An Exploratory Genomic and Transcriptomic Analysis Between Choloepus didactylus and Homo sapiens
Abstract
1. Introduction
2. Materials and Methods
2.1. Two-Toed Sloth and Human Volunteer Profiles
2.2. Collection and Processing of Blood Samples for Genetic Sequencing
2.3. RNA Extraction, Library Setup, and Sequencing
2.4. Preprocessing of RNA-Seq Data
2.5. Determination of Differentially Expressed Genes (DEGs)
2.6. Two-Toed Sloth Genome Assembly
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, S.; Jones, J.K., Jr. (Eds.) Orders and Families of Recent Mammals of the World; Wiley: Hoboken, NJ, USA, 1984. [Google Scholar]
- Kriegs, J.O.; Churakov, G.; Kiefmann, M.; Jordan, U.; Brosius, J.; Schmitz, J. Retroposed Elements as Archives for the Evolutionary History of Placental Mammals. PLoS Biol. 2006, 4, e91. [Google Scholar] [CrossRef] [PubMed]
- Elgar, M.A.; Harvey, P.H. Basal metabolic rates in mammals: Allometry, phylogeny and ecology. Funct. Ecol. 1987, 1, 25–36. [Google Scholar] [CrossRef]
- Presslee, S.; Slater, G.J.; Pujos, F.; Forasiepi, A.M.; Fischer, R.; Molloy, K.; Mackie, M.; Olsen, J.V.; Kramarz, A.; Taglioretti, M.; et al. Palaeoproteomics resolves sloth relationships. Nat. Ecol. Evol. 2019, 3, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Mayr, E. Animal Species and Evolution; Harvard University Press: Cambridge, MA, USA, 1963. [Google Scholar]
- San Diego Zoo Wildlife Alliance. Two-Toed Sloths (Choloepus spp.) Fact Sheet. Available online: https://ielc.libguides.com/sdzg/factsheets/twotoedsloths/ (accessed on 3 September 2024).
- Gross, M. How sloths got their sloth. Curr. Biol. 2019, 29, R603–R606. [Google Scholar] [CrossRef]
- Cliffe, R.N.; Haupt, R.J.; Kennedy, S.; Felton, C.; Williams, H.J.; Avey-Arroyo, J.; Wilson, R. The behaviour and activity budgets of two sympatric sloths; Bradypus variegatus and Choloepus hoffmanni. PeerJ 2023, 11, e15430. [Google Scholar] [CrossRef]
- Japanese Ministry of the Environment. Doubutsu No Aigo Oyobi Kanri Ni Kansuru Houritsu [Act on Welfare and Management of Animals]. Available online: https://www.env.go.jp/nature/dobutsu/aigo/1_law/files/aigo_kanri_1973_105_en.pdf (accessed on 17 February 2025).
- Britten, R.J. Divergence between samples of chimpanzee and human DNA sequences is 5%, counting indels. Proc. Natl. Acad. Sci. USA 2002, 99, 13633–13635. [Google Scholar] [CrossRef]
- Locke, D.P.; Hillier, L.D.W.; Warren, W.C.; Worley, K.C.; Nazareth, L.V.; Muzny, D.M.; Yang, S.-P.; Wang, Z.; Chinwalla, A.T.; Minx, P.; et al. Comparative and demographic analysis of orang-utan genomes. Nature 2011, 469, 529–533. [Google Scholar] [CrossRef]
- Pontius, J.U.; Mullikin, J.C.; Smith, D.R.; Agencourt Sequencing Team; Lindblad-Toh, K.; Gnerre, S.; Clamp, M.; Chang, J.; Stephens, R.; Neelam, B.; et al. Initial sequence and comparative analysis of the cat genome. Genome Res. 2007, 17, 1675–1689. [Google Scholar] [CrossRef]
- Mouse Genome Sequencing Consortium. Initial sequencing and comparative analysis of the mouse genome. Nature 2002, 420, 520–562. [Google Scholar] [CrossRef]
- Bovine Genome Sequencing and Analysis Consortium; Elsik, C.G.; Tellam, R.L.; Worley, K.C. The Genome Sequence of Taurine Cattle: A Window to Ruminant Biology and Evolution. Science 2009, 324, 522–528. [Google Scholar]
- International Chicken Genome Sequencing Consortium. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 2004, 432, 695–716. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Chung, R.; Vazquez, J.M.; Sheng, H.; Steinberg, P.H.; Ioannidis, N.M.; Sudmant, P.H. Tissue-specific impacts of aging and genetics on gene expression patterns in humans. Nat. Commun. 2022, 13, 5803. [Google Scholar] [CrossRef] [PubMed]
- Japanese Ministry of Agriculture, Forestry, and Fisheries. Shokuji Baransu Gaido Hayawakari [Novice’s Guide to a Balanced Diet]. Available online: https://www.maff.go.jp/j/syokuiku/zissen_navi/balance/required.html (accessed on 4 September 2024).
- Rouillard, A.D.; Gundersen, G.W.; Fernandez, N.F.; Wang, Z.; Monteiro, C.D.; McDermott, M.G.; Ma’ayan, A. The harmonizome: A collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, 2016, baw100. [Google Scholar] [CrossRef] [PubMed]
- Szewczuk, M.; Boguszewska, K.; Kaźmierczak-Barańska, J.; Karwowski, B.T. The role of AMPK in metabolism and its influence on DNA damage repair. Mol. Biol. Rep. 2020, 47, 9075–9086. [Google Scholar] [CrossRef]
- Muntean, C.; Sasaran, M.O.; Crisan, A.; Banescu, C. Effects of PPARG and PPARGC1A gene polymorphisms on obesity markers. Front. Public Health 2022, 10, 962852. [Google Scholar] [CrossRef]
- Vendl, C.; Frei, S.; Dittmann, M.T.; Furrer, S.; Osmann, C.; Ortmann, S.; Munn, A.; Kreuzer, M.; Clauss, M. Digestive physiology, metabolism and methane production of captive Linné’s two-toed sloths (Choloepus didactylus). J. Anim. Physiol. Anim. Nutr. 2016, 100, 552–564. [Google Scholar] [CrossRef]
- Milton, A.S. Chapter 8: Prostaglandins and fever. In Progress in Brain Research; Sharma, H.S., Westman, J., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 129–139. [Google Scholar]
- Ha, C.-E.; Bhagavan, N.V. Chapter 29—Endocrine metabolism IV: Thyroid gland. In Essentials of Medical Biochemistry, 3rd ed.; Academic Press: Cambridge, MA, USA, 2023; pp. 661–673. [Google Scholar]
- Montgomery, G.G.; Sunquist, M.E. Habitat selection and use by two-toed and three-toed sloths. In The Ecology of Arboreal Folivores; Smithsonian University Press: Washington, DC, USA, 1978; pp. 329–359. [Google Scholar]
- Britton, S.W.; Atkinson, W.E. Poikilothermism in the Sloth. J. Mammal. 1938, 19, 94–99. [Google Scholar] [CrossRef]
- Muramatsu, D.; Vidal, L.V.; Costa, E.R.; Toda, K.; Yabe, T.; Gordo, M. Low-cost thermoregulation of wild sloths revealed by heart rate and temperature loggers. J. Therm. Biol. 2022, 110, 103387. [Google Scholar] [CrossRef]
- Tchakarska, G.; Sola, B. The double dealing of cyclin D1. Cell Cycle 2020, 19, 163–178. [Google Scholar] [CrossRef]
- Zalmas, L.-P.; Coutts, A.S.; Helleday, T.; La Thangue, N.B. E2F-7 couples DNA damage-dependent transcription with the DNA repair process. Cell Cycle 2013, 12, 3037–3051. [Google Scholar] [CrossRef]
- Smith, M.J.; Urquhart, J.E.; Harkness, E.F.; Miles, E.K.; Bowers, N.L.; Byers, H.J.; Bulman, M.; Gokhale, C.; Wallace, A.J.; Newman, W.G.; et al. The Contribution of Whole Gene Deletions and Large Rearrangements to the Mutation Spectrum in Inherited Tumor Predisposing Syndromes. Hum. Mutat. 2016, 37, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.M.; Pena, M.T.; Muhammad, B.; Kraft, M.; Adams, L.B.; Lynch, V.J. Parallel evolution of reduced cancer risk and tumor suppressor duplications in Xenarthra. eLife 2022, 11, e82558. [Google Scholar] [CrossRef] [PubMed]
- Caulin, A.F.; Maley, C.C. Peto’s Paradox: Evolution’s prescription for cancer prevention. Trends Ecol. Evol. 2011, 26, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Costello, H.M.; Gumz, M.L. Circadian Rhythm, Clock Genes and Hypertension: Recent Advances in Hypertension. Hypertension 2021, 78, 1185–1196. [Google Scholar] [CrossRef]
- Ono, D.; Honma, K.; Yanagawa, Y.; Yamanaka, A.; Honma, S. Role of GABA in the regulation of the central circadian clock of the suprachiasmatic nucleus. J. Physiol. Sci. 2018, 68, 333–343. [Google Scholar] [CrossRef]
- Patton, A.P.; Morris, E.L.; McManus, D.; Wang, H.; Li, Y.; Chin, J.W.; Hastings, M.H. Astrocytic control of extracellular GABA drives circadian timekeeping in the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA 2023, 120, e2301330120. [Google Scholar] [CrossRef]
- Kucher, A.N.; Cherevko, N.A. Genes of the Histamine Pathway and Common Diseases. Russ. J. Genet. 2018, 54, 12–26. [Google Scholar] [CrossRef]
- Siegel, J.M. The neurotransmitters of sleep. J. Clin. Psychiatry 2004, 65 (Suppl. S16), 4–7. [Google Scholar]
- Voirin, B.; Scriba, M.F.; Martinez-Gonzalez, D.; Vyssotski, A.L.; Wikelski, M.; Rattenborg, N.C. Ecology and Neurophysiology of Sleep in Two Wild Sloth Species. Sleep 2014, 37, 753–761. [Google Scholar] [CrossRef]
- Savage, S.A.; Niewisch, M.R. Dyskeratosis Congenita and Related Telomere Biology Disorders. In GeneReviews [Internet]; Adam, M.P., Feldman, J.F., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2009; pp. 1–34. [Google Scholar]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Hayflick, L. The Cell Biology of Aging. J. Investig. Dermatol. 1979, 73, 8–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Counter, C.M. The roles of telomeres and telomerase in cell life span. Mutat. Res. 1996, 366, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Andrews, Z.B. Uncoupling Protein-2 and the Potential Link Between Metabolism and Longevity. Curr. Aging Sci. 2010, 3, 102–112. [Google Scholar] [CrossRef]
- Mannick, J.B.; Lamming, D.W. Targeting the biology of aging with mTOR inhibitors. Nat. Aging 2023, 3, 642–660. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Lin, M.; Levine, A.J. The Regulation of Aging and Longevity. Genes Cancer 2011, 2, 443–452. [Google Scholar] [CrossRef]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. ROLE of IGF-1 System in the Modulation of Longevity: Controversies and New Insights from a Centenarians’ Perspective. Front. Endocrinol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Sebastiani, P.; Gurinovich, A.; Nygaard, M.; Sasaki, T.; Sweigart, B.; Bae, H.; Andersen, S.L.; Villa, F.; Atzmon, G.; Christensen, K.; et al. APOE Alleles and Extreme Human Longevity. J. Gerontol. Ser. A 2018, 74, 44–51. [Google Scholar] [CrossRef]
- Espuch-Oliver, A.; Vázquez-Lorente, H.; Jurado-Fasoli, L.; de Haro-Muñoz, T.; Díaz-Alberola, I.; López-Velez, M.D.S.; de Haro-Romero, T.; Castillo, M.J.; Amaro-Gahete, F.J. Reference Values of Soluble α-Klotho Serum Levels Using an Enzyme-Linked Immunosorbent Assay in Healthy Adults Aged 18–85 Years. J. Clin. Med. 2022, 11, 2415. [Google Scholar] [CrossRef]
- Viñuela, A.; Brown, A.A.; Buil, A.; Tsai, P.-C.; Davies, M.N.; Bell, J.T.; Dermitzakis, E.T.; Spector, T.D.; Small, K.S. Age-dependent changes in mean and variance of gene expression across tissues in a twin cohort. Hum. Mol. Genet. 2017, 27, 732–741. [Google Scholar] [CrossRef]
- National Institutes of Health (US). Understanding Human Genetic Variation. NIH Curriculum Supplement Series [Internet], Biological Sciences Curriculum Study. 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK20363/ (accessed on 17 February 2025).
- Saleh, M.C.; Wheeler, M.B.; Chan, C.B. Endogenous islet uncoupling protein-2 expression and loss of glucose homeostasis in ob/ob mice. J. Endocrinol. 2006, 190, 659–667. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, W.; Teresky, A.K.; Hernando, E.; Cordon-Cardo, C.; Levine, A.J. Declining p53 function in the aging process: A possible mechanism for the increased tumor incidence in older populations. Proc. Natl. Acad. Sci. USA 2007, 104, 16633–16638. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.W.; Fellows, A.D.; Pilling, L.C.; Hernandez, D.; Singleton, A.; Bandinelli, S.; Curalnik, J.; Powell, J.; Ferrucci, L.; Melzer, D. Advancing age is associated with gene expression changes resembling mTOR inhibition: Evidence from two human populations. Mech. Ageing Dev. 2012, 133, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Ashpole, N.M.; Sanders, J.E.; Hodges, E.L.; Yan, H.; Sonntag, W.E. Growth hormone, insulin-like growth factor-1 and the aging brain. Exp. Gerontol. 2015, 68, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Devanney, N.A.; Golden, L.R.; Smith, C.T.; Schwartz, J.L.; Walsh, A.E.; Clarke, H.A.; Goulding, D.S.; Allenger, E.J.; Morillo-Segovia, G.; et al. APOE modulates microglial immunometabolism in response to age, amyloid pathology, and inflammatory challenge. Cell Rep. 2023, 42, 112196. [Google Scholar] [CrossRef]
- Speakman, J.R.; Colin, S.; McLaren, J.S.; Jean, H.E. Living Fast, Dying When? The Link between Aging and Energetics. J. Nutr. 2002, 132, 1583S–1597S. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Yu, Z.; Seim, I.; Yin, M.; Tian, R.; Sun, D.; Ren, W.; Yang, G.; Xu, S. Comparative analyses of aging-related genes in long-lived mammals provide insights into natural longevity. Innovation 2021, 2, 100108. [Google Scholar] [CrossRef]
- Cliffe, R.N.; Ewart, H.E.; Scantlebury, D.M.; Kennedy, S.; Avey-Arroyo, J.; Mindich, D.; Wilson, R.P. Sloth metabolism may make survival untenable under climate change scenarios. PeerJ 2024, 12, e18168. [Google Scholar] [CrossRef]
- Cliffe, R.N.; Scantlebury, D.M.; Kennedy, S.J.; Avey-Arroyo, J.; Mindich, D.; Wilson, R.P. The metabolic response of the Bradypus sloth to temperature. PeerJ 2018, 6, e5600. [Google Scholar] [CrossRef]
- Raines, J. Captive health and husbandry of the Bradypodidae. Zoo Biol. 2005, 24, 557–568. [Google Scholar] [CrossRef]
- Higginbotham, S.; Wong, W.R.; Linington, R.G.; Spadafora, C.; Iturrado, L.; Arnold, E.A. Sloth Hair as a Novel Source of Fungi with Potent Anti-Parasitic, Anti-Cancer and Anti-Bacterial Bioactivity. PLoS ONE 2014, 9, e84549. [Google Scholar] [CrossRef]
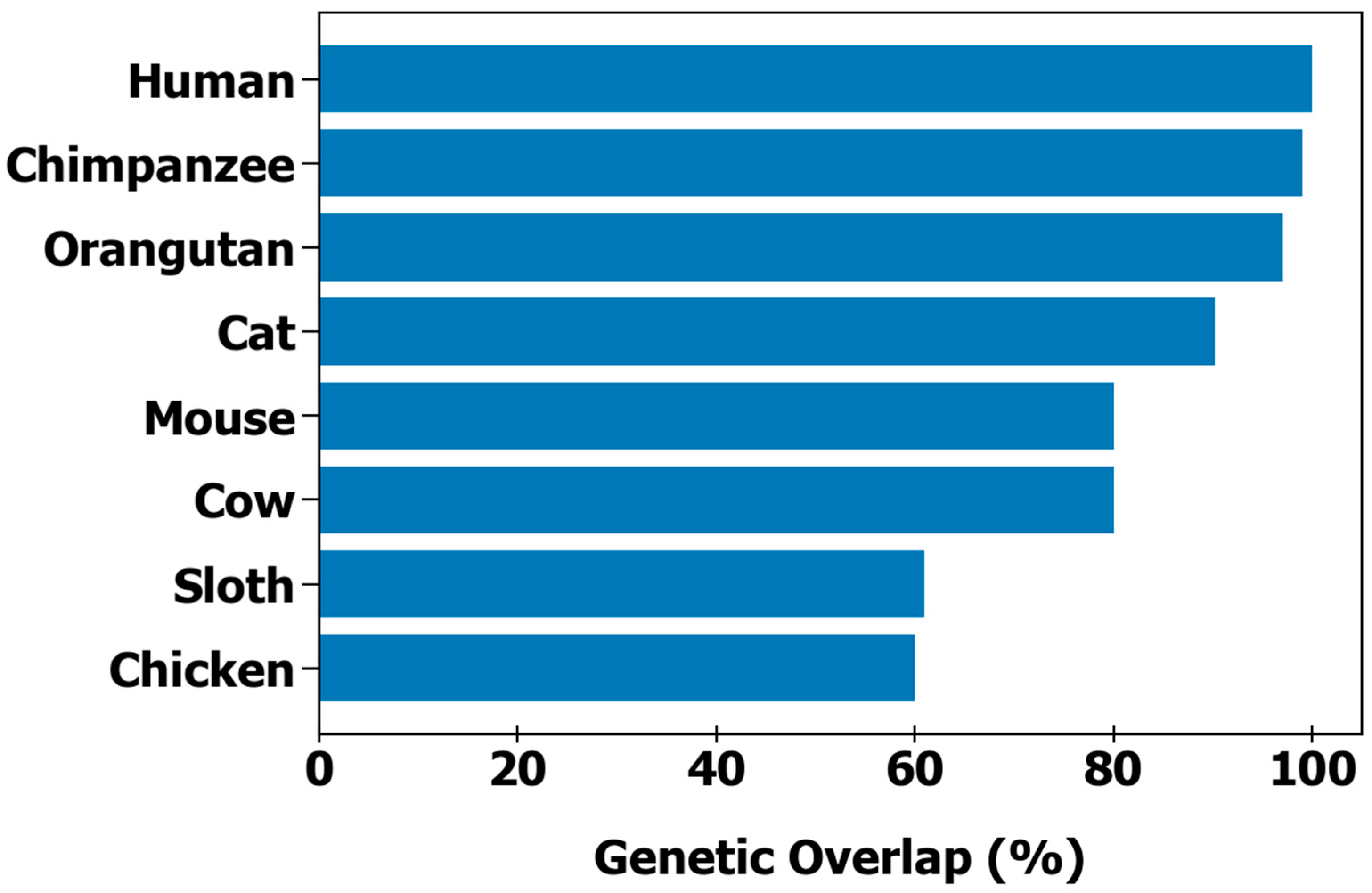
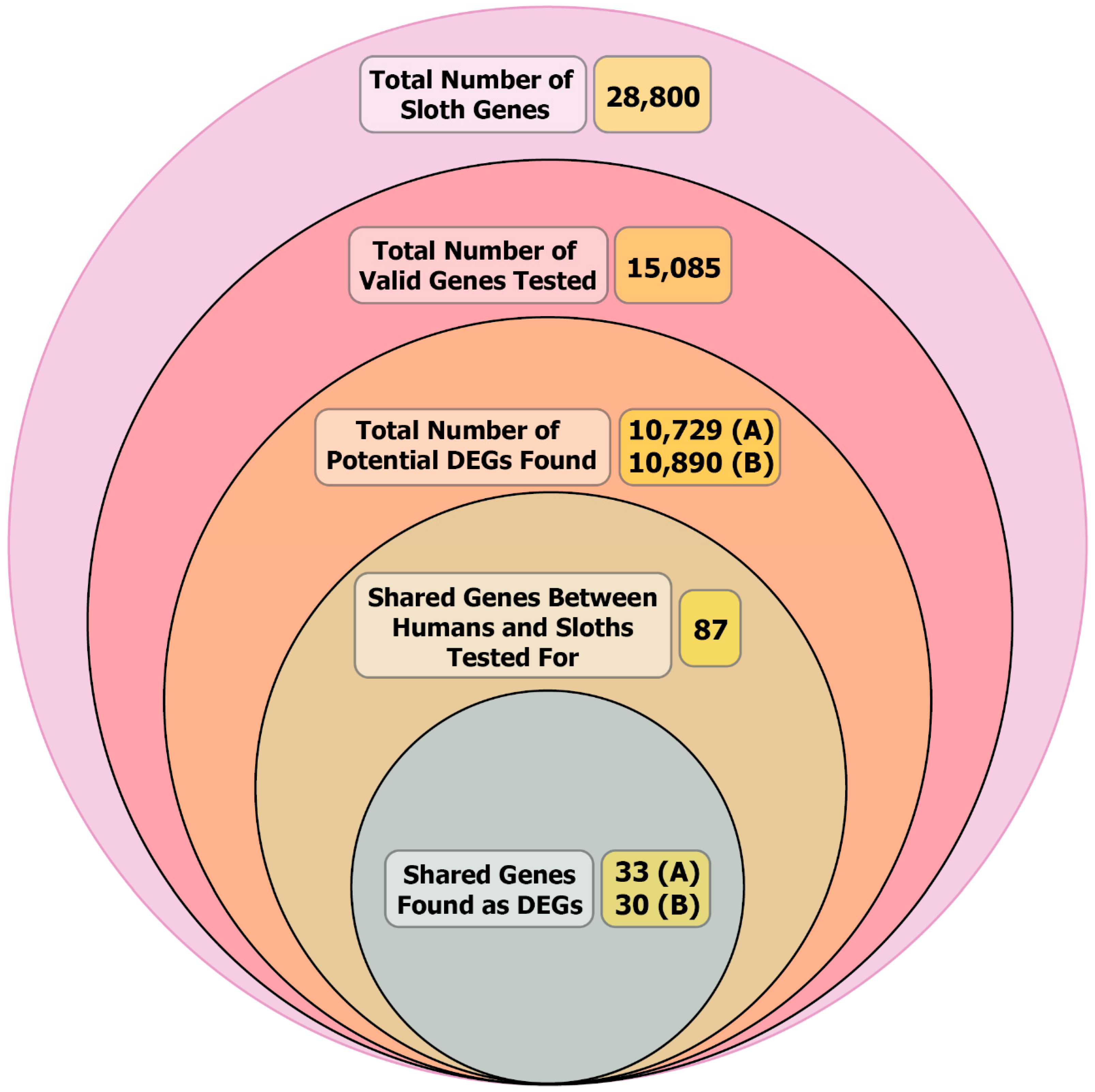
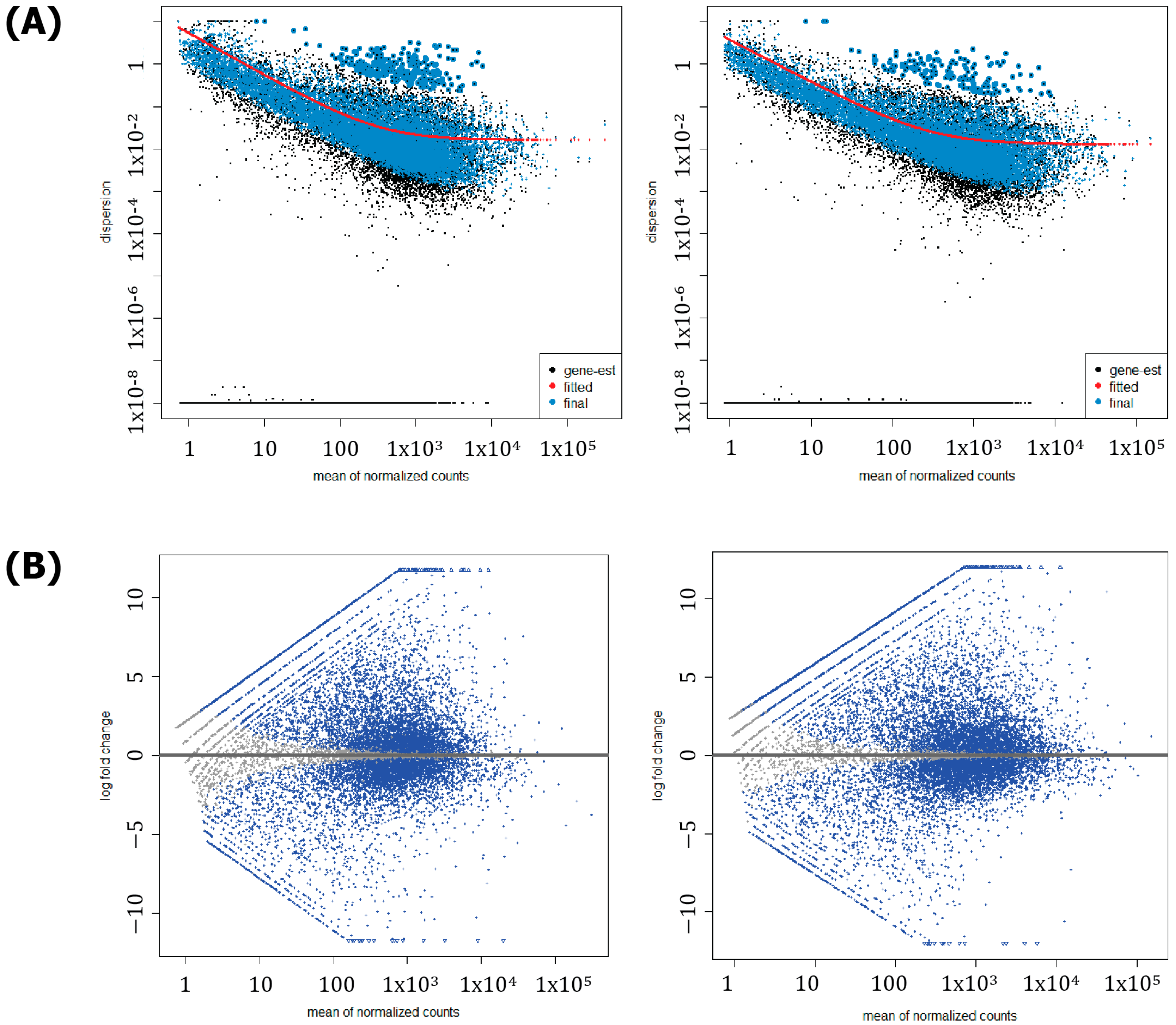


| Species | Number of Chromosomes | Length (bp) | Number of Genes (Protein Coding) | Shared Orthologs |
|---|---|---|---|---|
| Choloepus didactylus | 28 | 3,130,174,040 | 23,626 | 14,320 |
| Homo sapiens | 23 | 3,088,286,401 | 23,306 |
| Category | Two-Toed Sloth | Human (Female) | Difference (%) * |
|---|---|---|---|
| Caloric Intake (kcal/day) [kcal/day/kg] | 228.79 [38.78] | 2000 [37.74] | +3% |
| Feces (g/day) [g/day/kg] | 33.64 [5.7] | 99 [1.9] | +205% |
| Urine (mL/day) [mL/day/kg] | 81.82 [13.87] | 1400 [26.42] | −48% |
| Category | Gene | Log2 Fold Change | Adjusted p-Value | ||
|---|---|---|---|---|---|
| Human A | Human B | Human A | Human B | ||
| Metabolism | PKLR | 8.5 | 5.9 | <0.001 | <0.001 |
| PRKAA1 | 6.0 | 6.0 | <0.001 | <0.001 | |
| PPARGC1A | 5.2 | 6.3 | <0.001 | <0.001 | |
| HK1 | 3.5 | 5.8 | <0.001 | <0.001 | |
| HK2 | 2.2 | 3.6 | <0.001 | <0.001 | |
| PKM | −2.0 | - | <0.001 | - | |
| PPARA | −3.1 | −3.2 | <0.001 | <0.001 | |
| THRB | −7.3 | −6.0 | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, A.; Ibrahim, A.; Nakano, Y.; Aoshima, H.; Ozeki, T.; Sato-Baran, I.; Ordinario, D.D. An Exploratory Genomic and Transcriptomic Analysis Between Choloepus didactylus and Homo sapiens. Genes 2025, 16, 272. https://doi.org/10.3390/genes16030272
Baran A, Ibrahim A, Nakano Y, Aoshima H, Ozeki T, Sato-Baran I, Ordinario DD. An Exploratory Genomic and Transcriptomic Analysis Between Choloepus didactylus and Homo sapiens. Genes. 2025; 16(3):272. https://doi.org/10.3390/genes16030272
Chicago/Turabian StyleBaran, Ariella, Antony Ibrahim, Yuka Nakano, Hideyuki Aoshima, Takeshi Ozeki, Iri Sato-Baran, and David D. Ordinario. 2025. "An Exploratory Genomic and Transcriptomic Analysis Between Choloepus didactylus and Homo sapiens" Genes 16, no. 3: 272. https://doi.org/10.3390/genes16030272
APA StyleBaran, A., Ibrahim, A., Nakano, Y., Aoshima, H., Ozeki, T., Sato-Baran, I., & Ordinario, D. D. (2025). An Exploratory Genomic and Transcriptomic Analysis Between Choloepus didactylus and Homo sapiens. Genes, 16(3), 272. https://doi.org/10.3390/genes16030272





