Understanding Heritable Variation Among Hosts in Infectious Diseases Through the Lens of Twin Studies
Abstract
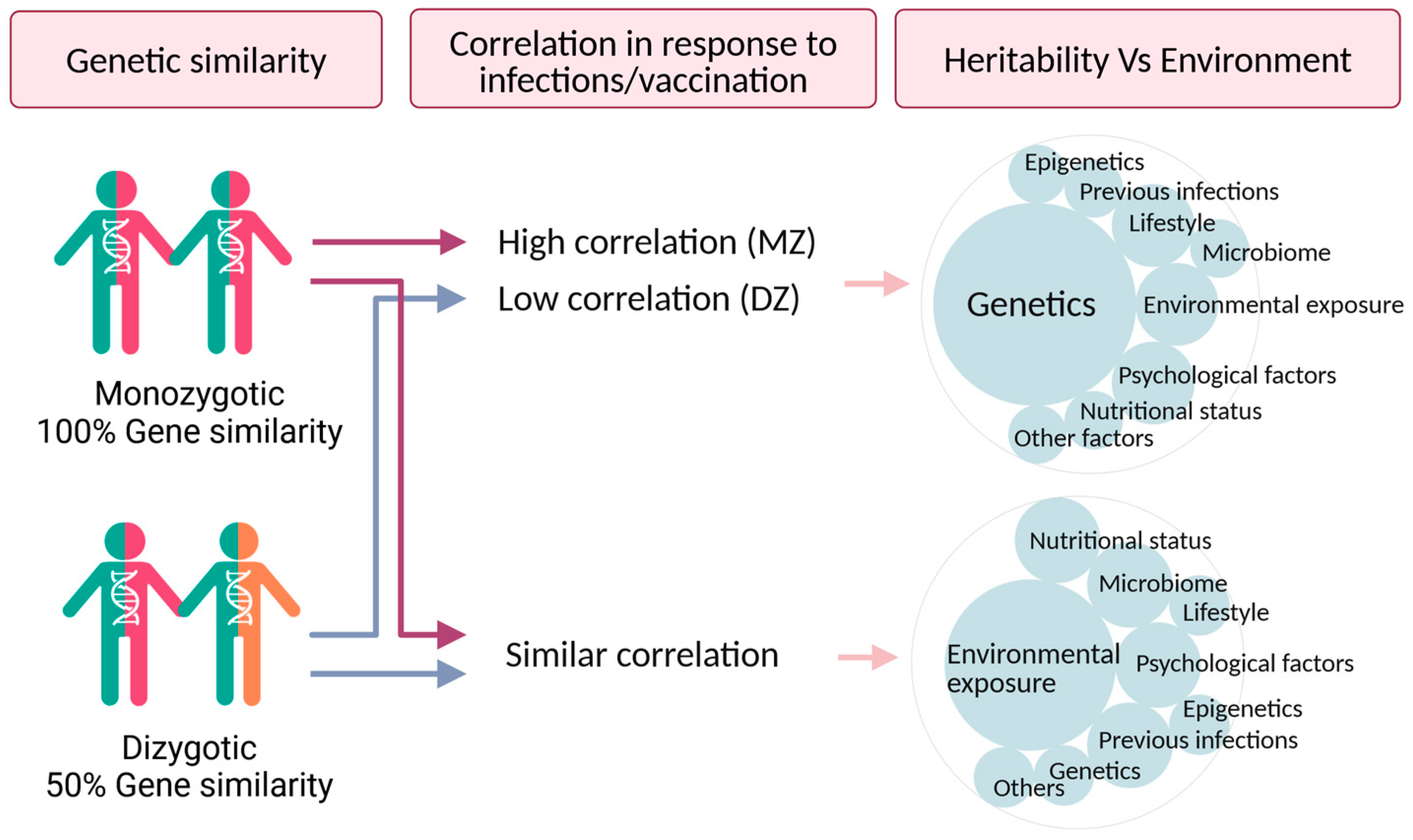
1. Introduction
2. Twin Studies of Susceptibility and Response to Infections
| Infection/Condition | Study Title | Year | Sample Size | Main Findings | Ref. | |
|---|---|---|---|---|---|---|
| 1 | Tuberculosis | Der erbeinfluss bei der tuberkulose (zwillingstuberkulose II) | 1936 | 205 twin pairs | Concordance: MZ: 65%–DZ: 25% | [10] |
| Twin Studies on the Significance of Genetic Factors in Tuberculosis | 1943 | 308 twin pairs | Concordance: MZ: 66.7%–DZ: 23% | [11] | ||
| Tuberculosis in Twins | 1963 | 205 twin pairs | Concordance: MZ: 32%–DZ: 14% | [12] | ||
| Tuberculosis in Twins: A Re-Analysis of the Prophit Survey | 1978 | 205 twin pairs | Concordance: MZ: 32.7%–DZ: 14% | [13] | ||
| 2 | Hepatitis B virus | Hepatitis B virus markers in Chinese twins | 1989 | 391 twin pairs 375 pairs matched singleton | Concordance: MZ: 35%–DZ: 4 | [16] |
| The primary comparative analysis between the host genetic factors and their relationships with clinical phenotype of HBV infected twins | 2004 | 20 pairs (HBV-infected and high risk twins) | Concordance: Significant difference in concordance between MZ and controls, and between MZ and DZ. | [17] | ||
| 3 | Otitis media | Distribution and Heritability of Recurrent Ear Infections | 1997 | 2750 twin pairs | Heritability: 0.74 in females and 0.45 in males | [20] |
| Otitis media: genetic factors and sex differences | 2004 | 4247 twin pairs | Heritability: 72% in females and 61% in males | [21] | ||
| The heritability of otitis media: a twin and triplet study | 1999 | 140 twin pairs | Heritability: 0.73 0.64 in males and 0.79 in females | [22] | ||
| Heritability of Symptom Domains in Otitis Media: A Longitudinal Study of 1373 Twin Pairs | 2002 | 1373 Twin pairs | Correlation: MZ: 0.9–DZ: <0.65 Heritability: 0.49–0.66, and 0.71, for different age groups | [24] | ||
| 4 | Plasmodium falciparum | Genetic regulation of fever in Plasmodium falciparum malaria in Gambian twin children | 1995 | 258 twin pairs | Fever in malaria is genetically regulated | [26] |
| Heritability of antibody isotype and subclass responses to Plasmodium falciparum antigens | 2009 | 519 twin pairs | Heritability: up to 48% for different plasma antibody isotypes and subclasses | [27] | ||
| 5 | Meningococcal disease | Genetic influence on cytokine production and fatal meningococcal disease | 1997 | 190 first-degree relatives 26 MZ twins | Heritability: MZ: 0.60 for TNF and 0.75 for IL-10 | [28] |
| 6 | Pliomyelitis | A twin-family study of susceptibility to poliomyelitis | 1951 | 46 families who had twins or triplets | Concordance: MZ: 36%–DZ: 6% | [29] |
| 7 | Cytomegalovirus | Neonatal cytomegalic inclusion disease in a set of twins one member of whom was a hydropic stillbirth the other completely uninfected | 1983 | 3 twin pairs | Concordanct in one case of MZ twins while discordant in two cases of DZ twin pairs | [30] |
| 8 | Multiple infections | Heredity and IDs: a twin study | 1984 | 656 twin pairs | Heritability: variable 86% of measles 0% scarlet fever | [31] |
| 9 | Helicobacter pylori | Helicobacter pylori infection: genetic and environmental influences. A study of twins | 1994 | 269 pairs: 36 MZ pairs reared apart 64 MZ pairs reared together 88 DZ pairs reared apart 81 DZ pairs reared together | Correlation: MZ (reared apart): 0.66 Concordance: MZ: 81%–DZ: 63% MZ reared apart: 82% DZ reared apart: 62% Heritability: 0.57 | [32] |
| 10 | Leprosy | A Twin Study on Leprosy | 1975 | 102 twin pairs | Concordance: MZ: 60% | [33] |
| 11 | Tonsillectomy | Iatrogenic influences on the heritability of childhood tonsillectomy: cohort differences in twin concordance | 1991 | NA | Heritability: In the 1950s: 29% Early 1960s: 82% | [34] |
| 12 | Epstein–Barr virus | Evidence of genetic susceptibility to infectious mononucleosis: a twin study | 2012 | 6926 twin pairs | Concordance: DZ: 12.1–MZ: 6.1 | [35] |
| 13 | Human papillomavirus | Cervix smear abnormalities: linking pathology data in female twins, their mothers and sisters | 2011 | 2020 twins 671 sisters of twins 416 mothers of twins 71 female spouses of male twins. | Correlation: MZ: 0.37–DZ and other first-degree relatives: 0.14 Heritability: 37% | [37] |
| 14 | Death due to infections | Genetic and Environmental Influences on Risk of Death due to Infections Assessed in Danish Twins, 1943–2001 | 2010 | 44,005 same-sex twin | Concordance: MZ vs. DZ: 9% vs. 0%, 10% vs. 3%, and 19% vs. 15% | [42] |
| 15 | SARS-CoV-2 | Self-Reported Symptoms of COVID-19, Including Symptoms Most Predictive of SARS-CoV-2 Infection, Are Heritable | 2020 | 3261 twins | Heritability: 19% to 49% for different SARS-CoV-2 symptoms | [38] |
| COVID-19 in twins: What can we learn from them | 2021 | 10 twin pairs | Higher concordance rates in the MZ twin pairs | [39] |
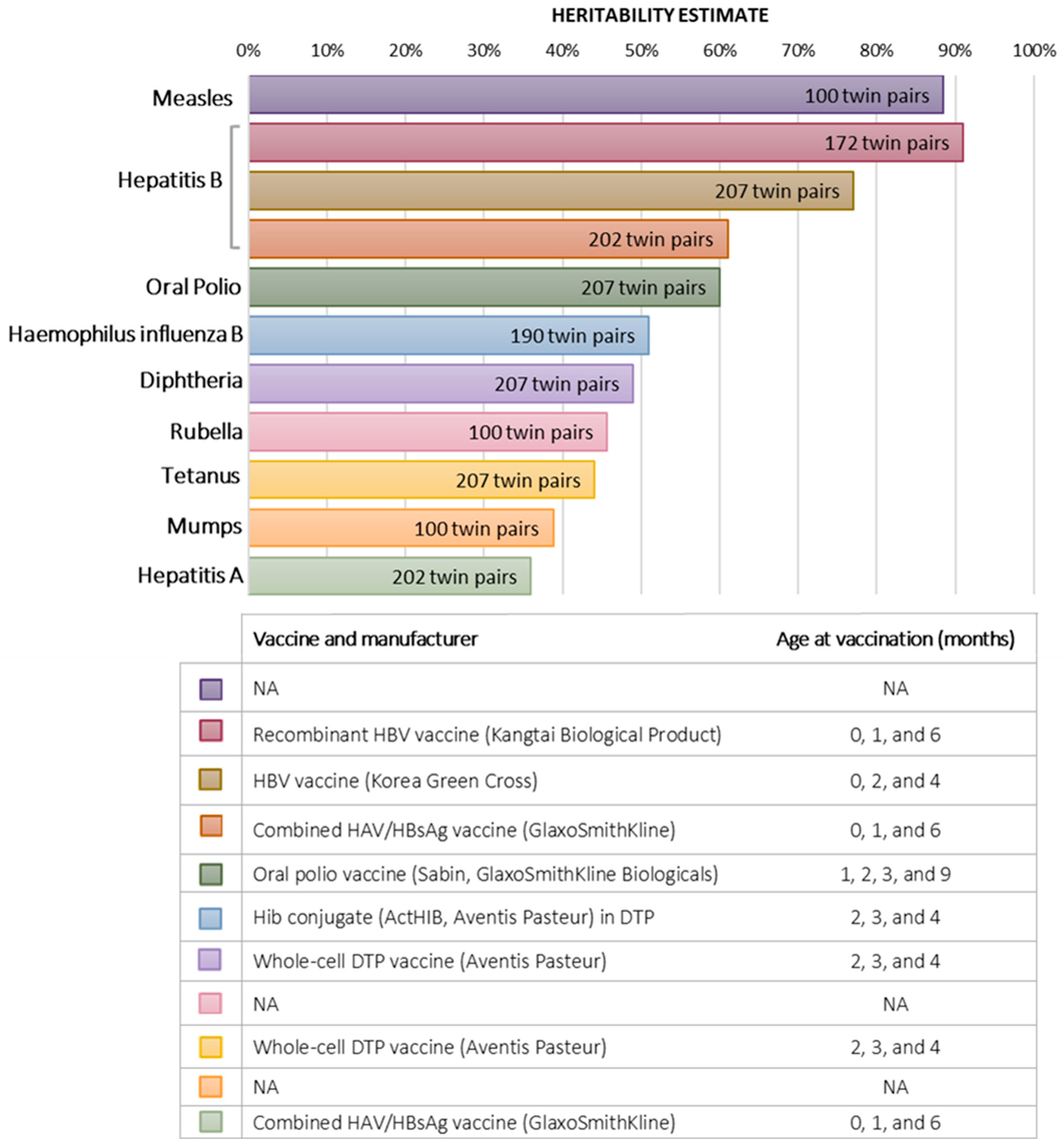
3. Twin Studies of Response to Vaccination
4. Twin Studies of General Immune Response
5. Twin Studies of Microbiome Composition
6. Advantages of the Twin Model in the IDs Field
7. Challenges for the Twin Model in the IDs Field
8. The Twin Model in the Modern Genomic Era
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Casanova, J.L.; Abel, L. Human genetics of infectious diseases: Unique insights into immunological redundancy. Semin. Immunol. 2018, 36, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Klebanov, N. Genetic Predisposition to Infectious Disease. Cureus 2018, 10, e3210. [Google Scholar] [CrossRef]
- Burbridge, D. Francis Galton on twins, heredity and social class. Br. J. Hist. Sci. 2001, 34, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Mayo, O. Early research on human genetics using the twin method: Who really invented the method? Twin Res. Hum. Genet. 2009, 12, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Boomsma, D.; Machin, G. A twin-pronged attack on complex traits. Nat. Genet. 1997, 17, 387–392. [Google Scholar] [CrossRef]
- Sahu, M.; Prasuna, J.G. Twin Studies: A Unique Epidemiological Tool. Indian. J. Community Med. 2016, 41, 177–182. [Google Scholar] [CrossRef]
- McGue, M. When assessing twin concordance, use the probandwise not the pairwise rate. Schizophr. Bull. 1992, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Falconer, D.S. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Ann. Hum. Genet. 1965, 29, 51–76. [Google Scholar] [CrossRef]
- Friedman, N.P.; Banich, M.T.; Keller, M.C. Twin studies to GWAS: There and back again. Trends Cogn. Sci. 2021, 25, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.G. Der erbeinfluss bei der tuberkulose (zwillingstuberkulose II). Eugen. Rev. 1937, 29, 207. [Google Scholar]
- Kallmann, F.J.; Reisner, D. Twin Studies on the Significance of Genetic Factors in Tuberculosis. Am. Rev. Tuberc. 1943, 47, 549–574. [Google Scholar] [CrossRef]
- Simonds, B. Tuberculosis in Twins; Pitman Medical Publishing Company: London, UK, 1963. [Google Scholar]
- Comstock, G.W. Tuberculosis in twins: A re-analysis of the Prophit survey. Am. Rev. Respir. Dis. 1978, 117, 621–624. [Google Scholar] [CrossRef] [PubMed]
- van der Eijk, E.A.; van de Vosse, E.; Vandenbroucke, J.P.; van Dissel, J.T. Heredity versus environment in tuberculosis in twins: The 1950s United Kingdom Prophit Survey Simonds and Comstock revisited. Am. J. Respir. Crit. Care Med. 2007, 176, 1281–1288. [Google Scholar] [CrossRef]
- Rieder, H.L. Of contagion and inherited susceptibility: An epidemiologic tribute to George W. Comstock. Am. J. Respir. Crit. Care Med. 2007, 176, 1176–1177. [Google Scholar] [CrossRef]
- Lin, T.M.; Chen, C.J.; Wu, M.M.; Yang, C.S.; Chen, J.S.; Lin, C.C.; Kwang, T.Y.; Hsu, S.T.; Lin, S.Y.; Hsu, L.C. Hepatitis B virus markers in Chinese twins. Anticancer. Res. 1989, 9, 737–741. [Google Scholar]
- Xu, B.Y.; Wang, Y.M.; Deng, G.H.; Huang, Y.P.; Zhong, L.H.; Liu, G.D.; Tan, Z.X.; Fan, Y.; Ding, S.T. The primary comparative analysis between the host genetic factors and their relationships with clinical phenotype of HBV infected twins. Zhonghua Yi Xue Za Zhi 2004, 84, 189–193. [Google Scholar] [PubMed]
- Jiang, Q.; Liu, Y.; Xu, B.; Zheng, W.; Xiang, X.; Tang, X.; Dong, H.; Chen, Y.; Wang, C.; Deng, G.; et al. Analysis of T cell receptor repertoire in monozygotic twins concordant and discordant for chronic hepatitis B infection. Biochem. Biophys. Res. Commun. 2018, 497, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Massa, H.M.; Cripps, A.W.; Lehmann, D. Otitis media: Viruses, bacteria, biofilms and vaccines. Med. J. Aust. 2009, 191, S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Kvaerner, K.J.; Tambs, K.; Harris, J.R.; Magnus, P. Distribution and heritability of recurrent ear infections. Ann. Otol. Rhinol. Laryngol. 1997, 106, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Kvestad, E.; Kvaerner, K.J.; Røysamb, E.; Tambs, K.; Harris, J.R.; Magnus, P. Otitis media: Genetic factors and sex differences. Twin Res. 2004, 7, 239–244. [Google Scholar] [CrossRef]
- Casselbrant, M.L.; Mandel, E.M.; Fall, P.A.; Rockette, H.E.; Kurs-Lasky, M.; Bluestone, C.D.; Ferrell, R.E. The heritability of otitis media: A twin and triplet study. JAMA 1999, 282, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Casselbrant, M.L.; Mandel, E.M.; Rockette, H.E.; Kurs-Lasky, M.; Fall, P.A.; Bluestone, C.D.; Ferrell, R.E. The genetic component of middle ear disease in the first 5 years of life. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rovers, M.; Haggard, M.; Gannon, M.; Koeppen-Schomerus, G.; Plomin, R. Heritability of symptom domains in otitis media: A longitudinal study of 1373 twin pairs. Am. J. Epidemiol. 2002, 155, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Wang, Q.; Chen, E.; Zheng, Q.Y. Current Understanding of Host Genetics of Otitis Media. Front. Genet. 2019, 10, 1395. [Google Scholar] [CrossRef]
- Jepson, A.P.; Banya, W.A.; Sisay-Joof, F.; Hassan-King, M.; Bennett, S.; Whittle, H.C. Genetic regulation of fever in Plasmodium falciparum malaria in Gambian twin children. J. Infect. Dis. 1995, 172, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Duah, N.O.; Weiss, H.A.; Jepson, A.; Tetteh, K.K.; Whittle, H.C.; Conway, D.J. Heritability of antibody isotype and subclass responses to Plasmodium falciparum antigens. PLoS ONE 2009, 4, e7381. [Google Scholar] [CrossRef]
- Westendorp, R.G.; Langermans, J.A.; Huizinga, T.W.; Elouali, A.H.; Verweij, C.L.; Boomsma, D.I.; Vandenbroucke, J.P.; Vandenbrouke, J.P. Genetic influence on cytokine production and fatal meningococcal disease. Lancet 1997, 349, 170–173. [Google Scholar] [CrossRef]
- Herndon, C.N.; Jennings, R.G. A twin-family study of susceptibility to poliomyelitis. Am. J. Hum. Genet. 1951, 3, 17–46. [Google Scholar] [PubMed]
- Morton, R.; Mitchell, I.; McNicol, E. Neonatal cytomegalic inclusion disease in a set of twins one member of whom was a hydropic stillbirth the other completely uninfected. Case report. BJOG Int. J. Obstet. Gynaecol. 1983, 90, 276–279. [Google Scholar] [CrossRef]
- Gedda, L.; Rajani, G.; Brenci, G.; Lun, M.T.; Talone, C.; Oddi, G. Heredity and infectious diseases: A twin study. Acta Genet. Med. Gemellol. 1984, 33, 497–500. [Google Scholar] [CrossRef]
- Malaty, H.M.; Engstrand, L.; Pedersen, N.L.; Graham, D.Y. Helicobacter pylori infection: Genetic and environmental influences. A study of twins. Ann. Intern. Med. 1994, 120, 982–986. [Google Scholar] [CrossRef]
- A Twin Study on Leprosy, By M.R. Chakravartti (Waltair, AP, India) and F. Vogel (Heidelberg, GFR). Georg Thieme Verlag, Stuttgart 1973. Vol. 1 in the series, Topics in Human Genetics, edited by P.E. Becker, W. Lenz, F. Vogel, G.G. Wendt. Soft cover, 17 × 24 cm, IX + 124 pp, 111 illustrations and 12 tables. Price: DM 54 (approximately US $ 22.00). Acta Genet. Med. Gemellol. 1975, 24, 179. [CrossRef]
- Martin, N.G.; Kehren, U.; Battistutta, D.; Mathews, J.D. Iatrogenic influences on the heritability of childhood tonsillectomy: Cohort differences in twin concordance. Acta Genet. Med. Gemellol. 1991, 40, 165–172. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hwang, A.E.; Hamilton, A.S.; Cockburn, M.G.; Ambinder, R.; Zadnick, J.; Brown, E.E.; Mack, T.M.; Cozen, W. Evidence of genetic susceptibility to infectious mononucleosis: A twin study. Epidemiol. Infect. 2012, 140, 2089–2095. [Google Scholar] [CrossRef]
- Couto, E.; Hemminki, K. Heritable and environmental components in cervical tumors. Int. J. Cancer 2006, 119, 2699–2701. [Google Scholar] [CrossRef]
- Vink, J.M.; van Kemenade, F.J.; Meijer, C.J.; Casparie, M.K.; Meijer, G.A.; Boomsma, D.I. Cervix smear abnormalities: Linking pathology data in female twins, their mothers and sisters. Eur. J. Hum. Genet. 2011, 19, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Williams, F.M.K.; Freidin, M.B.; Mangino, M.; Couvreur, S.; Visconti, A.; Bowyer, R.C.E.; Le Roy, C.I.; Falchi, M.; Mompeó, O.; Sudre, C.; et al. Self-Reported Symptoms of COVID-19, Including Symptoms Most Predictive of SARS-CoV-2 Infection, Are Heritable. Twin Res. Hum. Genet. 2020, 23, 316–321. [Google Scholar] [CrossRef] [PubMed]
- de Castro, M.V.; Silva, M.V.R.; Naslavsky, M.S.; Santos, K.S.; Magawa, J.Y.; Neto, E.C.; Passos-Bueno, M.R.; Zatz, M. COVID-19 in twins: What can we learn from them? medRxiv 2021. [Google Scholar] [CrossRef]
- de Castro, M.V.; Silva, M.V.R.; Soares, F.B.; Cória, V.R.; Naslavsky, M.S.; Scliar, M.O.; Castelli, E.C.; de Oliveira, J.R.; de Medeiros, G.X.; Sasahara, G.L.; et al. Corrigendum: Follow-up of young adult monozygotic twins after simultaneous critical coronavirus disease 2019: A case report. Front. Med. 2023, 10, 1185833. [Google Scholar] [CrossRef] [PubMed]
- Rupp, S.K.; Weimer, K.; Goebel-Stengel, M.; Enck, P.; Zipfel, S.; Stengel, A. Genetics, shared environment, or individual experience? A cross-sectional study of the health status following SARS-CoV-2 infection in monozygotic and dizygotic twins. Front. Psychiatry 2022, 13, 1048676. [Google Scholar] [CrossRef] [PubMed]
- Obel, N.; Christensen, K.; Petersen, I.; Sørensen, T.I.; Skytthe, A. Genetic and environmental influences on risk of death due to infections assessed in Danish twins, 1943–2001. Am. J. Epidemiol. 2010, 171, 1007–1013. [Google Scholar] [CrossRef]
- Smatti, M.K.; Alkhatib, H.A.; Al Thani, A.A.; Yassine, H.M. Will Host Genetics Affect the Response to SARS-CoV-2 Vaccines? Historical Precedents. Front. Med. 2022, 9, 802312. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Jacobson, R.M. Application of pharmacogenomics to vaccines. Pharmacogenomics 2009, 10, 837–852. [Google Scholar] [CrossRef]
- Newport, M.J.; Goetghebuer, T.; Weiss, H.A.; Whittle, H.; Siegrist, C.A.; Marchant, A.; Group, M.G.T.S. Genetic regulation of immune responses to vaccines in early life. Genes. Immun. 2004, 5, 122–129. [Google Scholar] [CrossRef]
- Marchant, A.; Pihlgren, M.; Goetghebuer, T.; Weiss, H.A.; Ota, M.O.; Schlegel-Hauter, S.E.; Whittle, H.; Lambert, P.H.; Newport, M.J.; Siegrist, C.A.; et al. Predominant influence of environmental determinants on the persistence and avidity maturation of antibody responses to vaccines in infants. J. Infect. Dis. 2006, 193, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.L.; Jacobson, R.M.; Poland, G.A.; Jacobsen, S.J.; Pankratz, V.S. Twin studies of immunogenicity--determining the genetic contribution to vaccine failure. Vaccine 2001, 19, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Sugiyama, M.; Sawai, H.; Nishina, S.; Sakai, A.; Ohashi, J.; Khor, S.S.; Kakisaka, K.; Tsuchiura, T.; Hino, K.; et al. Key HLA-DRB1-DQB1 haplotypes and role of the BTNL2 gene for response to a hepatitis B vaccine. Hepatology 2018, 68, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Höhler, T.; Reuss, E.; Evers, N.; Dietrich, E.; Rittner, C.; Freitag, C.M.; Vollmar, J.; Schneider, P.M.; Fimmers, R. Differential genetic determination of immune responsiveness to hepatitis B surface antigen and to hepatitis A virus: A vaccination study in twins. Lancet 2002, 360, 991–995. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Cai, W.; Cao, F.; Sun, H.; Chen, S.; Xu, R.; Wei, X.; Shi, X.; Yan, W. Genetic effects have a dominant role on poor responses to infant vaccination to hepatitis B virus. J. Hum. Genet. 2013, 58, 293–297. [Google Scholar] [CrossRef]
- Lee, Y.C.; Newport, M.J.; Goetghebuer, T.; Siegrist, C.A.; Weiss, H.A.; Pollard, A.J.; Marchant, A.; Group, M.T.S. Influence of genetic and environmental factors on the immunogenicity of Hib vaccine in Gambian twins. Vaccine 2006, 24, 5335–5340. [Google Scholar] [CrossRef] [PubMed]
- Jepson, A.; Banya, W.; Sisay-Joof, F.; Hassan-King, M.; Nunes, C.; Bennett, S.; Whittle, H. Quantification of the relative contribution of major histocompatibility complex (MHC) and non-MHC genes to human immune responses to foreign antigens. Infect. Immun. 1997, 65, 872–876. [Google Scholar] [CrossRef]
- Jepson, A.; Fowler, A.; Banya, W.; Singh, M.; Bennett, S.; Whittle, H.; Hill, A.V. Genetic regulation of acquired immune responses to antigens of Mycobacterium tuberculosis: A study of twins in West Africa. Infect. Immun. 2001, 69, 3989–3994. [Google Scholar] [CrossRef]
- Orrù, V.; Steri, M.; Sole, G.; Sidore, C.; Virdis, F.; Dei, M.; Lai, S.; Zoledziewska, M.; Busonero, F.; Mulas, A.; et al. Genetic variants regulating immune cell levels in health and disease. Cell 2013, 155, 242–256. [Google Scholar] [CrossRef]
- Glanville, J.; Kuo, T.C.; von Büdingen, H.C.; Guey, L.; Berka, J.; Sundar, P.D.; Huerta, G.; Mehta, G.R.; Oksenberg, J.R.; Hauser, S.L.; et al. Naive antibody gene-segment frequencies are heritable and unaltered by chronic lymphocyte ablation. Proc. Natl. Acad. Sci. USA 2011, 108, 20066–20071. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Cavanagh, M.M.; Le Saux, S.; Qi, Q.; Roskin, K.M.; Looney, T.J.; Lee, J.Y.; Dixit, V.; Dekker, C.L.; et al. B-cell repertoire responses to varicella-zoster vaccination in human identical twins. Proc. Natl. Acad. Sci. USA 2015, 112, 500–505. [Google Scholar] [CrossRef] [PubMed]
- de Craen, A.J.; Posthuma, D.; Remarque, E.J.; van den Biggelaar, A.H.; Westendorp, R.G.; Boomsma, D.I. Heritability estimates of innate immunity: An extended twin study. Genes. Immun. 2005, 6, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M.; Quaye, L.; Mangino, M.; Beddall, M.H.; Mahnke, Y.; Chattopadhyay, P.; Tosi, I.; Napolitano, L.; Terranova Barberio, M.; Menni, C.; et al. The genetic architecture of the human immune system: A bioresource for autoimmunity and disease pathogenesis. Cell 2015, 161, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Brodin, P.; Jojic, V.; Gao, T.; Bhattacharya, S.; Angel, C.J.; Furman, D.; Shen-Orr, S.; Dekker, C.L.; Swan, G.E.; Butte, A.J.; et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 2015, 160, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.D.; Jackson, K.J.L. Predicting vaccine responsiveness. Cell Host Microbe 2015, 17, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Dzierozynski, L.; Queen, J.; Sears, C.L. Subtle, persistent shaping of the gut microbiome by host genes: A critical determinant of host biology. Cell Host Microbe 2023, 31, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Igartua, C.; Davenport, E.R.; Gilad, Y.; Nicolae, D.L.; Pinto, J.; Ober, C. Host genetic variation in mucosal immunity pathways influences the upper airway microbiome. Microbiome 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Davenport, E.R.; Beaumont, M.; Jackson, M.A.; Knight, R.; Ober, C.; Spector, T.D.; Bell, J.T.; Clark, A.G.; Ley, R.E. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe 2016, 19, 731–743. [Google Scholar] [CrossRef]
- Stewart, J.A.; Chadwick, V.S.; Murray, A. Investigations into the influence of host genetics on the predominant eubacteria in the faecal microflora of children. J. Med. Microbiol. 2005, 54, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Zoetendal, E.G.; Akkermans, A.D.; Akkermans-van Vliet, W.M.; de Visser, J.A.G.; de Vos, W.M. The Host Genotype Affects the Bacterial Community in the Human Gastronintestinal Tract. Microb. Ecol. Health Dis. 2001, 13, 129–134. [Google Scholar] [CrossRef]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the human infant intestinal microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human genetics shape the gut microbiome. Cell 2014, 159, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Vilchez-Vargas, R.; Skieceviciene, J.; Lehr, K.; Varkalaite, G.; Thon, C.; Urba, M.; Morkūnas, E.; Kucinskas, L.; Bauraite, K.; Schanze, D.; et al. Gut microbial similarity in twins is driven by shared environment and aging. EBioMedicine 2022, 79, 104011. [Google Scholar] [CrossRef]
- Freire, M.; Moustafa, A.; Harkins, D.M.; Torralba, M.G.; Zhang, Y.; Leong, P.; Saffery, R.; Bockmann, M.; Kuelbs, C.; Hughes, T.; et al. Longitudinal Study of Oral Microbiome Variation in Twins. Sci. Rep. 2020, 10, 7954. [Google Scholar] [CrossRef] [PubMed]
- Rahmioğlu, N.; Ahmadi, K.R. Classical twin design in modern pharmacogenomics studies. Pharmacogenomics 2010, 11, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Plomin, R.; DeFries, J.C.; Loehlin, J.C. Genotype-environment interaction and correlation in the analysis of human behavior. Psychol. Bull. 1977, 84, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Hagenbeek, F.A.; Hirzinger, J.S.; Breunig, S.; Bruins, S.; Kuznetsov, D.V.; Schut, K.; Odintsova, V.V.; Boomsma, D.I. Maximizing the value of twin studies in health and behaviour. Nat. Hum. Behav. 2023, 7, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Gonggrijp, B.M.A.; van de Weijer, S.G.A.; Bijleveld, C.C.J.H.; van Dongen, J.; Boomsma, D.I. The Co-Twin Control Design: Implementation and Methodological Considerations. Twin Res. Hum. Genet. 2023, 26, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Hwang, L.D.; Mitchell, B.L.; Medland, S.E.; Martin, N.G.; Neale, M.C.; Evans, D.M. Correction to: The Augmented Classical Twin Design: Incorporating Genome-Wide Identity by Descent Sharing Into Twin Studies in Order to Model Violations of the Equal Environments Assumption. Behav. Genet. 2021, 51, 441–442. [Google Scholar] [CrossRef]
- Eaves, L. The use of twins in the analysis of assortative mating. Heredity 1979, 43, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Border, R.; O’Rourke, S.; de Candia, T.; Goddard, M.E.; Visscher, P.M.; Yengo, L.; Jones, M.; Keller, M.C. Author Correction: Assortative mating biases marker-based heritability estimators. Nat. Commun. 2022, 13, 1903. [Google Scholar] [CrossRef]
- Martin, A.R.; Gignoux, C.R.; Walters, R.K.; Wojcik, G.L.; Neale, B.M.; Gravel, S.; Daly, M.J.; Bustamante, C.D.; Kenny, E.E. Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am. J. Hum. Genet. 2017, 100, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Schaid, D.J.; Haralambieva, I.H.; Larrabee, B.R.; Ovsyannikova, I.G.; Kennedy, R.B.; Poland, G.A. Heritability of vaccine-induced measles neutralizing antibody titers. Vaccine 2017, 35, 1390–1394. [Google Scholar] [CrossRef]
- COVID-19 Host Genetic Initiative. Available online: https://www.covid19hg.org/blog/ (accessed on 1 December 2023).
- Minică, C.C.; Dolan, C.V.; Boomsma, D.I.; de Geus, E.; Neale, M.C. Extending Causality Tests with Genetic Instruments: An Integration of Mendelian Randomization with the Classical Twin Design. Behav. Genet. 2018, 48, 337–349. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smatti, M.K.; Yassine, H.M.; Mbarek, H.; Boomsma, D.I. Understanding Heritable Variation Among Hosts in Infectious Diseases Through the Lens of Twin Studies. Genes 2025, 16, 177. https://doi.org/10.3390/genes16020177
Smatti MK, Yassine HM, Mbarek H, Boomsma DI. Understanding Heritable Variation Among Hosts in Infectious Diseases Through the Lens of Twin Studies. Genes. 2025; 16(2):177. https://doi.org/10.3390/genes16020177
Chicago/Turabian StyleSmatti, Maria K., Hadi M. Yassine, Hamdi Mbarek, and Dorret I. Boomsma. 2025. "Understanding Heritable Variation Among Hosts in Infectious Diseases Through the Lens of Twin Studies" Genes 16, no. 2: 177. https://doi.org/10.3390/genes16020177
APA StyleSmatti, M. K., Yassine, H. M., Mbarek, H., & Boomsma, D. I. (2025). Understanding Heritable Variation Among Hosts in Infectious Diseases Through the Lens of Twin Studies. Genes, 16(2), 177. https://doi.org/10.3390/genes16020177






