Exploring the Role of IRF6 in Perinatal Arterial Ischemic Stroke: A Case of a Newborn with Craniofacial Malformations
Abstract
1. Introduction
2. Diagnostic Assessment—Materials and Methods
2.1. DNA Samples Preparation
2.2. Exome Sequencing
2.3. Filtering and Variant Prioritization
3. Results
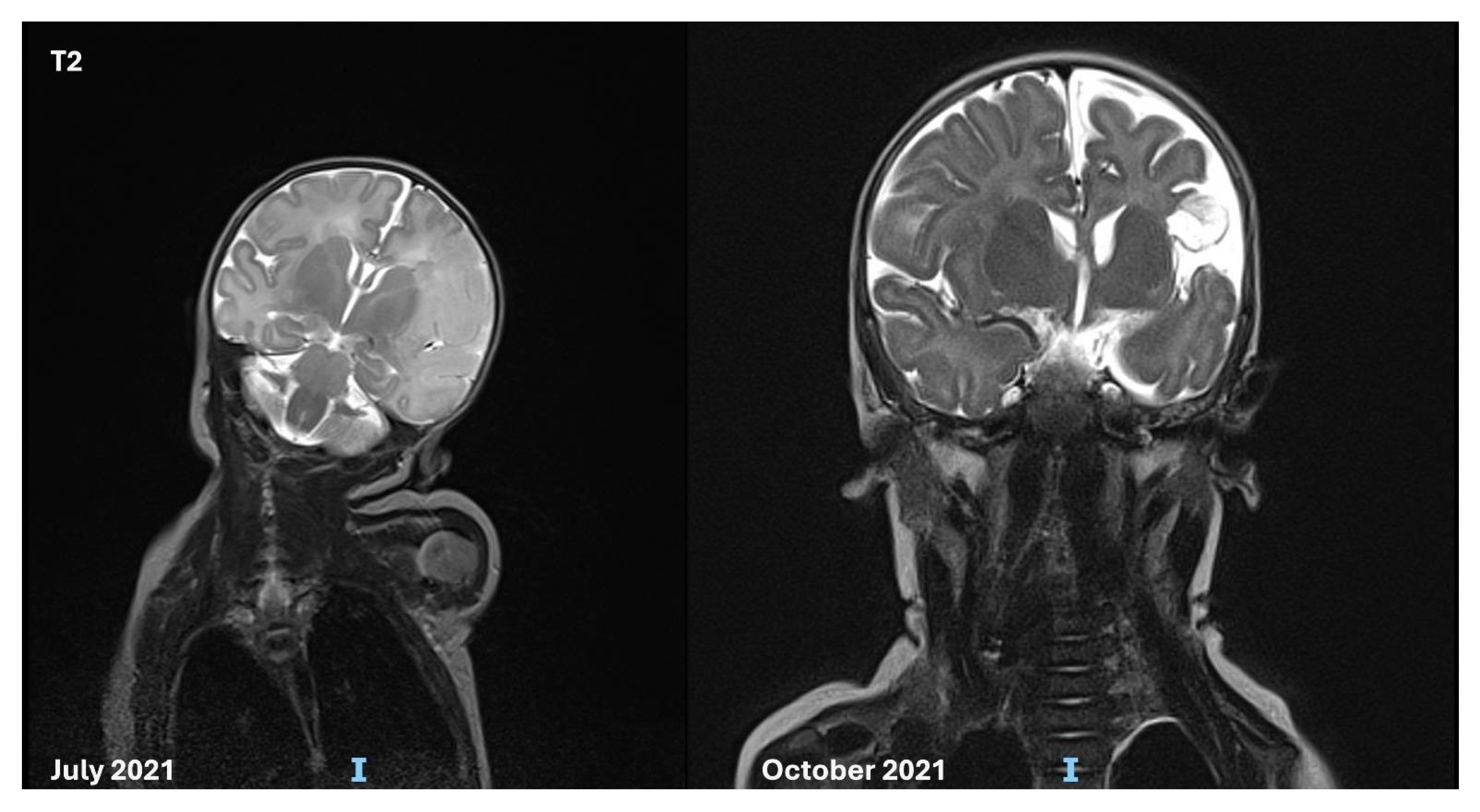
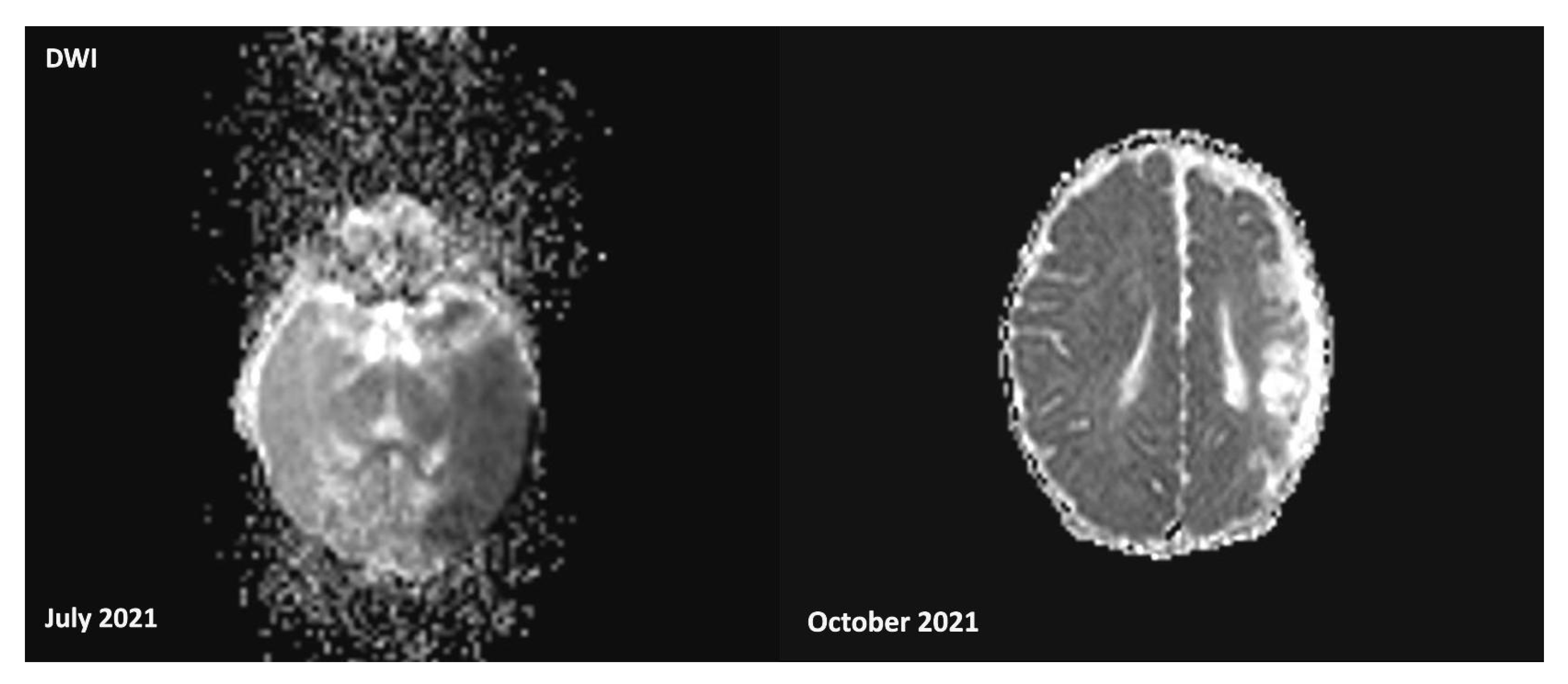
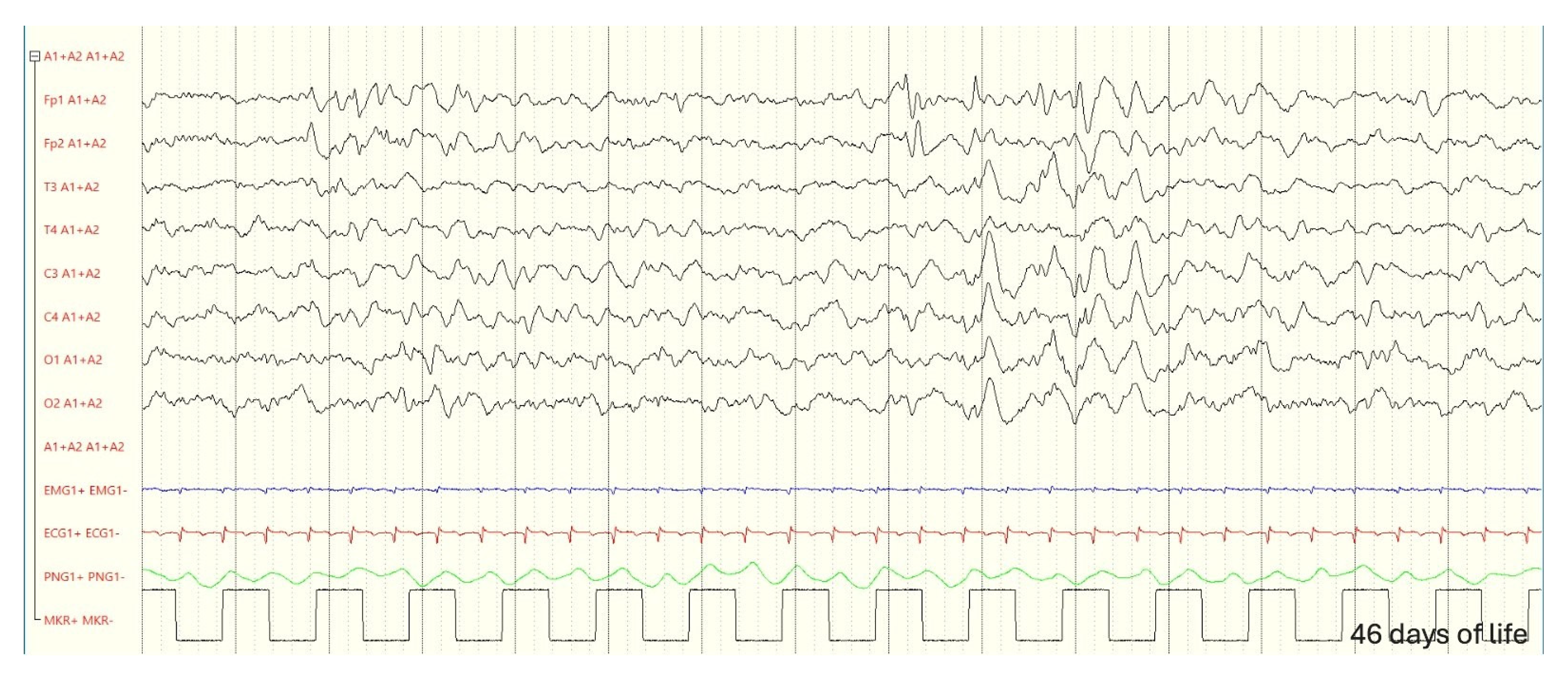
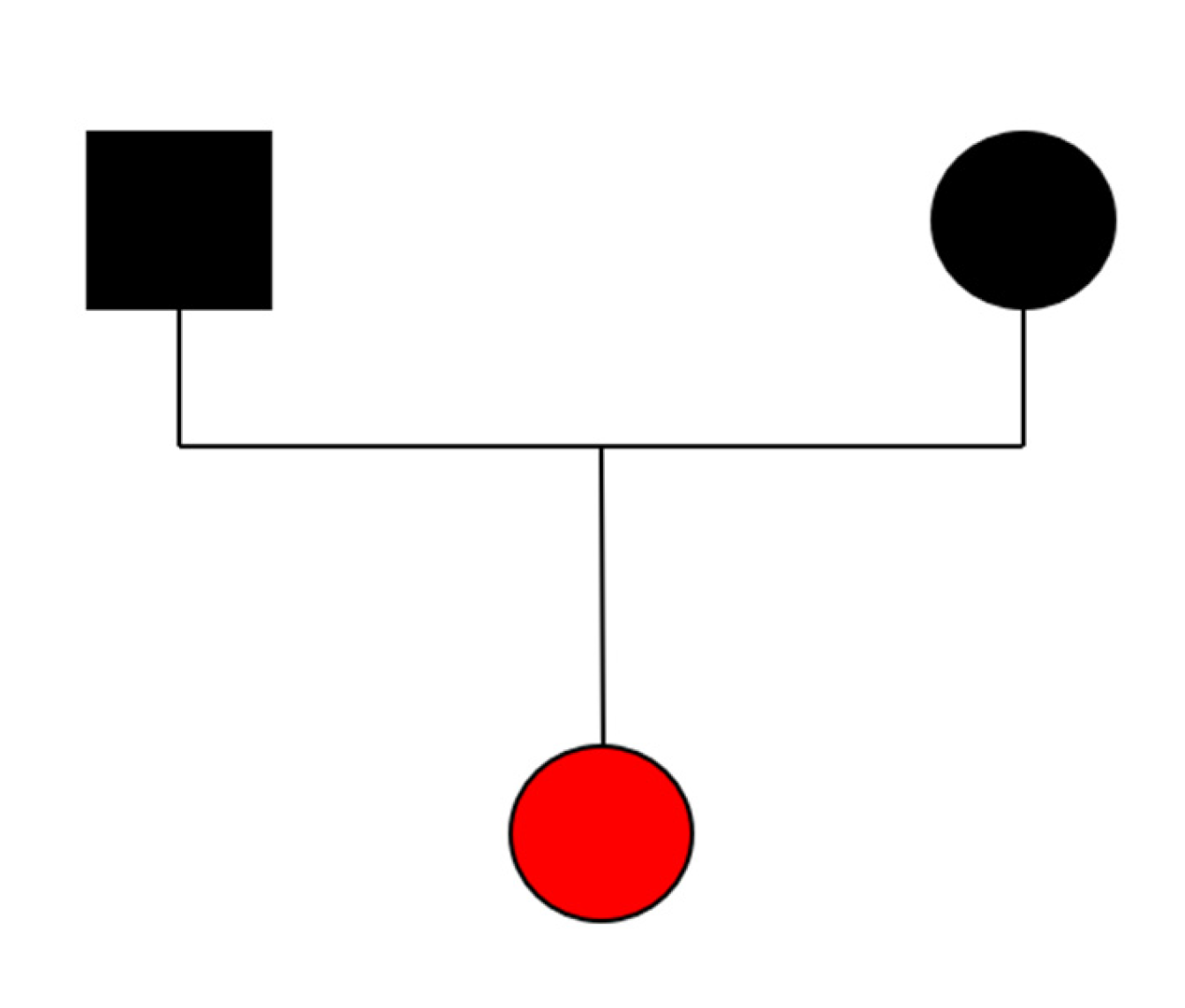
3.1. Case Description
3.2. Audiological Evaluation
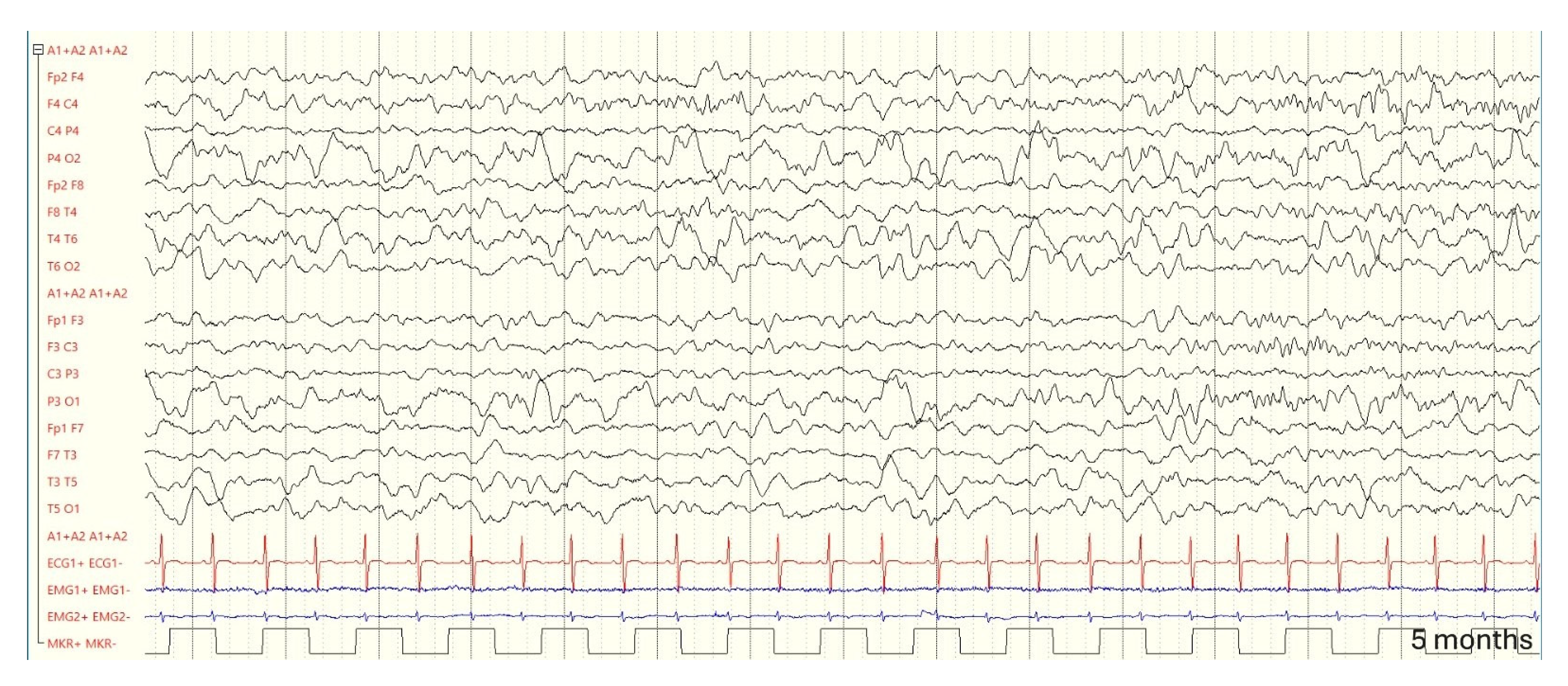
3.3. Follow-up and Neurodevelopmental Outcomes
3.4. Genetic
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VDWS | Von der Woude syndrome |
| PPS | Popliteal Pterygium Syndrome |
| NSCP | Non-syndromic cleft palate |
| eQTL | expression quantitative trait locus |
| GWAS | Genome-wide association study |
| aEEG | Amplitude electroencephalogram |
| AIS | ischaemic perinatal arterial stroke |
| PPAR-γ | Peroxisome proliferator-activated receptor gamma |
| IRF6 | Interferon Regulatory Factor 6 |
| aCGH | Array comparative genomic hybridization |
References
- Mastrangelo, M.; Bove, R.; Ricciardi, G.; Giordo, L.; Papoff, P.; Turco, E.; Lucente, M.; Pisani, F. Clinical profiles of acute arterial ischemic neonatal stroke. Minerva Pediatr. 2023; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bernson-Leung, M.E.; Rivkin, M.J. Stroke in neonates and children. Pediatr. Rev. 2016, 37, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Ferriero, D.M.; Fullerton, H.J.; Bernard, T.J.; Billinghurst, L.; Daniels, S.R.; DeBaun, M.R.; Deveber, G.; Ichord, R.N.; Jordan, L.C.; Massicotte, P.; et al. Management of Stroke in Neonates and Children: A Scientific Statement from the American Heart Association/ American Stroke Association. Stroke 2019, 50, e51–e96. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, M.; Kirton, A. Perinatal Stroke. Semin. Pediatr. Neurol. 2019, 32, 100767. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.B.; Lynch, J.K. Stroke in newborn infants. Lancet Neurol. 2004, 3, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.; Yau, I.; Moharir, M. Current Concepts in Pediatric Stroke. Indian J. Pediatr. 2014, 82, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, M.; Petrovic, B.; Novakovic, I.; Brankovic, S.; Radosavljevic, N.; Nikolic, D. The Genetic Basis of Strokes in Pediatric Populations and Insight into New Therapeutic Options. Int. J. Mol. Sci. 2022, 23, 1601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gelfand, A.A.; Croen, L.A.; Torres, A.R.; Wu, Y.W. Genetic risk factors for perinatal arterial ischemic stroke. Pediatr. Neurol. 2013, 48, 36–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Plaisier, E.; Ronco, P. COL4A1-Related Disorders. Updated 2016. In GeneReviews® [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Eds.; University of Washington: Seattle, WA, USA, 2009; pp. 1993–2021. [Google Scholar]
- Kondo, S.; Schutte, B.C.; Richardson, R.J.; Bjork, B.C.; Knight, A.S.; Watanabe, Y.; Howard, E.; Ferreira de Lima, R.L.; Daack-Hirsch, S.; Sander, A.; et al. Mutations in IRF6 cause Van der Woude and popliteal pterygium syndromes. Nat. Genet. 2002, 32, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Mukohda, M.; Stump, M.; Ketsawatsomkron, P.; Hu, C.; Quelle, F.W.; Sigmund, C.D. Endothelial PPAR-γ provides vascular protection from IL-1β-induced oxidative stress. Am. J. Physiol.-Heart Circ. Physiol. 2016, 310, H39–H48. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Gebska, M.A.; Blokhin, I.O.; Wilson, K.M.; Ketsawatsomkron, P.; Chauhan, A.K.; Keen, H.L.; Sigmund, C.D.; Lentz, S.R. Endothelial PPAR-γ protects against vascular thrombosis by downregulating P-selectin expression. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Hu, Z.; Feng, Y.; Yu, L.; Li, X. The Transcription Factor IRF6 Co-Represses PPARγ-Mediated Cytoprotection in Ischemic Cerebrovascular Endothelial Cells. Sci. Rep. 2017, 7, 2150. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.M.; Chen, B.; Lv, J.M.; Lei, Q.; Pan, Y.J.; Yang, Q. Knockdown of IRF6 Attenuates Hydrogen Dioxide-Induced Oxidative Stress via Inhibiting Mitochondrial Dysfunction in HT22 Cells. Cell. Mol. Neurobiol. 2016, 36, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.B.; Lei, S.F.; Zhang, Y.H.; Zhang, H. Integrative Analysis Identified IRF6 and NDST1 as Potential Causal Genes for Ischemic Stroke. Front. Neurol. 2019, 10, 517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, S.; Li, Z.Z.; Jiang, D.S.; Lu, Y.Y.; Liu, Y.; Gao, L.; Zhang, S.M.; Lei, H.; Zhu, L.H.; Zhang, X.D.; et al. IRF4 is a novel mediator for neuronal survival in ischaemic stroke. Cell Death Differ. 2014, 21, 888–903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiang, M.; Wang, L.; Guo, S.; Lu, Y.Y.; Lei, H.; Jiang, D.S.; Zhang, Y.; Liu, Y.; Zhou, Y.; Zhang, X.D.; et al. Interferon regulatory factor 8 protects against cerebral ischaemic-reperfusion injury. J. Neurochem. 2014, 129, 988–1001. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Guo, S.; Li, Z.Z.; Lu, Y.; Jiang, D.S.; Zhang, R.; Lei, H.; Gao, L.; Zhang, X.; Zhang, Y.; et al. A critical role for interferon regulatory factor 9 in cerebral ischemic stroke. J. Neurosci. 2014, 34, 11897–11912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perilli, L.; Negro, S.; Carbone, S.; Minerva, M.; Curcio, M.R.; Lotti, F.; Mencarelli, M.A.; Ariani, F.; Renieri, A.; Tomasini, B.; et al. Exploring the Role of IRF6 in Perinatal Arterial Ischemic Stroke: A Case of a Newborn with Craniofacial Malformations. Genes 2025, 16, 271. https://doi.org/10.3390/genes16030271
Perilli L, Negro S, Carbone S, Minerva M, Curcio MR, Lotti F, Mencarelli MA, Ariani F, Renieri A, Tomasini B, et al. Exploring the Role of IRF6 in Perinatal Arterial Ischemic Stroke: A Case of a Newborn with Craniofacial Malformations. Genes. 2025; 16(3):271. https://doi.org/10.3390/genes16030271
Chicago/Turabian StylePerilli, Lorenzo, Simona Negro, Samanta Carbone, Michele Minerva, Maria Rosaria Curcio, Federica Lotti, Maria Antonietta Mencarelli, Francesca Ariani, Alessandra Renieri, Barbara Tomasini, and et al. 2025. "Exploring the Role of IRF6 in Perinatal Arterial Ischemic Stroke: A Case of a Newborn with Craniofacial Malformations" Genes 16, no. 3: 271. https://doi.org/10.3390/genes16030271
APA StylePerilli, L., Negro, S., Carbone, S., Minerva, M., Curcio, M. R., Lotti, F., Mencarelli, M. A., Ariani, F., Renieri, A., Tomasini, B., & Grosso, S. (2025). Exploring the Role of IRF6 in Perinatal Arterial Ischemic Stroke: A Case of a Newborn with Craniofacial Malformations. Genes, 16(3), 271. https://doi.org/10.3390/genes16030271






