Effects of Combined Transcriptome and Metabolome Analysis Training on Athletic Performance of 2-Year-Old Trot-Type Yili Horses
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Animals and Training Programmes
2.2. Sample Collection
2.3. Transcriptome Analysis
2.3.1. RNA Isolation, Library Preparation and Sequencing
2.3.2. Differentially Expressed Genes Analysis
2.3.3. Functional Enrichment Analysis
2.3.4. Protein Network Interactions Analysis
2.4. Metabolome Analysis
2.5. Combined Transcriptome and Metabolome Analysis
2.6. Statistical Analysis
3. Results
3.1. Changes in Race Performance of Horses at Different Stages of Training
3.2. Transcriptome Results Analysis
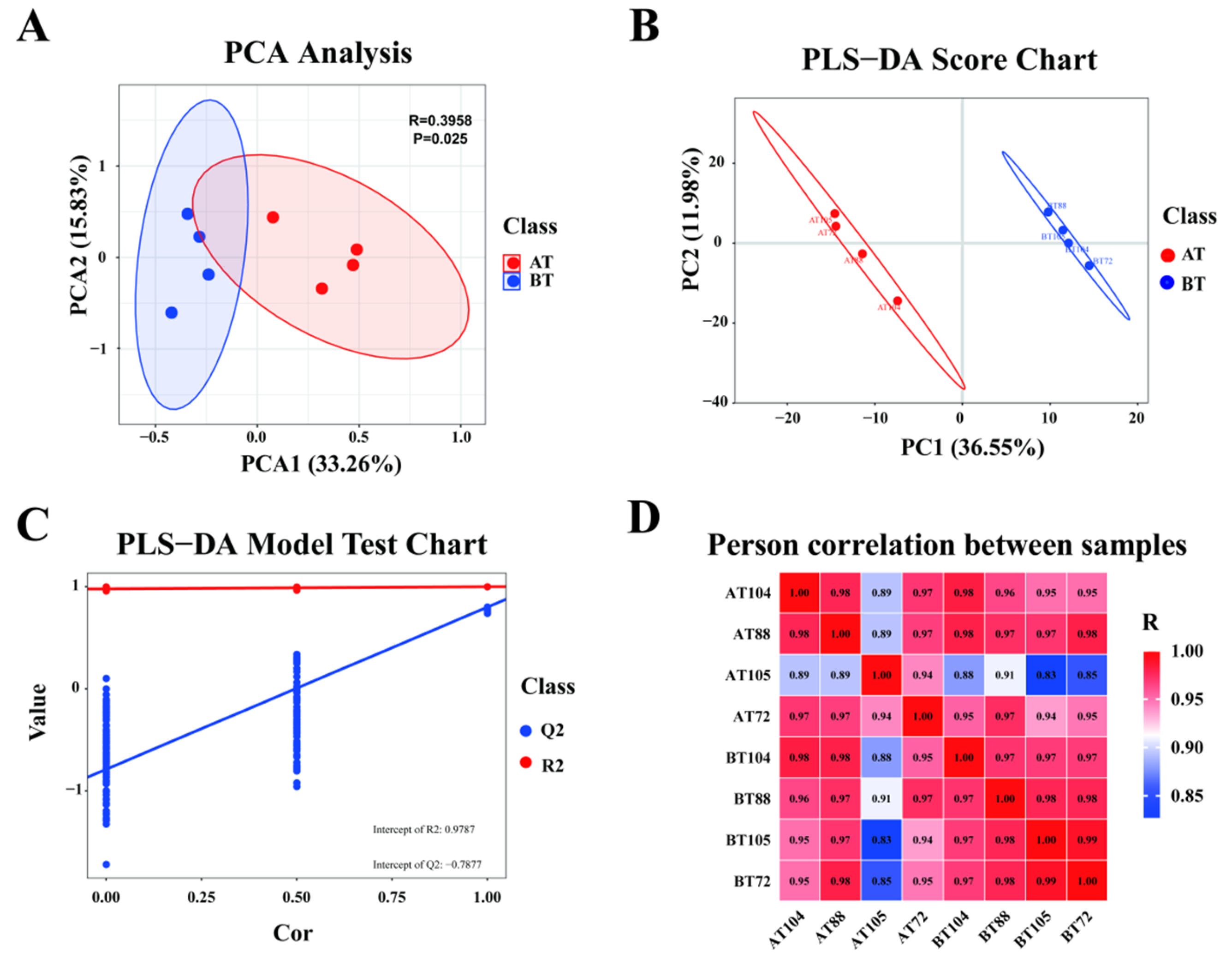
3.2.1. Transcriptome Quality Control Analysis
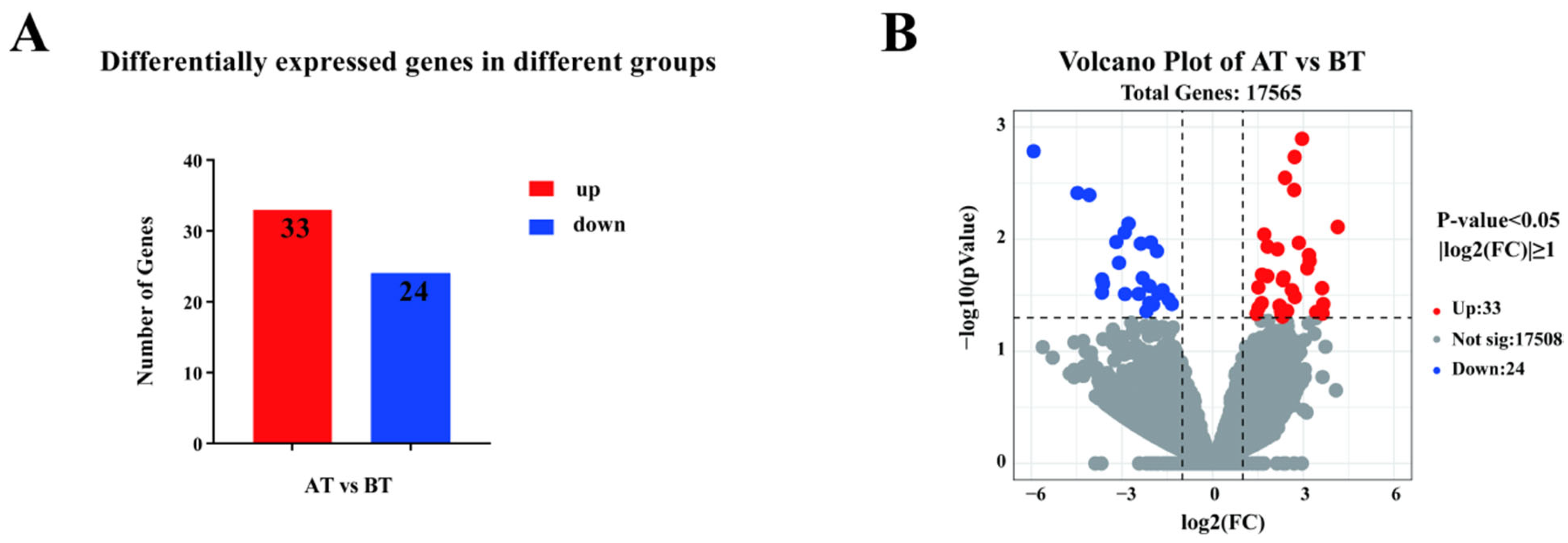
3.2.2. Differential Genes Analysis
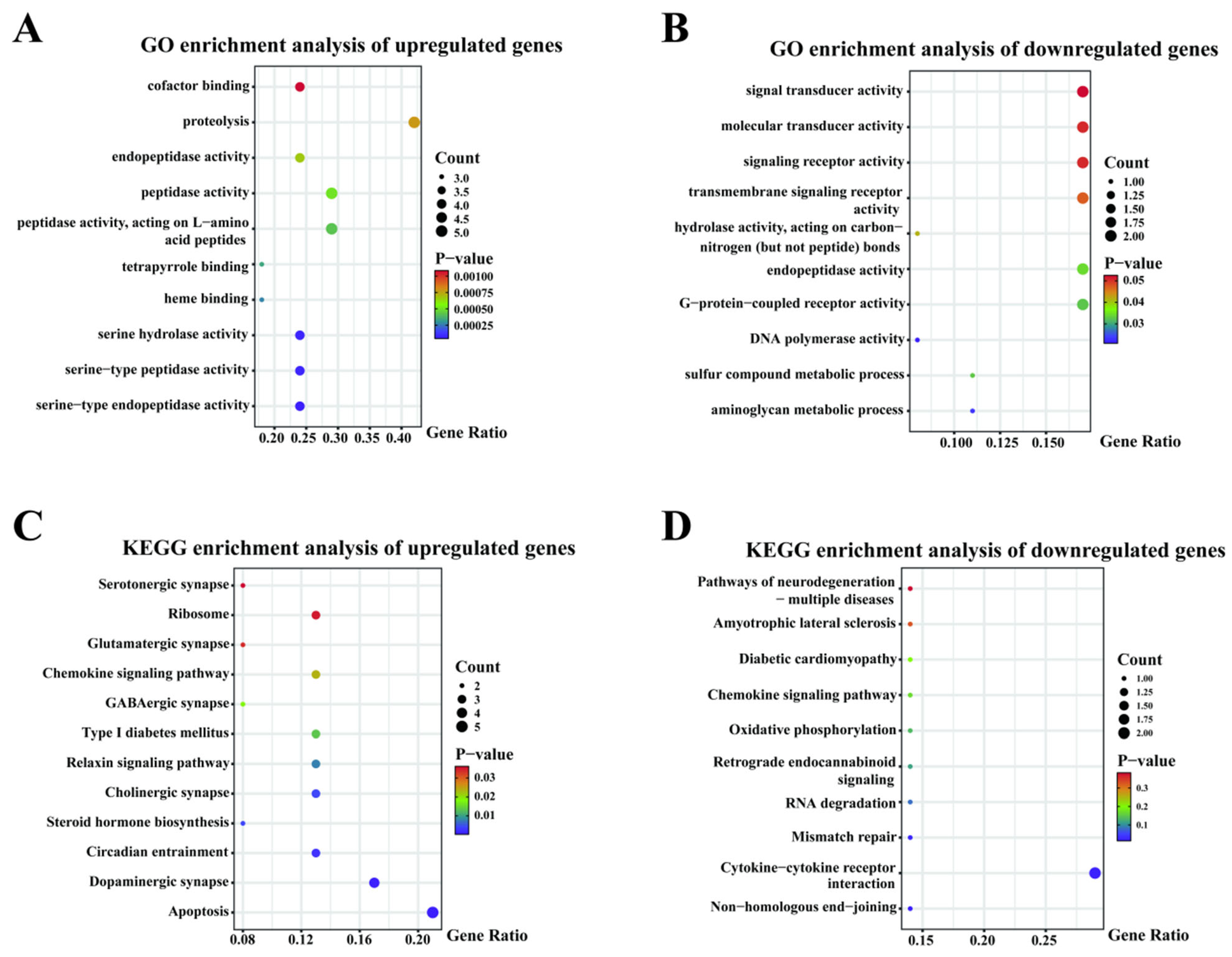
3.2.3. Transcriptome Pathway Enrichment Analysis
3.2.4. Protein Network Interactions Results Analysis
3.3. Metabolome Results Analysis
3.3.1. Metabolome Quality Control Analysis
3.3.2. Differential Metabolites Analysis
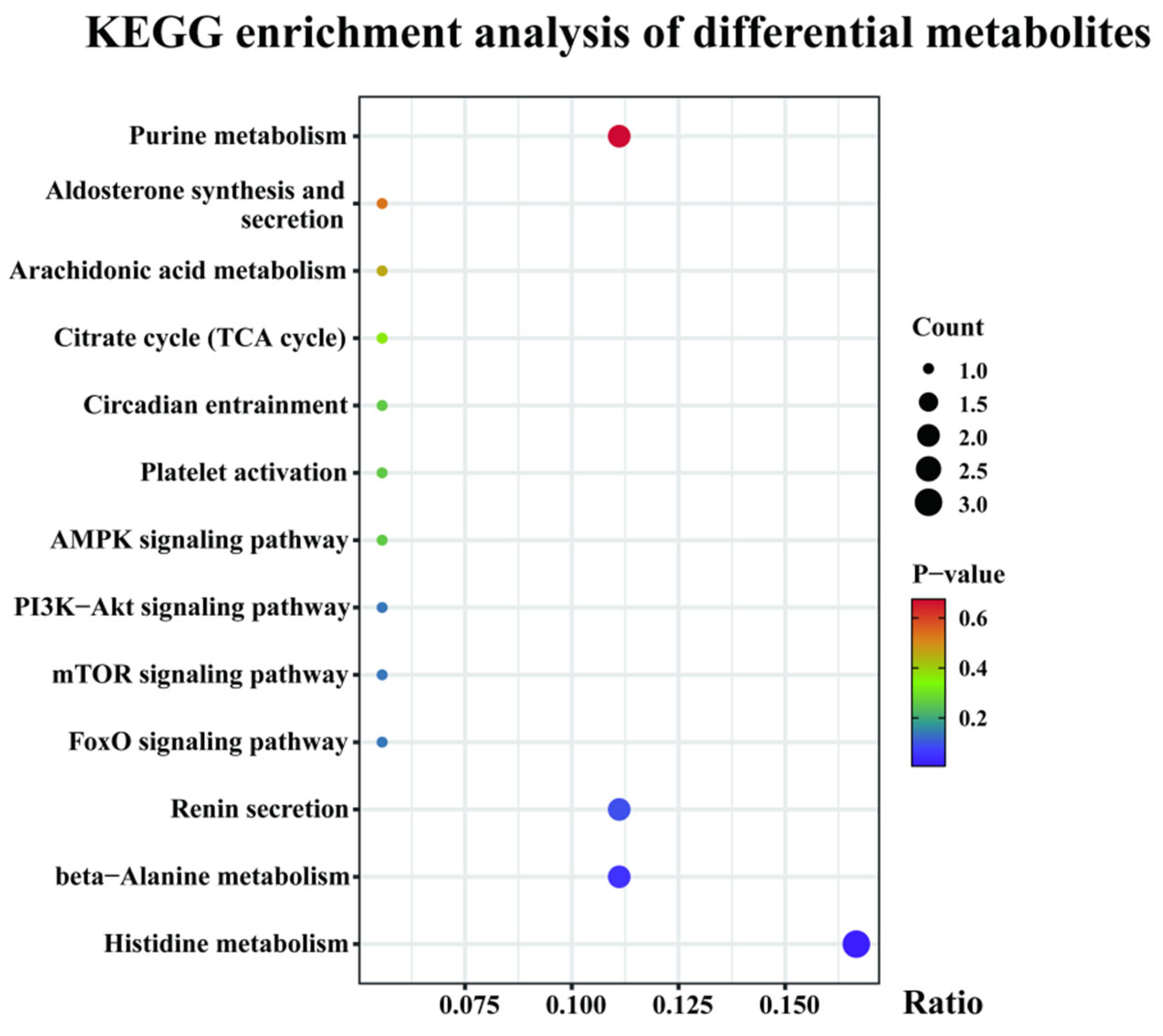
3.3.3. Metabolome Pathway Enrichment Analysis
3.4. Association Analysis Between Transcriptomic and Metabolomic Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.X.; Sánchez-García, R. Chinese equestrian policy development: A narrative review. Front. Vet. Sci. 2024, 10, 1281019. [Google Scholar] [CrossRef] [PubMed]
- di Corcia, M.; Tartaglia, N.; Polito, R.; Ambrosi, A.; Messina, G.; Francavilla, V.C.; Cincione, R.I.; Della Malva, A.; Ciliberti, M.G.; Sevi, A.; et al. Functional properties of meat in athletes’ performance and recovery. Int. J. Environ. Res. Public Health 2022, 19, 5145. [Google Scholar] [CrossRef] [PubMed]
- Phomsoupha, M.; Berger, Q.; Laffaye, G. Multiple repeated sprint ability test for badminton players involving four changes of direction: Validity and reliability (part 1). J. Strength Cond. Res. 2018, 32, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, C.Q.; Bi, Y.; Chen, L.X. Effect of integrative neuromuscular training for injury prevention and sports performance of female badminton players. BioMed Res. Int. 2021, 2021, 5555853. [Google Scholar] [CrossRef]
- Fiorenza, M.; Hostrup, M.; Gunnarsson, T.P.; Shirai, Y.; Schena, F.; Iaia, F.M.; Bangsbo, J. Neuromuscular fatigue and metabolism during high-intensity intermittent exercise. Med. Sci. Sports Exerc. 2019, 51, 1642–1652. [Google Scholar] [CrossRef]
- Kowalik, S.; Wiśniewska, A.; Kędzierski, W.; Janczarek, L. Concentrations of circulating irisin and myostatin in race and endurace purebred Arabian horses-preliminary study. Animals 2020, 10, 2268. [Google Scholar] [CrossRef]
- Fernandez, J.; Granacher, U.; Sanz-Rivas, D.; Marín, J.M.S.; Hernandez-Davo, J.L.; Moya, M. Sequencing effects of neuromuscular training on physical fitness in youth elite tennis players. J. Strength Cond. Res. 2018, 32, 849–856. [Google Scholar] [CrossRef]
- Jing, H.; Ding, Y.; Jiang, X.; Liu, G.; Sha, Y. RNA-Seq reveals ACTH-induced steroid hormone pathway participating in goat adrenal gland response to castration. Sci. Rep. 2023, 13, 14025. [Google Scholar] [CrossRef]
- Su, L.; Yang, C.L.; Meng, J.Y.; Zhou, L.; Zhang, C.Y. Comparative transcriptome and metabolome analysis of Ostrinia furnacalis female adults under UV-A exposure. Sci. Rep. 2021, 11, 6797. [Google Scholar] [CrossRef]
- Li, G.S.; Yu, X.T.; Portela Fontoura, A.B.; Javaid, A.; Maza-Escolà, V.S.; Salandy, N.S.; Fubini, S.L.; Grilli, E.; McFadden, J.W.; Duan, J.E. Transcriptomic regulations of heat stress response in the liver of lactating dairy cows. BMC Genom. 2023, 24, 410. [Google Scholar] [CrossRef]
- Bello, S.F.; Xu, H.P.; Guo, L.J.; Li, K.; Zheng, M.; Xu, Y.B.; Zhang, S.Y.; Bekele, E.J.; Bahareldin, A.A.; Zhu, W.J.; et al. Hypothalamic and ovarian transcriptome profiling reveals potential candidate genes in low and high egg production of white Muscovy ducks (Cairina moschata). Poult. Sci. 2021, 100, 101310. [Google Scholar] [CrossRef] [PubMed]
- Yee, E.M.; Hauser, C.T.; Petrocelli, J.J.; de Hart, N.M.M.P.; Ferrara, P.J.; Bombyck, P.; Fennel, Z.J.; Onselen, L.V.; Mookerjee, S.; Funai, K.; et al. Treadmill training does not enhance skeletal muscle recovery following disuse atrophy in older male mice. Front. Physiol. 2023, 14, 1263500. [Google Scholar] [CrossRef] [PubMed]
- McGivney, B.A.; McGettigan, P.A.; Browne, J.A.; Evans, A.C.O.; Fonseca, R.G.; Lohan, A.J. Characterization of the equine skeletal muscle transcriptome identifies novel functional responses to exercise training. BMC Genom. 2010, 11, 1–17. [Google Scholar] [CrossRef]
- Núñez, Y.; Radović, Č.; Savić, R.; García-Casco, J.M.; Čandek-Potokar, M.; Benítez, R.; Radojković, D.; Lukić, M.; Gogić, M.; Muñoz, M.; et al. Muscle transcriptome analysis reveals molecular pathways related to oxidative phosphorylation, antioxidant defense, fatness and growth in mangalitsa and moravka pigs. Animals 2021, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Schaar, A.E.; Gustafson, G.E.; Smith, A.; Howell, P.R.; Greenman, A.C.; Baum, S.; Colman, R.J.; Lamming, D.W.; Diffee, G.M.; et al. Adiponectin receptor agonist AdipoRon improves skeletal muscle function in aged mice. eLife 2022, 11, e71282. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Min, Y.; Jeong, Y.; Ramani, S.; Lim, H.; Jo, Y.; Kim, W.; Choi, Y.J.; Park, S. Comparison of growth performance and related gene expression of muscle and fat from Landrace, Yorkshire, and Duroc and Woori black pigs. J. Anim. Sci. Technol. 2023, 65, 160. [Google Scholar] [CrossRef]
- Hou, J.L.; Yang, W.Y.; Zhang, Q.; Feng, H.; Wang, X.B.; Li, H.; Zhou, S.; Xiao, S.M. Integration of metabolomics and transcriptomics to reveal the metabolic characteristics of exercise-improved bone mass. Nutrients 2023, 15, 1694. [Google Scholar] [CrossRef]
- Isung, J.; Granqvist, M.; Trepci, A.; Huang, J.; Schwieler, L.; Kierkegaard, M.; Erhardt, S.; Jokinen, J.; Piehl, F. Differential effects on blood and cerebrospinal fluid immune protein markers and kynurenine pathway metabolites from aerobic physical exercise in healthy subjects. Sci. Rep. 2021, 11, 1669. [Google Scholar] [CrossRef]
- Briand, J.; Deguire, S.; Gaudet, S.; Bieuzen, F. Monitoring variables influence on random forest models to forecast injuries in short-track speed skating. Front. Sports Act. Living 2022, 4, 896828. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, J.W.; Yao, X.K.; Zeng, Y.Q.; Wang, C.K.; Ren, W.L.; Yuan, X.X.; Wang, T.L.; Meng, J. Transcriptome blood profile of the Yili horse before and after training. Acta Vet. Brno 2024, 93, 159–167. [Google Scholar] [CrossRef]
- Khan, I.M.; Cao, Z.; Liu, H.; Khan, A.; Rahman, S.U.; Khan, M.Z.; Sathanawongs, A.; Zhang, Y.H. Impact of cryopreservation on spermatozoa freeze-thawed traits and relevance OMICS to assess sperm Cryo-tolerance in farm animals. Front. Vet. Sci. 2021, 8, 609180. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, Y.; Yan, Y.; Terashvili, M.; Wells, C.; Horikoshi, H.; Fujita, S.; Bošnjak, Ž.J.; Bai, X.W. Fatty acid-treated induced pluripotent stem cell-derived human cardiomyocytes exhibit adult cardiomyocyte-like energy metabolism phenotypes. Cells 2019, 8, 1095. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, L.; Wang, J.; Cui, H.; Zhao, G.P.; Wen, J. FOSL2 is involved in the regulation of glycogen content in chicken breast muscle tissue. Front. Physiol. 2021, 12, 682441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Chen, Y.X.; Chen, T.F.; Miao, B.C.; Tang, Z.F.; Hu, X.; Luo, Y.L.; Zheng, T.; Na, N. Single-cell transcriptomics provides new insights into the role of fibroblasts during peritoneal fibrosis. Clin. Transl. Med. 2021, 11, e321. [Google Scholar] [CrossRef]
- Arisi, G.M.; Foresti, M.L.; Katki, K.; Shapiro, L.A. Increased CCL2, CCL3, CCL5, and IL-1β cytokine concentration in piriform cortex, hippocampus, and neocortex after pilocarpine-induced seizures. J. Neuroinflamm. 2015, 12, 129. [Google Scholar] [CrossRef]
- Szalay, G.; Martinecz, B.; Lénárt, N.; Környei, Z.; Orsolits, B.; Judák, L.; Császár, E.; Fekete, R.; West, B.L.; Katona, G.; et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat. Commun. 2016, 7, 11499. [Google Scholar] [CrossRef]
- Reißmann, M.; Rajavel, A.; Kokov, Z.A.; Schmitt, A.O. Identification of differentially expressed genes after endurance runs in Karbadian horses to determine candidates for stress indicators and performance capability. Genes 2023, 14, 1982. [Google Scholar] [CrossRef]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef]
- Torre-Villalvazo, I.; Alemán-Escondrillas, G.; Valle-Ríos, R.; Noriega, L.G. Protein intake and amino acid supplementation regulate exercise recovery and performance through the modulation of mTOR, AMPK, FGF21, and immunity. Nutr. Res. 2019, 72, 1–17. [Google Scholar] [CrossRef]
- McGivney, B.A.; Eivers, S.S.; MacHugh, D.E.; MacLeod, J.N.; O’Gorman, G.M.; Park, S.D.; Katz, L.M.; Hill, E.W. Transcriptional adaptations following exercise in thoroughbred horse skeletal muscle highlights molecular mechanisms that lead to muscle hypertrophy. BMC Genom. 2009, 10, 638. [Google Scholar] [CrossRef]
- Takegaki, J.; Ogasawara, R.; Tamura, Y.; Takagi, R.; Arihara, Y.; Tsutaki, A.; Nakazato, K.; Ishii, N. Repeated bouts of resistance exercise with short recovery periods activates mTOR signaling, but not protein synthesis, in mouse skeletal muscle. Physiol. Rep. 2017, 5, e13515. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Piazuelo, D.; Criado-Mesas, L.; Revilla, M.; Castelló, A.; Fernández, A.; Folch, J.M.; Ballester, M. Indel detection from whole genome sequencing data and association with lipid metabolism in pigs. PLoS ONE 2019, 14, e0218862. [Google Scholar] [CrossRef] [PubMed]
- Fadaei, R.; Moradi, N.; Kazemi, T.; Chamani, E.; Azdaki, N.; Moezibady, S.A.; Shahmohamadnejad, S.; Fallah, S. Decreased serum levels of CTRP12/adipolin in patients with coronary artery disease in relation to inflammatory cytokines and insulin resistance. Cytokine 2019, 113, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Smajdor, J.; Paczosa-Bator, B.; Piech, R. Advances on hormones and steroids determination: A review of voltammetric methods since 2000. Membranes 2022, 12, 1225. [Google Scholar] [CrossRef]
- Azhar, S.; Dong, D.; Shen, W.J.; Hu, Z.; Kraemer, F.B. The role of miRNAs in regulating adrenal and gonadal steroidogenesis. J. Mol. Endocrinol. 2020, 64, R21–R43. [Google Scholar] [CrossRef]
- Harahap, N.S.; Lelo, A.; Purba, A.; Sibuea, A.; Amelia, R.; Zulaini, Z. The effect of red-fleshed pitaya (Hylocereus polyrhizus) on heat shock protein 70 and cortisol expression in strenuous exercise induced rats. F1000Research 2019, 8, 130. [Google Scholar] [CrossRef]
- Hilborn, E.; Stål, O.; Alexeyenko, A.; Jansson, A. The regulation of hydroxysteroid 17β-dehydrogenase type 1 and 2 gene expression in breast cancer cell lines by estradiol, dihydrotestosterone, microRNAs, and genes related to breast cancer. Oncotarget 2017, 8, 62183. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: Roles and integration for cellular development and growth with exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef]
- Golds, G.; Houdek, D.; Arnason, T. Male hypogonadism and osteoporosis: The effects, clinical consequences, and treatment of testosterone deficiency in bone health. Int. J. Endocrinol. 2017, 2017, 4602129. [Google Scholar] [CrossRef]
- Hackney, A.C.; Willett, H.N. Testosterone responses to intensive, prolonged endurance exercise in women. Endocrines 2020, 1, 119–124. [Google Scholar] [CrossRef]
- Whitham, J.C.; Bryant, J.L.; Miller, L.J. Beyond glucocorticoids: Integrating dehydroepiandrosterone (DHEA) into animal welfare research. Animals 2020, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, A.A.; Truter, C.; Viljoen, F.P.; Myburgh, J.G.; Harvey, B.H. Temporal dynamics of plasma catecholamines, metabolic and immune markers, and the corticosterone: DHEA Ratio in farmed crocodiles before and after an acute stressor. Animals 2024, 14, 2236. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.J.; Kang, J.; Han, N.N.; Ma, H.W. Effect of dehydroepiandrosterone treatment on hormone levels and antioxidant parameters in aged rats. Genet. Mol. Res. 2015, 14, 11300–11311. [Google Scholar] [CrossRef] [PubMed]
- Chaterjee, S.; Mondal, S. Effect of regular yogic training on growth hormone and dehydroepiandrosterone sulfate as an endocrine marker of aging. Evid. Based Complement. Altern. Med. 2014, 2014, 240581. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef]
- Gu, M.; Wang, S.; Di, A.; Wu, D.; Hai, C.; Liu, X.F.; Bai, C.L.; Su, G.H.; Yang, L.; Li, G.P. Combined transcriptome and metabolome analysis of smooth muscle of myostatin knockout cattle. Int. J. Mol. Sci. 2023, 24, 8120. [Google Scholar] [CrossRef]
- Halliwell, B.; Cheah, I.K.; Drum, C.L. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 2016, 470, 245–250. [Google Scholar] [CrossRef]
- Cheah, I.K.; Tang, R.M.Y.; Yew, T.S.Z.; Lim, K.H.C.; Halliwelll, B. Administration of pure ergothioneine to healthy human subjects: Uptake, metabolism, and effects on biomarkers of oxidative damage and inflammation. Antioxid. Redox Signal. 2017, 26, 193–206. [Google Scholar] [CrossRef]
- Fovet, T.; Guilhot, C.; Delobel, P.; Chopard, A.; Py, G.; Brioche, T. Ergothioneine improves aerobic performance without any negative effect on early muscle recovery signaling in response to acute exercise. Front. Physiol. 2022, 13, 834597. [Google Scholar] [CrossRef]
- Church, D.D.; Hoffman, J.R.; Varanoske, A.N.; Wang, R.; Baker, K.M.; La Monica, M.B.; Beyer, K.S.; Dodd, S.J.; Oliveira, L.P.; Harris, R.C.; et al. Comparison of two β-alanine dosing protocols on muscle carnosine elevations. J. Am. Coll. Nutr. 2017, 36, 608–616. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Landau, G.; Stout, J.R.; Hoffman, M.W.; Sharvit, N.; Rosen, P.; Moran, D.S.; Fukuda, D.H.; Shelef, I.; Carmom, E.; et al. β-Alanine ingestion increases muscle carnosine content and combat specific performance in tactical athletes. Med. Sci. Sports Exerc. 2015, 47, 581. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ito, O.; Mukai, N.; Takahashi, H.; Takamats, K. High level of skeletal muscle carnosine contributes to the latter half of exercise performance during 30-s maximal cycle ergometer sprinting. Jpn. J. Physiol. 2002, 52, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Baguet, A.; Bourgois, J.; Vanhee, L.; Achten, E.; Derave, W. Important role of muscle carnosine in rowing performance. J. Appl. Physiol. 2010, 109, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Hoffman, J.R.; Fragala, M.S.; Oliveira, L.P.; Jajtner, A.R.; Fukuda, D.H.; Stout, J.R. A microbiopsy method for immunohistological and morphological analysis: A pilot study. Med. Sci. Sports Exerc. 2016, 48, 331–335. [Google Scholar] [CrossRef]
- Kuleš, J.; Rubić, I.; Beer Ljubić, B.; Bilić, P.; Barić Rafaj, R.; Brkljačić, M.; Burchmore, R.; Eckersall, D.; Mrljak, V. Combined untargeted and targeted metabolomics approaches reveal urinary changes of amino acids and energy metabolism in canine babesiosis with different levels of kidney function. Front. Microbiol. 2021, 12, 715701. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, M.; Jiang, Z.; Lan, Y.X.; Chen, L.; Chen, Y.J.; Li, H.L.; Hui, J.W.; Zhang, L.J.; Hu, X.L.; et al. Integrated transcriptomic and metabolomic profiling reveals dysregulation of purine metabolism during the acute phase of spinal cord injury in rats. Front. Neurosci. 2022, 16, 1066528. [Google Scholar] [CrossRef]
- Rodgers, R.L. Glucagon, cyclic AMP, and hepatic glucose mobilization: A half-century of uncertainty. Physiol. Rep. 2022, 10, e15263. [Google Scholar] [CrossRef]
- Gaitán, J.M.; Moon, H.Y.; Stremlau, M.; Dubal, D.B.; Cook, D.B.; Okonkwo, O.C.; Praag, H.V. Effects of aerobic exercise training on systemic biomarkers and cognition in late middle-aged adults at risk for Alzheimer’s disease. Front. Endocrinol. 2021, 12, 660181. [Google Scholar] [CrossRef]
- Rathmacher, J.A.; Fuller, J.C.; Baier, S.M.; Abumrad, N.N.; Angus, H.F.; Sharp, R.L. Adenosine-5′-triphosphate (ATP) supplementation improves low peak muscle torque and torque fatigue during repeated high intensity exercise sets. J. Int. Soc. Sports Nutr. 2012, 9, 48. [Google Scholar] [CrossRef]
- Polčic, P. Naming the Cycle: On the Etymology of the Citric Acid Cycle Intermediates. J. Chem. Educ. 2018, 95, 1894–1896. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, W.T.; Hsu, F.L.; Tsai, P.W.; Hou, C.C. Metabolomics investigation of exercise-modulated changes in metabolism in rat liver after exhaustive and endurance exercises. Eur. J. Appl. Physiol. 2010, 108, 557–566. [Google Scholar] [CrossRef]



| Training Stage | Time | Training Content |
|---|---|---|
| Before training | Week 1 | Completion of horse affinity training. |
| Week 2 | Single rein conditioning circle training 20 min/day: 10 min each on the right and left inner arms. | |
| Week 3 | Single rein conditioning circle training 20 min/day: after 10 min each in the right and left inner arms, saddle preparation training. | |
| Week 4 | Double rein conditioning circle training 30 min/day: 15 min each in the right and left inner arms. | |
| Mid-training | Week 5 | Double rein conditioning circle training 40 min/day: 20 min each in the right and left inner arms. |
| Week 6 | Warm-up training for the conditioning circle, 5 min each on the right and the left side of the body. Walk grass training, two laps each of the forward and reverse lines. | |
| Week 7 | Warm-up training for the conditioning circle, 5 min each on the right and the left side of the body. Grass route training, 15 min for each route. | |
| Week 8 | Warm-up training for the conditioning circle, 5 min each on the right and the left side of the body. One lap each of positive and negative lines for sand slow backpedal training for sand lines. | |
| After training | Week 9 | Warm-up training for the conditioning circle, 5 min each on the right and the left side of the body. Riding training walk for 2000 m. |
| Week 10 | Warm-up training for the conditioning circle, 5 min each on the right and the left side of the body. Riding training walk for 2000 m, trot for 1000 m. | |
| Week 11 | Warm-up training for the conditioning circle, 5 min each on the right and the left side of the body. Riding training (walk + trot) for 2000 m. | |
| Week 12 | Warm-up training for the conditioning circle, 5 min each on the right and the left side of the body. Riding training (walk + trot) for 2000 m. |
| Items | Before-Training | Mid-Training | After-Training |
|---|---|---|---|
| Race time (s) | 336.62 ± 36.06 Aa | 316.01 ± 26.35 ABb | 303.48 ± 25.51 Bb |
| Sample | Raw Reads | Clean Reads | Error Rate | Q20% | Q30% | GC Content% | Mapped Reads |
|---|---|---|---|---|---|---|---|
| BT88 | 46,659,636 | 72,290 | 0.03 | 96.49 | 91.77 | 53.13 | 16,742 (23.16%) |
| BT104 | 47,835,518 | 45,103,352 | 0.03 | 97.10 | 92.16 | 52.60 | 36,975,171 (81.98%) |
| BT105 | 47,867,242 | 194,802 | 0.03 | 96.78 | 92.11 | 57.96 | 163,550 (83.96%) |
| BT72 | 49,818,952 | 1,360,372 | 0.03 | 97.23 | 92.74 | 56.90 | 1,028,107 (75.58%) |
| AT88 | 31,903,812 | 31,808,328 | 0.03 | 96.84 | 91.77 | 51.34 | 21,458,031 (67.46%) |
| AT104 | 46,635,024 | 156,744 | 0.03 | 96.78 | 92.15 | 58.40 | 96,856 (61.79%) |
| AT105 | 45,383,768 | 612,552 | 0.03 | 96.66 | 92.10 | 56.32 | 506,631 (82.71%) |
| AT72 | 43,102,918 | 40,677,736 | 0.03 | 97.06 | 92.03 | 52.23 | 38,359,904 (94.3%) |
| Genes | log2FC | p-Value | Up/Down |
|---|---|---|---|
| CCL5 | 1.513 | 0.027 | Up |
| FOS | 3.193 | 0.001 | Up |
| CD3E | 1.819 | 0.012 | Up |
| HSD17B1 | 3.635 | 0.046 | Up |
| CCR3 | −2.316 | 0.022 | Down |
| C1QTNF12 | −3.185 | 0.011 | Down |
| GATA1 | −3.098 | 0.016 | Down |
| TMPRSS6 | 2.310 | 0.049 | Up |
| CLEC1A | −3.624 | 0.025 | Down |
| PRF1 | 2.711 | 0.002 | Up |
| ND5 | −1.657 | 0.029 | Down |
| Metabolites | VIP | FC | log2FC | p-Value | Up/Down |
|---|---|---|---|---|---|
| N-Acetyl-L-leucine | 1.402 | 1.585 | 0.664 | 0.023 | Up |
| Dehydroepiandrosterone (DHEA) | 1.308 | 1.780 | 0.832 | 0.041 | Up |
| cis-Aconitic acid | 1.342 | 3.437 | 1.781 | 0.033 | Up |
| Pentadecanoic Acid | 1.278 | 0.416 | −1.266 | 0.014 | Down |
| Indoxyl-β-D-glucuronide | 1.594 | 20.878 | 4.384 | 0.029 | Up |
| Palmitoyl sphingomyelin | 1.486 | 1.702 | 0.768 | 0.041 | Up |
| 3′-Adenosine monophosphate (3′-AMP) | 1.485 | 0.082 | −3.612 | 0.001 | Down |
| Proline-hydroxyproline | 1.691 | 1.662 | 0.733 | 0.005 | Up |
| Asparagine | 1.116 | 0.659 | −0.601 | 0.014 | Down |
| Acetaminophen glucuronide | 1.310 | 2.664 | 1.413 | 0.001 | Up |
| Carnosine | 1.647 | 1.874 | 0.906 | 0.021 | Up |
| Pantothenic acid | 1.177 | 1.560 | 0.642 | 0.024 | Up |
| Ergothioneine | 1.665 | 0.476 | −1.070 | 0.022 | Down |
| Androsterone | 1.618 | 0.177 | −2.498 | 0.016 | Down |
| Nicotinuric Acid | 1.342 | 0.439 | −1.188 | 0.023 | Down |
| Fmoc-L-Isoleucine | 1.504 | 1.634 | 0.708 | 0.027 | Up |
| Prostaglandin J2 | 1.235 | 0.382 | −1.388 | 0.013 | Down |
| cGMP | 1.141 | 1.507 | 0.592 | 0.028 | Up |
| 1-Methylhistidine | 1.709 | 2.223 | 1.152 | 0.008 | Up |
| Adenosine 5′-monophosphate | 1.456 | 0.116 | −3.109 | 0.001 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Li, P.; Huang, X.; Wang, C.; Zeng, Y.; Wang, J.; Yao, X.; Meng, J. Effects of Combined Transcriptome and Metabolome Analysis Training on Athletic Performance of 2-Year-Old Trot-Type Yili Horses. Genes 2025, 16, 197. https://doi.org/10.3390/genes16020197
Yang L, Li P, Huang X, Wang C, Zeng Y, Wang J, Yao X, Meng J. Effects of Combined Transcriptome and Metabolome Analysis Training on Athletic Performance of 2-Year-Old Trot-Type Yili Horses. Genes. 2025; 16(2):197. https://doi.org/10.3390/genes16020197
Chicago/Turabian StyleYang, Liping, Pengcheng Li, Xinxin Huang, Chuankun Wang, Yaqi Zeng, Jianwen Wang, Xinkui Yao, and Jun Meng. 2025. "Effects of Combined Transcriptome and Metabolome Analysis Training on Athletic Performance of 2-Year-Old Trot-Type Yili Horses" Genes 16, no. 2: 197. https://doi.org/10.3390/genes16020197
APA StyleYang, L., Li, P., Huang, X., Wang, C., Zeng, Y., Wang, J., Yao, X., & Meng, J. (2025). Effects of Combined Transcriptome and Metabolome Analysis Training on Athletic Performance of 2-Year-Old Trot-Type Yili Horses. Genes, 16(2), 197. https://doi.org/10.3390/genes16020197





