MicroRNAs in the Mitochondria–Telomere Axis: Novel Insights into Cancer Development and Potential Therapeutic Targets
Abstract
1. Introduction
2. Cellular Organization and Energy Metabolism
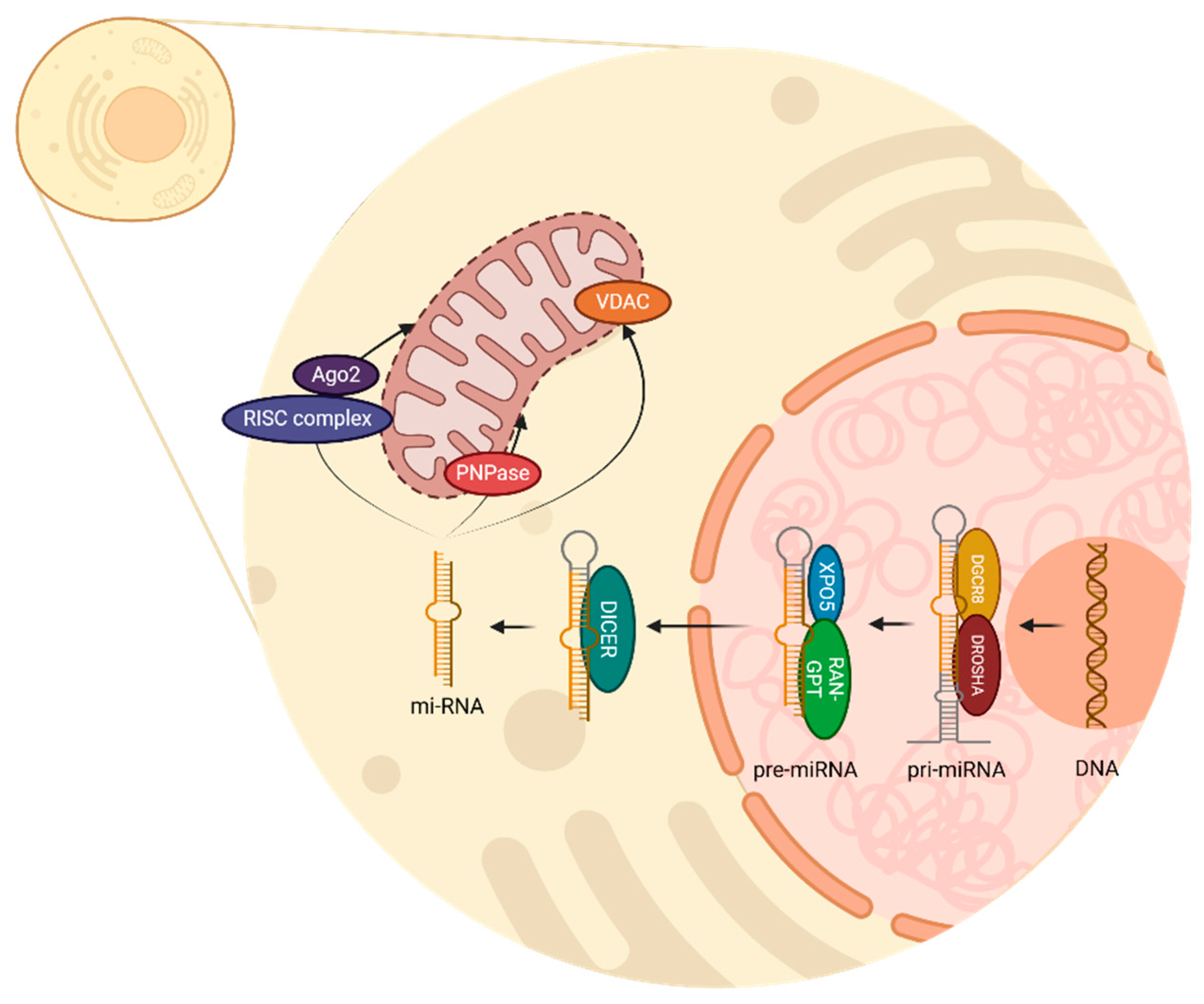
3. The miRNA: Biogenesis in Animal Cells and Its Relationship with Mitochondria
4. Fusion and Fission Mitochondrial Processes
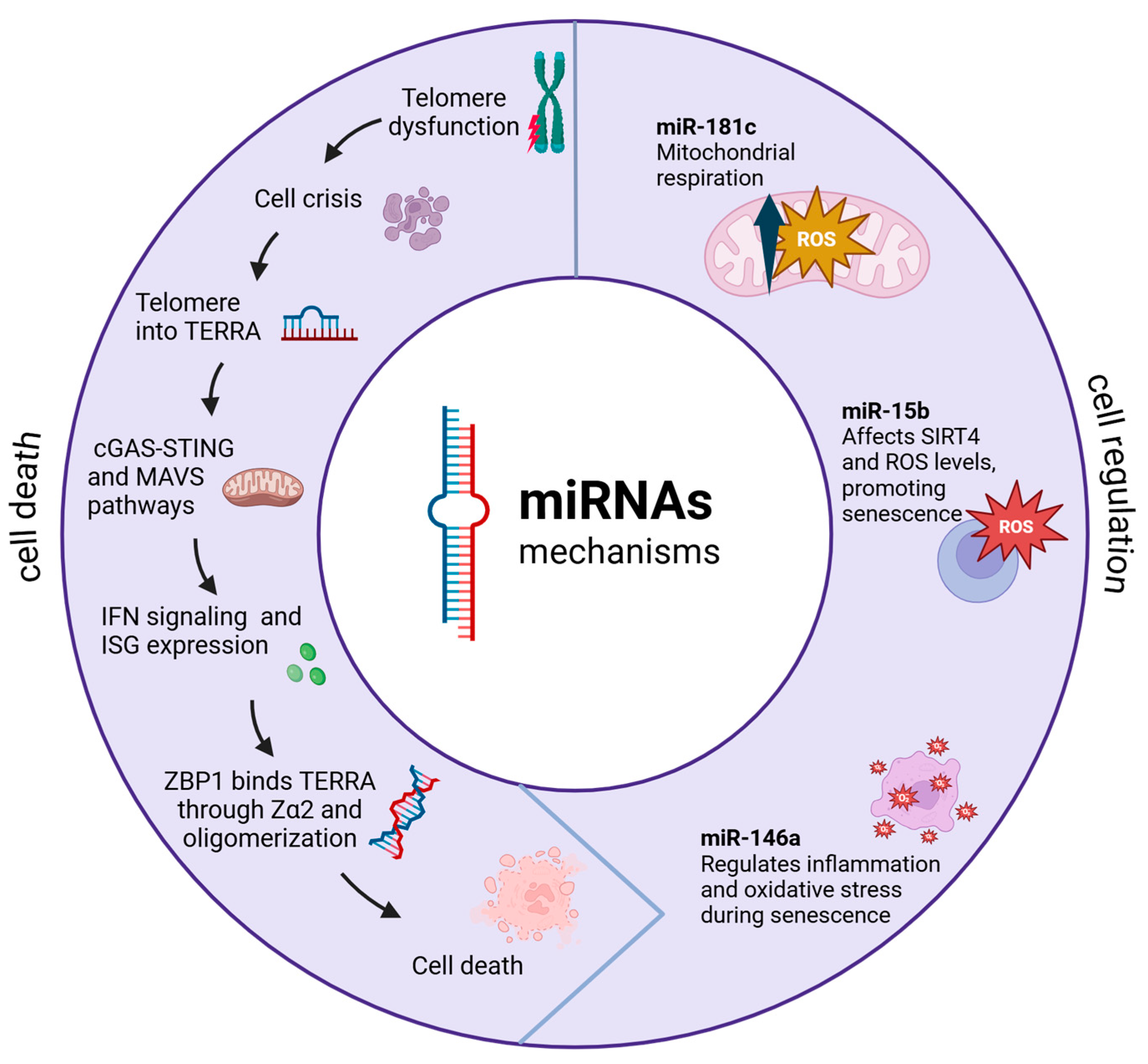
5. Mitochondrial–Telomere Communications in Cancer
5.1. Molecular Basis of Crosstalk Communication Between the Mitochondria and Telomeres
5.2. Mitochondrial–Telomere Communication via Non-Coding RNAs
5.3. Mitochondrial Nuclear-Encoded MitomiRs
5.4. Mitochondria-Encoded miRNAs
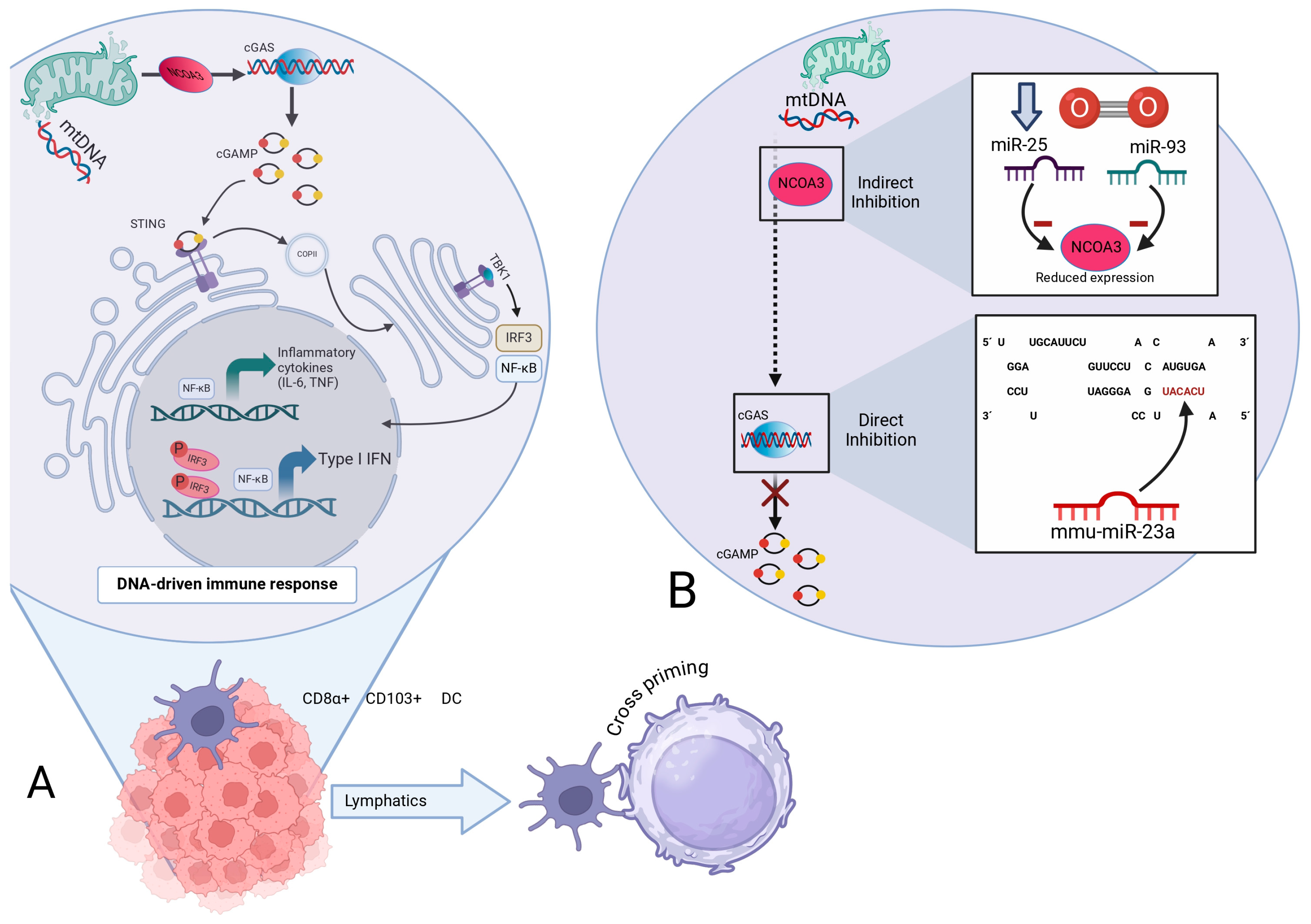
5.5. RNA-Dependent Modulation of the cGAS/STING Axis: Convergence of Mitochondrial Dynamics, Telomeric Integrity, and Programmed Cell Death Pathways
6. Metabolic Reprogramming in Cancer
7. Clinical Implications and Therapeutic Applications in Cancer
8. Current Challenges and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, X.; Yu, X.; Zhang, C.; Wang, Y.; Sun, Y.; Sun, H.; Zhang, H.; Shi, Y.; He, X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-Related Diseases. Stem Cell Rev. Rep. 2022, 18, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Assalve, G.; Lunetti, P.; Rocca, M.S.; Cosci, I.; Di Nisio, A.; Ferlin, A.; Zara, V.; Ferramosca, A. Exploring the Link Between Telomeres and Mitochondria: Mechanisms and Implications in Different Cell Types. Int. J. Mol. Sci. 2025, 26, 993. [Google Scholar] [CrossRef] [PubMed]
- Cusanelli, E.; Chartrand, P. Telomeric Repeat-Containing RNA TERRA: A Noncoding RNA Connecting Telomere Biology to Genome Integrity. Front. Genet. 2015, 6, 143. [Google Scholar] [CrossRef]
- Brosnan, C.A.; Voinnet, O. The Long and the Short of Noncoding RNAs. Curr. Opin. Cell Biol. 2009, 21, 416–425. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-Coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Khan, A.Q.; Ahmed, E.I.; Elareer, N.R.; Junejo, K.; Steinhoff, M.; Uddin, S. Role of miRNA-Regulated Cancer Stem Cells in the Pathogenesis of Human Malignancies. Cells 2019, 8, 840. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of Triple-Negative Breast Cancer Molecular Subtypes: Implications for Neoadjuvant Chemotherapy Selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A.; Tan, A.R. Triple-Negative Breast Cancer: Molecular Subtypes and New Targets for Therapy. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, e31–e39. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial Dysfunction: Mechanisms and Advances in Therapy. Sig Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial Dynamics in Health and Disease: Mechanisms and Potential Targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Cai, X.; Ng, C.P.; Jones, O.; Fung, T.S.; Ryu, K.W.; Li, D.; Thompson, C.B. Lactate Activates the Mitochondrial Electron Transport Chain Independently of Its Metabolism. Mol. Cell 2023, 83, 3904–3920.e7. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, C.M.; Falkenberg, M.; Larsson, N.-G. Maintenance and Expression of Mammalian Mitochondrial DNA. Annu. Rev. Biochem. 2016, 85, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; An, Y.; Ren, M.; Wang, H.; Bai, J.; Du, W.; Kong, D. The Mechanisms of Action of Mitochondrial Targeting Agents in Cancer: Inhibiting Oxidative Phosphorylation and Inducing Apoptosis. Front. Pharmacol. 2023, 14, 1243613. [Google Scholar] [CrossRef]
- Zou, W.; Rohatgi, N.; Brestoff, J.R.; Li, Y.; Barve, R.A.; Tycksen, E.; Kim, Y.; Silva, M.J.; Teitelbaum, S.L. Ablation of Fat Cells in Adult Mice Induces Massive Bone Gain. Cell Metab. 2020, 32, 801–813.e6. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. miRBase: Tools for microRNA Genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Chiang, H.R.; Schoenfeld, L.W.; Ruby, J.G.; Auyeung, V.C.; Spies, N.; Baek, D.; Johnston, W.K.; Russ, C.; Luo, S.; Babiarz, J.E.; et al. Mammalian microRNAs: Experimental Evaluation of Novel and Previously Annotated Genes. Genes. Dev. 2010, 24, 992–1009. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Han, J.; Lee, Y.; Yeom, K.-H.; Kim, Y.-K.; Jin, H.; Kim, V.N. The Drosha-DGCR8 Complex in Primary microRNA Processing. Genes. Dev. 2004, 18, 3016–3027. [Google Scholar] [CrossRef]
- Luo, L.; An, X.; Xiao, Y.; Sun, X.; Li, S.; Wang, Y.; Sun, W.; Yu, D. Mitochondrial-Related microRNAs and Their Roles in Cellular Senescence. Front. Physiol. 2024, 14, 1279548. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. VDAC, a Multi-Functional Mitochondrial Protein Regulating Cell Life and Death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, H.-W.; Oktay, Y.; Zhang, J.; Allen, E.L.; Smith, G.M.; Fan, K.C.; Hong, J.S.; French, S.W.; McCaffery, J.M.; et al. PNPASE Regulates RNA Import into Mitochondria. Cell 2010, 142, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Kuthethur, R.; Shukla, V.; Mallya, S.; Adiga, D.; Kabekkodu, S.P.; Ramachandra, L.; Saxena, P.U.P.; Satyamoorthy, K.; Chakrabarty, S. Expression Analysis and Function of Mitochondrial Genome-Encoded microRNAs. J. Cell Sci. 2022, 135, jcs258937. [Google Scholar] [CrossRef]
- Li, P.; Jiao, J.; Gao, G.; Prabhakar, B.S. Control of Mitochondrial Activity by miRNAs. J. Cell Biochem. 2012, 113, 1104–1110. [Google Scholar] [CrossRef]
- Wu, Z.; Xiao, C.; Long, J.; Huang, W.; You, F.; Li, X. Mitochondrial Dynamics and Colorectal Cancer Biology: Mechanisms and Potential Targets. Cell Commun. Signal 2024, 22, 91. [Google Scholar] [CrossRef]
- Darvin, P.; Sasidharan Nair, V. Editorial: Understanding Mitochondrial Dynamics and Metabolic Plasticity in Cancer Stem Cells: Recent Advances in Cancer Treatment and Potential Therapeutic Approaches. Front. Oncol. 2023, 13, 1155774. [Google Scholar] [CrossRef]
- van Soest, D.M.K.; Polderman, P.E.; den Toom, W.T.F.; Keijer, J.P.; van Roosmalen, M.J.; Leyten, T.M.F.; Lehmann, J.; Zwakenberg, S.; De Henau, S.; van Boxtel, R.; et al. Mitochondrial H2O2 Release Does Not Directly Cause Damage to Chromosomal DNA. Nat. Commun. 2024, 15, 2725. [Google Scholar] [CrossRef]
- Jagaraj, C.J.; Shadfar, S.; Kashani, S.A.; Saravanabavan, S.; Farzana, F.; Atkin, J.D. Molecular Hallmarks of Ageing in Amyotrophic Lateral Sclerosis. Cell. Mol. Life Sci. 2024, 81, 111. [Google Scholar] [CrossRef]
- Zeinoun, B.; Teixeira, M.T.; Barascu, A. Hog1 Acts in a Mec1-Independent Manner to Counteract Oxidative Stress Following Telomerase Inactivation in Saccharomyces Cerevisiae. Commun. Biol. 2024, 7, 761. [Google Scholar] [CrossRef]
- Sung, J.Y.; Kim, S.G.; Park, S.-Y.; Kim, J.-R.; Choi, H.C. Telomere Stabilization by Metformin Mitigates the Progression of Atherosclerosis via the AMPK-Dependent p-PGC-1α Pathway. Exp. Mol. Med. 2024, 56, 1967–1979. [Google Scholar] [CrossRef]
- Nassour, J.; Aguiar, L.G.; Correia, A.; Schmidt, T.T.; Mainz, L.; Przetocka, S.; Haggblom, C.; Tadepalle, N.; Williams, A.; Shokhirev, M.N.; et al. Telomere-to-Mitochondria Signalling by ZBP1 Mediates Replicative Crisis. Nature 2023, 614, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Azzalin, C.M.; Reichenbach, P.; Khoriauli, L.; Giulotto, E.; Lingner, J. Telomeric Repeat–Containing RNA and RNA Surveillance Factors at Mammalian Chromosome Ends. Science 2007, 318, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Strzyz, P. From Shortening Telomeres to Replicative Crisis. Nat. Rev. Mol. Cell Biol. 2023, 24, 239. [Google Scholar] [CrossRef]
- Gaela, V.M.; Chen, L.-Y. Ends End It via Mitochondria: A Telomere-Dependent Tumor Suppressive Mechanism Acts during Replicative Crisis. Mol. Cell 2023, 83, 1027–1029. [Google Scholar] [CrossRef]
- Nassour, J.; Radford, R.; Correia, A.; Fusté, J.M.; Schoell, B.; Jauch, A.; Shaw, R.J.; Karlseder, J. Autophagic Cell Death Restricts Chromosomal Instability during Replicative Crisis. Nature 2019, 565, 659–663. [Google Scholar] [CrossRef]
- Zhan, J.; Wang, J.; Liang, Y.; Wang, L.; Huang, L.; Liu, S.; Zeng, X.; Zeng, E.; Wang, H. Apoptosis Dysfunction: Unravelling the Interplay between ZBP1 Activation and Viral Invasion in Innate Immune Responses. Cell Commun. Signal. 2024, 22, 149. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Wu, J.; Qi, N. MAVS: A Two-Sided CARD Mediating Antiviral Innate Immune Signaling and Regulating Immune Homeostasis. Front. Microbiol. 2021, 12, 744348. [Google Scholar] [CrossRef]
- Wallace, L.; Aikhionbare, K.; Banerjee, S.; Peagler, K.; Pitts, M.; Yao, X.; Aikhionbare, F. Differential Expression Profiles of Mitogenome Associated MicroRNAs Among Colorectal Adenomatous Polyps. Cancer Res. J. 2021, 9, 23–33. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Paul, C.; Liu, X.; Sadayappan, S.; Wang, Y.; Pauklin, S. Mitochondrial Nucleoid in Cardiac Homeostasis: Bidirectional Signaling of Mitochondria and Nucleus in Cardiac Diseases. Basic. Res. Cardiol. 2021, 116, 49. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Lin, Y.-C.; Lo, Y.K.; Lu, Y.-Z.; Babuharisankar, A.P.; Lien, H.-W.; Chou, H.-Y.; Lee, A.Y.-L. The Mitochondrial Stress Signaling Tunes Immunity from a View of Systemic Tumor Microenvironment and Ecosystem. iScience 2024, 27, 110710. [Google Scholar] [CrossRef]
- Maurya, A.K.; Rabina, P.; Kumar, V.B.S. In-Silico Analysis of Important Mitochondrial microRNAs and Their Differential Expression in Mitochondria. bioRxiv 2024. [Google Scholar] [CrossRef]
- Liu, R.-M. Aging, Cellular Senescence, and Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 1989. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.; Grether-Beck, S.; Singh, M.; Kuck, F.; Jakob, S.; Kefalas, A.; Altinoluk-Hambüchen, S.; Graffmann, N.; Schneider, M.; Lindecke, A.; et al. MicroRNA-15b Regulates Mitochondrial ROS Production and the Senescence-Associated Secretory Phenotype through Sirtuin 4/SIRT4. Aging 2016, 8, 484–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, I.C.; Meng, S.; Xu, J. miR-146a Decreases Inflammation and ROS Production in Aged Dermal Fibroblasts. Int. J. Mol. Sci. 2024, 25, 6821. [Google Scholar] [CrossRef]
- Fan, S.; Tian, T.; Chen, W.; Lv, X.; Lei, X.; Zhang, H.; Sun, S.; Cai, L.; Pan, G.; He, L.; et al. Mitochondrial miRNA Determines Chemoresistance by Reprogramming Metabolism and Regulating Mitochondrial Transcription. Cancer Res. 2019, 79, 1069–1084. [Google Scholar] [CrossRef]
- Dasgupta, N.; Peng, Y.; Tan, Z.; Ciraolo, G.; Wang, D.; Li, R. miRNAs in mtDNA-Less Cell Mitochondria. Cell Death Discov. 2015, 1, 15004. [Google Scholar] [CrossRef]
- Das, S.; Ferlito, M.; Kent, O.A.; Fox-Talbot, K.; Wang, R.; Liu, D.; Raghavachari, N.; Yang, Y.; Wheelan, S.J.; Murphy, E.; et al. Nuclear miRNA Regulates the Mitochondrial Genome in the Heart. Circ. Res. 2012, 110, 1596–1603. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, B.; Huang, Y.; Zhang, Y.; Jiang, Y.; Ma, L.; Shen, Y.-Q. Mitochondrial DNA-Targeted Therapy: A Novel Approach to Combat Cancer. Cell Insight 2023, 2, 100113. [Google Scholar] [CrossRef]
- Lopez Sanchez, M.I.G.; Cipullo, M.; Gopalakrishna, S.; Khawaja, A.; Rorbach, J. Methylation of Ribosomal RNA: A Mitochondrial Perspective. Front. Genet. 2020, 11, 761. [Google Scholar] [CrossRef]
- Qin, X.-W.; He, J.; Yu, Y.; Liu, C.; Luo, Z.-Y.; Li, Z.-M.; Weng, S.-P.; Guo, C.-J.; He, J.-G. The Roles of Mandarin Fish STING in Innate Immune Defense against Infectious Spleen and Kidney Necrosis Virus Infections. Fish. Shellfish. Immunol. 2020, 100, 80–89. [Google Scholar] [CrossRef]
- Gareev, I.; de Jesus Encarnacion Ramirez, M.; Goncharov, E.; Ivliev, D.; Shumadalova, A.; Ilyasova, T.; Wang, C. MiRNAs and lncRNAs in the Regulation of Innate Immune Signaling. Noncoding RNA Res. 2023, 8, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.E.; Winship, D.; Snyder, J.M.; Child, S.J.; Geballe, A.P.; Stetson, D.B. The AIM2-like Receptors Are Dispensable for the Interferon Response to Intracellular DNA. Immunity 2016, 45, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Hartmann, R.; Ablasser, A.; Hopfner, K.-P. OAS Proteins and cGAS: Unifying Concepts in Sensing and Responding to Cytosolic Nucleic Acids. Nat. Rev. Immunol. 2014, 14, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS–STING Pathway as a Therapeutic Target in Inflammatory Diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Izquierdo, J.M. Mitochondria-cGAS-STING Axis Is a Potential Therapeutic Target for Senescence-Dependent Inflammaging-Associated Neurodegeneration. Neural Regen. Res. 2025, 20, 805–807. [Google Scholar] [CrossRef]
- He, X.; Wedn, A.; Wang, J.; Gu, Y.; Liu, H.; Zhang, J.; Lin, Z.; Zhou, R.; Pang, X.; Cui, Y. IUPHAR ECR Review: The cGAS-STING Pathway: Novel Functions beyond Innate Immune and Emerging Therapeutic Opportunities. Pharmacol. Res. 2024, 201, 107063. [Google Scholar] [CrossRef]
- Nicolai, C.J.; Wolf, N.; Chang, I.-C.; Kirn, G.; Marcus, A.; Ndubaku, C.O.; McWhirter, S.M.; Raulet, D.H. NK Cells Mediate Clearance of CD8+ T Cell-Resistant Tumors in Response to STING Agonists. Sci. Immunol. 2020, 5, eaaz2738. [Google Scholar] [CrossRef]
- Nakamura, T.; Miyabe, H.; Hyodo, M.; Sato, Y.; Hayakawa, Y.; Harashima, H. Liposomes Loaded with a STING Pathway Ligand, Cyclic Di-GMP, Enhance Cancer Immunotherapy against Metastatic Melanoma. J. Control. Release 2015, 216, 149–157. [Google Scholar] [CrossRef]
- Yu, Q.; Chu, L.; Li, Y.; Wang, Q.; Zhu, J.; Wang, C.; Cui, S. miR-23a/b Suppress cGAS-Mediated Innate and Autoimmunity. Cell Mol. Immunol. 2021, 18, 1235–1248. [Google Scholar] [CrossRef]
- Wu, M.-Z.; Cheng, W.-C.; Chen, S.-F.; Nieh, S.; O’Connor, C.; Liu, C.-L.; Tsai, W.-W.; Wu, C.-J.; Martin, L.; Lin, Y.-S.; et al. miR-25/93 Mediates Hypoxia-Induced Immunosuppression by Repressing cGAS. Nat. Cell Biol. 2017, 19, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. The Metabolism of Carcinoma Cells1. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, M.; Wang, R.; Man, Y.; Zhou, H.; Xu, Z.-X.; Wang, Y. The Crosstalk between Glucose Metabolism and Telomerase Regulation in Cancer. Biomed. Pharmacother. 2024, 175, 116643. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The Sirtuin Family in Health and Disease. Sig Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Wang, H.; Guo, M.; Wei, H.; Chen, Y. Targeting P53 Pathways: Mechanisms, Structures and Advances in Therapy. Sig Transduct. Target. Ther. 2023, 8, 92. [Google Scholar] [CrossRef]
- Mantovani, F.; Collavin, L.; Del Sal, G. Mutant P53 as a Guardian of the Cancer Cell. Cell Death Differ. 2019, 26, 199–212. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, X.; Yi, J.; Huang, J.; He, J.; Tao, Y. Sirtuins in Metabolism, DNA Repair and Cancer. J. Exp. Clin. Cancer Res. 2016, 35, 182. [Google Scholar] [CrossRef]
- Yamakuchi, M. MicroRNA Regulation of SIRT1. Front. Physio. 2012, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Chen, Q.; Ma, L.; Liu, J.; Yang, Z.; Shen, J.; Cui, Y.; Bian, X.; Qian, C. Oncolytic Adenovirus Co-Expressing miRNA-34a and IL-24 Induces Superior Antitumor Activity in Experimental Tumor Model. J. Mol. Med. 2013, 91, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Noh, J.H.; Kim, J.K.; Eun, J.W.; Jung, K.H.; Kim, M.G.; Chang, Y.G.; Shen, Q.; Kim, S.-J.; Park, W.S.; et al. MicroRNA-29c Functions as a Tumor Suppressor by Direct Targeting Oncogenic SIRT1 in Hepatocellular Carcinoma. Oncogene 2014, 33, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Qi, X.; Hu, Y.; Wang, Y.; Zhang, J.; Liu, Z.; Qin, Z. Targeting Sirtuins for Cancer Therapy: Epigenetics Modifications and Beyond. Theranostics 2024, 14, 6726–6767. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.; Han, M.-J.; Cha, H.-J.; Zoldan, J.; Burkart, A.; Jung, J.H.; Jang, Y.; Kim, C.-H.; Jeong, H.-C.; Kim, B.-G.; et al. Metabolic Control of Primed Human Pluripotent Stem Cell Fate and Function by the miR-200c–SIRT2 Axis. Nat. Cell Biol. 2017, 19, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, M.; Kussainova, A.; Aripova, A.; Bersimbaev, R.; Bulgakova, O. The Molecular Mechanisms in Senescent Cells Induced by Natural Aging and Ionizing Radiation. Cells 2024, 13, 550. [Google Scholar] [CrossRef]
- Roy, B.; Dwivedi, Y. An Insight into the Sprawling Microverse of microRNAs in Depression Pathophysiology and Treatment Response. Neurosci. Biobehav. Rev. 2023, 146, 105040. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as Anticancer Mechanism: Function and Dysfunction of Its Modulators and Targeted Therapeutic Strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor Biomarkers for Diagnosis, Prognosis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef]
- Boccardi, V.; Marano, L. Aging, Cancer, and Inflammation: The Telomerase Connection. Int. J. Mol. Sci. 2024, 25, 8542. [Google Scholar] [CrossRef]
- Witten, J.; Hu, Y.; Langer, R.; Anderson, D.G. Recent Advances in Nanoparticulate RNA Delivery Systems. Proc. Natl. Acad. Sci. USA 2024, 121, e2307798120. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug Delivery Systems for RNA Therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.N.; Dahlman, J.E. RNA Delivery Systems. Proc. Natl. Acad. Sci. USA 2024, 121, e2315789121. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA Therapeutics—Challenges and Potential Solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef]
- Zarnack, K.; Eyras, E. Artificial Intelligence and Machine Learning in RNA Biology. Brief. Bioinform. 2023, 24, bbad415. [Google Scholar] [CrossRef]
- Martinelli, D.D. From Sequences to Therapeutics: Using Machine Learning to Predict Chemically Modified siRNA Activity. Genomics 2024, 116, 110815. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Ramos, J.A.; de la Mora-Jiménez, E.; Llanes-Cervantes, B.A.; Damián-Mejía, M.Á. MicroRNAs in the Mitochondria–Telomere Axis: Novel Insights into Cancer Development and Potential Therapeutic Targets. Genes 2025, 16, 268. https://doi.org/10.3390/genes16030268
Cruz-Ramos JA, de la Mora-Jiménez E, Llanes-Cervantes BA, Damián-Mejía MÁ. MicroRNAs in the Mitochondria–Telomere Axis: Novel Insights into Cancer Development and Potential Therapeutic Targets. Genes. 2025; 16(3):268. https://doi.org/10.3390/genes16030268
Chicago/Turabian StyleCruz-Ramos, José Alfonso, Emmanuel de la Mora-Jiménez, Beatriz Alejandra Llanes-Cervantes, and Miguel Ángel Damián-Mejía. 2025. "MicroRNAs in the Mitochondria–Telomere Axis: Novel Insights into Cancer Development and Potential Therapeutic Targets" Genes 16, no. 3: 268. https://doi.org/10.3390/genes16030268
APA StyleCruz-Ramos, J. A., de la Mora-Jiménez, E., Llanes-Cervantes, B. A., & Damián-Mejía, M. Á. (2025). MicroRNAs in the Mitochondria–Telomere Axis: Novel Insights into Cancer Development and Potential Therapeutic Targets. Genes, 16(3), 268. https://doi.org/10.3390/genes16030268





