18 genome based on integrated databases. (A) The pie chart shows comparable percentage of gene functions distributed in four main functional categories. (B) The horizontal bar chart shows the comparable number of gene functions devoted to different metabolic functional categories.
Further, we identified abundance of genes in the histidine metabolic pathway (13 genes), lysine biosynthetic pathway (13 genes), purine metabolism (38 genes), and pyrimidine metabolism (24 genes). Additionally, 13 genes were associated with oxidative phosphorylation, 10 genes in nitrogen metabolism, 26 genes in glycolysis/gluconeogenesis, and 20 genes in pyruvate metabolism (Supplementary Table S5). Oxidative phosphorylation is a major ATP-producing pathway in many organisms, but heterofermentative lactobacilli, which do not favor oxygen, primarily rely on fermentation for energy production [34]. In the context of its probiotic function, these genes may support the strain’s survival in the less anaerobic part of the human gut. Additionally, they may contribute to enhancing oxidative phosphorylation in the host, potentially improving energy metabolism and nutrient utilization [35]. Further analysis of these metabolic genes and transport systems is needed to understand the strain-specific traits of L. fermentum KUB-D18.
3.2. Exploring the Metabolic Genes Revealing Core and Strain-Specific Characteristics of Probiotic L. fermentum KUB-D18
To investigate core and strain-specific genes in relation to probiotic properties of L. fermentum KUB-D18, 705 metabolic genes were initially explored for functions related to acid resistance, bile tolerance, antioxidant function, antimicrobial function, and anti-inflammatory property against the related L. fermentum strains (Materials and Methods). Regarding comparative genes analysis between KUB-D18 across these selected strains, a total of 50 core genes were identified, namely acid resistance, bile tolerance and metabolic adaptability (16 genes), antioxidant functions (21 genes), antimicrobial functions (5 genes), and anti-inflammatory properties (8 genes) (Table 2, Supplementary Table S6). Promisingly, L. fermentum KUB-D18 displayed 12 strain-specific genes related to metabolic adaptability (11 genes), and antioxidant functions (1 gene), as detailed in Table 2.

Table 2.
List of core and strain-specific genes related to probiotic functions of L. fermentum KUB-D18 in comparative analysis with other relevant strains.
Comparative genes analysis revealed that L. fermentum KUB-D18 exhibits acid resistance and bile tolerance properties, supported by the presence of 8 core atp genes, i.e., atpA gene (GH00514), atpB (GH00510), atpC (GH00517), atpD (GH00516), atpE (GH00511), atpF (GH00512), atpG (GH00515), and atpH (GH00513). The atp genes in L. fermentum strains suggest an increased ATP production capacity, which likely supports its survival under acidic stress condition [36,37]. Moreover, six core ldh genes (GH01323, GH01664, GH01127, GH00286, GH00416, GH00725) encoding L-lactate dehydrogenase (EC: 1.1.1.27), which is the key enzyme of fermentation by lactic acid bacteria, and two core bsh genes (GH00033, GH01152) encoding bile salt hydrolase (EC: 3.5.1.24) were identified in L. fermentum KUB-D18. These results are consistent with Phujumpa et al. [2]. Accordingly, the presence of multiple ldh gene copies may enhance enzyme activity, improving environmental adaptability and antioxidant potential [37,55]. Altogether, L. fermentum KUB-D18 demonstrates acid resistance and bile tolerance and cholesterol-lowering properties, making it a candidate for industrial probiotic yogurt production, which maintains viability and functionality under acidic conditions [56].
For ula genes, notable ones are ulaA (GH01065, GH02018) encoding ascorbate PTS system EIIC component, ulaB (GH01066, GH02017) encoding ascorbate PTS system EIIB component (EC: 2.7.1.194), ulaC (GH01064, GH02019) encoding ascorbate PTS system EIIA or EIIAB component (EC: 2.7.1.194), and others crucial for L-ascorbic acid metabolism, i.e., ulaD (GH02016) encoding 3-dehydro-L-gulonate-6-phosphate decarboxylase (EC: 4.1.1.85), ulaE (GH02030) encoding L-ribulose-5-phosphate 3-epimerase (EC: 5.1.3.22), ulaF (GH02029) encoding L-ribulose-5-phosphate 4-epimerase (EC: 5.1.3.4), and ulaG (GH01067, GH02020) encoding L-ascorbate 6-phosphate lactonase (EC: 3.1.1.-).
The complete ula gene cluster in L. fermentum KUB-D18, absent in other strains, highlights its potential for L-ascorbic acid metabolism. Compared with orthologous 75 strains, as shown in Supplementary Table S7, this is supported by the annotated function of the ula-encoded phosphotransferase transport system for ascorbic acid catabolism and metabolic capability [56]. This result suggests a potential role in the high metabolic capability of L. fermentum KUB-D18, as seen in high copy numbers of genes, e.g., genes encoding the ascorbate PTS system [40].
Based on our findings, L. fermentum KUB-D18 harbors genes associated with antioxidant functions, such as folate (vitamin B9) [41,42]. The identification of comparative genes revealed eight core fol genes involved in folate biosynthesis, including folA (GH01227), folB (GH01551), folC (GH00605, GH01548), folD (GH01469), folK (GH01550), folP (GH01546), and folE (GH01549) (Supplementary Table S6) [2]. The folate regulates homocysteine levels, supporting cysteine and glutathione synthesis to combat oxidative stress [57]. Functional genes analysis identified three core glutamate-cysteine ligase (gshA) genes (EC: 6.3.2.2) (GH00749, GH01002, GH01072) essential for glutathione synthesis [43,58], along with glutathione reductase genes, i.e., two core genes (GH01873, GH02130) and a strain-specific gene (GH01400) [44]. A core gene (GH00480) encoding NADH-dependent peroxiredoxin subunit C and seven thioredoxin system genes (trxA: GH00635, GH01874, GH01968, GH02124; trxB: GH00410, GH00481, GH02127) further support redox balance and oxidative damage repair in L. fermentum KUB-D18 [45,46,47].
Furthermore, we also identified three core genes related to exopolysaccharide (EPS) biosynthesis in L. fermentum KUB-D18, including epsA (GH00095, GH01646) encoding protein tyrosine kinase modulator and epsB (GH00096) encoding protein tyrosine kinase. EPS forms a dense extracellular matrix that serves as a protective barrier, preventing pathogenic adhesion and biofilm formation [50,59]. Antimicrobial functions in L. fermentum KUB-D18 were linked to two core genes involved in the acetic acid biosynthetic pathway: GH00336 encoding acetaldehyde dehydrogenase/alcohol dehydrogenase (EC: 1.2.1.10; 1.1.1.1) and GH00451 encoding phosphate acetyltransferase (EC: 2.3.1.8) [48,49]. Acetic acid is a primary short-chain fatty acid (SCFA); this suggests that it might be a key role in maintaining energy balance and metabolic homeostasis in L. fermentum KUB-D18 [60].
Considering anti-inflammatory properties, we identified five core genes related to histidine metabolism in L. fermentum KUB-D18: hisF (GH00861, GH00869), hisH (GH00859, GH00867), and hisJ (GH00862), encoding essential enzymes, e.g., imidazole glycerol-phosphate synthase (EC: 4.3.2.10) and histidinol-phosphatase (EC: 3.1.3.15) involved in the production of histidinol phosphate, a key intermediate in histamine biosynthesis [51]. Histidine-derived histamine modulates immune cell activity, helping maintain immune balance and exerting anti-inflammatory effects [61,62]. Previous studies have shown that his genes exert anti-inflammatory effects supported by animal model experiments [62] by regulating pro-inflammatory mediators, such as tumor necrosis factor (Tnf)-α and interleukin (Il)-6.
Additionally, genes linked to glutamate and glutamine metabolism, including GH01482 (glutamine synthetase, EC: 6.3.1.2) and GH01059, GH01060 (glutamate:GABA antiporter/glutamate decarboxylase, EC: 4.1.1.15), indicate the presence of a metabolic pathway for GABA synthesis, suggesting roles in stress tolerance, anti-inflammatory effects, and gut–brain axis modulation [52,53,63,64,65]. Previous studies have shown that L. fermentum KUB-D18 exhibits high resistance in an in vitro gastrointestinal model, surviving stomach and small intestinal conditions while modulating the gut microbiota of overweight individuals in a colon fermentation model. This adaptability suggests its ability to adjust to metabolic activity in response to environmental changes. This metabolic flexibility was observed in conjunction with L. reuteri KUB-AC5, indicating a synergistic effect rather than an isolated trait of L. fermentum KUB-D18 alone [66].
Altogether, these findings not only highlight L. fermentum KUB-D18′s probiotic properties but also provide a foundation for use in functional foods and probiotic supplements. Since the probiotic functionality often depends on their sugar uptake and metabolism, which provide energy to cells, drive key metabolic pathways, and support the probiotic functions, further studies on of L. fermentum KUB-D18′s metabolic transports are essential for understanding probiotic effects.
3.3. Identification of Metabolic Transports of L. fermentum KUB-D18 Using Transportome Analysis
Apart from the metabolic genes relevant to metabolism, how metabolic genes are transported across the cellular membrane or between compartments remains unknown in L. fermentum KUB-D18. To explore the metabolic transporters in L. fermentum KUB-D18, transportome analysis was performed on 2079 protein-encoding genes, with 14.76% (307 genes) encoding transmembrane proteins. These were annotated using TCDB (155 genes), Pfam (127 genes), eggNOG (58 genes), and InterPro (44 genes) (Table 3). Across integrated databases, 259 genes were related to metabolic transports (Supplementary Table S8). A maximum likelihood tree classified these into seven transporters’ categories: ATP-binding cassette (ABC) superfamily (97 genes, 37.5%), major facilitator superfamily (MFS) (82 genes, 31.7%), transport-related enzymes (30 genes, 11.6%), energy-coupling transporter (ECT) superfamily (20 genes, 7.7%), secondary transporter superfamily (16 genes, 6.2%), protein transport and secretion (7 genes, 2.7%), and iron transporter superfamily (7 genes, 2.7%) (Figure 3).

Table 3.
List of metabolic transporter genes across integrated protein and transporter databases.
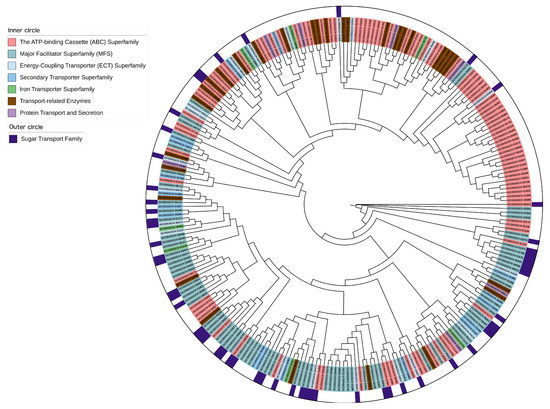
Figure 3.
The maximum likelihood phylogenetic tree of metabolic transporter genes distributing in different transporter categories of L. fermentum KUB-D18. The phylogenetic tree was initially produced using RAxML–HPC BlackBox through the CIPRES Science Gateway with default parameters and then visualized by the online tool iTOL version 6 (https://itol.embl.de/, accessed on 26 December 2024).
In lactic acid bacteria, the sugar transporters were promisingly required to study in L. fermentum, and thus the term of sugar transportome has been raised to represent whole sugar transporters, which were investigated through advanced bioinformatics and next generation sequencing technology [29,67].
3.3.1. Sugar Transportome Analysis Towards Alternative Carbon Substrate Utilization of L. fermentum KUB-D18
In this study, a number of sugar transporters (57 of 259 metabolic transporter genes) were identified in the L. fermentum KUB-D18 genome (Table 4). They were classified into three groups, including secondary carriers (43 genes), e.g., the Major Facilitator Superfamily (MFS) and drug/metabolite transporter (DMT) superfamilies (Table 5), PTS systems (11 genes), and ABC carriers (3 genes) (Supplementary Table S9). These transporters play key roles in carbohydrate metabolism. MFS transporters facilitate the passive diffusion of sugars along their concentration gradient [68], while the PTS family actively couples sugar entry with phosphorylation, altering sugar structures [69]. ABC transporters, energy-dependent systems, enable sugar uptake via ATP hydrolysis, typically with high substrate specificity. As illustrated in Figure 3, phylogenetic analysis also revealed that genes from the same family did not cluster in a single branch, suggesting evolutionary divergence. This diversity is likely due to factors such as varying substrate specificity [70] and/or environmental adaptations [71]. These findings highlight the complexity and evolutionary diversity of sugar transporter functions in L. fermentum KUB-D18.

Table 4.
List of sugar transporter genes across integrated protein and transporter databases.

Table 5.
List of sugar transporter genes categorized into secondary carriers, PTS systems, and ABC carriers.
3.3.2. Probing Carbon Utilization in L. fermentum KUB-D18
Based on the sugar transportome analysis, hereby we mapped the 57 sugar transporter genes in the metabolism of L. fermentum KUB-D18. As illustrated in Figure 4, the transport genes of the following carbon as substrate including C3 (glycerol-3-phosphate, glycerol), C4 (L-malate), C5 (2-oxoglutarate, D-ribose), C6 (D-glucose, D-fructose, D-galactose, D-glucosamine, D-gluconate, D-mannose, D-mannitol, D-sorbitol, lactose), C8 (n-acetyl-D-glucosamine), C12 (trehalose, arbutin, maltose, sucrose, cellobiose), C13 (salicin), and C18 (raffinose) were mapped.
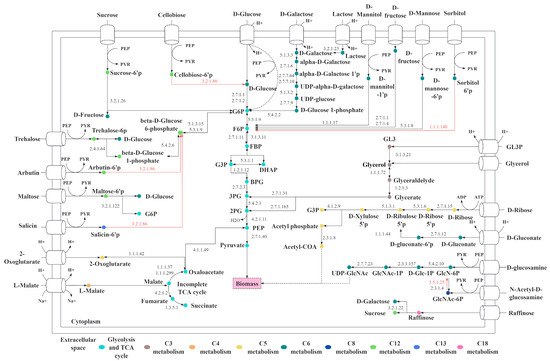
Figure 4.
The proposed core metabolic functions illustrate diverse carbon utilization through various sugar transporters in L. fermentum KUB-D18. The red line indicates the missing gene encoding the enzyme. Abbreviated metabolite names are as follows: G6P, glucose 6-phosphate; F6P, β-fructose 6-phosphate; G3P, glyceraldehyde 3-phosphate; DHAP, dihydroxyacetone phosphate; GL3P, glycerol 3-phosphate; PEP, phosphoenolpyruvate; PYR, pyruvate; 2PG, 2-phospho-glycerate; 3PG, 3-phospho-glycerate.
It is worth noting that lactose and raffinose are key carbon sources for probiotics, offering distinct benefits beyond industrial staples, like glucose, sucrose, or maltose. Lactose utilization helps prevent lactose allergies and supports lactose-intolerant individuals, making L. fermentum KUB-D18 a promising candidate for probiotic products targeting this demographic, with added industrial potential [72]. Raffinose promotes beneficial gut bacteria, improves gut health, and it is low-calorie and non-digestible by human, enhancing the health benefits and market value of probiotic products [73]. Understanding these pathways highlights L. fermentum KUB-D18′s genetic basis for probiotic properties, supporting its optimization for functional foods and health-related applications. Comparing with other strains, L. fermentum CECT5716, for example, was experimentally validated to utilize similar carbon sources, e.g., glucose, maltose, lactose, and raffinose for growth [26]. In addition to specific carbon sources used, a presence of PTS system identified in genome could guide the ability to uptake carbon substrates.
Interestingly, 11 out of 57 genes were also related to the PTS system (Figure 4), revealing its critical role in carbon metabolism. Many carbon substrates, such as sucrose, D-mannose, and maltose, enter the cell via the PTS pathway. This system is specialized with phosphorylate substrates during transport, enabling their direct entry into subsequent metabolic pathways. This process enhances the efficiency of carbon substrate utilization [74]. Furthermore, the PTS system regulates substrate priority, allowing cells to preferentially metabolize glucose while inhibiting the utilization of other carbon sources. This mechanism optimizes metabolic efficiency under resource-limited conditions, maximizing microbial growth rates [75]. Carbon utilization analysis in L. fermentum KUB-D18 revealed potential transport pathways for glycerol, D-mannitol, and trehalose (Figure 4), while the strain was unable to metabolize arbutin, salicin, cellobiose, sorbitol, or glucosamine, as confirmed by API 50 CH tests (listed in Supplementary Table S10). Most of the carbon sources that cannot be utilized may be due to the absence of metabolic genes or the fact that the associated genes have not been linked to the metabolism of those carbon sources. In addition, it might be because of enzyme preference and substrate specificity [76].
It is worth noting that although transporter genes for sorbitol have been identified, an enzyme required to convert sorbitol 6-phosphate into fructose-6-phosphate is missing (Figure 4). Thus, strain KUB-D18 cannot grow using sorbitol as a specific source. These findings highlight its adaptation and growth characteristics. Functional studies of metabolic transporters together with metabolic genes are crucial for engineering L. fermentum KUB-D18 to improve interactions with gut microbiota, including nutrient acquisition (e.g., sugars) and metabolism, adhesion to gut surfaces, as well as key in maintaining gut homeostasis and preventing dysbiosis [77,78].
4. Conclusions
A complete genome of L. fermentum KUB-D18 was successfully assembled using integrative next-generation and third-generation sequencing. Key genes linked to probiotic properties, for instances acid resistance, bile tolerance, antioxidant functions, and anti-inflammatory properties, were identified. This supports the annotation and analysis of a prior result from L. fermentum KUB-D18 [2]. The sugar transportome analysis revealed sugar transporter genes, indicating diverse carbon utilization and PTS systems, consistent with API 50 CHL test results. This study provides valuable insight into the genotype–phenotype relationship of L. fermentum KUB-D18, supporting its potential for industrial and engineered probiotic applications. Future research should focus on comprehensive in vitro and in vivo investigations to validate the probiotic potential of L. fermentum KUB-D18, particularly its metabolic function, activity and impact on gut health, e.g., L-ascorbic acid metabolism through physiological studies, transcriptomics, proteomics or metabolomics. Additionally, engineering L. fermentum KUB-D18 to enhance the production of beneficial metabolites could improve its potential for treating inflammatory bowel diseases and balancing gut microbiota. Finally, exploring L. fermentum KUB-D18′s application in functional foods and probiotics provides valuable insights into optimizing gut health and antioxidant capacity. Ultimately, this research contributes to the development of next-generation probiotics with enhanced therapeutic properties, paving the way for novel dietary interventions that promote long-term gut health and overall well-being.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/genes16030348/s1, Table S1: The COG category classification of L. fermentum KUB-D18. Table S2: The KEGG annotation of L. fermentum KUB-D18. Table S3: The Pfam annotation of L. fermentum KUB-D18. Table S4: The eggNOG annotation of L. fermentum KUB-D18. Table S5: The gene list for the primary subcategories within the main functional categories of L. fermentum KUB-D18. Table S6: List of core and strain-specific genes related to probiotic functions of L. fermentum KUB-D18 in comparative analysis with other strains. Table S7: List of core and strain-specific genes-related to ula gene cluster of L. fermentum KUB-D18 in comparative analysis with other strains. Table S8: The genes related to transmembrane protein of L. fermentum KUB-D18. Table S9: The sugar transport related genes of L. fermentum KUB-D18. Table S10: The API 50 CHL carbohydrate metabolism test result of L. fermentum KUB-D18.
Author Contributions
Y.H. analyzed the data, prepared Figures and Tables, and wrote the manuscript; K.M. assisted in experiments, analyzed the data, and performed writing—review and editing of the manuscript; P.C. assisted in analyzed the data, and performed writing—review and editing of the manuscript; M.N. conceived and designed the experiments, supervised throughout the study and performed writing—review and editing of the manuscript; and W.V. conceived and designed all experiments, interpreted all results, supervised throughout the study and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Kasetsart University Research and Development Institute (KURDI) (FF(KU)29.67), International SciKU Branding (ISB), Department of Zoology, Interdisciplinary Graduate Program in Bioscience, Faculty of Science, Kasetsart University, and Kasetsart University through the Graduate School Fellowship Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the sequences have been deposited in the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra, accessed on 19 November 2024) under the accession no. PRJNA1187331 (SRA number: SRR31373359).
Acknowledgments
The authors would like to thank the Department of Biotechnology, Faculty of Agro-Industry, Kasetsart University, for laboratory facilities and resources. Y.H. would like to thank the Interdisciplinary Graduate Program in Bioscience, Faculty of Science, Kasetsart University and Kasetsart University through the Graduate School Fellowship Program. W.V. would like to thank the Department of Zoology, SciKU Biodata Server, and International SciKU Branding (ISB) and Kasetsart University Research and Development Institute (KURDI) (FF(KU)29.67) for their supports.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siezen, R.J.; Kok, J.; Abee, T.; Schaafsma, G. Lactic Acid Bacteria: Genetics, Metabolism and Applications; Springer Science & Business Media: Dordrecht, The Netherlands, 2002; Volume 82. [Google Scholar]
- Phujumpa, P.; Muangham, S.; Jatuponwiphat, T.; Koffas, M.; Nakphaichit, M.; Vongsangnak, W. Comparative genomics-based probiotic relevance of Limosilactobacillus fermentum KUB-D18. Gene 2022, 840, 146747. [Google Scholar] [CrossRef]
- Kim, B.; Meng, Z.; Xu, X.; Baek, S.; Pathiraja, D.; Choi, I.-G.; Oh, S. Complete genome sequence of Limosilactobacillus fermentum JNU532 as a probiotic candidate for the functional food and feed supplements. J. Anim. Sci. Technol. 2023, 65, 271. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-B.; Lew, L.-C.; Yeo, S.-K.; Nair Parvathy, S.; Liong, M.-T. Probiotics and the BSH-related cholesterol lowering mechanism: A Jekyll and Hyde scenario. Crit. Rev. Biotechnol. 2015, 35, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef]
- Saier, M.H., Jr.; Paulsen, I.T. Phylogeny of multidrug transporters. Semin. Cell Dev. Biol. 2001, 12, 205–213. [Google Scholar] [CrossRef]
- Kang, X.; Xu, J.; Luo, X.; Schonhuth, A. Hybrid-hybrid correction of errors in long reads with HERO. Genome Biol. 2023, 24, 275. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Erickson, D.L.; Meng, J. Benchmarking hybrid assembly approaches for genomic analyses of bacterial pathogens using Illumina and Oxford Nanopore sequencing. BMC Genom. 2020, 21, 631. [Google Scholar] [CrossRef]
- Khezri, A.; Avershina, E.; Ahmad, R. Hybrid Assembly Provides Improved Resolution of Plasmids, Antimicrobial Resistance Genes, and Virulence Factors in Escherichia coli and Klebsiella pneumoniae Clinical Isolates. Microorganisms 2021, 9, 2560. [Google Scholar] [CrossRef]
- Baptista, R.P.; Li, Y.; Sateriale, A.; Sanders, M.J.; Brooks, K.L.; Tracey, A.; Ansell, B.R.; Jex, A.R.; Cooper, G.W.; Smith, E.D. Long-read assembly and comparative evidence-based reanalysis of Cryptosporidium genome sequences reveal expanded transporter repertoire and duplication of entire chromosome ends including subtelomeric regions. Genome Res. 2022, 32, 203–213. [Google Scholar] [CrossRef]
- Pozdnyakov, I.R.; Potapenko, E.V.; Nassonova, E.S.; Babenko, V.V.; Boldyreva, D.I.; Tcvetkova, V.S.; Karpov, S.A. To the Origin of Fungi: Analysis of MFS Transporters of First Assembled Aphelidium Genome Highlights Dissimilarity of Osmotrophic Abilities between Aphelida and Fungi. J. Fungi 2023, 9, 1021. [Google Scholar] [CrossRef]
- Wongrattanapipat, S.; Chiracharoenchitta, A.; Choowongwitthaya, B.; Komsathorn, P.; La-Ongkham, O.; Nitisinprasert, S.; Tunsagool, P.; Nakphaichit, M. Selection of potential probiotics with cholesterol-lowering properties for probiotic yoghurt production. Food Sci. Technol. Int. 2022, 28, 353–365. [Google Scholar] [CrossRef] [PubMed]
- De Coster, W.; D’Hert, S.; Schultz, D.T.; Cruts, M.; Van Broeckhoven, C. NanoPack: Visualizing and processing long-read sequencing data. Bioinformatics 2018, 34, 2666–2669. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Robertson, J.; Nash, J.H.E. MOB-suite: Software tools for clustering, reconstruction and typing of plasmids from draft assemblies. Microb. Genom. 2018, 4, e000206. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353–D361. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Heller, D.; Hernandez-Plaza, A.; Forslund, S.K.; Cook, H.; Mende, D.R.; Letunic, I.; Rattei, T.; Jensen, L.J.; et al. eggNOG 5.0: A hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019, 47, D309–D314. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, W.; Bilige, M.; Zhang, H. Complete genome sequence of the probiotic Lactobacillus fermentum F-6 isolated from raw milk. J. Biotechnol. 2015, 194, 110–111. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Piloquet, H.; Schaubeck, M. Limosilactobacillus fermentum CECT5716: Clinical potential of a probiotic strain isolated from human Milk. Nutrients 2023, 15, 2207. [Google Scholar] [CrossRef]
- Abramov, V.M.; Kosarev, I.V.; Machulin, A.V.; Priputnevich, T.V.; Chikileva, I.O.; Deryusheva, E.I.; Abashina, T.N.; Donetskova, A.D.; Panin, A.N.; Melnikov, V.G.; et al. Limosilactobacillus fermentum Strain 3872: Antibacterial and Immunoregulatory Properties and Synergy with Prebiotics against Socially Significant Antibiotic-Resistant Infections of Animals and Humans. Antibiotics 2022, 11, 1437. [Google Scholar] [CrossRef]
- Cárdenas, N.; Laiño, J.E.; Delgado, S.; Jiménez, E.; Juárez del Valle, M.; Savoy de Giori, G.; Sesma, F.; Mayo, B.; Fernández, L.; LeBlanc, J.G. Relationships between the genome and some phenotypical properties of Lactobacillus fermentum CECT 5716, a probiotic strain isolated from human milk. Appl. Microbiol. Biotechnol. 2015, 99, 4343–4353. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H., Jr.; Reddy, V.S.; Moreno-Hagelsieb, G.; Hendargo, K.J.; Zhang, Y.; Iddamsetty, V.; Lam, K.J.K.; Tian, N.; Russum, S.; Wang, J. The transporter classification database (TCDB): 2021 update. Nucleic Acids Res. 2021, 49, D461–D467. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.; Apweiler, R.; Attwood, T.K.; Bairoch, A.; Bateman, A.; Binns, D.; Bork, P.; Das, U.; Daugherty, L.; Duquenne, L.; et al. InterPro: The integrative protein signature database. Nucleic Acids Res. 2009, 37, D211–D215. [Google Scholar] [CrossRef]
- Zaunmüller, T.; Unden, G. Transport of sugars and sugar alcohols by lactic acid bacteria. In Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 149–163. [Google Scholar]
- Katoh, K.; Standley, D.M. A simple method to control over-alignment in the MAFFT multiple sequence alignment program. Bioinformatics 2016, 32, 1933–1942. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Moretti, S.; Tran, V.D.T.; Mehl, F.; Ibberson, M.; Pagni, M. MetaNetX/MNXref: Unified namespace for metabolites and biochemical reactions in the context of metabolic models. Nucleic Acids Res. 2021, 49, D570–D574. [Google Scholar] [CrossRef] [PubMed]
- Maresca, D.; Zotta, T.; Mauriello, G. Adaptation to aerobic environment of Lactobacillus johnsonii/gasseri strains. Front. Microbiol. 2018, 9, 157. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, G.; Noh, M.-G.; Park, J.-H.; Jang, M.; Fang, S.; Park, H. Lactobacillus fermentum promotes adipose tissue oxidative phosphorylation to protect against diet-induced obesity. Exp. Mol. Med. 2020, 52, 1574–1586. [Google Scholar] [CrossRef]
- Sun, Y.; Fukamachi, T.; Saito, H.; Kobayashi, H. ATP requirement for acidic resistance in Escherichia coli. J. Bacteriol. 2011, 193, 3072–3077. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Y.; Qu, X. Mechanisms and improvement of acid resistance in lactic acid bacteria. Arch. Microbiol. 2018, 200, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Kusada, H.; Morinaga, K.; Tamaki, H. Identification of Bile Salt Hydrolase and Bile Salt Resistance in a Probiotic Bacterium Lactobacillus gasseri JCM1131(T). Microorganisms 2021, 9, 1011. [Google Scholar] [CrossRef]
- Bustos, A.Y.; de Valdez, G.F.; Fadda, S.; Taranto, M.P. New insights into bacterial bile resistance mechanisms: The role of bile salt hydrolase and its impact on human health. Food Res. Int. 2018, 112, 250–262. [Google Scholar] [CrossRef]
- Linares, D.; Michaud, P.; Delort, A.-M.; Traikia, M.; Warrand, J. Catabolism of L-ascorbate by Lactobacillus rhamnosus GG. J. Agric. Food Chem. 2011, 59, 4140–4147. [Google Scholar] [CrossRef]
- Bailey, L.B. Folate in Health and Disease; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Rezk, B.M.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. Tetrahydrofolate and 5-methyltetrahydrofolate are folates with high antioxidant activity. Identification of the antioxidant pharmacophore. FEBS Lett. 2003, 555, 601–605. [Google Scholar] [CrossRef]
- Kong, M.; Wang, F.; Tian, L.; Tang, H.; Zhang, L. Functional identification of glutamate cysteine ligase and glutathione synthetase in the marine yeast Rhodosporidium diobovatum. Sci. Nat. 2018, 105, 4. [Google Scholar] [CrossRef]
- Sannasimuthu, A.; Sharma, D.; Paray, B.A.; Al-Sadoon, M.K.; Arockiaraj, J. Intracellular oxidative damage due to antibiotics on gut bacteria reduced by glutathione oxidoreductase-derived antioxidant molecule GM15. Arch. Microbiol. 2020, 202, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- La Carbona, S.; Sauvageot, N.; Giard, J.C.; Benachour, A.; Posteraro, B.; Auffray, Y.; Sanguinetti, M.; Hartke, A. Comparative study of the physiological roles of three peroxidases (NADH peroxidase, Alkyl hydroperoxide reductase and Thiol peroxidase) in oxidative stress response, survival inside macrophages and virulence of Enterococcus faecalis. Mol. Microbiol. 2007, 66, 1148–1163. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Zeller, T.; Klug, G. Thioredoxins in bacteria: Functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften 2006, 93, 259–266. [Google Scholar] [CrossRef]
- Ryssel, H.; Kloeters, O.; Germann, G.; Schafer, T.; Wiedemann, G.; Oehlbauer, M. The antimicrobial effect of acetic acid—An alternative to common local antiseptics? Burns 2009, 35, 695–700. [Google Scholar] [CrossRef]
- Moynihan, P.J.; Clarke, A.J. Assay for peptidoglycan O-acetyltransferase: A potential new antibacterial target. Anal. Biochem. 2013, 439, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Mahdhi, A.; Leban, N.; Chakroun, I.; Chaouch, M.A.; Hafsa, J.; Fdhila, K.; Mahdouani, K.; Majdoub, H. Extracellular polysaccharide derived from potential probiotic strain with antioxidant and antibacterial activities as a prebiotic agent to control pathogenic bacterial biofilm formation. Microb. Pathog. 2017, 109, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Major, A.; Rendon, D.; Lugo, M.; Jackson, V.; Shi, Z.; Mori-Akiyama, Y.; Versalovic, J. Histamine H2 Receptor-Mediated Suppression of Intestinal Inflammation by Probiotic Lactobacillus reuteri. mBio 2015, 6, e01358-15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Lu, X.; Yang, S.; Zou, Y.; Zeng, F.; Xiong, S.; Cao, Y.; Zhou, W. The anti-inflammatory activity of GABA-enriched Moringa oleifera leaves produced by fermentation with Lactobacillus plantarum LK-1. Front. Nutr. 2023, 10, 1093036. [Google Scholar] [CrossRef] [PubMed]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. γ-aminobutyric acid as a potential postbiotic mediator in the gut–brain axis. NPJ Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef]
- Leng, Y.; Jiang, C.; Xing, X.; Tsai, M.-S.; Snyder, M.; Zhai, A.; Yao, G. Prevention of severe intestinal barrier dysfunction through a single-species probiotics is associated with the activation of microbiome-mediated glutamate–glutamine biosynthesis. Shock 2021, 55, 128–137. [Google Scholar] [CrossRef]
- Peetermans, A.; Foulquié-Moreno, M.R.; Thevelein, J.M. Mechanisms underlying lactic acid tolerance and its influence on lactic acid production in Saccharomyces cerevisiae. Microb. Cell 2021, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Wongrattanapipat, S.; Nakphaichit, M. Selection and Potential Evaluation of Probiotic for Cholesterol Lowering Effect in Intestinal Cell Line. Ph.D. Thesis, Kasetsart University, Bangkok, Thailand, 2021. [Google Scholar]
- Kaye, A.D.; Jeha, G.M.; Pham, A.D.; Fuller, M.C.; Lerner, Z.I.; Sibley, G.T.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kevil, C.G. Folic Acid Supplementation in Patients with Elevated Homocysteine Levels. Adv. Ther. 2020, 37, 4149–4164. [Google Scholar] [CrossRef]
- Averill-Bates, D.A. The antioxidant glutathione. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2023; Volume 121, pp. 109–141. [Google Scholar]
- Deng, Z.; Luo, X.M.; Liu, J.; Wang, H. Quorum Sensing, Biofilm, and Intestinal Mucosal Barrier: Involvement the Role of Probiotic. Front. Cell Infect Microbiol. 2020, 10, 538077. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Bai, Y.; Zha, L.; Ullah, N.; Ullah, H.; Shah, S.R.H.; Sun, H.; Zhang, C. Mechanism of the gut microbiota colonization resistance and enteric pathogen infection. Front. Cell. Infect. Microbiol. 2021, 11, 716299. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.; Yoshikawa, F.S.Y.; Pietrobon, A.J.; Sato, M.N. Role of Histamine in Modulating the Immune Response and Inflammation. Mediat. Inflamm. 2018, 2018, 9524075. [Google Scholar] [CrossRef]
- Holeček, M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- Mazzoli, R.; Pessione, E. The Neuro-endocrinological Role of Microbial Glutamate and GABA Signaling. Front. Microbiol. 2016, 7, 1934. [Google Scholar] [CrossRef]
- Yogeswara, I.B.A.; Maneerat, S.; Haltrich, D. Glutamate Decarboxylase from Lactic Acid Bacteria-A Key Enzyme in GABA Synthesis. Microorganisms 2020, 8, 1923. [Google Scholar] [CrossRef]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Pusuntisumpun, N.; Tunsagool, P.; Nitisinprasert, S.; Nakphaichit, M. Impacts of combining Limosilactobacillus reuteri KUB-AC5 and Limosilactobacillus fermentum KUB-D18 on overweight gut microbiota using a simulated human colon model. Int. J. Food Sci. Tech. 2024, 59, 1898–1910. [Google Scholar] [CrossRef]
- Poolman, B. Energy transduction in lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 125–147. [Google Scholar] [CrossRef] [PubMed]
- Pao, S.S.; Paulsen, I.T.; Saier, M.H., Jr. Major facilitator superfamily. Microbiol. Mol. Biol. Rev. 1998, 62, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.W.; Lengeler, J.W.; Jacobson, G.R. Phosphoenolpyruvate: Carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 1993, 57, 543–594. [Google Scholar] [CrossRef]
- Higgins, C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992, 8, 67–113. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Oak, S.J.; Jha, R. The effects of probiotics in lactose intolerance: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1675–1683. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Frias, J.; Vidal-Valverde, C. α-galactosides: Antinutritional factors or functional ingredients? Crit. Rev. Food Sci. Nutr. 2008, 48, 301–316. [Google Scholar] [CrossRef]
- Deutscher, J.; Francke, C.; Postma, P.W. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 2006, 70, 939–1031. [Google Scholar] [CrossRef]
- Görke, B.; Stülke, J. Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat. Rev. Microbiol. 2008, 6, 613–624. [Google Scholar] [CrossRef]
- Ramsey, M.; Hartke, A.; Huycke, M. The physiology and metabolism of enterococci. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection [Internet]; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Judkins, T.C.; Archer, D.L.; Kramer, D.C.; Solch, R.J. Probiotics, Nutrition, and the Small Intestine. Curr. Gastroenterol. Rep. 2020, 22, 2. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Comparative Genomics of the Transport Proteins of Ten Lactobacillus Strains. Genes 2020, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).