Characterization of Metabolites and Transcriptome of Pepper Accessions from Four Southern Provinces of China
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Widely Targeted Metabolomics Analysis
2.3. RNA-Seq and Resequencing
2.4. Stastatistical Analysis
3. Results
3.1. Genome, Transcriptome and Metabolome Analyses
3.2. Characteristic Differential Metabolites and Expressed Genes of Pepper Accessions from Hunan Group and Yun_Gui_Chuan Group
3.3. Characteristic Differential Metabolites and Expressed Genes in Pepepr Accessions from Yunnan, Guizhou and Sichuan Provinces
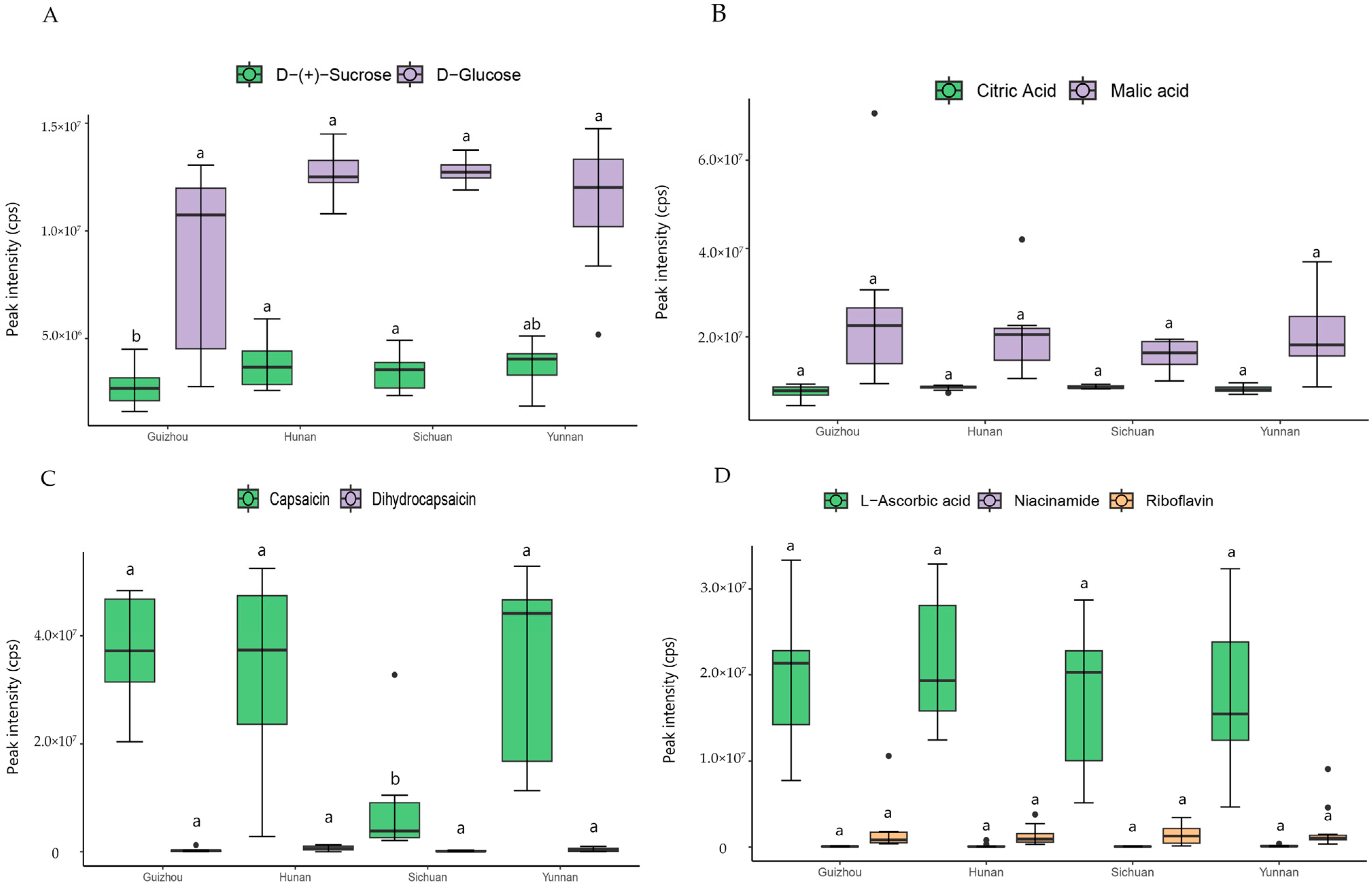
3.4. Taste and Nutrition Related Metabolites in Pepper Accession from Four Provinces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAO | Food and Agriculture Organization of the United Nations |
| PCA | Principal Component Analysis |
| FC | Fold Change |
| FDR | False Discovery Rate |
| VIP | Variable Importance in the Projection |
| oPLS-DA | orthogonal Partial Least Squares Discriminant Analysis |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| CV | Coefficients of Variation |
| QC | Quality Control |
References
- Andrews, J. Peppers: The Domesticated Capsicums; University of Texas Press: Austin, TX, USA, 1984. [Google Scholar]
- Perry, L.R.; Dickau, S.; Zarrillo, I.; Holst, D.M.; Pearsall, D.R.; Piperno, M.J.; Berman, M.J.; Cooke, R.G.; Rademaker, K.; Ranere, A.J.; et al. Starch Fossils and the Domestication and Dispersal of Chili Peppers (Capsicum Spp. L.) in the Americas. Science 2007, 315, 986–988. [Google Scholar] [CrossRef] [PubMed]
- Burger, C.P.J.; Smit, H.P. The World Pepper Economy—Developments and Outlook; Economisch en Sociaal Instituut (ESI): Prague, Czech Republic, 1997. [Google Scholar]
- Pickersgill, B. Domestication of plants in the Americas: Insights from mendelian and molecular genetics. Ann. Bot. 2007, 100, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Carrizo García, C.; Barfuss, M.H.; Sehr, E.M.; Barboza, G.E.; Samuel, R.; Moscone, E.A.; Ehrendorfer, F. Phylogenetic relationships, diversification and expansion of chili peppers (Capsicum, Solanaceae). Ann. Bot. 2016, 118, 35–51. [Google Scholar] [CrossRef]
- Smith, S.H. In the shadow of a pepper-centric historiography: Understanding the global diffusion of capsicums in the sixteenth and seventeenth centuries. J. Ethnopharmacol 2015, 167, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. The Spread of Pepper and Its Influence in China. In Agricultural History of China; China Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Batiha, G.E.-S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Ochoa-Alejo, N. Capsaicin accumulation in Capsicum spp. suspension cultures. Methods Mol. Biol. 2006, 318, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.B.; Gomes, V.M.; Moraes, T.M.; Zottich, U.P.; Rabelo, G.R.; Carvalho, A.O.; Moulin, M.; Gonçalves, L.S.; Rodrigues, R.; Da Cunha, M. Characterization of Capsicum species using anatomical and molecular data. Genet. Mol. Res. 2013, 12, 6488–6501. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Shishehbor, F.; Behroo, L.; Ghafouriyan Broujerdnia, M.; Namjoyan, F.; Latifi, S.-M. Quercetin effectively quells peanut-induced anaphylactic reactions in the peanut sensitized rats. Iran. J. Allergy Asthma Immunol. 2010, 9, 27–34. [Google Scholar]
- Xia, R.; Xu, G.; Huang, Y.; Sheng, X.; Xu, X.; Lu, H. Hesperidin suppresses the migration and invasion of non-small cell lung cancer cells by inhibiting the SDF-1/CXCR-4 pathway. Life Sci. 2018, 201, 111–120. [Google Scholar] [CrossRef]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. Mini Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, X.; Xu, J.; Wang, T.; Liang, T.; Xu, X.; Ma, C.; Xu, Z.; Wang, W.; Li, H.; et al. Luteolin alleviates neuroinflammation via downregulating the TLR4/TRAF6/NF-κB pathway after intracerebral hemorrhage. Biomed. Pharmacother. 2020, 126, 110044. [Google Scholar] [CrossRef] [PubMed]
- Pott, D.; Osorio, S.; Vallarino, J. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Perla, V.; Nimmakayala, P.; Nadimi, M.; Alaparthi, S.; Hankins, G.R.; Ebert, A.W.; Reddy, U.K. Vitamin C and reducing sugars in the world collection of Capsicum baccatum L. genotypes. Food Chem. 2016, 202, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Martínez, E.; García-Martínez, M.D.; Adalid-Martínez, A.M.; Pereira-Dias, L.; Casanova, C.; Soler, E.; Figàs, M.R.; Raigón, M.D.; Plazas, M.; Soler, S.; et al. Fruit composition profile of pepper, tomato and eggplant varieties grown under uniform conditions. Food Res. Int. 2021, 147, 110531. [Google Scholar] [CrossRef] [PubMed]
- Naves, E.R.; de Ávila Silva, L.; Sulpice, R.; Araújo, W.L.; Nunes-Nesi, A.; Peres, L.E.; Zsögön, A. Capsaicinoids: Pungency beyond Capsicum. Trends Plant Sci. 2019, 24, 109–120. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Shultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef]
- Gil Martínez, V.; Avedillo Salas, A.; Santander Ballestín, S. Vitamin Supplementation and Dementia: A Systematic Review. Nutrients 2022, 14, 1033. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Fan, X.; Zhang, S.; Wu, T.; Bai, L.; Wang, H.; Ma, Y.; Guan, X.; Wang, C.; Yang, H. Effects of variety and origin on the metabolic and texture characteristics of quinoa seeds based on ultrahigh-performance liquid chromatography coupled with high-field quadrupole-orbitrap high-resolution mass spectrometry. Food Res. Int. 2022, 162, 111693. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, H.; An, Y.; Zhang, S.; Sheng, Y.; Wang, C. Analysis on the Difference of Daohuaxiang Rice Metabolites in Different Producing Areas. Sci. Technol. Food Ind. 2022, 43, 10–20. [Google Scholar]
- Feng, X.; Yu, Q.; Li, B.; Kan, J. Comparative analysis of carotenoids and metabolite characteristics in discolored red pepper and normal red pepper based on non-targeted metabolomics. LWT 2022, 153, 112398. [Google Scholar] [CrossRef]
- Hu, H.; Du, L.; Zhang, R.; Zhong, Q.; Liu, F.; Li, W.; Gui, M. Dissection of Metabolome and Transcriptome—Insights into Capsaicin and Flavonoid Accumulation in Two Typical Yunnan Xiaomila Fruits. Int. J. Mol. Sci. 2024, 25, 7761. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Díaz-Valenzuela, E.; Sawers, R.H.; Cibrián-Jaramillo, A. Cis- and Trans-Regulatory Variations in the Domestication of the Chili Pepper. Mol. Biol. Evol. 2020, 37, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.S.; Purugganan, M.D. Evolution of crop species: Genetics of domestication and diversification. Nat. Rev. Genet. 2013, 14, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M.; Wagler, T.N.; Quijada, P.; Doebley, J. A distant upstream enhancer at the maize domestication gene tb1 has pleiotropic effects on plant and inflorescent architecture. Nat. Genet. 2006, 38, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J.F.; Gaut, B.S.; Smith, B.D. The molecular genetics of crop domestication. Cell 2006, 127, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Pu, K.; Ding, D.; Yang, Y.; Niu, T.; Li, J.; Xie, J. Foliar Spraying of Glycine Betaine Alleviated Growth Inhibition, Photoinhibition, and Oxidative Stress in Pepper (Capsicum annuum L.) Seedlings under Low Temperatures Combined with Low Light. Plants 2023, 12, 2563. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, J.; Xiang, Z.; Xi, P.; Zhao, D. Physiological Changes and Differential Gene Expression of Tea Plants (Camellia sinensis (L.) Kuntze var. niaowangensis Q.H. Chen) Under Cold Stress. DNA Cell Biol. 2021, 40, 906–920. [Google Scholar] [CrossRef]
- Choi, J.Y.; Cho, J.S.; Park, K.J.; Choi, J.H.; Lim, J.H. Effect of Moisture Content Difference on the Analysis of Quality Attributes of Red Pepper (Capsicum annuum L.) Powder Using a Hyperspectral System. Foods 2022, 11, 4086. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, Y.; Tan, F.; Chen, W.; Ou, L. Effects of Soil Type on Trace Element Absorption and Fruit Quality of Pepper. Front. Plant Sci. 2021, 12, 698796. [Google Scholar] [CrossRef]
- Mahmood, T.; Rana, R.M.; Ahmar, S.; Saeed, S.; Gulzar, A.; Khan, M.A.; Wattoo, F.M.; Wang, X.; Branca, F.; Mora-Poblete, F.; et al. Effect of Drought Stress on Capsaicin and Antioxidant Contents in Pepper Genotypes at Reproductive Stage. Plants 2021, 10, 1286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Yin, W.; Yang, B.; Ma, Y. The Development Status, Problems and Suggestions of Hunan Pepper Industry. J. China Capsicum 2020, 18, 8–13. [Google Scholar]
- Gui, M.; Du, L.; Zhang, R.; Zhong, Q.; Long, H. Development of Processing Pepper Industry in Yunnan Province. Agric. Eng. 2019, 9, 70–73. [Google Scholar]
- Lu, M.; Tong, S.Q.; Peng, G.H.; Zhou, M.; Wu, Y.J. Processing status and development suggestions on pepper industry in Guizhou. China Brew. 2021, 40, 221–224. [Google Scholar]
- Xu, Y.; Li, H.; Fang, H.; Gong, X.; Chen, X.; Song, Z. Development Status and Suggestions on Pepper Industry in Sichuan Province. J. China Capsicum 2020, 18, 1–5. [Google Scholar]
- Gong, C.; Guo, G.; Pan, B.; Gao, C.; Zhu, X.; Liu, G.; Wang, S.; Diao, W. Global Transcription and Metabolic Profiles of Five Tissues in Pepper Fruits. Sci. Data 2024, 11, 1129. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, Q.; Zhang, A.; Li, D.; Liu, Y.; Xu, H.; Yang, Q.; Liu, F.; Han, T.; Tang, X.; et al. Integrated metabolome and transcriptome analysis provides clues to fruit color formation of yellow, orange, and red bell pepper. Sci. Rep. 2024, 14, 29737. [Google Scholar] [CrossRef] [PubMed]
- Von Steimker, J.; Tripodi, P.; Wendenburg, R.; Tringovska, I.; Nankar, A.N.; Stoeva, V.; Pasev, G.; Klemmer, A.; Todorova, V.; Bulut, M.; et al. The genetic architecture of the pepper metabolome and the biosynthesis of its signature capsianoside metabolites. Curr. Biol. 2024, 34, 4209–4223.e4203. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.; Zhang, H.; Liu, X.; Yu, S.; Xiong, L.; Luo, J. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-discovery approach for sample matching of a nerve-agent precursor using liquid chromatography-mass spectrometry, XCMS, and chemometrics. Anal. Chem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Kattupalli, D.; Pinski, A.; Sreekumar, S.; Usha, A.; Girija, A.; Beckmann, M.; Mur, L.A.J.S.; Vasudevan, E. Non-Targeted Metabolite Profiling Reveals Host Metabolomic Reprogramming during the Interaction of Black Pepper with Phytophthora capsici. Int. J. Mol. Sci. 2021, 22, 11433. [Google Scholar] [CrossRef]
- Abdelhafez, O.H.; Othman, E.M.; Fahim, J.R.; Desoukey, S.Y.; Pimentel-Elardo, S.M.; Nodwell, J.R.; Schirmeister, T.; Tawfike, A.; Abdelmohsen, U.R. Metabolomics analysis and biological investigation of three Malvaceae plants. Phytochem. Anal. 2020, 31, 204–214. [Google Scholar] [CrossRef]
- Fischer, S.; Flis, P.; Zhao, F.J.; Salt, D.E. Transcriptional network underpinning ploidy-related elevated leaf potassium in neo-tetraploids. Plant Physiol. 2022, 190, 1715–1730. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Zhang, X.; Chen, Z.; Xia, Y.; Wang, L.; Sun, Y.; Zhang, M.; Xiao, Y.; Han, Z.; et al. Plant receptor-like protein activation by a microbial glycoside hydrolase. Nature 2022, 610, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Baker, D.; van den Beek, M.; Blankenberg, D.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Eberhard, C.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses. Nucleic Acids Res. 2016, 44, W3–W10. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, K.; Yu, H.; Chen, S.; Xu, D.; Zhao, H.; Zhang, Z.; Yang, Y.; Gu, X.; Liu, X.; et al. Pepper variome reveals the history and key loci associated with fruit domestication and diversification. Mol. Plant 2022, 15, 1744–1758. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 28 October 2024).
- Tang, Q.; Zheng, X.; Chen, W.; Ye, X.; Tu, P. Metabolomics reveals key resistant responses in tomato fruit induced by Cryptococcus laurentii. Food Chem. Mol. Sci. 2021, 4, 100066. [Google Scholar] [CrossRef] [PubMed]
- Bouzayen, M.; Latché, A.; Nath, P.; Pech, J.C. (Eds.) Mechanism of Fruit Ripening; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Dillard, C.J.; German, J.B. Phytochemicals: Nutraceuticals and human health. J. Sci. Food Agric. 2000, 80, 1744–1756. [Google Scholar] [CrossRef]
- Olatunji, T.L.; Afolayan, A.J. The suitability of chili pepper (Capsicum annuum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr. 2018, 6, 2239–2251. [Google Scholar] [CrossRef]
- Goncalo, C.J. Flavonoids—From Biosynthesis to Human Health; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Zhang, K.L.; Leng, Y.N.; Hao, R.R.; Zhang, W.Y.; Li, H.F.; Chen, M.X.; Zhu, F.Y. Adaptation of High-Altitude Plants to Harsh Environments: Application of Phenotypic-Variation-Related Methods and Multi-Omics Techniques. Int. J. Mol. Sci. 2024, 25, 12666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kuang, T.; Dong, W.; Qian, Z.; Zhang, H.; Landis, J.B.; Feng, T.; Li, L.; Sun, Y.; Huang, J.; et al. Genomic convergence underlying high-altitude adaptation in alpine plants. J. Integr. Plant Biol. 2023, 65, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yu, S.; Zhang, W.; Zhang, S.; Fu, J.; Ying, H.; Pingcuo, G.; Liu, S.; Zhao, F.; Wu, Q.; et al. The content and diversity of carotenoids associated with high-altitude adaptation in Tibetan peach fruit. Food Chem. 2023, 398, 133909. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y.; Wu, X.; Wen, X.; Li, D.; Zhou, H.; Li, Z.; Liu, B.; Wei, J.; Chen, F.; et al. High-quality evergreen azalea genome reveals tandem duplication-facilitated low-altitude adaptability and floral scent evolution. Plant Biotechnol. J. 2021, 19, 2544–2560. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Zuo, H.; Zheng, W.; Zhang, S.; Huang, Y.; Pingcuo, G.; Ying, H.; Zhao, F.; Li, Y.; et al. Genomic basis of high-altitude adaptation in Tibetan Prunus fruit trees. Curr. Biol. 2021, 31, 3848–3860.e8. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H. Regional cultural influences on the introduction of chili peppers to China. Agric. Archaeol. 2013, 1, 221–224. (In Chinese) [Google Scholar]
- Sobczak, A.; Pióro-Jabrucka, E.; Gajc-Wolska, J.; Kowalczyk, K. Effect of Salicylic Acid and Calcium on Growth, Yield, and Fruit Quality of Pepper (Capsicum annuum L.) Grown Hydroponically. Agronomy 2024, 14, 329. [Google Scholar] [CrossRef]
- Lee, S.-J.; Hong, S.; Yoo, S.-H.; Kim, G.-W. Cyanidin-3-O-Sambubioside from Acanthopanax sessiliflorus Fruit Inhibits Metastasis by Downregulating MMP-9 in Breast Cancer Cells MDA-MB-231. Planta Med. 2013, 79, 1636–1640. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wu, Y.; Bu, Q.; Zhong, K.; Gao, H. Comparative study on antibacterial mechanism of shikimic acid and quinic acid against Staphylococcus aureus through transcriptomic and metabolomic approaches. Food Sci. Technol. 2022, 153, 112441. [Google Scholar] [CrossRef]
- Rivera-Pérez, A.; Romero-González, R.; Garrido Frenich, A. Application of an innovative metabolomics approach to discriminate geographical origin and processing of black pepper by untargeted UHPLC-Q-Orbitrap-HRMS analysis and mid-level data fusion. Food Res. Int. 2021, 150, 110722. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, Y.; Tan, J.; Lv, H.; Wang, Y.; Chen, D.-X. Metabolomics and transcriptomics reveal the mechanism of alkaloid synthesis in Corydalis yanhusuo bulbs. PLoS ONE 2024, 19, e0304258. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, R.; Chen, M.; Wang, X.; Yin, H.; Liu, Y.; Jiang, L. Comparison and Analysis of the Flavor Components of Chopped Peppers from Farmhouses in Different Regions of Hunan. Sci. Technol. Food Ind. 2022, 43, 310–318, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Dou, J. Comparisons of Yield and Disease Resistance of Different Dried Hot Pepper. China Fruit Veg. 2016, 35, 32–34, (In Chinese with English abstract). [Google Scholar]
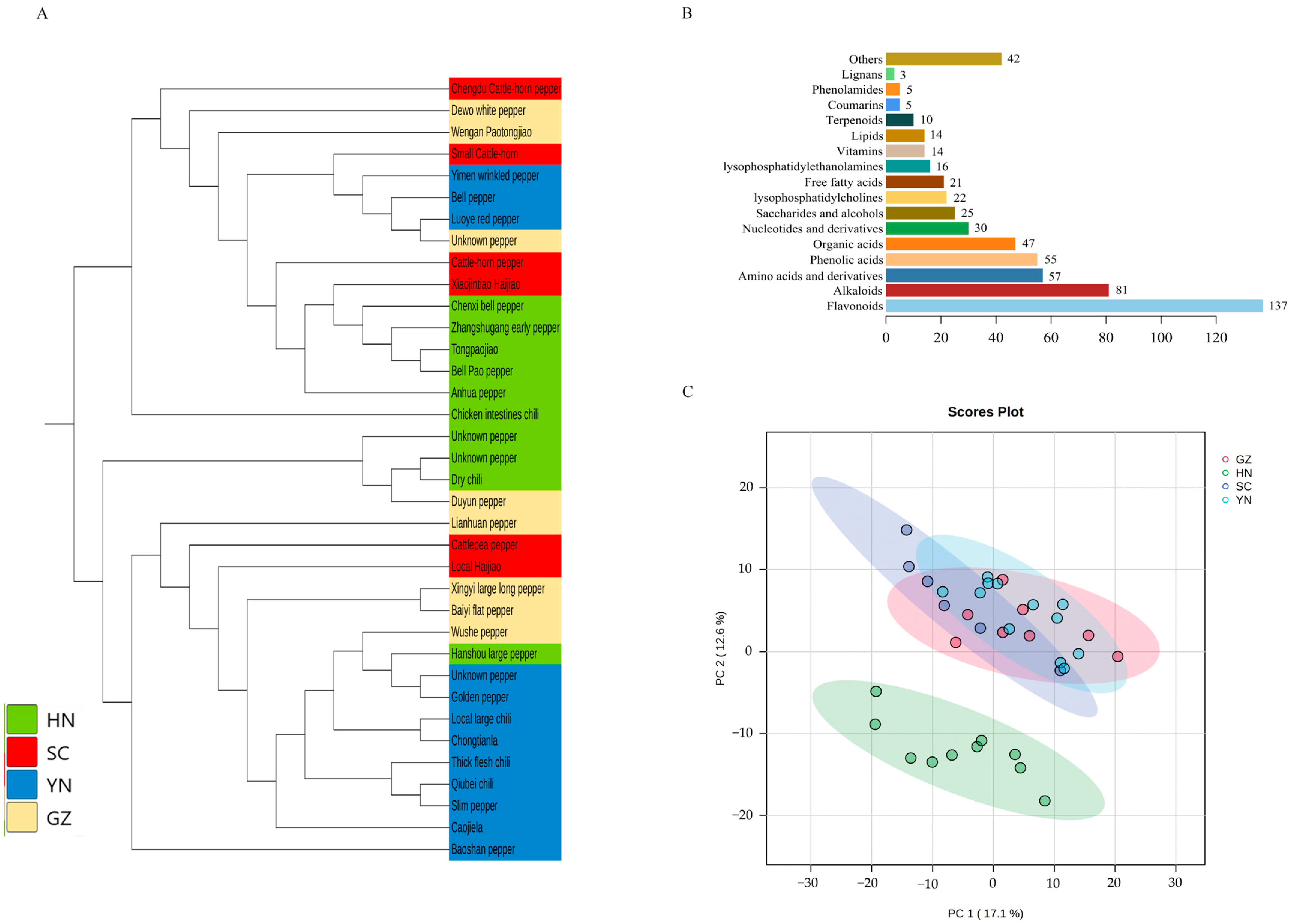
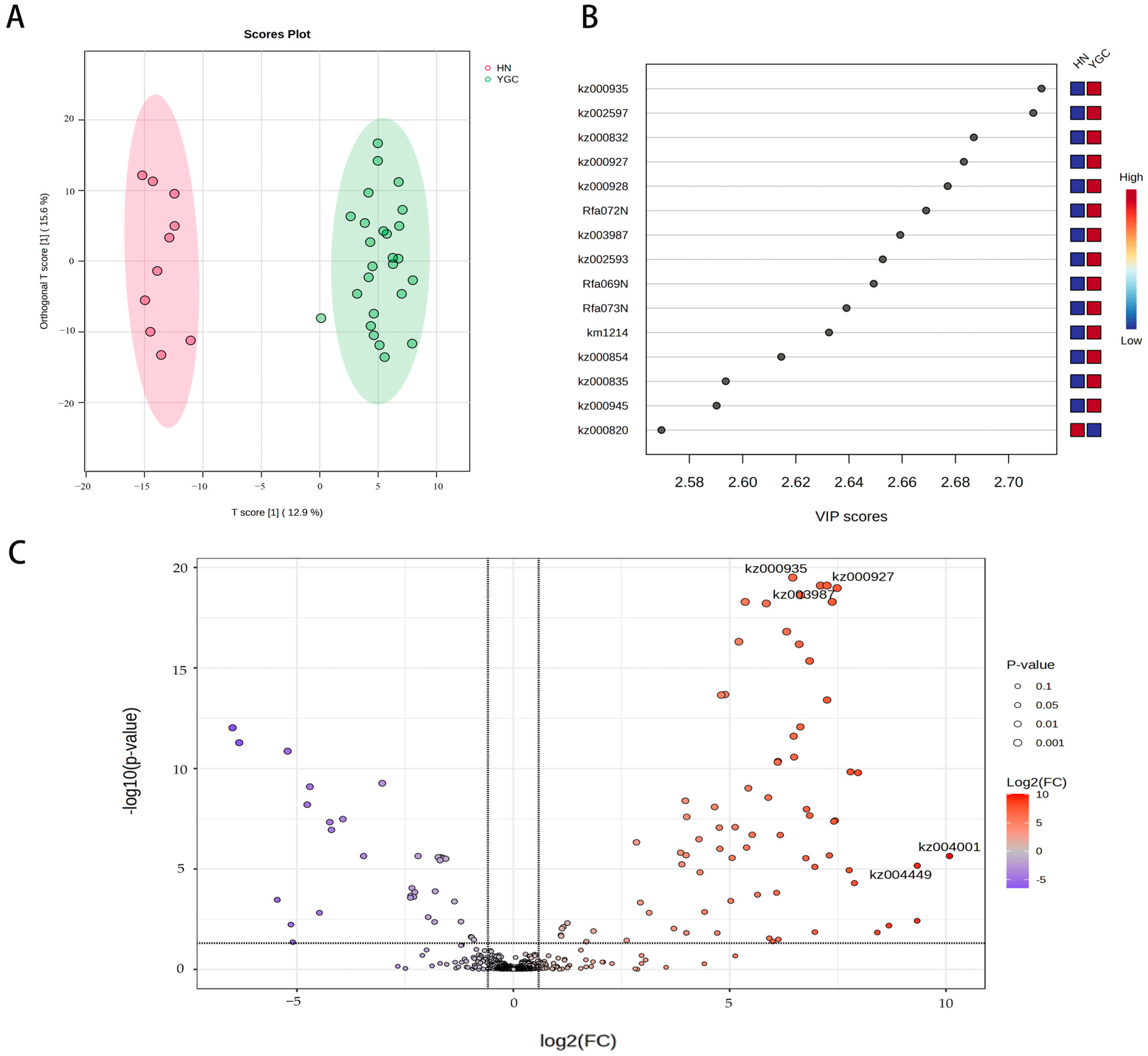
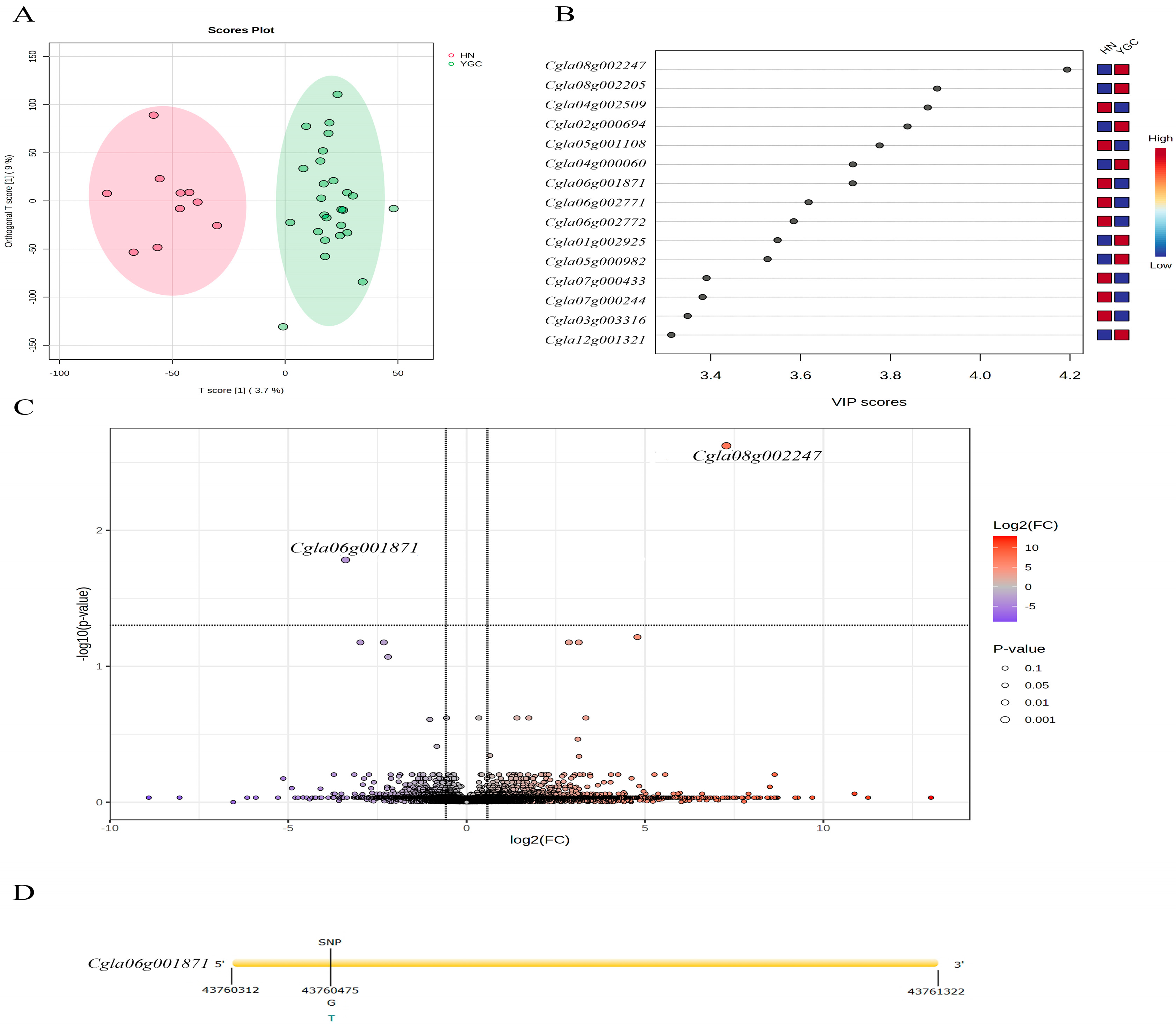
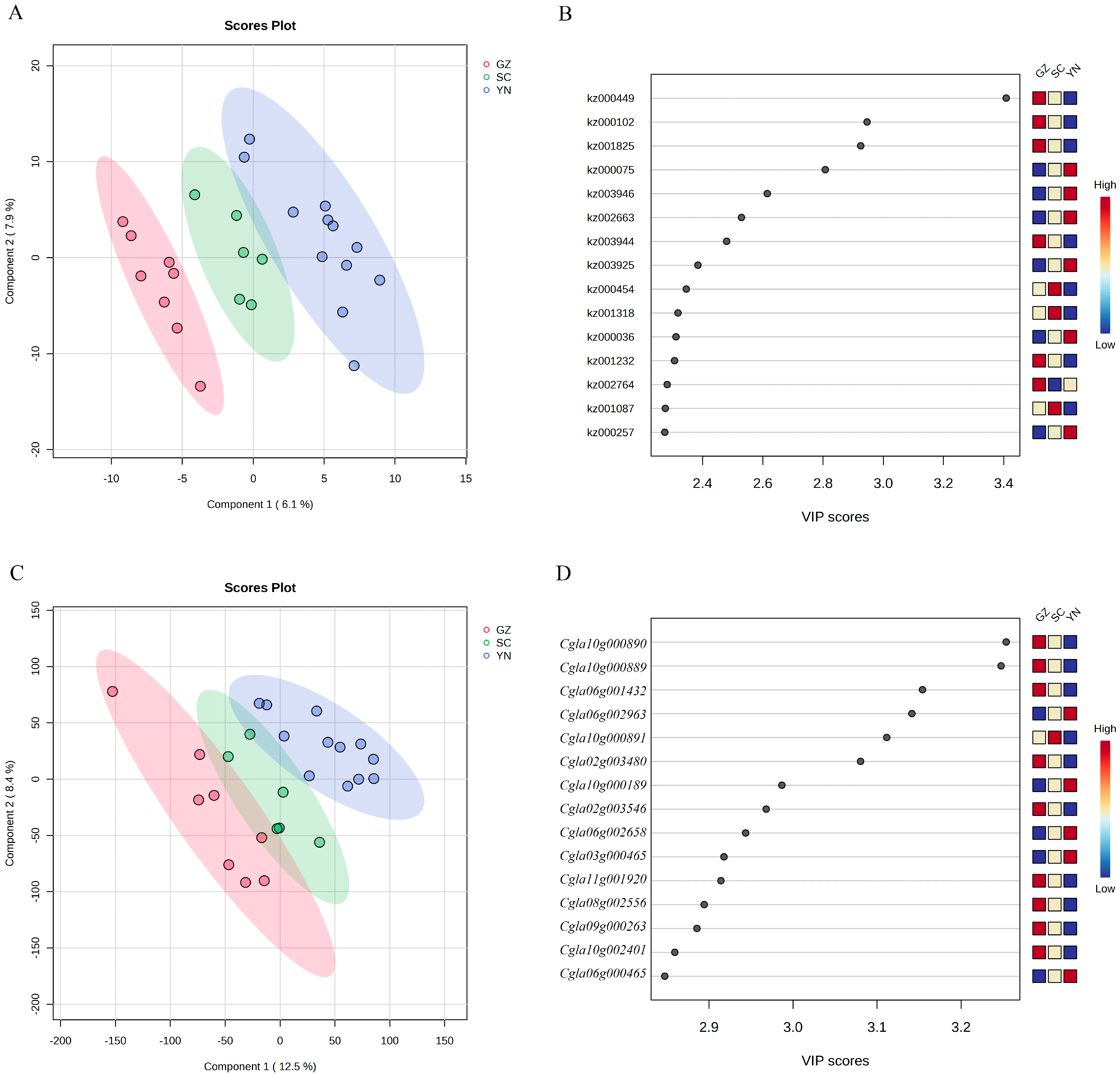
| KZ ID | Compounds | Class | The Group from Which Pepper Accessions with Higher Characteristic Metabolites Content Come |
|---|---|---|---|
| kz002597 | Kaempferol-C-glucoside-C-glucoside | Flavonoid carbonoside | Yun_Gui_Chuan |
| kz000935 | C-Hexosyl-luteolin C-pentoside | Flavonoid carbonoside | |
| kz000832 | Luteolin-6,8-di-C-glucoside | Flavonoid carbonoside | |
| kz002593 | luteolin-6-C-glucoside (Isoorientin) | Flavones | |
| km1214 | Chrysoeriol 8-C-hexoside | Flavonoid carbonoside | |
| kz000927 | Luteolin-6-C-glucoside (Isoorientin) | Flavonoid carbonoside | |
| Rfa072N | Luteolin 6-C-hexoside-8-C-pentoside | Flavonoid carbonoside | |
| kz000835 | Luteolin-6-C-2-glucuronylglucoside | Flavonoid carbonoside | |
| kz003987 | Luteolin-O-Malonyl-O-Hexoside-O-Pentoside | Flavones | |
| kz000820 | Apigenin O-hexosyl-O-pentoside | Flavones | Hunnan |
| Gene ID | Function Annotation | The Group from Which Pepper Accessions with Higher Characteristic Gene Expression Come |
|---|---|---|
| Cgla01g002925 | F-box domain-containing protein | Yun_Gui_Chuan |
| Cgla02g000694 | Transcription factor TCP17 | |
| Cgla04g000060 | RING-H2 finger protein ATL44 | |
| Cgla05g000982 | sulfated surface glycoprotein 185-like | |
| Cgla08g002205 | F-box domain-containing protein | |
| Cgla08g002247 | F-box domain-containing protein | |
| Cgla12g001321 | Rop guanine nucleotide exchange factor 9 | |
| Cgla03g003316 | Transposon MuDR mudrA-like protein, putative | Hunan |
| Cgla04g002509 | Chaperone protein dnaJ 2 | |
| Cgla05g001108 | protein PHYLLO, chloroplastic isoform X1 | |
| Cgla06g001871 | Phorbol-ester/DAG-type domain-containing protein | |
| Cgla06g002771 | Putative α-mannosidase I MNS5 | |
| Cgla06g002772 | Unknown function | |
| Cgla07g000244 | Pentatricopeptide repeat-containing protein, chloroplastic | |
| Cgla07g000433 | START domain-containing protein |
| Kz ID | Compounds | Class | The Province from Which Pepper Accessions with the Highest Characteristic Metabolites Come |
|---|---|---|---|
| kz000449 | p-Coumaroyl agmatine | Alkaloids | Guizhou |
| kz002764 | Cyanidin 3-Sambubioside | Anthocyanins | |
| kz000102 | 9-HpOTrE | Free fatty acids | |
| kz001232 | 13-HOTrE(r) | Free fatty acids | |
| kz001825 | p-coumaroyl malic acid | Phenolic acids | |
| kz003944 | Nordihydrocapsiate | Phenolic acids | |
| kz000454 | Fer-agmatine | Alkaloids | Sichuan |
| kz001318 | Quinic acid | Organic acids | |
| kz001087 | Maslinic acid | Terpenoids | |
| kz000075 | Caffeic aldehyde | Phenolic acids | Yunnan |
| kz003946 | Dihydrocaffeoylglucose | Phenolic acids | |
| kz002663 | Abscisic acid | Organic acids | |
| kz003925 | N’-p-Coumaroyl agmatine-glucoside | Alkaloids | |
| kz000036 | N-(4′-O-glycosyl)-p-coumaroyl agmatine | Alkaloids | |
| kz000257 | Ribulose-5-phosphate | Saccharides |
| Gene | Function Annotation | The Province from Which Pepper Accessions with the Highest Characteristic Metabolites Come |
|---|---|---|
| Cgla10g000889 | 3-deoxy-manno-octulosonate cytidylyltransferase, mitochondrial | Guizhou |
| Cgla02g003480 | Protein kinase domain-containing protein | |
| Cgla02g003546 | Putative pentatricopeptide repeat-containing protein At3g01580 | |
| Cgla06g001432 | gibberellin 3-β-dioxygenase 1-like | |
| Cgla08g002556 | Uncharacterized protein | |
| Cgla09g000263 | Uncharacterized protein | |
| Cgla10g000890 | DUF547 domain-containing protein | |
| Cgla10g002401 | GH10 domain-containing protein | |
| Cgla11g001920 | protein phytochrome kinase substrate 1 | |
| Cgla10g000891 | DUF547 domain-containing protein | Sichuan |
| Cgla03g000465 | Peptidase_M16_C domain-containing protein | Yunnan |
| Cgla06g000465 | CRAL-TRIO domain-containing protein | |
| Cgla06g002658 | Dehydroascorbate reductase | |
| Cgla06g002963 | Serine/threonine-protein kinase HT1-like (Fragment) | |
| Cgla10g000189 | Unknown function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yang, C.; He, J.; Zhang, L.; Xing, X.; Zhang, K.; Yu, H.; Zhang, Z.; Wu, H.; Cheng, F.; et al. Characterization of Metabolites and Transcriptome of Pepper Accessions from Four Southern Provinces of China. Genes 2025, 16, 137. https://doi.org/10.3390/genes16020137
Liu Z, Yang C, He J, Zhang L, Xing X, Zhang K, Yu H, Zhang Z, Wu H, Cheng F, et al. Characterization of Metabolites and Transcriptome of Pepper Accessions from Four Southern Provinces of China. Genes. 2025; 16(2):137. https://doi.org/10.3390/genes16020137
Chicago/Turabian StyleLiu, Zhuo, Chuangchuang Yang, Jianwen He, Lingkui Zhang, Xiaolin Xing, Kang Zhang, Hailong Yu, Zhenghai Zhang, Huamao Wu, Feng Cheng, and et al. 2025. "Characterization of Metabolites and Transcriptome of Pepper Accessions from Four Southern Provinces of China" Genes 16, no. 2: 137. https://doi.org/10.3390/genes16020137
APA StyleLiu, Z., Yang, C., He, J., Zhang, L., Xing, X., Zhang, K., Yu, H., Zhang, Z., Wu, H., Cheng, F., Cao, Y., & Wang, L. (2025). Characterization of Metabolites and Transcriptome of Pepper Accessions from Four Southern Provinces of China. Genes, 16(2), 137. https://doi.org/10.3390/genes16020137






